15cfb536f074de299a8630bd84bf1440.ppt
- Количество слайдов: 61
 Malignant Neoplasm of Lung Dr Edit Csada 13. 09. 2017. 1
Malignant Neoplasm of Lung Dr Edit Csada 13. 09. 2017. 1
 Epidemiology Globocan 2012. • Lung cancer is the most frequent malignant disease New cases: 1, 82 million/year (13%) Mortality: 1, 59 million/year Most frequent cause of death amoung malignant diseases>colon+prostate+breast Europe: ~1000 death/day Lung cancer fatality: breast cancer fatality: Male/female: 2, 4/1 1, 59/1, 82 = 0, 87 0, 35 2
Epidemiology Globocan 2012. • Lung cancer is the most frequent malignant disease New cases: 1, 82 million/year (13%) Mortality: 1, 59 million/year Most frequent cause of death amoung malignant diseases>colon+prostate+breast Europe: ~1000 death/day Lung cancer fatality: breast cancer fatality: Male/female: 2, 4/1 1, 59/1, 82 = 0, 87 0, 35 2
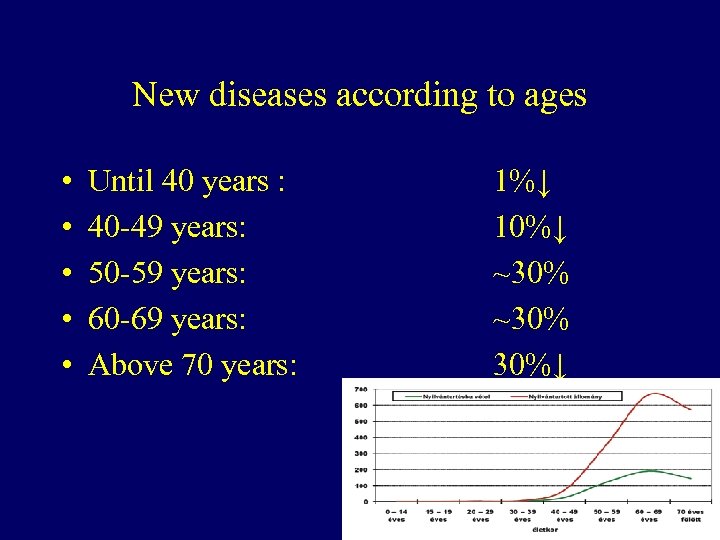 New diseases according to ages • • • Until 40 years : 40 -49 years: 50 -59 years: 60 -69 years: Above 70 years: 1%↓ 10%↓ ~30% 30%↓ 3
New diseases according to ages • • • Until 40 years : 40 -49 years: 50 -59 years: 60 -69 years: Above 70 years: 1%↓ 10%↓ ~30% 30%↓ 3
 Etiologic factors Smoking Athmospheric pollution Ionisation Occupational factors asbestos, radon, etc Other lung diseases tb, COPD, ILD Genetic events 4
Etiologic factors Smoking Athmospheric pollution Ionisation Occupational factors asbestos, radon, etc Other lung diseases tb, COPD, ILD Genetic events 4
 Smoking • 400 chemical materials • 60 carcinogens • Gas and particulate phase – Nitrosamines, aromatic amines, benzopyrene, CO 2, aldehids, nicotin, free radicals • Pack-year 5
Smoking • 400 chemical materials • 60 carcinogens • Gas and particulate phase – Nitrosamines, aromatic amines, benzopyrene, CO 2, aldehids, nicotin, free radicals • Pack-year 5
 Smoking and Lung Cancer • 85 -90% of lung cancer patients are smokers • Damages of 10 -15 gens have role in the development of lung cancer • 86% of smokers have damages of these gens 6
Smoking and Lung Cancer • 85 -90% of lung cancer patients are smokers • Damages of 10 -15 gens have role in the development of lung cancer • 86% of smokers have damages of these gens 6
 Molecular biology of lung cancer • Genetic damages – Deletion – Mutations – Amplifications Tumor suppressor gen injury (p 53, RB 1) Inhibation of proliferation Repair mechanism Induction of apoptosis Protooncogen abnormalities Autocrine growth factors membran receptors transcription factors 7
Molecular biology of lung cancer • Genetic damages – Deletion – Mutations – Amplifications Tumor suppressor gen injury (p 53, RB 1) Inhibation of proliferation Repair mechanism Induction of apoptosis Protooncogen abnormalities Autocrine growth factors membran receptors transcription factors 7
