
 M 6 X 8: 20 -24 e M 6 X 12: 14 -16 e
M 6 X 8: 20 -24 e M 6 X 12: 14 -16 e
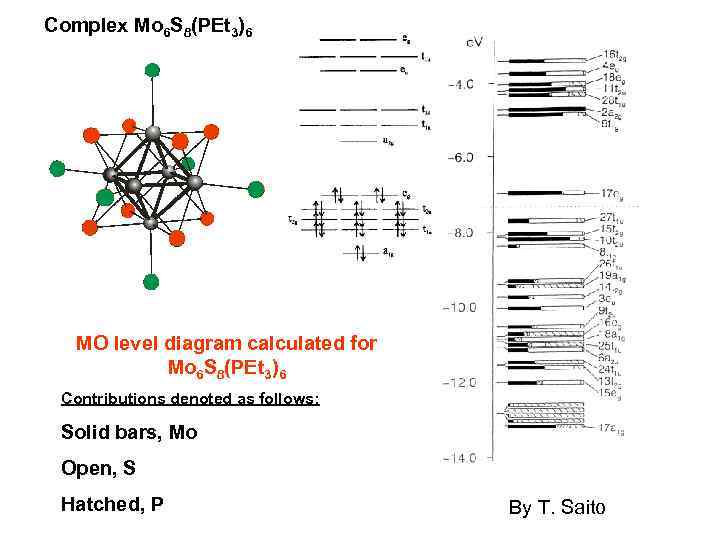 Complex Mo 6 S 8(PEt 3)6 MO level diagram calculated for Mo 6 S 8(PEt 3)6 Contributions denoted as follows: Solid bars, Mo Open, S Hatched, P By T. Saito
Complex Mo 6 S 8(PEt 3)6 MO level diagram calculated for Mo 6 S 8(PEt 3)6 Contributions denoted as follows: Solid bars, Mo Open, S Hatched, P By T. Saito
![E(e. V) Occ [Re 6 Se 8 F 6]4 - MO % SFO E(e. E(e. V) Occ [Re 6 Se 8 F 6]4 - MO % SFO E(e.](https://present5.com/presentation/5652994_182163158/image-4.jpg) E(e. V) Occ [Re 6 Se 8 F 6]4 - MO % SFO E(e. V) Occ Fragment (first member) 6. 895 2. 00 10 Eg: 1 53. 75% 33. 30% 11. 38% 5. 03% -3. 78% -1. 74% 1. 48% 1 D: z 2 -5. 514 1. 00 1 P: x -6. 626 1. 33 2 P: z -0. 551 0. 00 1 D: z 2 -5. 514 1. 00 2 D: z 2 4. 838 0. 00 2 D: z 2 7. 357 0. 00 3 Re 7 Se 1 Re 3 Re 7 Se 6. 895 2. 00 10 Eg: 2 53. 75% 33. 30% 11. 38% 5. 03% -3. 78% -1. 74% 1. 