545e64251f26682bd6ccbd362a3948e5.ppt
- Количество слайдов: 37
 Maintaining a Validated SAS Toolset Co-Presenters: Edward Helton and Patricia Halley Copyright © 2005, SAS Institute Inc. All rights reserved.
Maintaining a Validated SAS Toolset Co-Presenters: Edward Helton and Patricia Halley Copyright © 2005, SAS Institute Inc. All rights reserved.
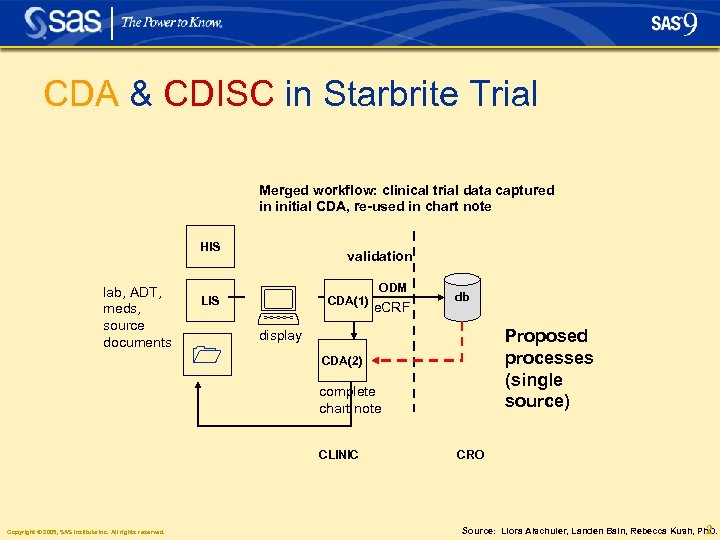 CDA & CDISC in Starbrite Trial Merged workflow: clinical trial data captured in initial CDA, re-used in chart note HIS lab, ADT, meds, source documents LIS validation CDA(1) ODM e. CRF db Proposed processes (single source) display CDA(2) complete chart note CLINIC Copyright © 2005, SAS Institute Inc. All rights reserved. CRO Source: Liora Alschuler, Landen Bain, Rebecca Kush, Ph. D. 2
CDA & CDISC in Starbrite Trial Merged workflow: clinical trial data captured in initial CDA, re-used in chart note HIS lab, ADT, meds, source documents LIS validation CDA(1) ODM e. CRF db Proposed processes (single source) display CDA(2) complete chart note CLINIC Copyright © 2005, SAS Institute Inc. All rights reserved. CRO Source: Liora Alschuler, Landen Bain, Rebecca Kush, Ph. D. 2
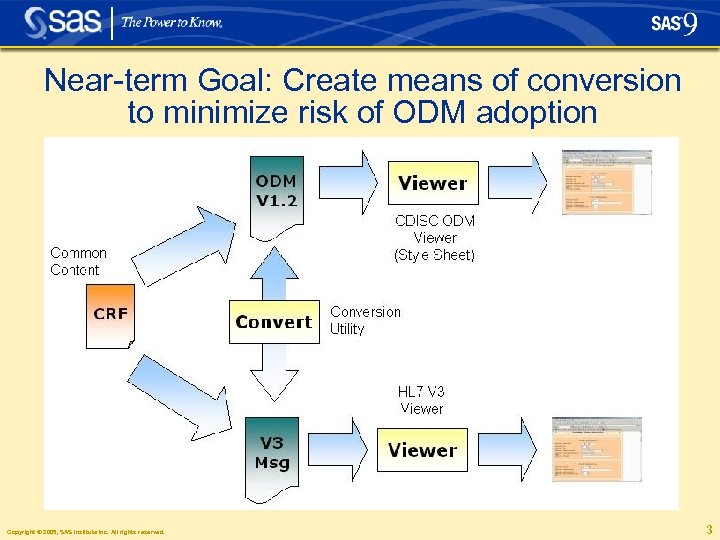 Near-term Goal: Create means of conversion to minimize risk of ODM adoption Copyright © 2005, SAS Institute Inc. All rights reserved. 3
Near-term Goal: Create means of conversion to minimize risk of ODM adoption Copyright © 2005, SAS Institute Inc. All rights reserved. 3
 Agenda § Part 11 and the Predicate Rules § Applying the Predicate Rules to Maintenance of a Validated SAS Toolset § Support from SAS (now and future) Copyright © 2005, SAS Institute Inc. All rights reserved. 4
Agenda § Part 11 and the Predicate Rules § Applying the Predicate Rules to Maintenance of a Validated SAS Toolset § Support from SAS (now and future) Copyright © 2005, SAS Institute Inc. All rights reserved. 4
 Common Threads of Gx. Ps § § § Calibrate Test Validate Standardize Retain Records Maintain Written Procedures Copyright © 2005, SAS Institute Inc. All rights reserved. 5