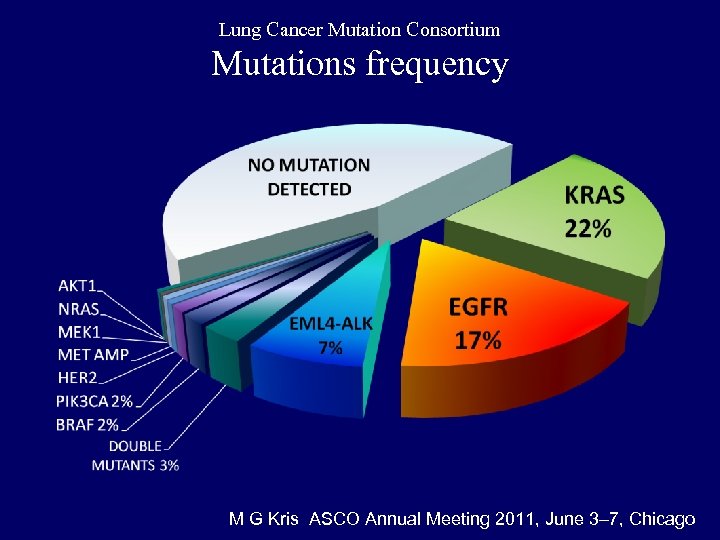 Lung Cancer Mutation Consortium Mutations frequency M G Kris ASCO Annual Meeting 2011, June 3– 7, Chicago
Lung Cancer Mutation Consortium Mutations frequency M G Kris ASCO Annual Meeting 2011, June 3– 7, Chicago
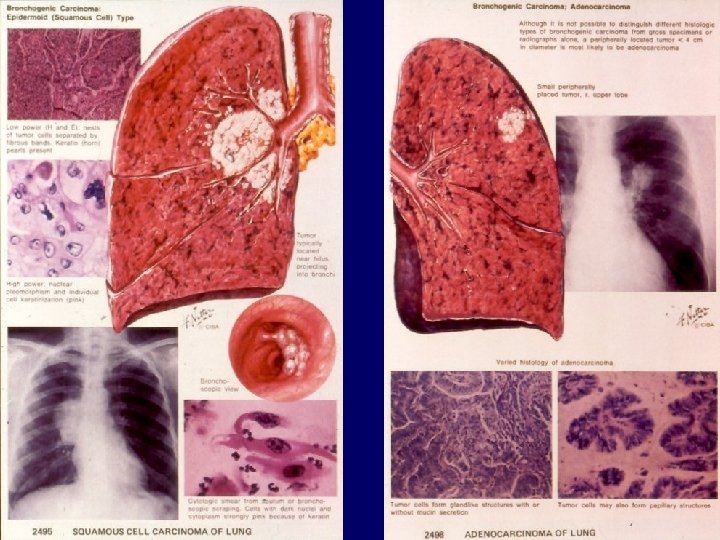
 Histology of lung cancer Non small cell lung cancer Squamous cell carcinoma (30%) Well, or less differentiated, with or without keratinisation Adenocancer (45%) acinar papillary bronchioloalveolar with mucus formation Large cell carcinoma (10%↓) clear cell giant cell 9
Histology of lung cancer Non small cell lung cancer Squamous cell carcinoma (30%) Well, or less differentiated, with or without keratinisation Adenocancer (45%) acinar papillary bronchioloalveolar with mucus formation Large cell carcinoma (10%↓) clear cell giant cell 9
 10
10
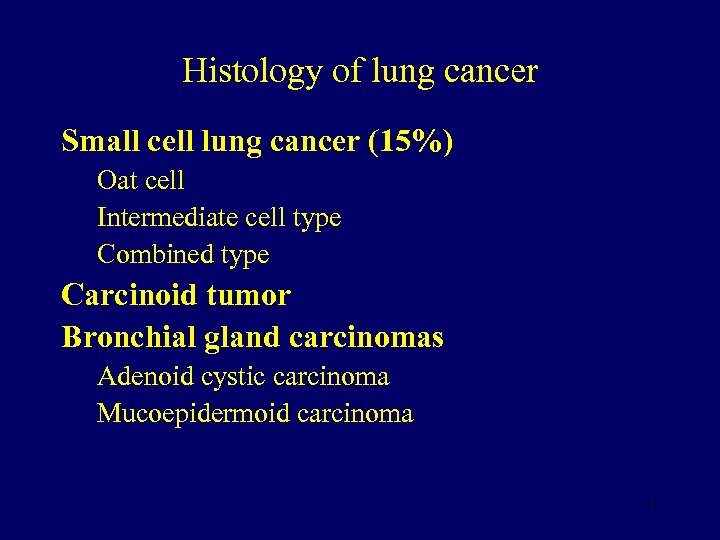 Histology of lung cancer Small cell lung cancer (15%) Oat cell Intermediate cell type Combined type Carcinoid tumor Bronchial gland carcinomas Adenoid cystic carcinoma Mucoepidermoid carcinoma 11
Histology of lung cancer Small cell lung cancer (15%) Oat cell Intermediate cell type Combined type Carcinoid tumor Bronchial gland carcinomas Adenoid cystic carcinoma Mucoepidermoid carcinoma 11
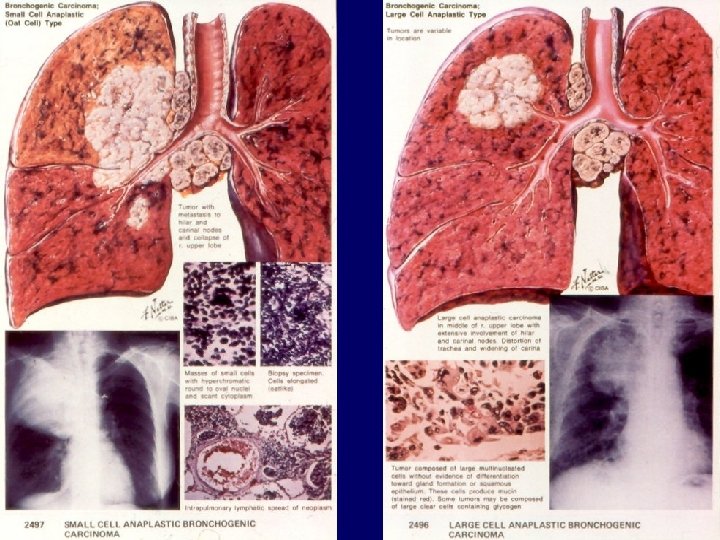 12
12
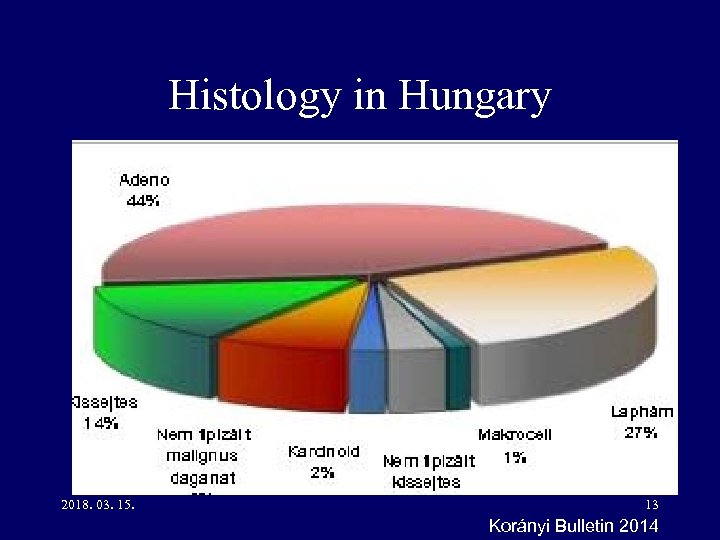 Histology in Hungary 2018. 03. 15. 13 Korányi Bulletin 2014
Histology in Hungary 2018. 03. 15. 13 Korányi Bulletin 2014
 Pathological prognostic factors • • TNM Histology Histological differentiation Invading vessels Necrosis Proliferation activity Prognostic proteins 14
Pathological prognostic factors • • TNM Histology Histological differentiation Invading vessels Necrosis Proliferation activity Prognostic proteins 14
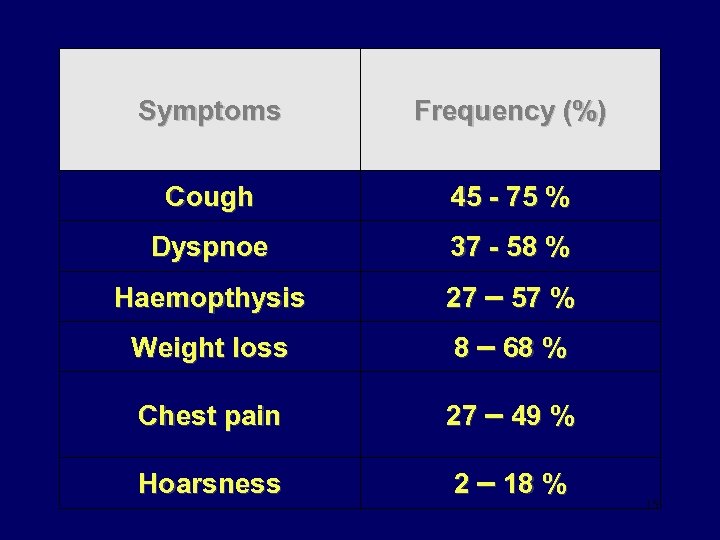 Symptoms Frequency (%) Cough 45 - 75 % Dyspnoe 37 - 58 % Haemopthysis 27 – 57 % Weight loss 8 – 68 % Chest pain 27 – 49 % Hoarsness 2 – 18 % 15
Symptoms Frequency (%) Cough 45 - 75 % Dyspnoe 37 - 58 % Haemopthysis 27 – 57 % Weight loss 8 – 68 % Chest pain 27 – 49 % Hoarsness 2 – 18 % 15
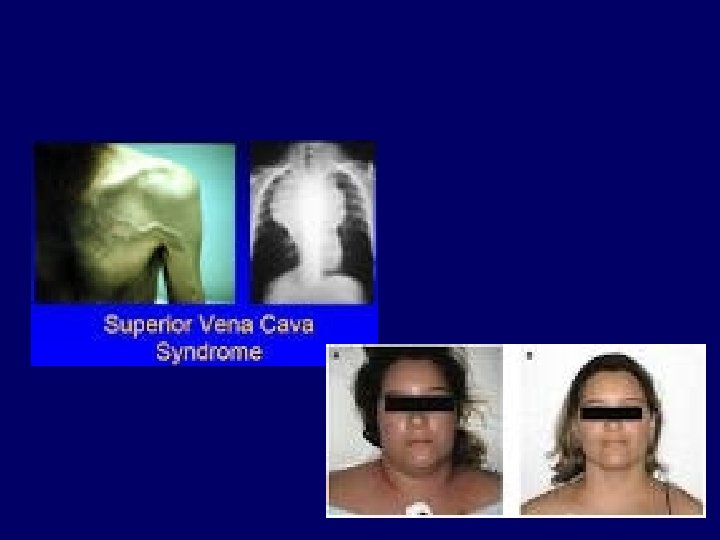
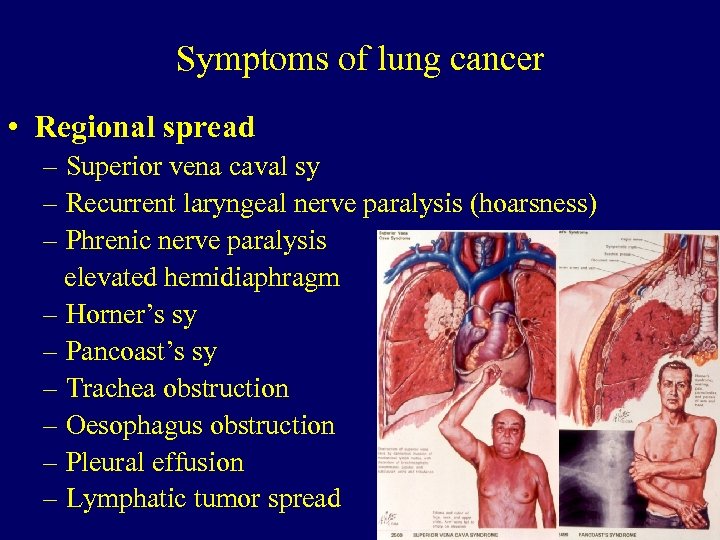 Symptoms of lung cancer • Regional spread – Superior vena caval sy – Recurrent laryngeal nerve paralysis (hoarsness) – Phrenic nerve paralysis elevated hemidiaphragm – Horner’s sy – Pancoast’s sy – Trachea obstruction – Oesophagus obstruction – Pleural effusion – Lymphatic tumor spread 16
