4366337e53ce5d9ea95245fafcef33a0.ppt
- Количество слайдов: 40
 MALDI-TOF Matrix assisted laser desorption ionization – time of flight MALDI-TOF: Bringing st Bacteriology into the 21 Century Ross Davidson Ph. D, FCCM, D(ABMM) Director, Bacteriology Dept. Of Pathology & Laboratory Medicine CDHA
MALDI-TOF Matrix assisted laser desorption ionization – time of flight MALDI-TOF: Bringing st Bacteriology into the 21 Century Ross Davidson Ph. D, FCCM, D(ABMM) Director, Bacteriology Dept. Of Pathology & Laboratory Medicine CDHA
 Disclosure • I have NO affiliation, financial or otherwise, with any company whose products or devices are discussed within this presentation.
Disclosure • I have NO affiliation, financial or otherwise, with any company whose products or devices are discussed within this presentation.
 MALDI-TOF At the end of this session, participants will be able to: • Understand the principles of MALDI-TOF • Understand the application and integration of MALDI -TOF into the clinical laboratory • Describe the benefits of MALDI-TOF for patient care and potential cost savings for the laboratory • Describe potential future applications of MALDI-TOF
MALDI-TOF At the end of this session, participants will be able to: • Understand the principles of MALDI-TOF • Understand the application and integration of MALDI -TOF into the clinical laboratory • Describe the benefits of MALDI-TOF for patient care and potential cost savings for the laboratory • Describe potential future applications of MALDI-TOF
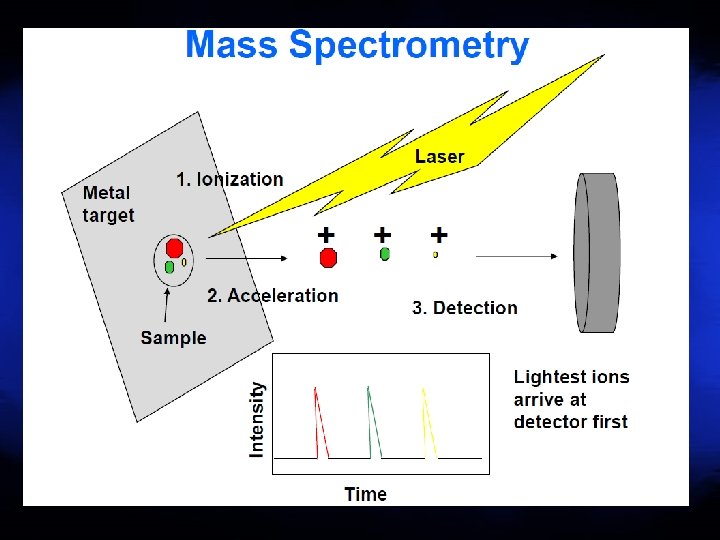
 Matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry
Matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry
 Advances in Bacterial Identification 1900 1970 2000
Advances in Bacterial Identification 1900 1970 2000
 Biochemical to MALDI-TOF Bacterial Identification • Most significant advance in Clinical Microbiology (Bacteriology) in 30 years! – Rapid and cost effective identification of bacteria directly from isolated colonies and positive culture bottles based on protein biomarkers • Protein biomarkers measured are highly expressed proteins responsible for housekeeping functions, such as ribosomal (16 S) and transcription/translation factor proteins 6 FASTER, BETTER, CHEAPER, BUT NOT PERFECT!
Biochemical to MALDI-TOF Bacterial Identification • Most significant advance in Clinical Microbiology (Bacteriology) in 30 years! – Rapid and cost effective identification of bacteria directly from isolated colonies and positive culture bottles based on protein biomarkers • Protein biomarkers measured are highly expressed proteins responsible for housekeeping functions, such as ribosomal (16 S) and transcription/translation factor proteins 6 FASTER, BETTER, CHEAPER, BUT NOT PERFECT!