Common Threads of Gx. Ps § § § Calibrate Test Validate Standardize Retain Records Maintain Written Procedures Copyright © 2005, SAS Institute Inc. All rights reserved. 5
 Update on Part 11 GMP by the Sea August 25, 2004 Rick Friedman, Team Leader Guidance & Policy Division of Manufacturing and Product Quality Office of Compliance, CDER 6
Update on Part 11 GMP by the Sea August 25, 2004 Rick Friedman, Team Leader Guidance & Policy Division of Manufacturing and Product Quality Office of Compliance, CDER 6
 FDA Regulation References - GCP § Strong New FDA Industry Guidances in GCPs § 50. 20 – 312. 56 ( c) – sponsors shall review and evaluate evidence related to safety and effectiveness § 312. 57 (a) – Records must be Accurate & Complete § 312. 62(a, b, c) – Investigator Recordkeeping - drug disposition, case histories, record retention Copyright © 2005, SAS Institute Inc. All rights reserved. 7
FDA Regulation References - GCP § Strong New FDA Industry Guidances in GCPs § 50. 20 – 312. 56 ( c) – sponsors shall review and evaluate evidence related to safety and effectiveness § 312. 57 (a) – Records must be Accurate & Complete § 312. 62(a, b, c) – Investigator Recordkeeping - drug disposition, case histories, record retention Copyright © 2005, SAS Institute Inc. All rights reserved. 7
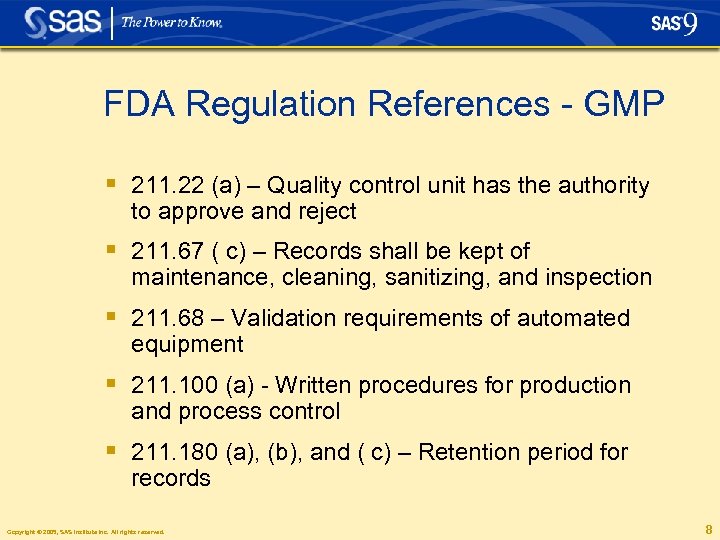 FDA Regulation References - GMP § 211. 22 (a) – Quality control unit has the authority to approve and reject § 211. 67 ( c) – Records shall be kept of maintenance, cleaning, sanitizing, and inspection § 211. 68 – Validation requirements of automated equipment § 211. 100 (a) - Written procedures for production and process control § 211. 180 (a), (b), and ( c) – Retention period for records Copyright © 2005, SAS Institute Inc. All rights reserved. 8
FDA Regulation References - GMP § 211. 22 (a) – Quality control unit has the authority to approve and reject § 211. 67 ( c) – Records shall be kept of maintenance, cleaning, sanitizing, and inspection § 211. 68 – Validation requirements of automated equipment § 211. 100 (a) - Written procedures for production and process control § 211. 180 (a), (b), and ( c) – Retention period for records Copyright © 2005, SAS Institute Inc. All rights reserved. 8