48% 1 D: x 2 -y 2 -5. 514 1 P: x -6. 626 2 P: y -0. 551 1 D: z 2 -5. 514 2 D: z 2 4. 838 2 D: x 2 -y 2 7. 357 1 Re 7 Se 3 Re 1 Re 7 Se 1. 00 1. 33 0. 00 1. 00 0. 00 9. 372 0. 00 2 A 2 g 108. 19% 1 D: x 2 -y 2 -5. 514 1. 00 1 Re -8. 09% 2 D: x 2 -y 2 4. 838 0. 00 1 Re
E(e. V) Occ [Re 6 Se 8 F 6]4 - MO % SFO E(e. V) Occ Fragment (first member) 6. 895 2. 00 10 Eg: 1 53. 75% 33. 30% 11. 38% 5. 03% -3. 78% -1. 74% 1. 48% 1 D: z 2 -5. 514 1. 00 1 P: x -6. 626 1. 33 2 P: z -0. 551 0. 00 1 D: z 2 -5. 514 1. 00 2 D: z 2 4. 838 0. 00 2 D: z 2 7. 357 0. 00 3 Re 7 Se 1 Re 3 Re 7 Se 6. 895 2. 00 10 Eg: 2 53. 75% 33. 30% 11. 38% 5. 03% -3. 78% -1. 74% 1. 48% 1 D: x 2 -y 2 -5. 514 1 P: x -6. 626 2 P: y -0. 551 1 D: z 2 -5. 514 2 D: z 2 4. 838 2 D: x 2 -y 2 7. 357 1 Re 7 Se 3 Re 1 Re 7 Se 1. 00 1. 33 0. 00 1. 00 0. 00 9. 372 0. 00 2 A 2 g 108. 19% 1 D: x 2 -y 2 -5. 514 1. 00 1 Re -8. 09% 2 D: x 2 -y 2 4. 838 0. 00 1 Re