 Conventional ID vs MALDI • • Monday, 12 pm, Mr. J’s blood culture flags positive Bottle removed, gram stain /culture prepared Gram negative rods seen, floor called at 1: 10 pm 3 pm – Mr. J started on Ceftriaxone • Tuesday, 10: 30 am P. aeruginosa identified • Floor called 10: 45 am • Mr J started on Pip/tazo • MALDI ID would have seen Mr J on appropriate anti. Pseudomonal therapy 20 -24 hours earlier
Conventional ID vs MALDI • • Monday, 12 pm, Mr. J’s blood culture flags positive Bottle removed, gram stain /culture prepared Gram negative rods seen, floor called at 1: 10 pm 3 pm – Mr. J started on Ceftriaxone • Tuesday, 10: 30 am P. aeruginosa identified • Floor called 10: 45 am • Mr J started on Pip/tazo • MALDI ID would have seen Mr J on appropriate anti. Pseudomonal therapy 20 -24 hours earlier
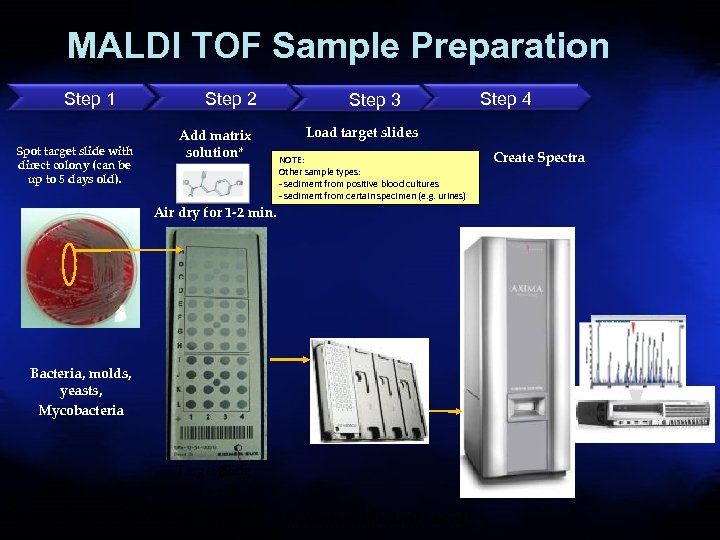 MALDI TOF Sample Preparation Step 1 Spot target slide with direct colony (can be up to 5 days old). Step 2 Add matrix solution* Step 3 Step 4 Load target slides NOTE: Other sample types: - sediment from positive blood cultures - sediment from certain specimen (e. g. urines) Air dry for 1 -2 min. Bacteria, molds, yeasts, Mycobacteria Target 8 Slide 48 wells Matrix Solution: (0. 5 µl -cyano-4 -hydroxycinnamic acid) Create Spectra
MALDI TOF Sample Preparation Step 1 Spot target slide with direct colony (can be up to 5 days old). Step 2 Add matrix solution* Step 3 Step 4 Load target slides NOTE: Other sample types: - sediment from positive blood cultures - sediment from certain specimen (e. g. urines) Air dry for 1 -2 min. Bacteria, molds, yeasts, Mycobacteria Target 8 Slide 48 wells Matrix Solution: (0. 5 µl -cyano-4 -hydroxycinnamic acid) Create Spectra
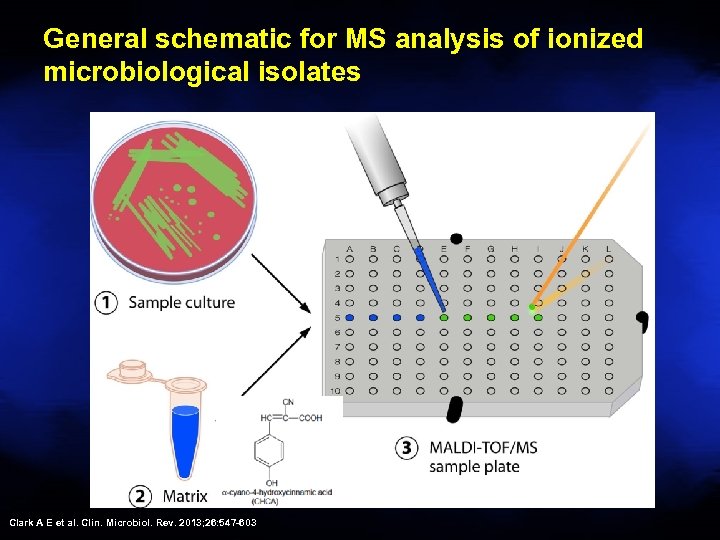 General schematic for MS analysis of ionized microbiological isolates Clark A E et al. Clin. Microbiol. Rev. 2013; 26: 547 -603
General schematic for MS analysis of ionized microbiological isolates Clark A E et al. Clin. Microbiol. Rev. 2013; 26: 547 -603
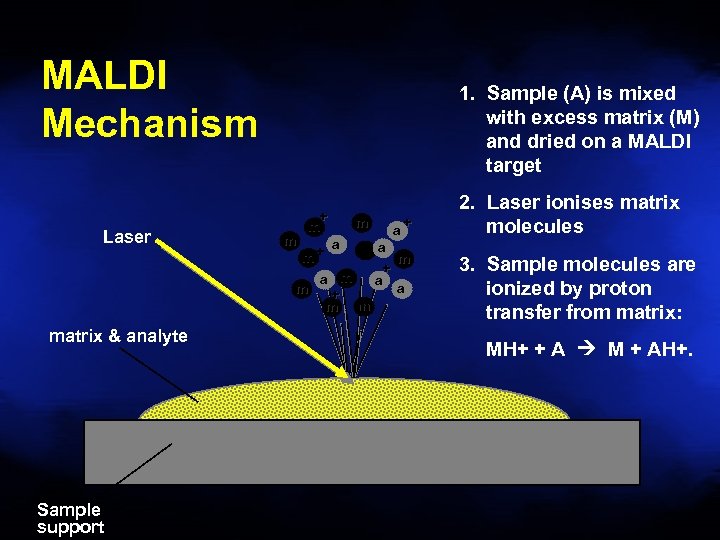 MALDI Mechanism Laser matrix & analyte Sample support 1. Sample (A) is mixed with excess matrix (M) and dried on a MALDI target m + m m a a + m a m + a m a a m + m m m+ 2. Laser ionises matrix molecules 3. Sample molecules are ionized by proton transfer from matrix: MH+ + A M + AH+.
MALDI Mechanism Laser matrix & analyte Sample support 1. Sample (A) is mixed with excess matrix (M) and dried on a MALDI target m + m m a a + m a m + a m a a m + m m m+ 2. Laser ionises matrix molecules 3. Sample molecules are ionized by proton transfer from matrix: MH+ + A M + AH+.