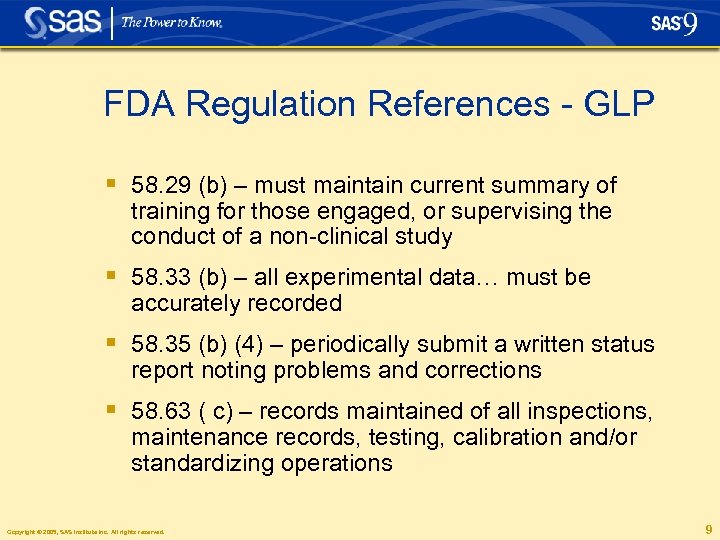 FDA Regulation References - GLP § 58. 29 (b) – must maintain current summary of training for those engaged, or supervising the conduct of a non-clinical study § 58. 33 (b) – all experimental data… must be accurately recorded § 58. 35 (b) (4) – periodically submit a written status report noting problems and corrections § 58. 63 ( c) – records maintained of all inspections, maintenance records, testing, calibration and/or standardizing operations Copyright © 2005, SAS Institute Inc. All rights reserved. 9
FDA Regulation References - GLP § 58. 29 (b) – must maintain current summary of training for those engaged, or supervising the conduct of a non-clinical study § 58. 33 (b) – all experimental data… must be accurately recorded § 58. 35 (b) (4) – periodically submit a written status report noting problems and corrections § 58. 63 ( c) – records maintained of all inspections, maintenance records, testing, calibration and/or standardizing operations Copyright © 2005, SAS Institute Inc. All rights reserved. 9
 FDA Regulation References Devices § 21 CFR 812. 145 (a) & (b) - Records must be Accurate & Complete § 21 CFR 812. 140 – must retain records required by 812 Copyright © 2005, SAS Institute Inc. All rights reserved. 10
FDA Regulation References Devices § 21 CFR 812. 145 (a) & (b) - Records must be Accurate & Complete § 21 CFR 812. 140 – must retain records required by 812 Copyright © 2005, SAS Institute Inc. All rights reserved. 10
 11
11
 21 CFR Part 11 - Upcoming Amendment § Comments collected on the existing rule § Support for Part 11 in general § It is not going away – expect rewrite in future Copyright © 2005, SAS Institute Inc. All rights reserved. 12