Symptoms of lung cancer • Regional spread – Superior vena caval sy – Recurrent laryngeal nerve paralysis (hoarsness) – Phrenic nerve paralysis elevated hemidiaphragm – Horner’s sy – Pancoast’s sy – Trachea obstruction – Oesophagus obstruction – Pleural effusion – Lymphatic tumor spread 16
 17
17
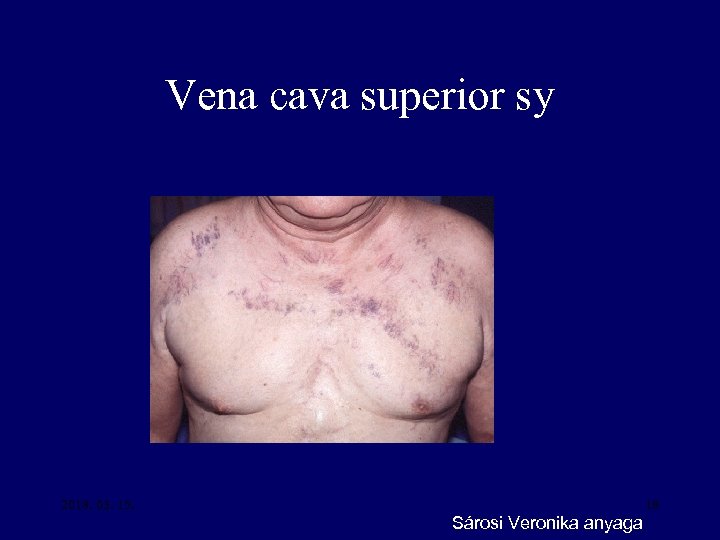 Vena cava superior sy 2018. 03. 15. 18 Sárosi Veronika anyaga
Vena cava superior sy 2018. 03. 15. 18 Sárosi Veronika anyaga
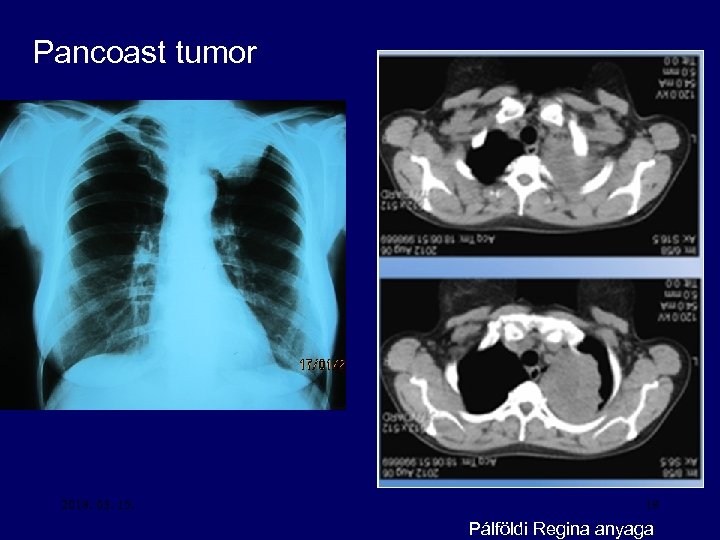 Pancoast tumor 2018. 03. 15. 19 Pálföldi Regina anyaga
Pancoast tumor 2018. 03. 15. 19 Pálföldi Regina anyaga
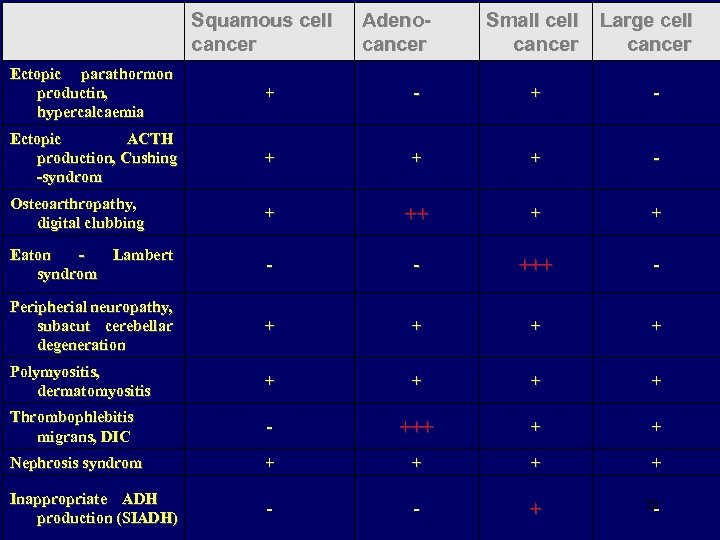 Squamous cell cancer Adenocancer Small cell cancer Large cell cancer Ectopic parathormon productin, hypercalcaemia + - Ectopic ACTH production, Cushing -syndrom + + + - Osteoarthropathy, digital clubbing + ++ + + Eaton Lambert syndrom - - +++ - Peripherial neuropathy, subacut cerebellar degeneration + + Polymyositis, dermatomyositis + + Thrombophlebitis migrans, DIC - +++ + + Nephrosis syndrom + + Inappropriate ADH production (SIADH) - - + 20 -
Squamous cell cancer Adenocancer Small cell cancer Large cell cancer Ectopic parathormon productin, hypercalcaemia + - Ectopic ACTH production, Cushing -syndrom + + + - Osteoarthropathy, digital clubbing + ++ + + Eaton Lambert syndrom - - +++ - Peripherial neuropathy, subacut cerebellar degeneration + + Polymyositis, dermatomyositis + + Thrombophlebitis migrans, DIC - +++ + + Nephrosis syndrom + + Inappropriate ADH production (SIADH) - - + 20 -
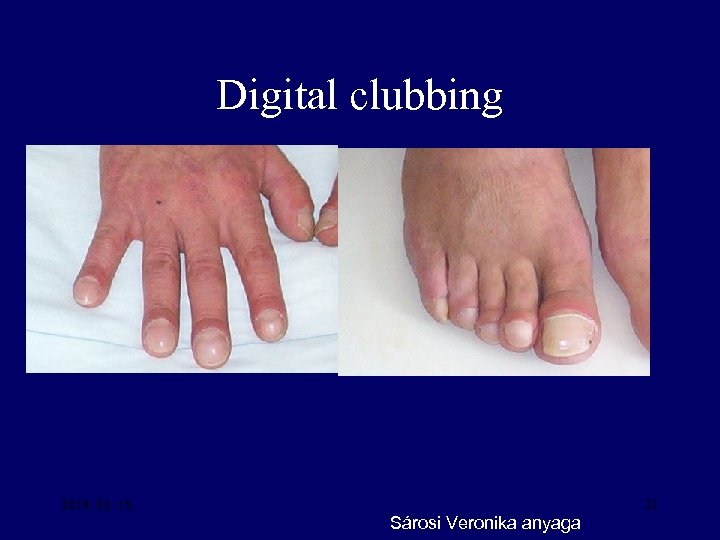 Digital clubbing 2018. 03. 15. 21 Sárosi Veronika anyaga
Digital clubbing 2018. 03. 15. 21 Sárosi Veronika anyaga
 Diagnostic procedures • • Imaging technics Endoscopy Pathology Laboratory tests 22
Diagnostic procedures • • Imaging technics Endoscopy Pathology Laboratory tests 22
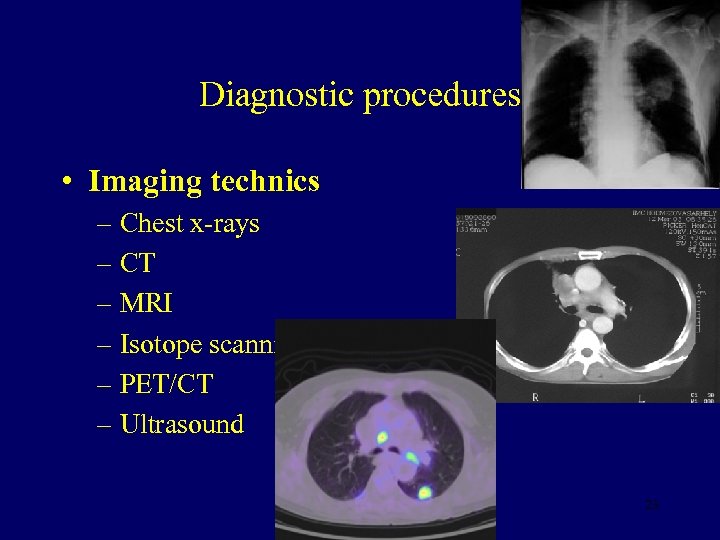 Diagnostic procedures • Imaging technics – Chest x-rays – CT – MRI – Isotope scanning – PET/CT – Ultrasound 23
Diagnostic procedures • Imaging technics – Chest x-rays – CT – MRI – Isotope scanning – PET/CT – Ultrasound 23
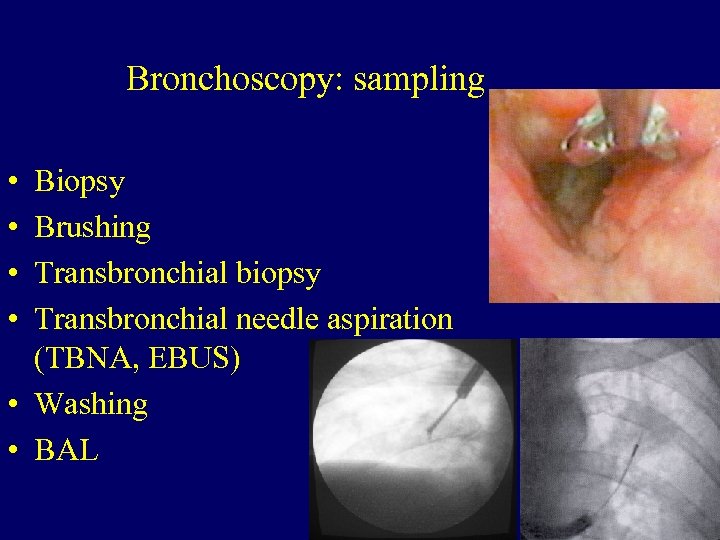 Bronchoscopy: sampling • • Biopsy Brushing Transbronchial biopsy Transbronchial needle aspiration (TBNA, EBUS) • Washing • BAL 24
Bronchoscopy: sampling • • Biopsy Brushing Transbronchial biopsy Transbronchial needle aspiration (TBNA, EBUS) • Washing • BAL 24
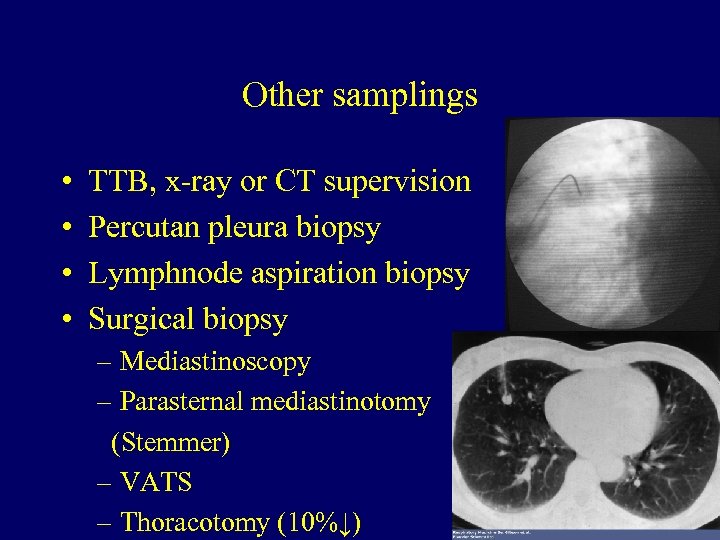 Other samplings • • TTB, x-ray or CT supervision Percutan pleura biopsy Lymphnode aspiration biopsy Surgical biopsy – Mediastinoscopy – Parasternal mediastinotomy (Stemmer) – VATS – Thoracotomy (10%↓) 25
Other samplings • • TTB, x-ray or CT supervision Percutan pleura biopsy Lymphnode aspiration biopsy Surgical biopsy – Mediastinoscopy – Parasternal mediastinotomy (Stemmer) – VATS – Thoracotomy (10%↓) 25