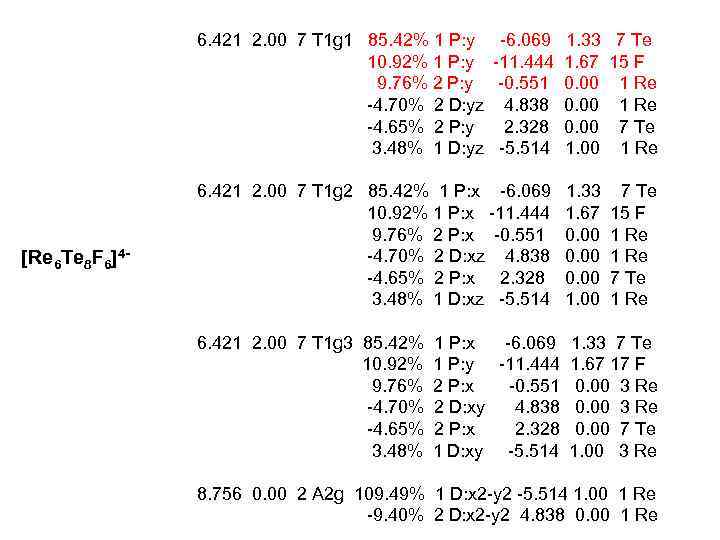 6. 421 2. 00 7 T 1 g 1 85. 42% 1 P: y 10. 92% 1 P: y 9. 76% 2 P: y -4. 70% 2 D: yz -4. 65% 2 P: y 3. 48% 1 D: yz [Re 6 Te 8 F 6]4 - -6. 069 -11. 444 -0. 551 4. 838 2. 328 -5. 514 6. 421 2. 00 7 T 1 g 2 85. 42% 1 P: x -6. 069 10. 92% 1 P: x -11. 444 9. 76% 2 P: x -0. 551 -4. 70% 2 D: xz 4. 838 -4. 65% 2 P: x 2. 328 3. 48% 1 D: xz -5. 514 6. 421 2. 00 7 T 1 g 3 85. 42% 10. 92% 9. 76% -4. 70% -4. 65% 3. 48% 1. 33 7 Te 1. 67 15 F 0. 00 1 Re 0. 00 7 Te 1. 00 1 Re 1. 33 1. 67 0. 00 1. 00 7 Te 15 F 1 Re 7 Te 1 Re 1 P: x -6. 069 1. 33 7 Te 1 P: y -11. 444 1. 67 17 F 2 P: x -0. 551 0. 00 3 Re 2 D: xy 4. 838 0. 00 3 Re 2 P: x 2. 328 0. 00 7 Te 1 D: xy -5. 514 1. 00 3 Re 8. 756 0. 00 2 A 2 g 109. 49% 1 D: x 2 -y 2 -5. 514 1. 00 1 Re -9. 40% 2 D: x 2 -y 2 4. 838 0. 00 1 Re
6. 421 2. 00 7 T 1 g 1 85. 42% 1 P: y 10. 92% 1 P: y 9. 76% 2 P: y -4. 70% 2 D: yz -4. 65% 2 P: y 3. 48% 1 D: yz [Re 6 Te 8 F 6]4 - -6. 069 -11. 444 -0. 551 4. 838 2. 328 -5. 514 6. 421 2. 00 7 T 1 g 2 85. 