21 CFR Part 11 - Upcoming Amendment § Comments collected on the existing rule § Support for Part 11 in general § It is not going away – expect rewrite in future Copyright © 2005, SAS Institute Inc. All rights reserved. 12
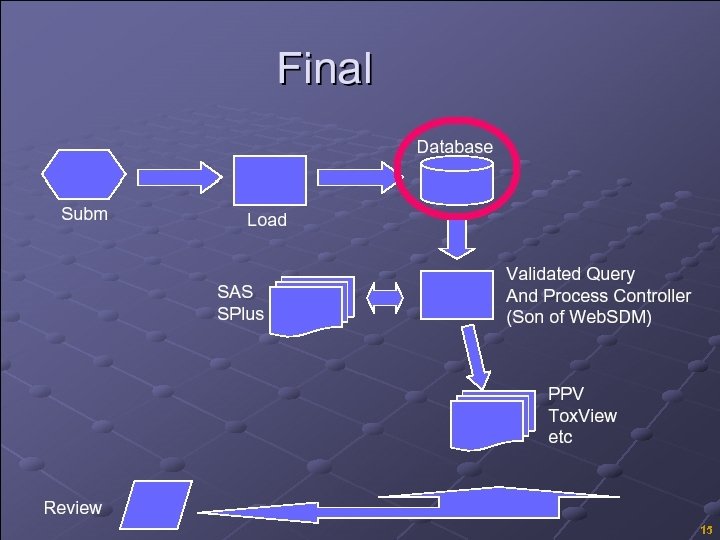
 Standard Data / JANUS § Tools to receive, upload analyze data § Unambiguous path of the source data to the analysis findings (metadata, derivations, flags, imputations, source code, analysis files) § Focus on standard data and standard analysis makes maintaining validation even more critical – valid interface, interaction between SAS systems § This makes your SAS configuration even more important! Copyright © 2005, SAS Institute Inc. All rights reserved. 13
Standard Data / JANUS § Tools to receive, upload analyze data § Unambiguous path of the source data to the analysis findings (metadata, derivations, flags, imputations, source code, analysis files) § Focus on standard data and standard analysis makes maintaining validation even more critical – valid interface, interaction between SAS systems § This makes your SAS configuration even more important! Copyright © 2005, SAS Institute Inc. All rights reserved. 13
 Copyright © 2005, SAS Institute Inc. All rights reserved. 14
Copyright © 2005, SAS Institute Inc. All rights reserved. 14
 Copyright © 2005, SAS Institute Inc. All rights reserved. 15
Copyright © 2005, SAS Institute Inc. All rights reserved. 15
 Standard Data / JANUS § Right now it takes analysis of many applications to find common trends. § One study took 10 person years. § We don’t have that kind of time to “find” the right data. § Standardization enhances the need for a validated SAS system. Copyright © 2005, SAS Institute Inc. All rights reserved. 16
Standard Data / JANUS § Right now it takes analysis of many applications to find common trends. § One study took 10 person years. § We don’t have that kind of time to “find” the right data. § Standardization enhances the need for a validated SAS system. Copyright © 2005, SAS Institute Inc. All rights reserved. 16