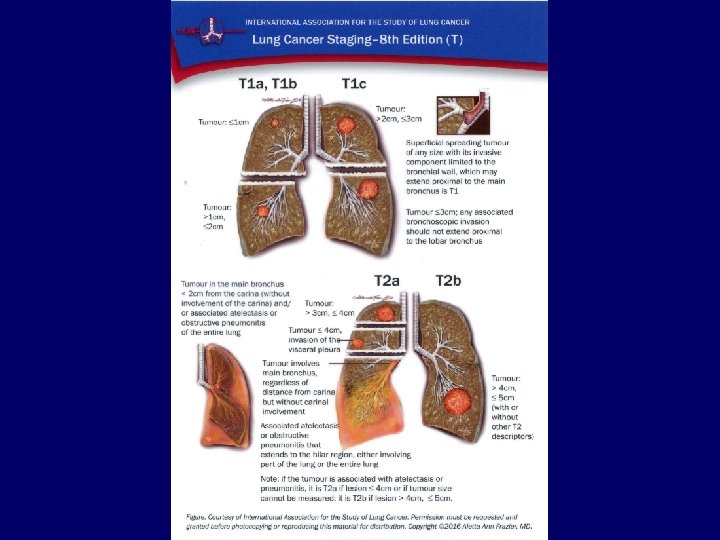
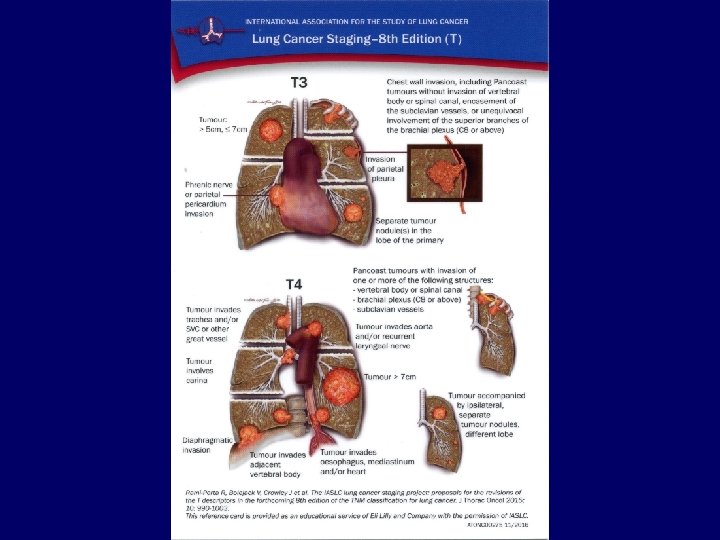
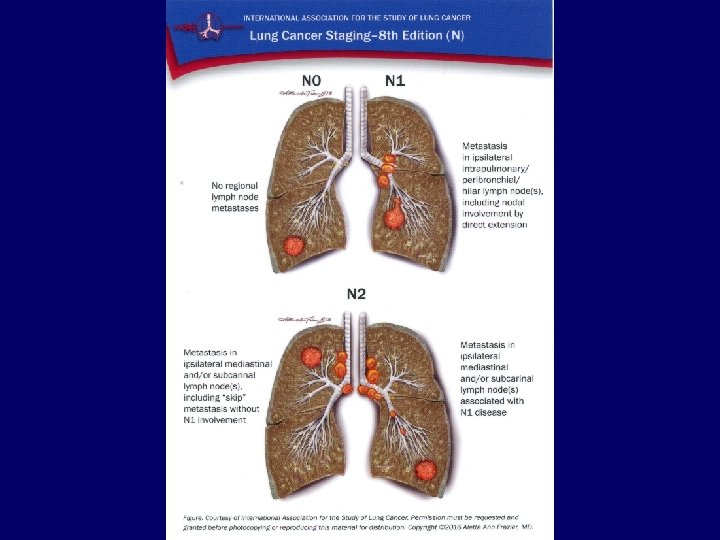
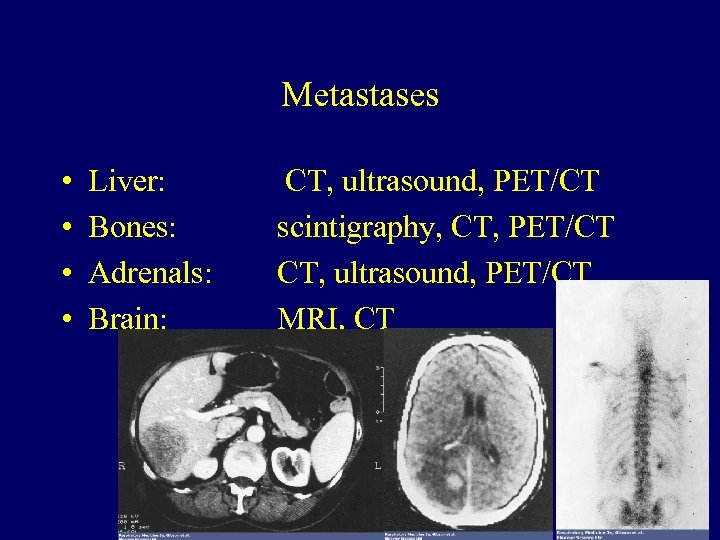 Metastases • • Liver: Bones: Adrenals: Brain: CT, ultrasound, PET/CT scintigraphy, CT, PET/CT CT, ultrasound, PET/CT MRI, CT 34
Metastases • • Liver: Bones: Adrenals: Brain: CT, ultrasound, PET/CT scintigraphy, CT, PET/CT CT, ultrasound, PET/CT MRI, CT 34
 Prognostic factors • Poor performance status – Karnofsky, WHO ECOG • • Weight loss, more than 10% Elevated LDH Elevated tumormarker (CEA, NSE, SCC) Old age 35
Prognostic factors • Poor performance status – Karnofsky, WHO ECOG • • Weight loss, more than 10% Elevated LDH Elevated tumormarker (CEA, NSE, SCC) Old age 35
 Performance status Grade ECOG 0 Fully active, able to carry on all pre-disease performance without restriction 1 Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e. g. , light house work, office work 2 Ambulatory and capable of all selfcare but unable to carry out any work activities. Up and about more than 50% of waking hours 3 Capable of only limited selfcare, confined to bed or chair more than 50% of waking hours 4 Completely disabled. Cannot carry on any selfcare. Totally confined to bed or chair 5 Dead 36
Performance status Grade ECOG 0 Fully active, able to carry on all pre-disease performance without restriction 1 Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e. g. , light house work, office work 2 Ambulatory and capable of all selfcare but unable to carry out any work activities. Up and about more than 50% of waking hours 3 Capable of only limited selfcare, confined to bed or chair more than 50% of waking hours 4 Completely disabled. Cannot carry on any selfcare. Totally confined to bed or chair 5 Dead 36
 Performance status Karnofsky scale Description 100 Normal; no complaints; no evidence of disease 90 Able to carry out normal activity; minor signs or symptoms of disease 80 Normal activity with effort; some signs or symptoms of disease 70 Cares for self; unable to carry on normal activity or do active work 60 Requires occasional assistance, but is able to care for most of his/her needs 50 Requires considerable assistance and frequent medical care 40 Disabled; requires special care and assistance 30 20 Severely disabled; hospitalization is indicated although death not imminent Very sick; hospitalization necessary, active supportive treatment necessary 10 Moribund; fatal processes progressing rapidly 0 Dead 37