42% 1 P: x -6. 069 10. 92% 1 P: x -11. 444 9. 76% 2 P: x -0. 551 -4. 70% 2 D: xz 4. 838 -4. 65% 2 P: x 2. 328 3. 48% 1 D: xz -5. 514 6. 421 2. 00 7 T 1 g 3 85. 42% 10. 92% 9. 76% -4. 70% -4. 65% 3. 48% 1. 33 7 Te 1. 67 15 F 0. 00 1 Re 0. 00 7 Te 1. 00 1 Re 1. 33 1. 67 0. 00 1. 00 7 Te 15 F 1 Re 7 Te 1 Re 1 P: x -6. 069 1. 33 7 Te 1 P: y -11. 444 1. 67 17 F 2 P: x -0. 551 0. 00 3 Re 2 D: xy 4. 838 0. 00 3 Re 2 P: x 2. 328 0. 00 7 Te 1 D: xy -5. 514 1. 00 3 Re 8. 756 0. 00 2 A 2 g 109. 49% 1 D: x 2 -y 2 -5. 514 1. 00 1 Re -9. 40% 2 D: x 2 -y 2 4. 838 0. 00 1 Re
 2+ 2+
2+ 2+
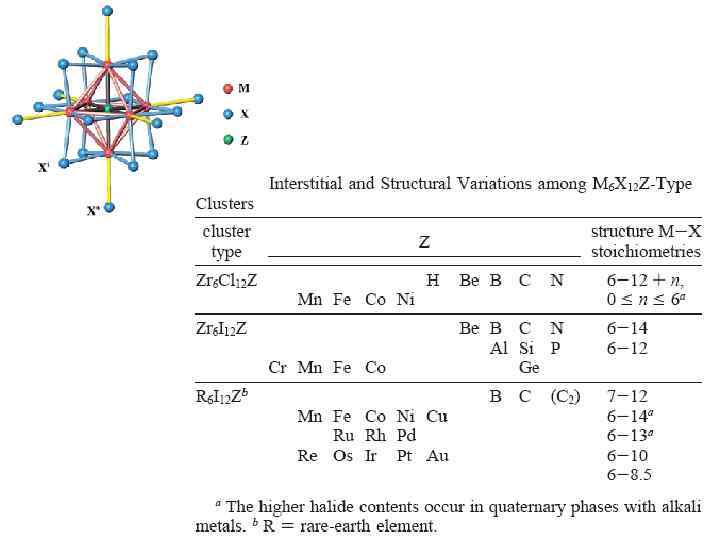
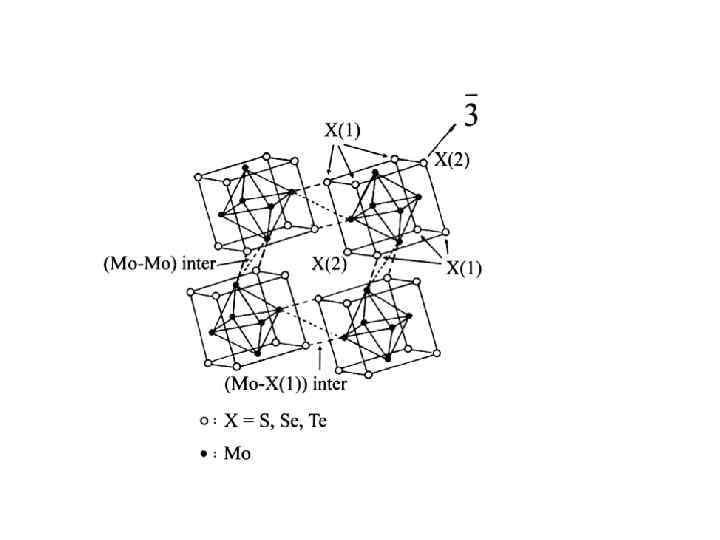
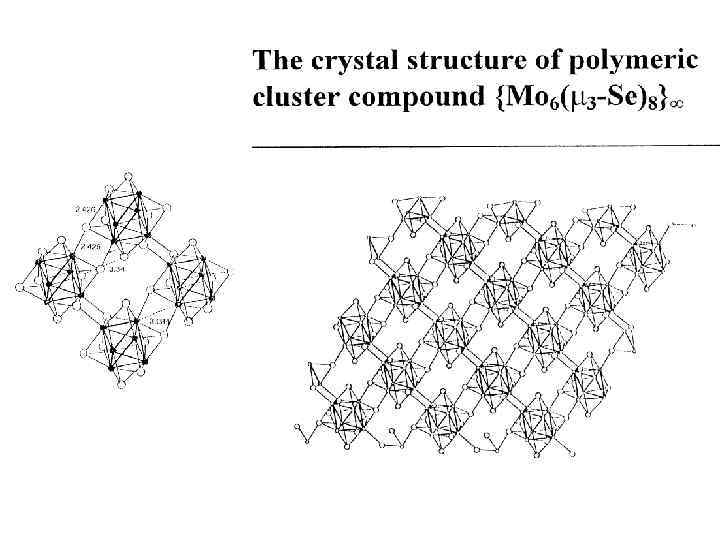
 M 6 Q i 8 L a 6
M 6 Q i 8 L a 6

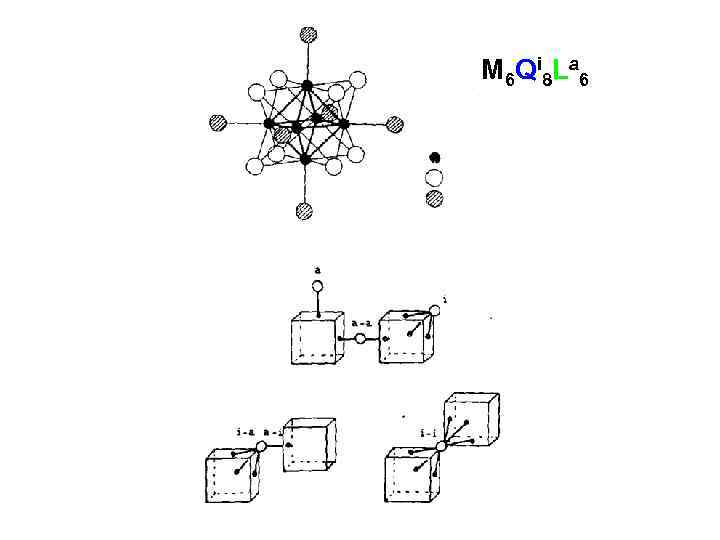
![[Mo 6 Cl 8]Cl 2 Cl 4/2 [Mo 6 Cl 8]Cl 2 Cl 4/2](https://present5.com/presentation/5652994_182163158/image-12.jpg) [Mo 6 Cl 8]Cl 2 Cl 4/2
[Mo 6 Cl 8]Cl 2 Cl 4/2