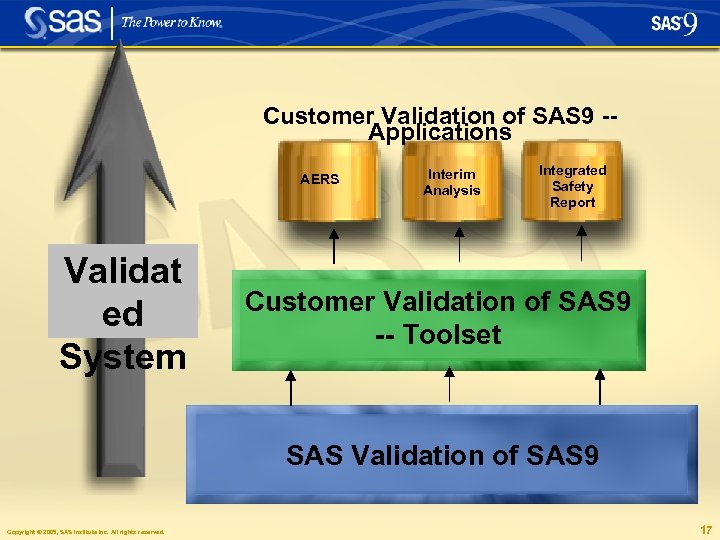 Customer Validation of SAS 9 -Applications AERS Validat ed System Interim Analysis Integrated Safety Report Customer Validation of SAS 9 -- Toolset SAS Validation of SAS 9 Copyright © 2005, SAS Institute Inc. All rights reserved. 17
Customer Validation of SAS 9 -Applications AERS Validat ed System Interim Analysis Integrated Safety Report Customer Validation of SAS 9 -- Toolset SAS Validation of SAS 9 Copyright © 2005, SAS Institute Inc. All rights reserved. 17
 Copyright © 2005, SAS Institute Inc. All rights reserved. 18
Copyright © 2005, SAS Institute Inc. All rights reserved. 18
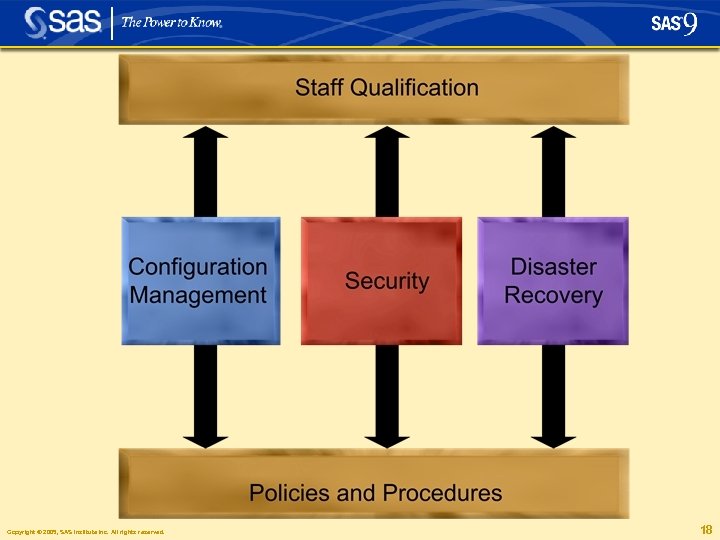
 Configuration Management § Identify functional and physical characteristics § Manage changes to those characteristics Source -- IEEE Copyright © 2005, SAS Institute Inc. All rights reserved. 19
Configuration Management § Identify functional and physical characteristics § Manage changes to those characteristics Source -- IEEE Copyright © 2005, SAS Institute Inc. All rights reserved. 19
 Copyright © 2005, SAS Institute Inc. All rights reserved. 20
Copyright © 2005, SAS Institute Inc. All rights reserved. 20
 Managing Changes Copyright © 2005, SAS Institute Inc. All rights reserved. 21
Managing Changes Copyright © 2005, SAS Institute Inc. All rights reserved. 21
 Identify and Describe Changes § Sources of Information • • Tech Support SAS Notes Mail & File Lists (TSNEWS-L) Monitoring Tools § Sources of SAS Updates • Hot Fixes • Service Packs Copyright © 2005, SAS Institute Inc. All rights reserved. 22
Identify and Describe Changes § Sources of Information • • Tech Support SAS Notes Mail & File Lists (TSNEWS-L) Monitoring Tools § Sources of SAS Updates • Hot Fixes • Service Packs Copyright © 2005, SAS Institute Inc. All rights reserved. 22
 Identify and Describe Changes Future Automatic Alert Notification Electronic Software Downloads Copyright © 2005, SAS Institute Inc. All rights reserved. 23
Identify and Describe Changes Future Automatic Alert Notification Electronic Software Downloads Copyright © 2005, SAS Institute Inc. All rights reserved. 23