Performance status Karnofsky scale Description 100 Normal; no complaints; no evidence of disease 90 Able to carry out normal activity; minor signs or symptoms of disease 80 Normal activity with effort; some signs or symptoms of disease 70 Cares for self; unable to carry on normal activity or do active work 60 Requires occasional assistance, but is able to care for most of his/her needs 50 Requires considerable assistance and frequent medical care 40 Disabled; requires special care and assistance 30 20 Severely disabled; hospitalization is indicated although death not imminent Very sick; hospitalization necessary, active supportive treatment necessary 10 Moribund; fatal processes progressing rapidly 0 Dead 37
 Defining treatment • Tumor specific factors – TNM stage – Histology – Molecular features • Patient specific factors – Age – Performance status – Concomitant diseases – gender, etnicity, smoking Based on these factors multidisciplinary tumour board decides on curative-palliative therapy
Defining treatment • Tumor specific factors – TNM stage – Histology – Molecular features • Patient specific factors – Age – Performance status – Concomitant diseases – gender, etnicity, smoking Based on these factors multidisciplinary tumour board decides on curative-palliative therapy
 Multimodality treatment Surgery Radiotherapy Chemotherapy Molecular target therapy Immunoncology! Supportive therapy
Multimodality treatment Surgery Radiotherapy Chemotherapy Molecular target therapy Immunoncology! Supportive therapy
 Surgery • Type of surgical procedure depends on – – Staging patient’s performance status cardiopulmonal function comorbidities. • Aim is radical resection • Sublobar resection may have a role in very early diseases. • Thoracotomy • Video assisted thoracoscopy (VATS) 40
Surgery • Type of surgical procedure depends on – – Staging patient’s performance status cardiopulmonal function comorbidities. • Aim is radical resection • Sublobar resection may have a role in very early diseases. • Thoracotomy • Video assisted thoracoscopy (VATS) 40
 Surgery • Absolute contraindications: – haematogen metastases in the lungs – pleuritis carcinomatosa – III. b stage disease – multiplex distant metastases • Relative contraindications 41
Surgery • Absolute contraindications: – haematogen metastases in the lungs – pleuritis carcinomatosa – III. b stage disease – multiplex distant metastases • Relative contraindications 41
 Surgery (20 -25%) • • • NSCLC IIIA stage Lobectomy, pulmonectomy, sleeve lobectomy, extensive resection – radical Segmentectomy, wedge resection – mostly non radical Early stage SCLC, as part of combined therapy Carina resection? Before surgery: lung function, Ecg, functional evaluation 42
Surgery (20 -25%) • • • NSCLC IIIA stage Lobectomy, pulmonectomy, sleeve lobectomy, extensive resection – radical Segmentectomy, wedge resection – mostly non radical Early stage SCLC, as part of combined therapy Carina resection? Before surgery: lung function, Ecg, functional evaluation 42