 Нужна была матрица перехода из моноклинной в ромбоэдрическую ячейку! Известия СО АН СССР, 1966
Нужна была матрица перехода из моноклинной в ромбоэдрическую ячейку! Известия СО АН СССР, 1966
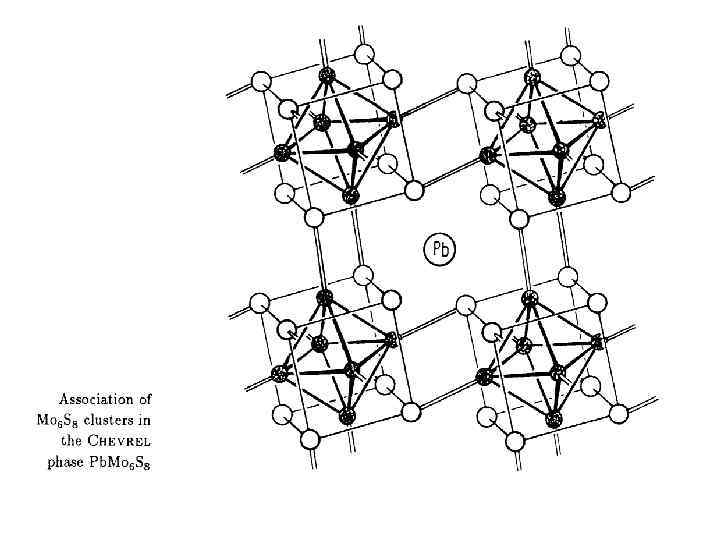
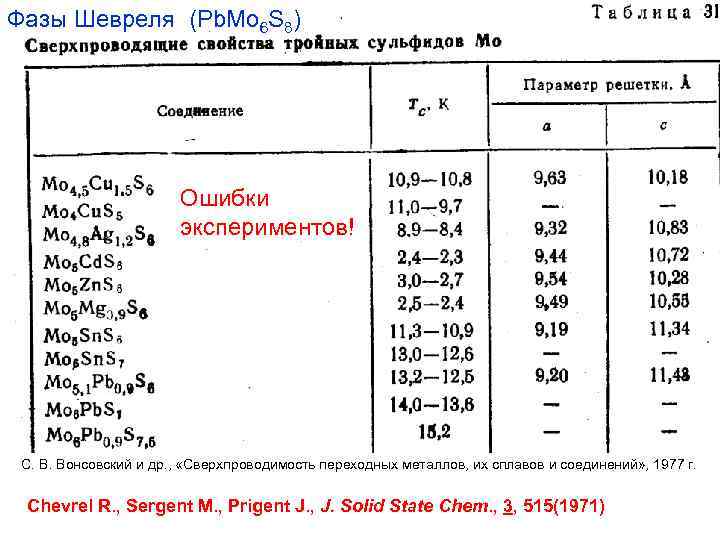 Фазы Шевреля (Pb. Mo 6 S 8) Ошибки экспериментов! C. В. Вонсовский и др. , «Сверхпроводимость переходных металлов, их сплавов и соединений» , 1977 г. Chevrel R. , Sergent M. , Prigent J. , J. Solid State Chem. , 3, 515(1971)
Фазы Шевреля (Pb. Mo 6 S 8) Ошибки экспериментов! C. В. Вонсовский и др. , «Сверхпроводимость переходных металлов, их сплавов и соединений» , 1977 г. Chevrel R. , Sergent M. , Prigent J. , J. Solid State Chem. , 3, 515(1971)
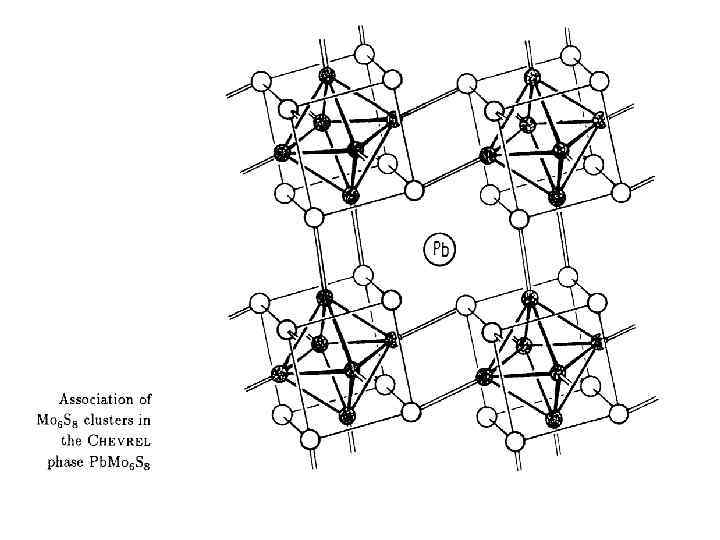
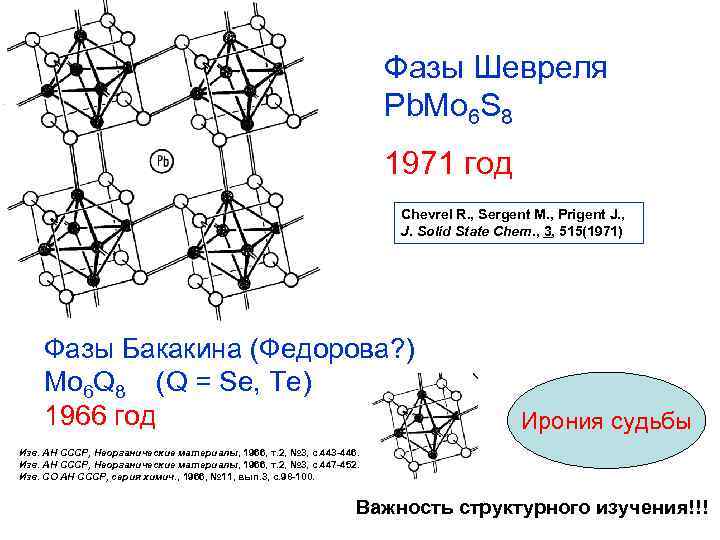 Фазы Шевреля Pb. Mo 6 S 8 1971 год Chevrel R. , Sergent M. , Prigent J. , J. Solid State Chem. , 3, 515(1971) Фазы Бакакина (Федорова? ) Mo 6 Q 8 (Q = Se, Te) 1966 год Ирония судьбы Изв. АН