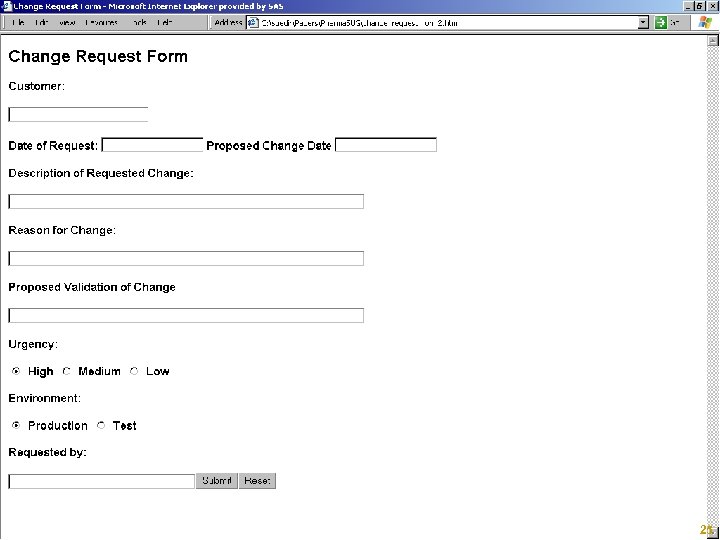
 Request and Approve Changes § Classify Change § Submit Request § Obtain Approval Copyright © 2005, SAS Institute Inc. All rights reserved. 24
Request and Approve Changes § Classify Change § Submit Request § Obtain Approval Copyright © 2005, SAS Institute Inc. All rights reserved. 24
 Copyright © 2005, SAS Institute Inc. All rights reserved. 25
Copyright © 2005, SAS Institute Inc. All rights reserved. 25
 Implement and Verify Changes § Test environment § SASIQ/SASOQ § Data Migration Copyright © 2005, SAS Institute Inc. All rights reserved. 26
Implement and Verify Changes § Test environment § SASIQ/SASOQ § Data Migration Copyright © 2005, SAS Institute Inc. All rights reserved. 26
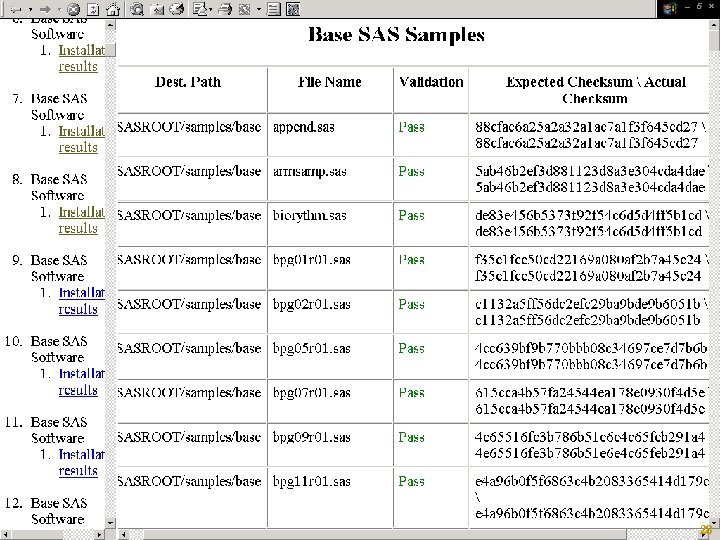
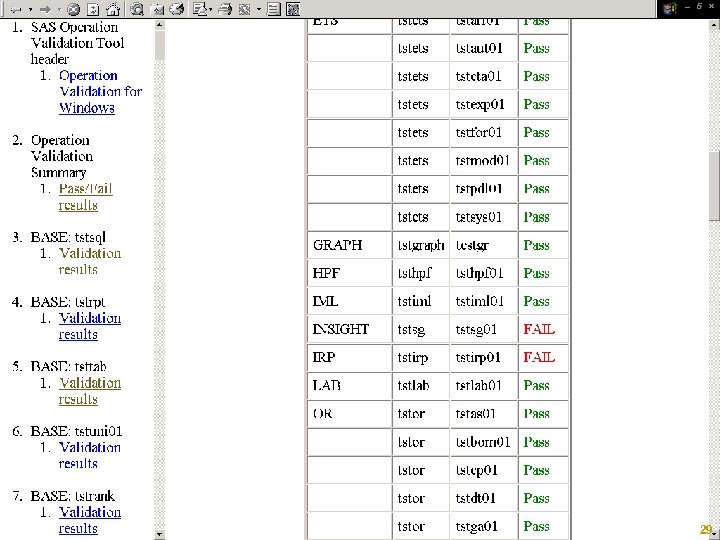
 SASIQ/SASOQ § § Automated Tested Repeatable Reports and Stores Results (XML) http: //support. sas. com/rnd/migration/planning/valid ation/ Copyright © 2005, SAS Institute Inc. All rights reserved. 2003, 27
SASIQ/SASOQ § § Automated Tested Repeatable Reports and Stores Results (XML) http: //support. sas. com/rnd/migration/planning/valid ation/ Copyright © 2005, SAS Institute Inc. All rights reserved. 2003, 27
 Copyright © 2005, SAS Institute Inc. All rights reserved. 28
Copyright © 2005, SAS Institute Inc. All rights reserved. 28
 Copyright © 2005, SAS Institute Inc. All rights reserved. 29
Copyright © 2005, SAS Institute Inc. All rights reserved. 29
 Data Migration § Proc Migrate § Migration Macros § Guidance and tools available at http: //support. sas. com/rnd/migration/fulltoc. html Copyright © 2005, SAS Institute Inc. All rights reserved. 30
Data Migration § Proc Migrate § Migration Macros § Guidance and tools available at http: //support. sas. com/rnd/migration/fulltoc. html Copyright © 2005, SAS Institute Inc. All rights reserved. 30
 Implement and Verify Changes – Future § IQ/OQ Enhancements § Install Enhancements § Less media (1 instead of 19) Copyright © 2005, SAS Institute Inc. All rights reserved. 31