 Radiation therapy • NSCLC: III. A, III. B stage • SCLC: combined with chemotherapy • Inoperable patient with resecable disease • Resected N 2 disease, in combined treatment • Metastasis palliation • Pancoast’s tu • Brain metastasis (stereotactic, whole brain) • PCI • Brachytherapy Radiochemotherapy! 43
Radiation therapy • NSCLC: III. A, III. B stage • SCLC: combined with chemotherapy • Inoperable patient with resecable disease • Resected N 2 disease, in combined treatment • Metastasis palliation • Pancoast’s tu • Brain metastasis (stereotactic, whole brain) • PCI • Brachytherapy Radiochemotherapy! 43
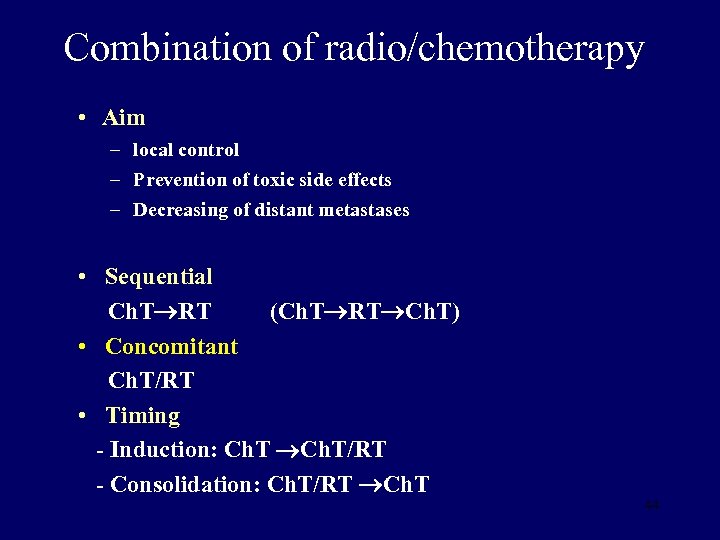 Combination of radio/chemotherapy • Aim – local control – Prevention of toxic side effects – Decreasing of distant metastases • Sequential Ch. T RT (Ch. T RT Ch. T) • Concomitant Ch. T/RT • Timing - Induction: Ch. T/RT - Consolidation: Ch. T/RT Ch. T 44
Combination of radio/chemotherapy • Aim – local control – Prevention of toxic side effects – Decreasing of distant metastases • Sequential Ch. T RT (Ch. T RT Ch. T) • Concomitant Ch. T/RT • Timing - Induction: Ch. T/RT - Consolidation: Ch. T/RT Ch. T 44
 Chemotherapy • Neoadjuvant treatment – Before surgery IIIa stage • Adjuvant treatment – After surgery II-IIIa stage • First-, second-, thirdline…. . – IIIb, IV stage 45
Chemotherapy • Neoadjuvant treatment – Before surgery IIIa stage • Adjuvant treatment – After surgery II-IIIa stage • First-, second-, thirdline…. . – IIIb, IV stage 45
 46
46
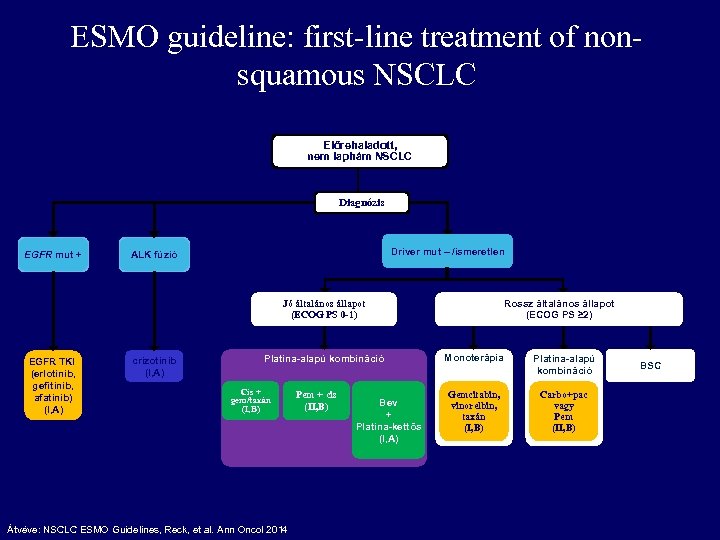 ESMO guideline: first-line treatment of nonsquamous NSCLC Előrehaladott, nem laphám NSCLC Diagnózis EGFR mut + Driver mut – /ismeretlen ALK fúzió Rossz általános állapot (ECOG PS ≥ 2) Jó általános állapot (ECOG PS 0 -1) EGFR TKI (erlotinib, gefitinib, afatinib) (I, A) crizotinib (I, A) Platina-alapú kombináció Cis + gem/taxán (I, B) Átvéve: NSCLC ESMO Guidelines, Reck, et al. Ann Oncol 2014 Pem + cis (II, B) Bev + Platina-kettős (I, A) Monoterápia Platina-alapú kombináció Gemcitabin, vinorelbin, taxán (I, B) Carbo+pac vagy Pem (II, B) BSC
ESMO guideline: first-line treatment of nonsquamous NSCLC Előrehaladott, nem laphám NSCLC Diagnózis EGFR mut + Driver mut – /ismeretlen ALK fúzió Rossz általános állapot (ECOG PS ≥ 2) Jó általános állapot (ECOG PS 0 -1) EGFR TKI (erlotinib, gefitinib, afatinib) (I, A) crizotinib (I, A) Platina-alapú kombináció Cis + gem/taxán (I, B) Átvéve: NSCLC ESMO Guidelines, Reck, et al. Ann Oncol 2014 Pem + cis (II, B) Bev + Platina-kettős (I, A) Monoterápia Platina-alapú kombináció Gemcitabin, vinorelbin, taxán (I, B) Carbo+pac vagy Pem (II, B) BSC