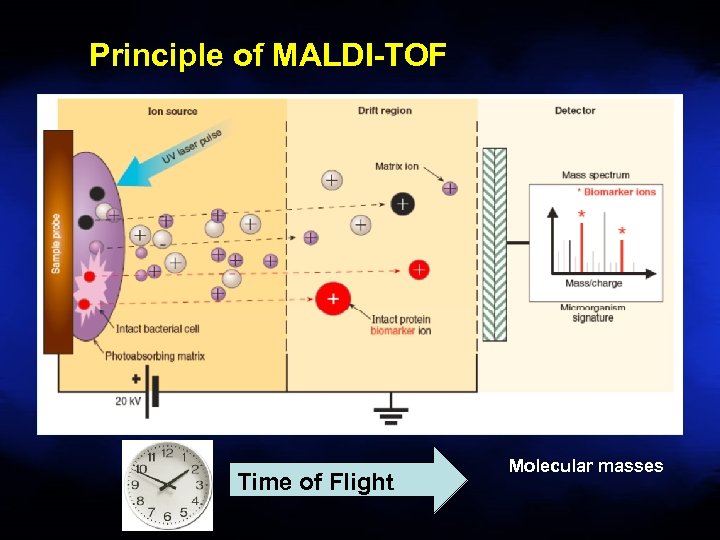 Principle of MALDI-TOF Time of Flight Molecular masses
Principle of MALDI-TOF Time of Flight Molecular masses
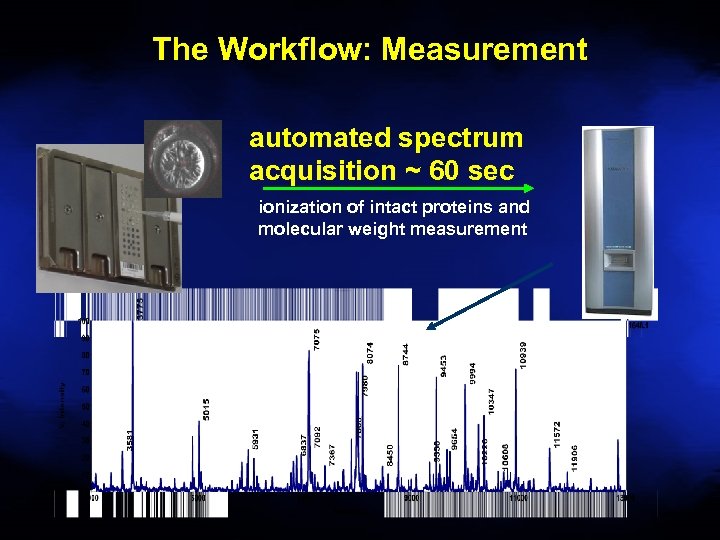 The Workflow: Measurement automated spectrum acquisition ~ 60 sec ionization of intact proteins and molecular weight measurement
The Workflow: Measurement automated spectrum acquisition ~ 60 sec ionization of intact proteins and molecular weight measurement
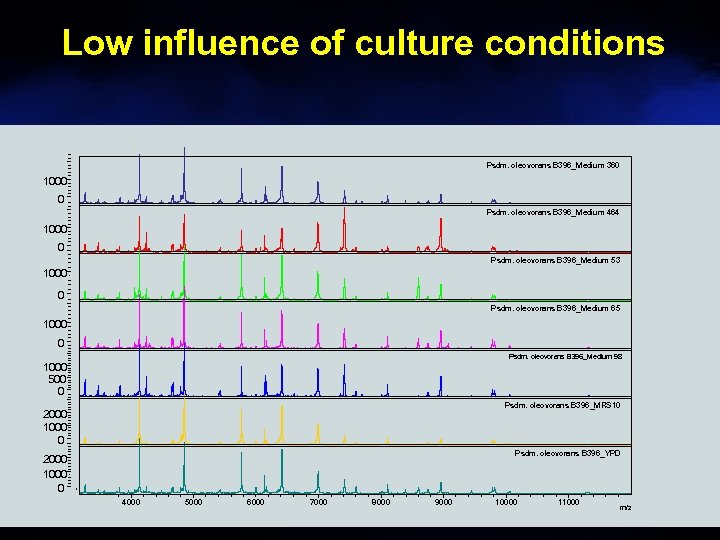 Low influence of culture conditions Psdm. oleovorans B 396_Medium 360 1000 0 Psdm. oleovorans B 396_Medium 464 1000 0 Psdm. oleovorans B 396_Medium 53 1000 0 Psdm. oleovorans B 396_Medium 65 1000 0 Psdm. oleovorans B 396_Medium 98 1000 500 0 Psdm. oleovorans B 396_MRS 10 2000 1000 0 Psdm. oleovorans B 396_YPD 2000 1000 0 4000 5000 6000 7000 8000 9000 10000 11000 m/z
Low influence of culture conditions Psdm. oleovorans B 396_Medium 360 1000 0 Psdm. oleovorans B 396_Medium 464 1000 0 Psdm. oleovorans B 396_Medium 53 1000 0 Psdm. oleovorans B 396_Medium 65 1000 0 Psdm. oleovorans B 396_Medium 98 1000 500 0 Psdm. oleovorans B 396_MRS 10 2000 1000 0 Psdm. oleovorans B 396_YPD 2000 1000 0 4000 5000 6000 7000 8000 9000 10000 11000 m/z
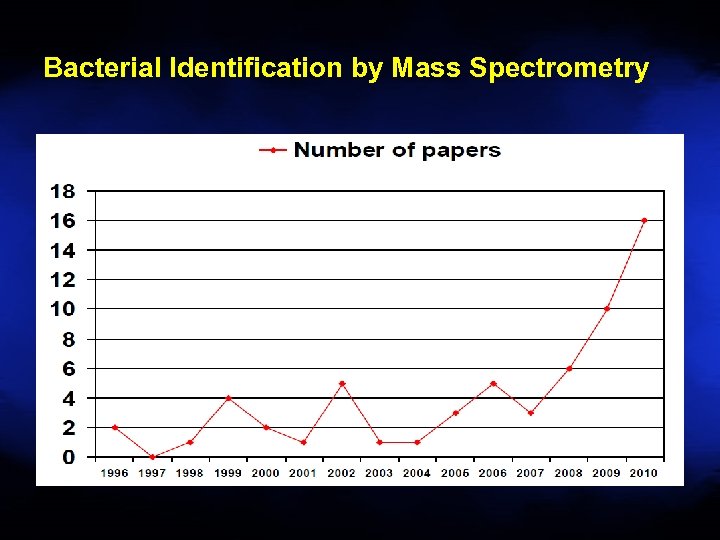 Bacterial Identification by Mass Spectrometry
Bacterial Identification by Mass Spectrometry
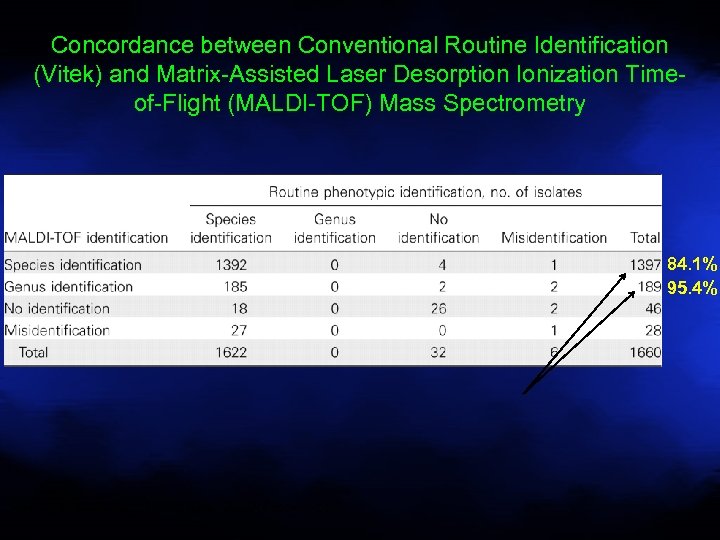 Concordance between Conventional Routine Identification (Vitek) and Matrix-Assisted Laser Desorption Ionization Timeof-Flight (MALDI-TOF) Mass Spectrometry 84. 1% 95. 4% Seng P et al. Clin Infect Dis. 2009; 49: 543 -551
Concordance between Conventional Routine Identification (Vitek) and Matrix-Assisted Laser Desorption Ionization Timeof-Flight (MALDI-TOF) Mass Spectrometry 84. 1% 95. 4% Seng P et al. Clin Infect Dis. 2009; 49: 543 -551
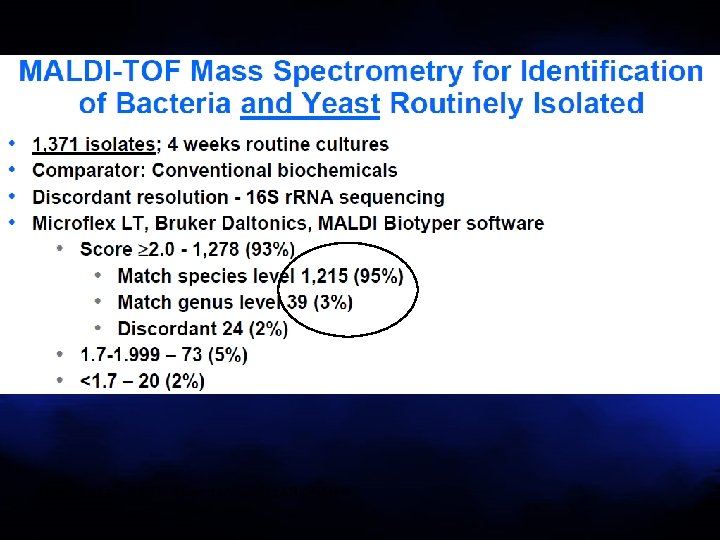 Bizzini et al. J Clin Microbiol 2010; 48: 1549
Bizzini et al. J Clin Microbiol 2010; 48: 1549
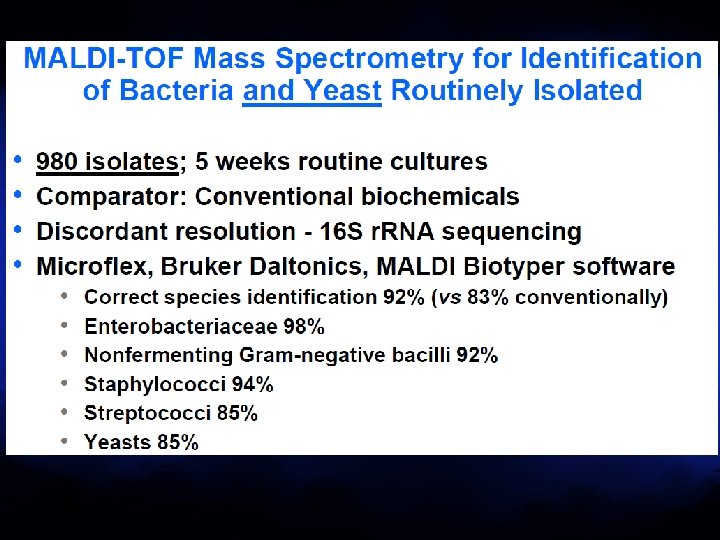 van Veen et al. J Clin Microbiol 2010: 48: 900
van Veen et al. J Clin Microbiol 2010: 48: 900
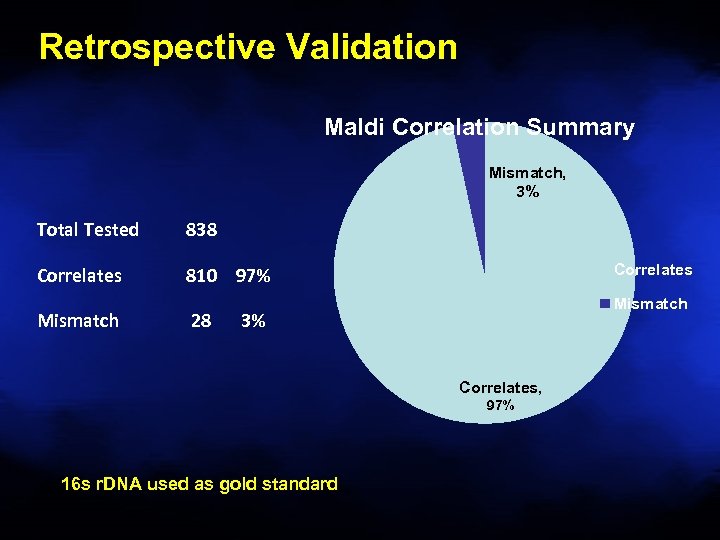 Retrospective Validation Maldi Correlation Summary Mismatch, 3% Total Tested 838 Correlates 810 97% Mismatch 28 Correlates Mismatch 3% Correlates, 97% 16 s r. DNA used as gold standard