Implement and Verify Changes – Future § IQ/OQ Enhancements § Install Enhancements § Less media (1 instead of 19) Copyright © 2005, SAS Institute Inc. All rights reserved. 31
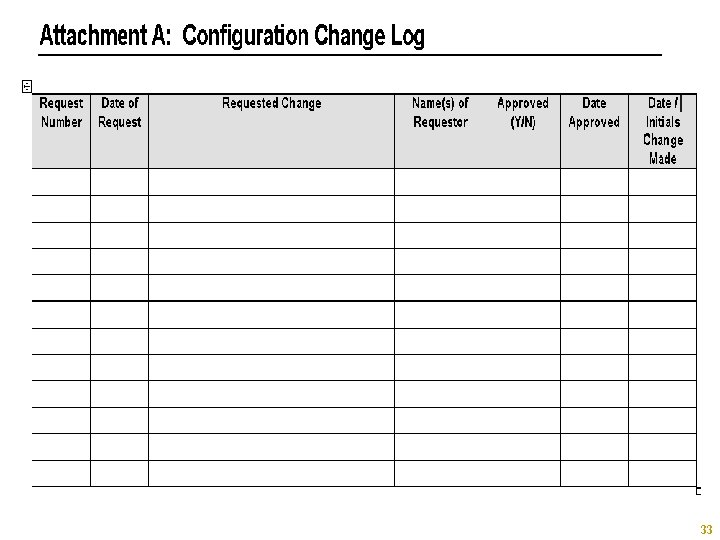
 Document § § § Initial Change Requests IQ/OQ Output SAS Logs SAS Registry (Future) Design / Configuration Specification Change Log Copyright © 2005, SAS Institute Inc. All rights reserved. 32
Document § § § Initial Change Requests IQ/OQ Output SAS Logs SAS Registry (Future) Design / Configuration Specification Change Log Copyright © 2005, SAS Institute Inc. All rights reserved. 32
 Copyright © 2005, SAS Institute Inc. All rights reserved. 33
Copyright © 2005, SAS Institute Inc. All rights reserved. 33
 Security § Physical § Logical • • Operating System Level Account Maintenance Internet Security SAS Management Console Copyright © 2005, SAS Institute Inc. All rights reserved. 34
Security § Physical § Logical • • Operating System Level Account Maintenance Internet Security SAS Management Console Copyright © 2005, SAS Institute Inc. All rights reserved. 34
 Business Continuity § § Backups Redundancy Environmental Controls Disaster Recovery Copyright © 2005, SAS Institute Inc. All rights reserved. 35
Business Continuity § § Backups Redundancy Environmental Controls Disaster Recovery Copyright © 2005, SAS Institute Inc. All rights reserved. 35
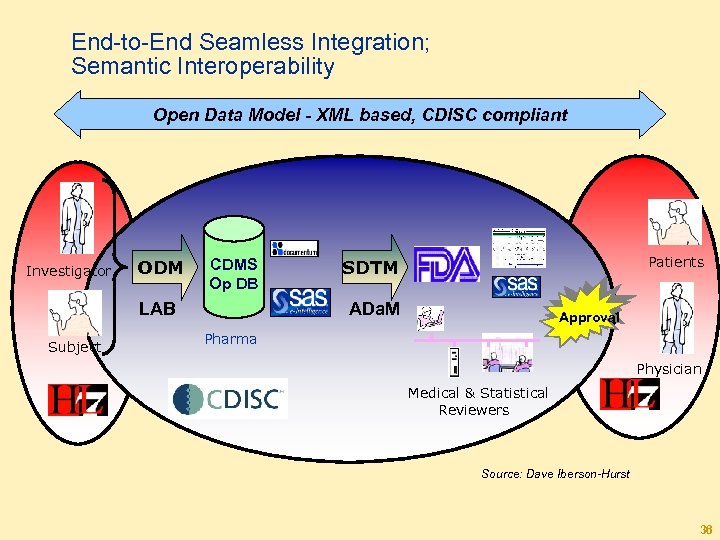 End-to-End Seamless Integration; Semantic Interoperability Open Data Model - XML based, CDISC compliant Investigator ODM CDMS Op DB LAB Subject Patients SDTM ADa. M Approval Pharma Physician Medical & Statistical Reviewers Source: Dave Iberson-Hurst 36
End-to-End Seamless Integration; Semantic Interoperability Open Data Model - XML based, CDISC compliant Investigator ODM CDMS Op DB LAB Subject Patients SDTM ADa. M Approval Pharma Physician Medical & Statistical Reviewers Source: Dave Iberson-Hurst 36
 Copyright © 2005, SAS Institute Inc. All rights reserved. 37
Copyright © 2005, SAS Institute Inc. All rights reserved. 37