 Chemotherapy of NSCLC • First-line – – – Cis-, carboplatin-gemcitabin Cis-, carboplatin-paclitaxel Cisplatin-docetaxel Cisplatin-vinorelbin Cisplatin-pemeterexed (non squamous c) Doublet+bevacizumab(adenoc) • Second-line – Pemetrexed – docetaxel 48
Chemotherapy of NSCLC • First-line – – – Cis-, carboplatin-gemcitabin Cis-, carboplatin-paclitaxel Cisplatin-docetaxel Cisplatin-vinorelbin Cisplatin-pemeterexed (non squamous c) Doublet+bevacizumab(adenoc) • Second-line – Pemetrexed – docetaxel 48
 Molecular target therapy • EGFR tirosin kinase inhibitors – erlotinib (Tarceva) – gefinitib (Iressa) – Afatinib (Giotrif) • Angiogenesis inhibitor – bevacizumab (Avastin) • Alk-EML 4 fusion gene inhibitor – Crizotinib (Xalkori) – Ceritinib – alectinib 49
Molecular target therapy • EGFR tirosin kinase inhibitors – erlotinib (Tarceva) – gefinitib (Iressa) – Afatinib (Giotrif) • Angiogenesis inhibitor – bevacizumab (Avastin) • Alk-EML 4 fusion gene inhibitor – Crizotinib (Xalkori) – Ceritinib – alectinib 49
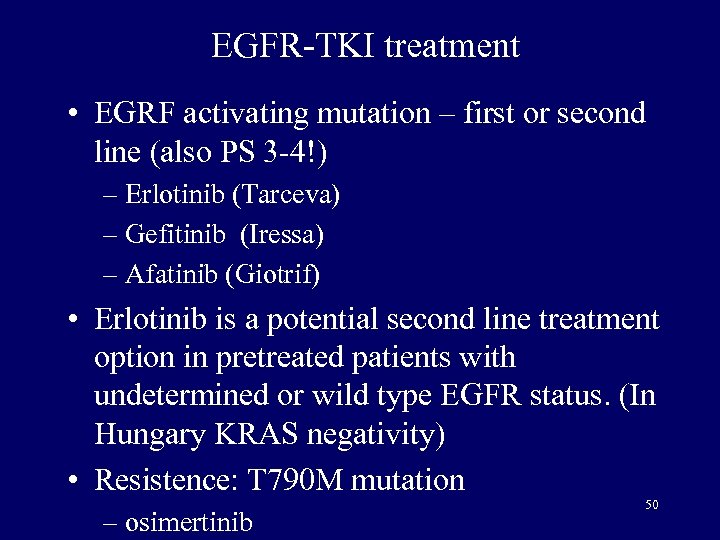 EGFR-TKI treatment • EGRF activating mutation – first or second line (also PS 3 -4!) – Erlotinib (Tarceva) – Gefitinib (Iressa) – Afatinib (Giotrif) • Erlotinib is a potential second line treatment option in pretreated patients with undetermined or wild type EGFR status. (In Hungary KRAS negativity) • Resistence: T 790 M mutation – osimertinib 50
EGFR-TKI treatment • EGRF activating mutation – first or second line (also PS 3 -4!) – Erlotinib (Tarceva) – Gefitinib (Iressa) – Afatinib (Giotrif) • Erlotinib is a potential second line treatment option in pretreated patients with undetermined or wild type EGFR status. (In Hungary KRAS negativity) • Resistence: T 790 M mutation – osimertinib 50
 Maintenance treatment Bevacizumab Pemetrexed erlotinib
Maintenance treatment Bevacizumab Pemetrexed erlotinib
 Immuntherapy • Immun check point inhibitors – PD-1 and PDL-1 inhibitors • Nivolumab: Opdivo – 2. and 3. line • Pembrolizumab: Keytruda – (1. ) and 2. line 52
Immuntherapy • Immun check point inhibitors – PD-1 and PDL-1 inhibitors • Nivolumab: Opdivo – 2. and 3. line • Pembrolizumab: Keytruda – (1. ) and 2. line 52
 Surgery in SCLC • I/A-I/B: resection • Postoperative chemotherapy • Adjuvant irradiation in positive node status • Induction chemotherapy
Surgery in SCLC • I/A-I/B: resection • Postoperative chemotherapy • Adjuvant irradiation in positive node status • Induction chemotherapy
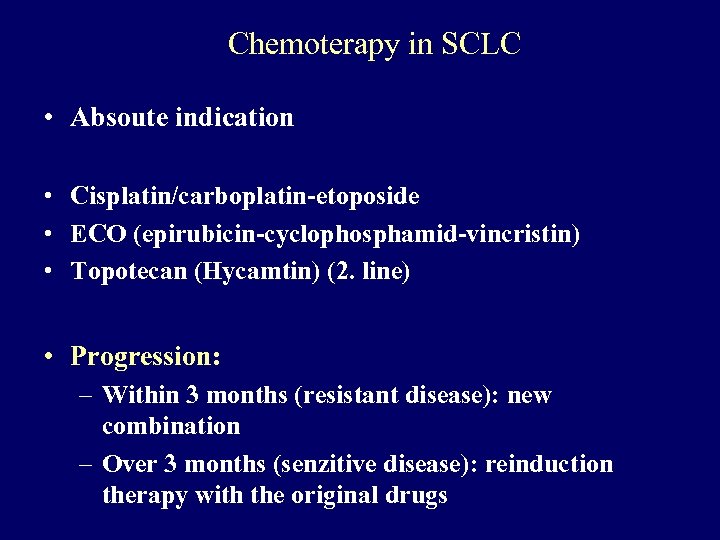 Chemoterapy in SCLC • Absoute indication • Cisplatin/carboplatin-etoposide • ECO (epirubicin-cyclophosphamid-vincristin) • Topotecan (Hycamtin) (2. line) • Progression: – Within 3 months (resistant disease): new combination – Over 3 months (senzitive disease): reinduction therapy with the original drugs