Retrospective Validation Maldi Correlation Summary Mismatch, 3% Total Tested 838 Correlates 810 97% Mismatch 28 Correlates Mismatch 3% Correlates, 97% 16 s r. DNA used as gold standard
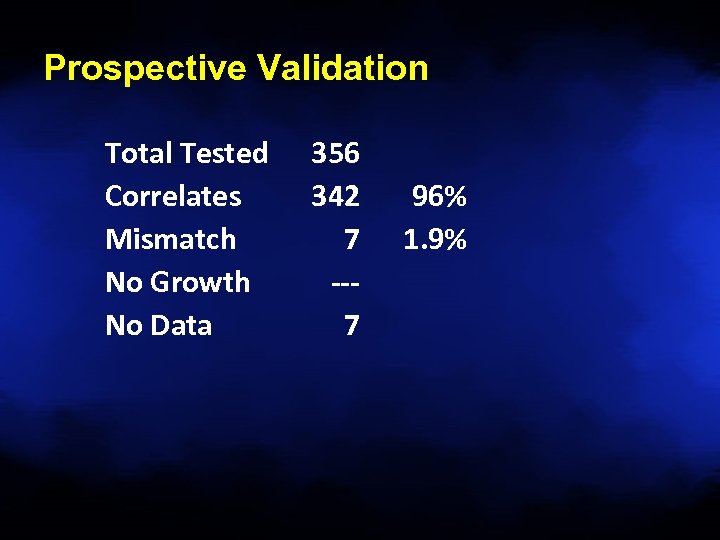 Prospective Validation Total Tested Correlates Mismatch No Growth No Data 356 342 7 --7 96% 1. 9%
Prospective Validation Total Tested Correlates Mismatch No Growth No Data 356 342 7 --7 96% 1. 9%
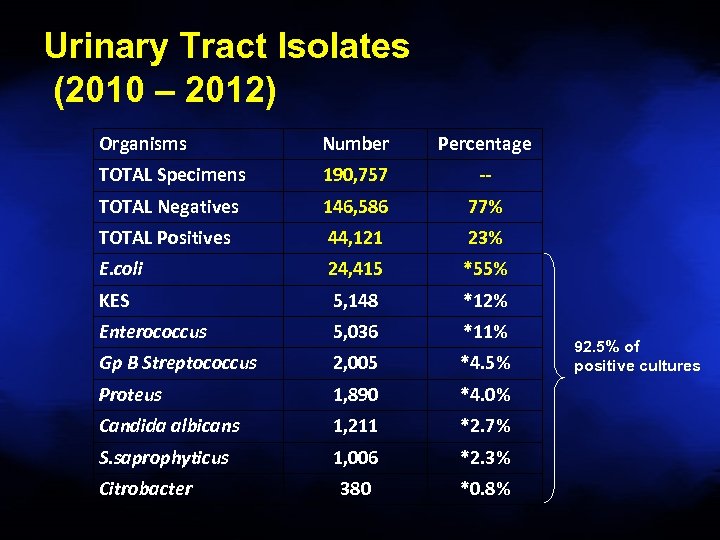 Urinary Tract Isolates (2010 – 2012) Organisms Number Percentage TOTAL Specimens 190, 757 -- TOTAL Negatives 146, 586 77% TOTAL Positives 44, 121 23% E. coli 24, 415 *55% KES 5, 148 *12% Enterococcus 5, 036 *11% Gp B Streptococcus 2, 005 *4. 5% Proteus 1, 890 *4. 0% Candida albicans 1, 211 *2. 7% S. saprophyticus 1, 006 *2. 3% 380 *0. 8% Citrobacter 92. 5% of positive cultures
Urinary Tract Isolates (2010 – 2012) Organisms Number Percentage TOTAL Specimens 190, 757 -- TOTAL Negatives 146, 586 77% TOTAL Positives 44, 121 23% E. coli 24, 415 *55% KES 5, 148 *12% Enterococcus 5, 036 *11% Gp B Streptococcus 2, 005 *4. 5% Proteus 1, 890 *4. 0% Candida albicans 1, 211 *2. 7% S. saprophyticus 1, 006 *2. 3% 380 *0. 8% Citrobacter 92. 5% of positive cultures
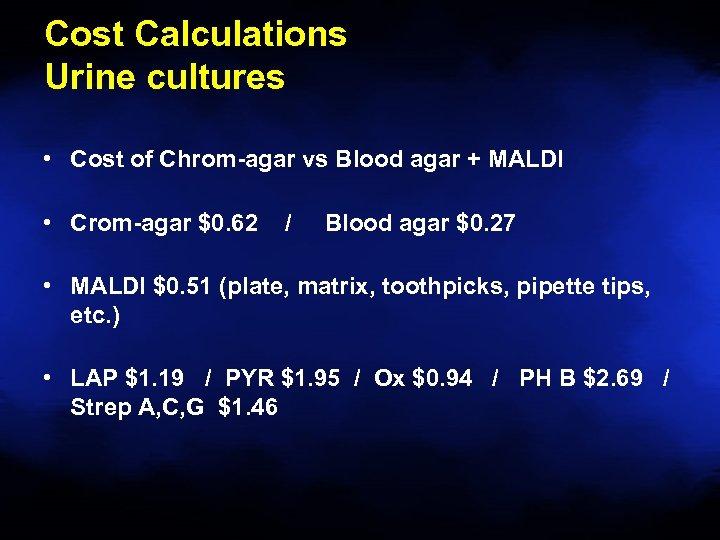 Cost Calculations Urine cultures • Cost of Chrom-agar vs Blood agar + MALDI • Crom-agar $0. 62 / Blood agar $0. 27 • MALDI $0. 51 (plate, matrix, toothpicks, pipette tips, etc. ) • LAP $1. 19 / PYR $1. 95 / Ox $0. 94 / PH B $2. 69 / Strep A, C, G $1. 46
Cost Calculations Urine cultures • Cost of Chrom-agar vs Blood agar + MALDI • Crom-agar $0. 62 / Blood agar $0. 27 • MALDI $0. 51 (plate, matrix, toothpicks, pipette tips, etc. ) • LAP $1. 19 / PYR $1. 95 / Ox $0. 94 / PH B $2. 69 / Strep A, C, G $1. 46
 Cost Calculations Urine cultures • • • • Total # of specimens / year: 63, 585 Total # of negatives / year: 48, 862 Total # of positives / year: 14, 707 Cost of a negative culture: 48, 862 x 0. 62 - 48, 862 x 0. 27 ($30, 294 - $13, 192 = $17, 102 SAV)
Cost Calculations Urine cultures • • • • Total # of specimens / year: 63, 585 Total # of negatives / year: 48, 862 Total # of positives / year: 14, 707 Cost of a negative culture: 48, 862 x 0. 62 - 48, 862 x 0. 27 ($30, 294 - $13, 192 = $17, 102 SAV)