СССР, Неорганические материалы, 1966, т. 2, № 3, с. 443 -446. Изв. АН СССР, Неорганические материалы, 1966, т. 2, № 3, с. 447 -452. Изв. СО АН СССР, серия химич. , 1966, № 11, вып. 3, с. 98 -100. Важность структурного изучения!!!
Фазы Шевреля Pb. Mo 6 S 8 1971 год Chevrel R. , Sergent M. , Prigent J. , J. Solid State Chem. , 3, 515(1971) Фазы Бакакина (Федорова? ) Mo 6 Q 8 (Q = Se, Te) 1966 год Ирония судьбы Изв. АН СССР, Неорганические материалы, 1966, т. 2, № 3, с. 443 -446. Изв. АН СССР, Неорганические материалы, 1966, т. 2, № 3, с. 447 -452. Изв. СО АН СССР, серия химич. , 1966, № 11, вып. 3, с. 98 -100. Важность структурного изучения!!!
 Universite de Rennes 1, October 18, 2007
Universite de Rennes 1, October 18, 2007
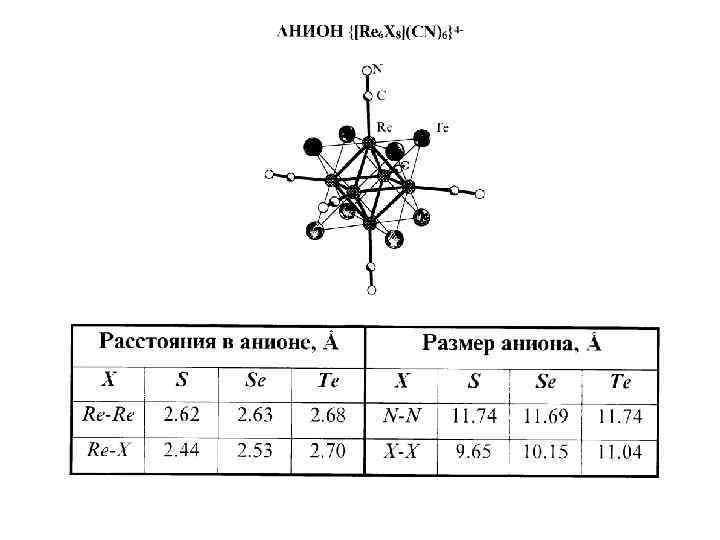
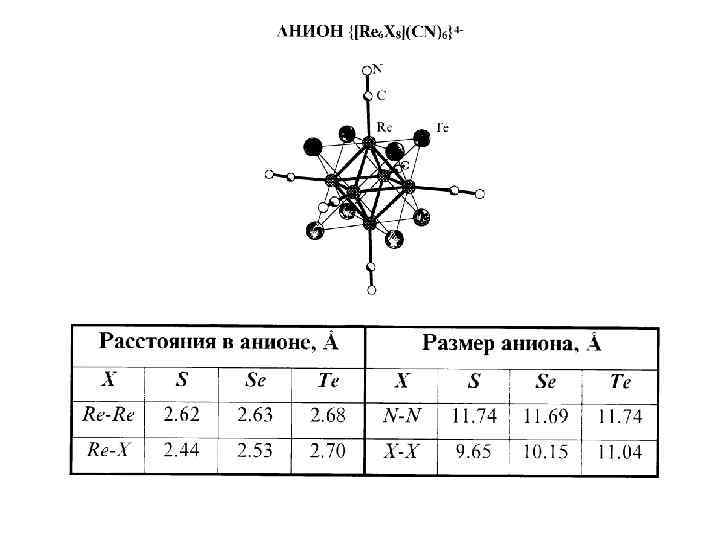
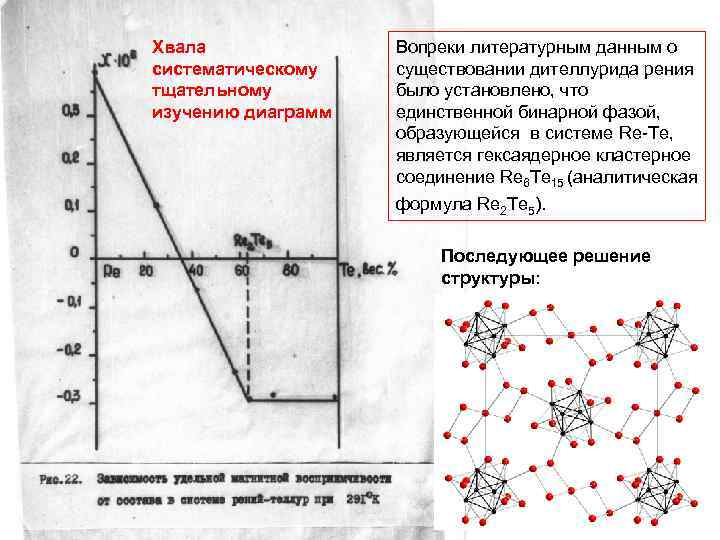 Хвала систематическому тщательному изучению диаграмм Вопреки литературным данным о существовании дителлурида