Chemoterapy in SCLC • Absoute indication • Cisplatin/carboplatin-etoposide • ECO (epirubicin-cyclophosphamid-vincristin) • Topotecan (Hycamtin) (2. line) • Progression: – Within 3 months (resistant disease): new combination – Over 3 months (senzitive disease): reinduction therapy with the original drugs
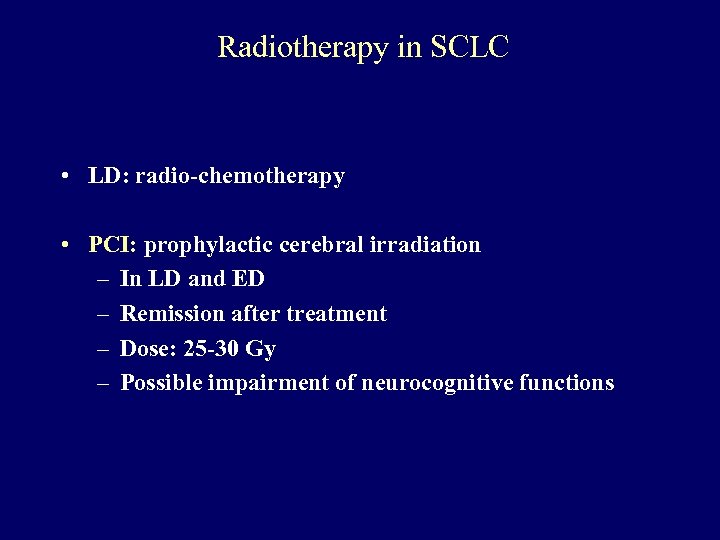 Radiotherapy in SCLC • LD: radio-chemotherapy • PCI: prophylactic cerebral irradiation – In LD and ED – Remission after treatment – Dose: 25 -30 Gy – Possible impairment of neurocognitive functions
Radiotherapy in SCLC • LD: radio-chemotherapy • PCI: prophylactic cerebral irradiation – In LD and ED – Remission after treatment – Dose: 25 -30 Gy – Possible impairment of neurocognitive functions
 Supportive treatment • Pain control – WHO suggestion • Adverse events control • Thrombosis prophylaxis • Malignant pleural fluid treatment • Bone metastases treatment • Endobronchial palliation • Nutrition 56
Supportive treatment • Pain control – WHO suggestion • Adverse events control • Thrombosis prophylaxis • Malignant pleural fluid treatment • Bone metastases treatment • Endobronchial palliation • Nutrition 56
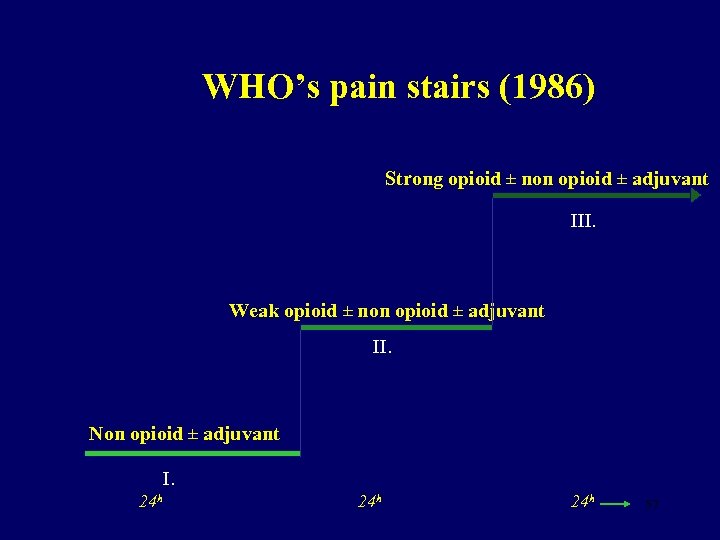 WHO’s pain stairs (1986) Strong opioid ± non opioid ± adjuvant III. Weak opioid ± non opioid ± adjuvant II. Non opioid ± adjuvant I. 24 h 24 h 57
WHO’s pain stairs (1986) Strong opioid ± non opioid ± adjuvant III. Weak opioid ± non opioid ± adjuvant II. Non opioid ± adjuvant I. 24 h 24 h 57
 Supportive treatment • Pain control • Adverse events control – febrile neutopenia – Anaemia (erythropoetin) – Nausea, vomiting • Thrombosis prophylaxis • Malignant pleural fluid treatment – pleurodesis • Bone metastases treatment – bisphonat • Endobronchial palliation 58
Supportive treatment • Pain control • Adverse events control – febrile neutopenia – Anaemia (erythropoetin) – Nausea, vomiting • Thrombosis prophylaxis • Malignant pleural fluid treatment – pleurodesis • Bone metastases treatment – bisphonat • Endobronchial palliation 58
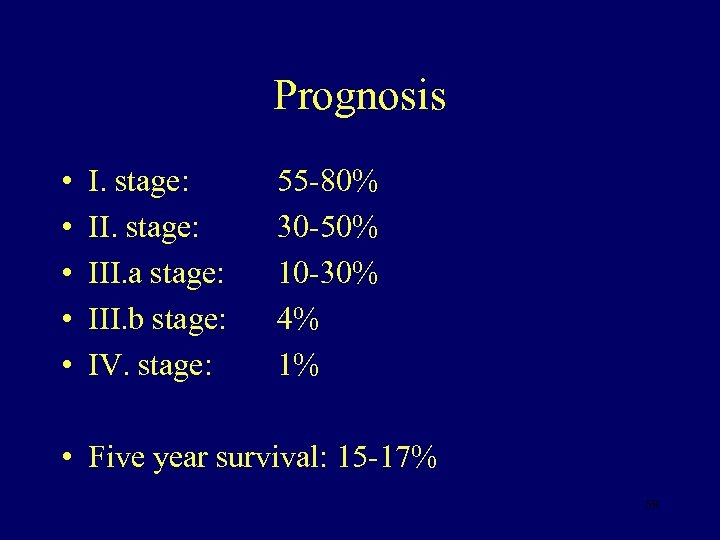 Prognosis • • • I. stage: III. a stage: III. b stage: IV. stage: 55 -80% 30 -50% 10 -30% 4% 1% • Five year survival: 15 -17% 59
Prognosis • • • I. stage: III. a stage: III. b stage: IV. stage: 55 -80% 30 -50% 10 -30% 4% 1% • Five year survival: 15 -17% 59
 Prevention • Primary – Smoking sessation • Secundary – Screening • X-ray • LDCT 60
Prevention • Primary – Smoking sessation • Secundary – Screening • X-ray • LDCT 60
 Thank you for your attention!
Thank you for your attention!