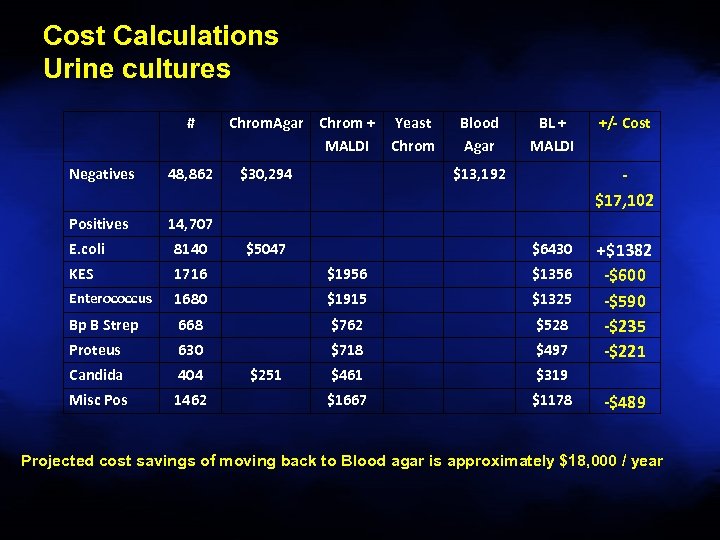 Cost Calculations Urine cultures # Negatives 48, 862 Positives Chrom. Agar Chrom + MALDI $30, 294 Yeast Chrom Blood Agar BL + MALDI $13, 192 +/- Cost 14, 707 $5047 $17, 102 E. coli 8140 KES 1716 $1956 $1356 Enterococcus 1680 $1915 $1325 Bp B Strep 668 $762 $528 Proteus 630 $718 $497 Candida 404 $461 $319 Misc Pos 1462 $1667 $1178 $251 $6430 +$1382 -$600 -$590 -$235 -$221 -$489 Projected cost savings of moving back to Blood agar is approximately $18, 000 / year
Cost Calculations Urine cultures # Negatives 48, 862 Positives Chrom. Agar Chrom + MALDI $30, 294 Yeast Chrom Blood Agar BL + MALDI $13, 192 +/- Cost 14, 707 $5047 $17, 102 E. coli 8140 KES 1716 $1956 $1356 Enterococcus 1680 $1915 $1325 Bp B Strep 668 $762 $528 Proteus 630 $718 $497 Candida 404 $461 $319 Misc Pos 1462 $1667 $1178 $251 $6430 +$1382 -$600 -$590 -$235 -$221 -$489 Projected cost savings of moving back to Blood agar is approximately $18, 000 / year
 Cost calculations Throat cultures • Gp A Strep $2996 (PYR) vs $1298 (MALDI) = $1698 SAV • Gp C Strep: Costs $534 for a neg PYR $445 extraction $650 Gp C latex = $1629 vs $231 (MALDI) = $1398 SAV • Gp G Strep: Costs $377 for a neg PYR $314 extraction $458 for a neg Gp C latex $458 Gp G latex = $1607 vs $163 (MALDI) = $1444 SAV • Approx $5000 / year Saving!!
Cost calculations Throat cultures • Gp A Strep $2996 (PYR) vs $1298 (MALDI) = $1698 SAV • Gp C Strep: Costs $534 for a neg PYR $445 extraction $650 Gp C latex = $1629 vs $231 (MALDI) = $1398 SAV • Gp G Strep: Costs $377 for a neg PYR $314 extraction $458 for a neg Gp C latex $458 Gp G latex = $1607 vs $163 (MALDI) = $1444 SAV • Approx $5000 / year Saving!!
 What Does an Instrument Cost? Instrument Service Contract Disposables ~ cost per test ~$ 220, 000 ~$ 20, 000 / yr ~ $3 -7, 000 / yr ~ $ 0. 51 / test We currently spend ~$ 65 – 75, 000 / yr on Vitek ID panels
What Does an Instrument Cost? Instrument Service Contract Disposables ~ cost per test ~$ 220, 000 ~$ 20, 000 / yr ~ $3 -7, 000 / yr ~ $ 0. 51 / test We currently spend ~$ 65 – 75, 000 / yr on Vitek ID panels