рения было установлено, что единственной бинарной фазой, образующейся в системе Re-Te, является гексаядерное кластерное соединение Re 6 Te 15 (аналитическая формула Re 2 Te 5). Последующее решение структуры:
Хвала систематическому тщательному изучению диаграмм Вопреки литературным данным о существовании дителлурида рения было установлено, что единственной бинарной фазой, образующейся в системе Re-Te, является гексаядерное кластерное соединение Re 6 Te 15 (аналитическая формула Re 2 Te 5). Последующее решение структуры:
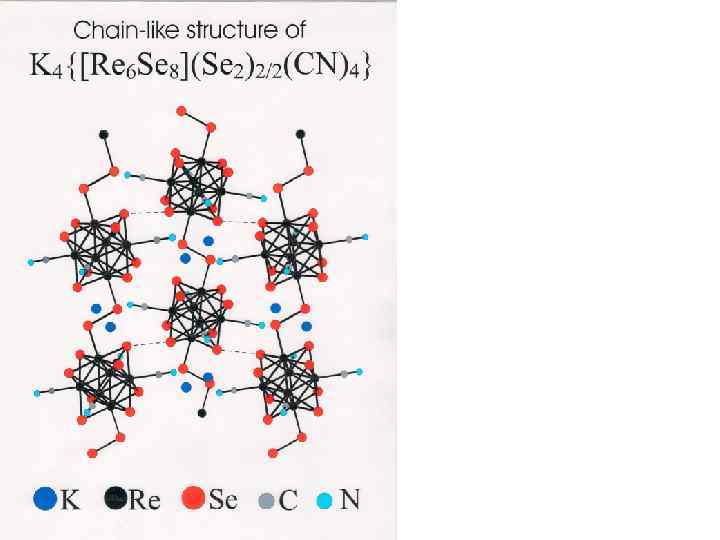
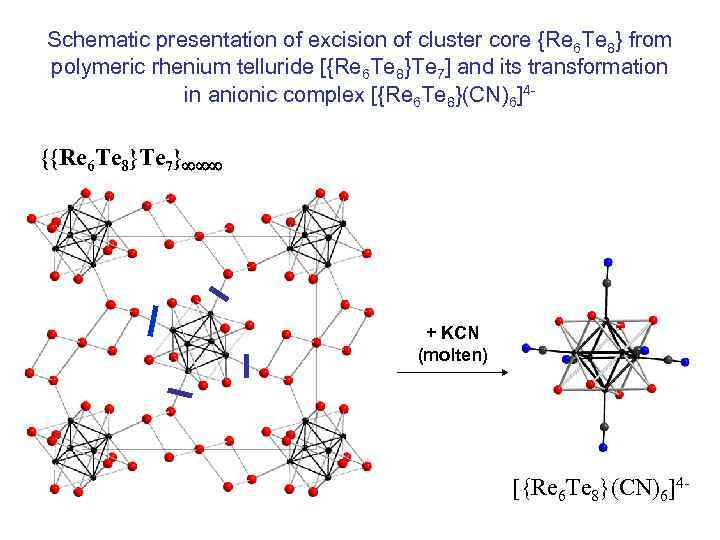 Schematic presentation of excision of cluster core {Re 6 Te 8} from polymeric rhenium telluride [{Re 6 Te 8}Te 7] and its transformation in anionic complex [{Re 6 Te 8}(CN)6]4 - {{Re 6 Te 8}Te 7} + KCN (molten) [{Re 6 Te 8}(CN)6]4 -
Schematic presentation of excision of cluster core {Re 6 Te 8} from polymeric rhenium telluride [{Re 6 Te 8}Te 7] and its transformation in anionic complex [{Re 6 Te 8}(CN)6]4 - {{Re 6 Te 8}Te 7} + KCN (molten) [{Re 6 Te 8}(CN)6]4 -