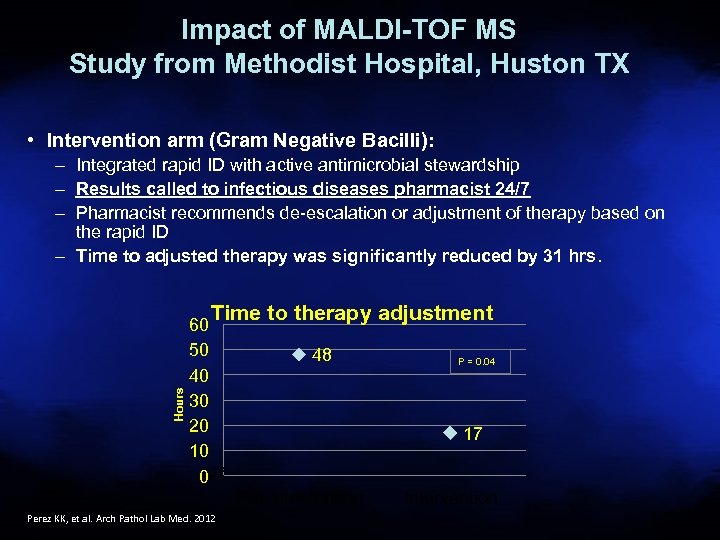 Impact of MALDI-TOF MS Study from Methodist Hospital, Huston TX • Intervention arm (Gram Negative Bacilli): – Integrated rapid ID with active antimicrobial stewardship – Results called to infectious diseases pharmacist 24/7 – Pharmacist recommends de-escalation or adjustment of therapy based on the rapid ID – Time to adjusted therapy was significantly reduced by 31 hrs. Hours Time to therapy adjustment 60 50 40 30 20 10 0 26 48 17 Pre-intervention Perez KK, et al. Arch Pathol Lab Med. 2012 P = 0. 04 Intervention
Impact of MALDI-TOF MS Study from Methodist Hospital, Huston TX • Intervention arm (Gram Negative Bacilli): – Integrated rapid ID with active antimicrobial stewardship – Results called to infectious diseases pharmacist 24/7 – Pharmacist recommends de-escalation or adjustment of therapy based on the rapid ID – Time to adjusted therapy was significantly reduced by 31 hrs. Hours Time to therapy adjustment 60 50 40 30 20 10 0 26 48 17 Pre-intervention Perez KK, et al. Arch Pathol Lab Med. 2012 P = 0. 04 Intervention
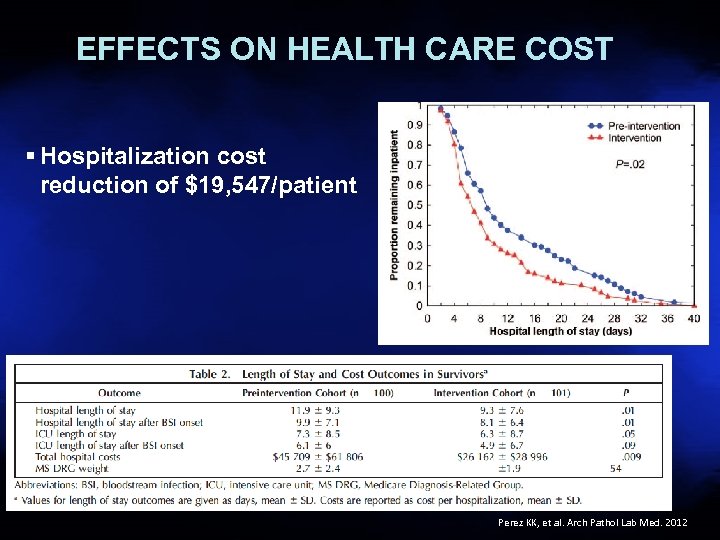 EFFECTS ON HEALTH CARE COST § Hospitalization cost reduction of $19, 547/patient 27 Perez KK, et al. Arch Pathol Lab Med. 2012
EFFECTS ON HEALTH CARE COST § Hospitalization cost reduction of $19, 547/patient 27 Perez KK, et al. Arch Pathol Lab Med. 2012
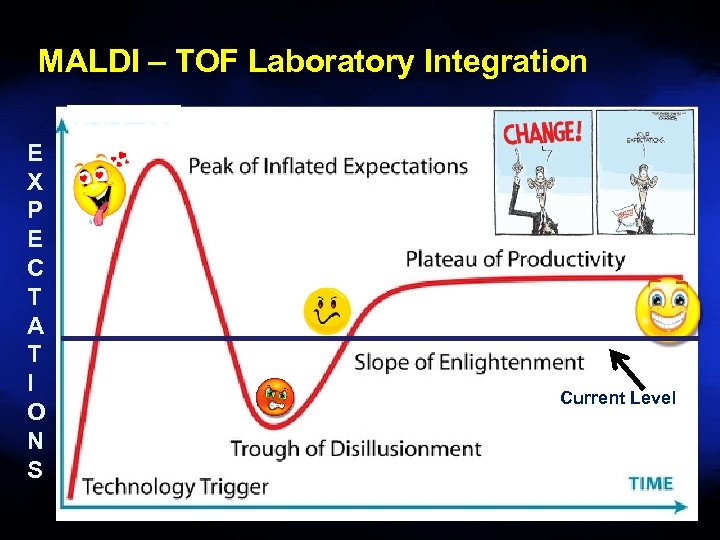 MALDI – TOF Laboratory Integration E X P E C T A T I O N S Current Level
MALDI – TOF Laboratory Integration E X P E C T A T I O N S Current Level
 MALDI – TOF Laboratory Integration Challenges • Technologist buy-in • Spotting plates an “art”, not a science • Updated nomenclature (New names) -Wohlfahrtiimonas chitinclastica (Wool farti WHAT? ? ) -isolated from 3 rd stage larvae of Wohlfahrtia magnifica • Workflow
MALDI – TOF Laboratory Integration Challenges • Technologist buy-in • Spotting plates an “art”, not a science • Updated nomenclature (New names) -Wohlfahrtiimonas chitinclastica (Wool farti WHAT? ? ) -isolated from 3 rd stage larvae of Wohlfahrtia magnifica • Workflow
 No Test is perfect! • E. coli vs Shigella • Acinetobacter baumanii-calcoaceticus complex (A. baumanii, A. calcoaceticus, A. genospecies 3, A. genospecies 13)
No Test is perfect! • E. coli vs Shigella • Acinetobacter baumanii-calcoaceticus complex (A. baumanii, A. calcoaceticus, A. genospecies 3, A. genospecies 13)
 Pre MALDI - Good Clinical Microbiology Begins With Good specimens – Garbage In = Garbage Out • Control of sample acceptability • • • Verification that appropriate sample(s) collected Correct volume submitted Sample placed promptly in correct transport media Optimal and timely transport conditions Sample handled properly in laboratory • • Shared samples Reflexed samples 31
Pre MALDI - Good Clinical Microbiology Begins With Good specimens – Garbage In = Garbage Out • Control of sample acceptability • • • Verification that appropriate sample(s) collected Correct volume submitted Sample placed promptly in correct transport media Optimal and timely transport conditions Sample handled properly in laboratory • • Shared samples Reflexed samples 31
 Future Direction • Direct specimen applications (already blood / urine data) • Ability to resolve poly-microbial specimens • Antimicrobial resistance determination (already MRSA, carbapenemase) • Strain typing
Future Direction • Direct specimen applications (already blood / urine data) • Ability to resolve poly-microbial specimens • Antimicrobial resistance determination (already MRSA, carbapenemase) • Strain typing
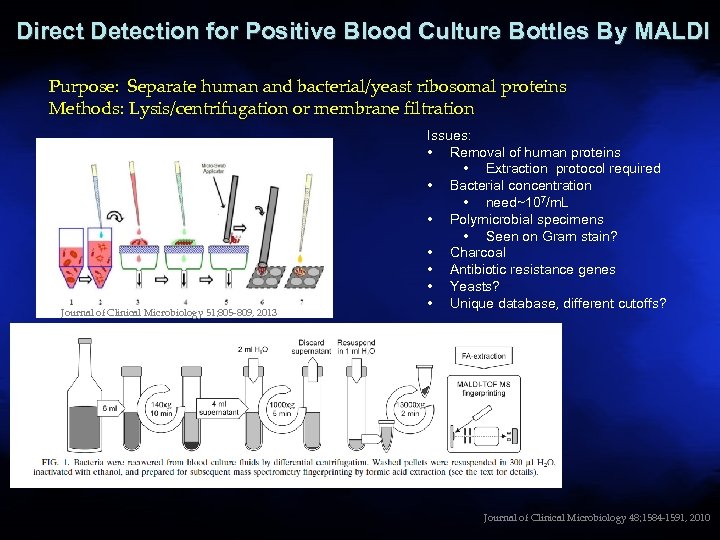 Direct Detection for Positive Blood Culture Bottles By MALDI Purpose: Separate human and bacterial/yeast ribosomal proteins Methods: Lysis/centrifugation or membrane filtration Journal of Clinical Microbiology 51; 805 -809, 2013 Issues: • Removal of human proteins • Extraction protocol required • Bacterial concentration • need~107/m. L • Polymicrobial specimens • Seen on Gram stain? • Charcoal • Antibiotic resistance genes • Yeasts? • Unique database, different cutoffs? 33 Journal of Clinical Microbiology 48; 1584 -1591, 2010
Direct Detection for Positive Blood Culture Bottles By MALDI Purpose: Separate human and bacterial/yeast ribosomal proteins Methods: Lysis/centrifugation or membrane filtration Journal of Clinical Microbiology 51; 805 -809, 2013 Issues: • Removal of human proteins • Extraction protocol required • Bacterial concentration • need~107/m. L • Polymicrobial specimens • Seen on Gram stain? • Charcoal • Antibiotic resistance genes • Yeasts? • Unique database, different cutoffs? 33 Journal of Clinical Microbiology 48; 1584 -1591, 2010
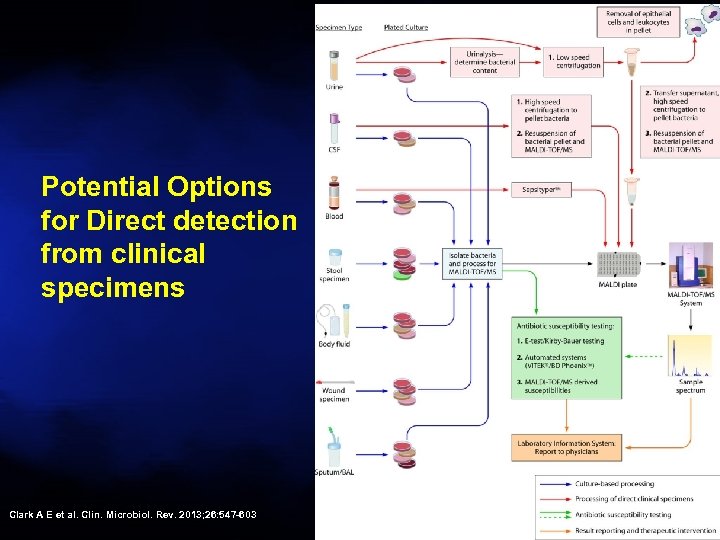 Potential Options for Direct detection from clinical specimens Clark A E et al. Clin. Microbiol. Rev. 2013; 26: 547 -603
Potential Options for Direct detection from clinical specimens Clark A E et al. Clin. Microbiol. Rev. 2013; 26: 547 -603
 MALDI-TOF Bacterial ID • Minimal sample preparation • Cost effective - low consumable cost • Powerful bioinformatic approaches • Species to strain resolution • Non-expert identification possible • Dedicated databases continue to expand
MALDI-TOF Bacterial ID • Minimal sample preparation • Cost effective - low consumable cost • Powerful bioinformatic approaches • Species to strain resolution • Non-expert identification possible • Dedicated databases continue to expand
 MALDI-TOF Limitations • Databases : still in their infancy • High initial capital expenditure • New approaches (business models) • Potential instrument downtime - single instrument
MALDI-TOF Limitations • Databases : still in their infancy • High initial capital expenditure • New approaches (business models) • Potential instrument downtime - single instrument
 MALDI-TOF in the Clinical Laboratory • Rapid turn around time, high throughput - impact on appropriate emperic therapy • Single colony requirement - direct from blood culture • Low exposure risk –sample inactivation • Broad applicability (all types bacteria including anaerobes, yeasts, fungi) • COST SAVINGS
MALDI-TOF in the Clinical Laboratory • Rapid turn around time, high throughput - impact on appropriate emperic therapy • Single colony requirement - direct from blood culture • Low exposure risk –sample inactivation • Broad applicability (all types bacteria including anaerobes, yeasts, fungi) • COST SAVINGS
 Questions
Questions
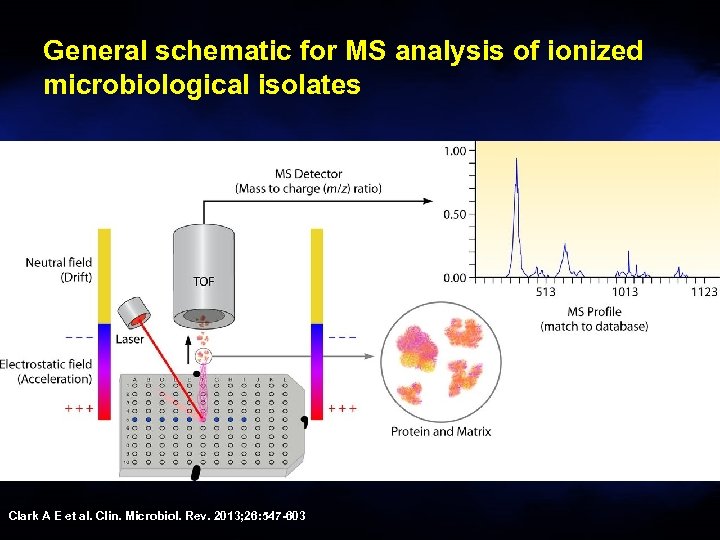 General schematic for MS analysis of ionized microbiological isolates Clark A E et al. Clin. Microbiol. Rev. 2013; 26: 547 -603
General schematic for MS analysis of ionized microbiological isolates Clark A E et al. Clin. Microbiol. Rev. 2013; 26: 547 -603