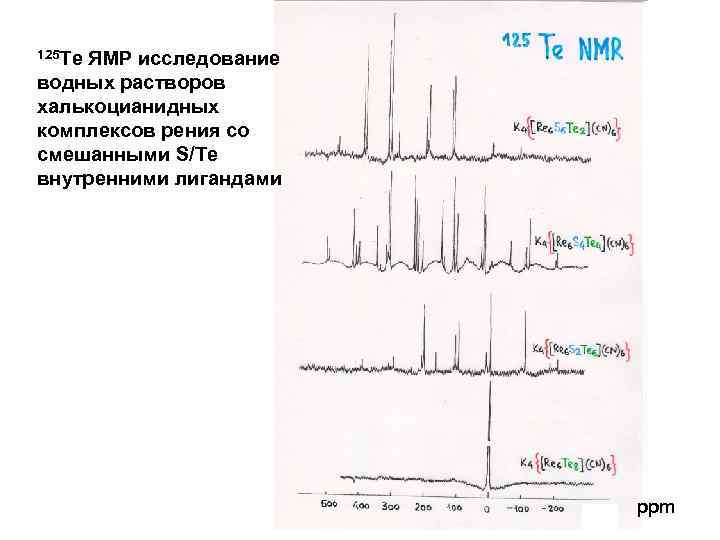 125 Te ЯМР исследование водных растворов халькоцианидных комплексов рения со смешанными S/Te внутренними лигандами ppm
125 Te ЯМР исследование водных растворов халькоцианидных комплексов рения со смешанными S/Te внутренними лигандами ppm
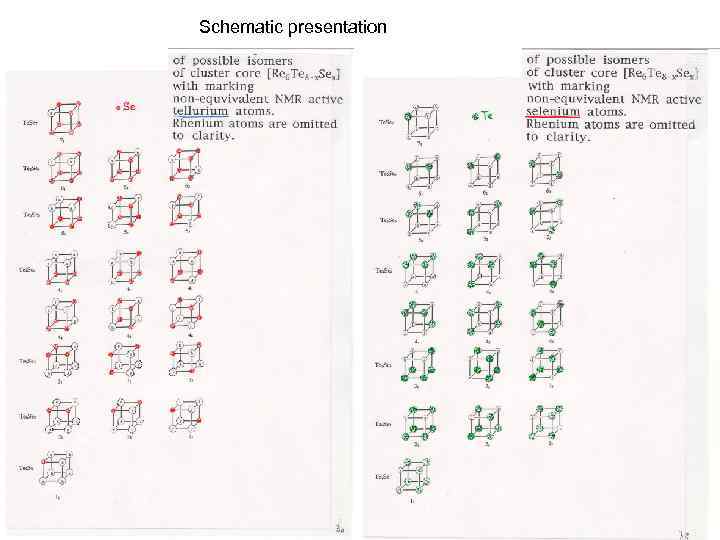 Schematic presentation
Schematic presentation
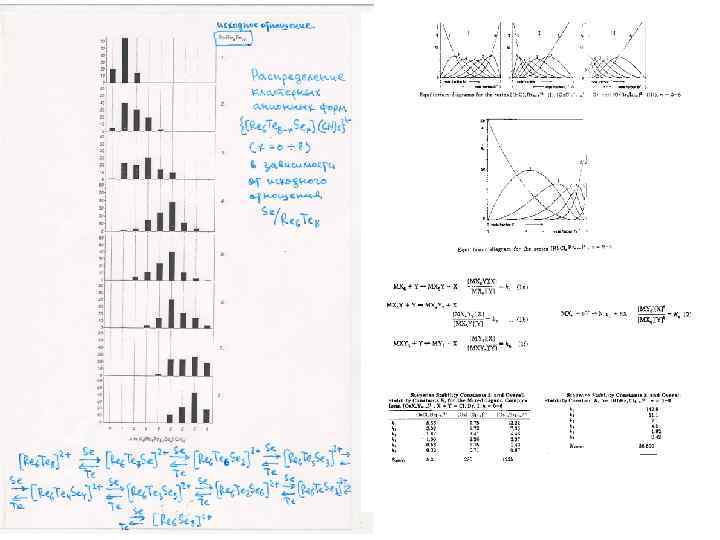
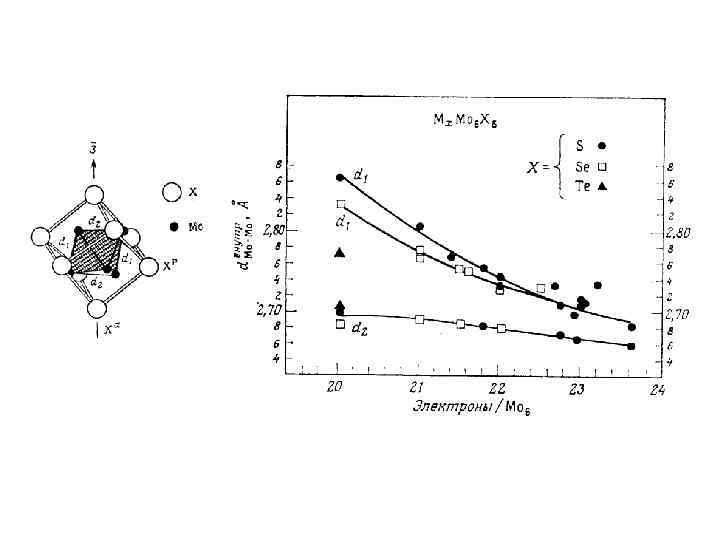
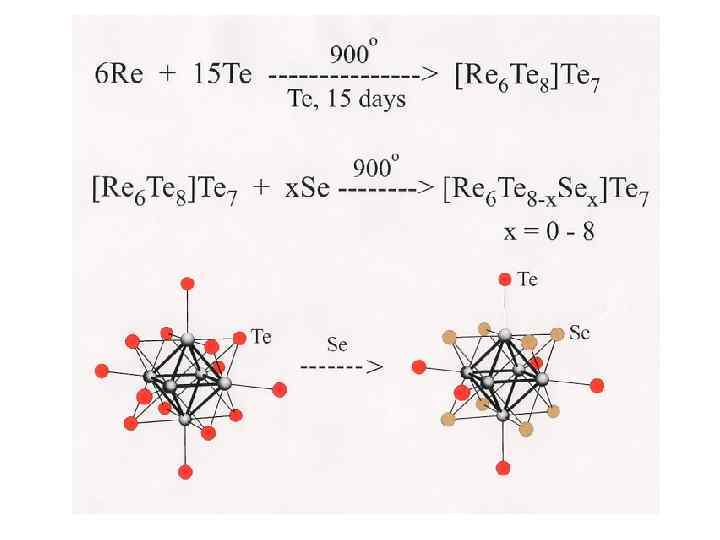
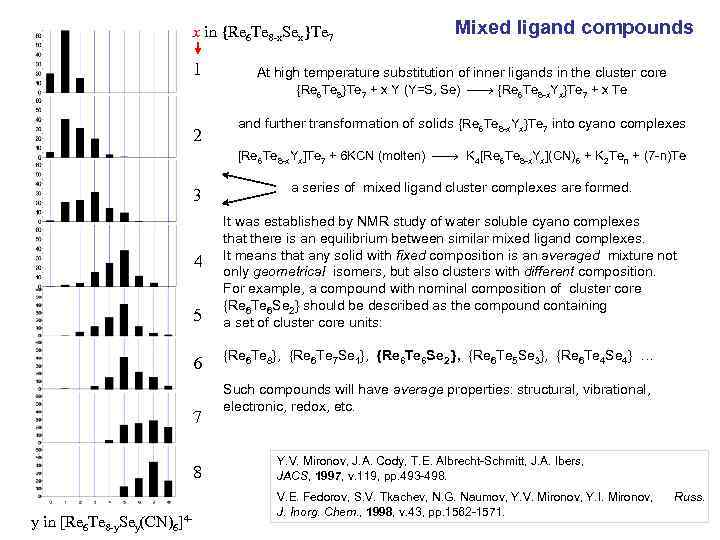 x in {Re 6 Te 8 -x. Sex}Te 7 1 Mixed ligand compounds At high temperature substitution of inner ligands in the cluster core {Re 6 Te 8}Te 7 + x Y (Y=S, Se) {Re 6 Te 8 -x. Yx}Te 7 + x Te 2 and further transformation of solids {Re 6 Te 8 -x. Yx}Te 7 into cyano complexes [Re 6 Te 8 -x. Yx]Te 7 + 6 KCN (molten) K 4[Re 6 Te 8 -x. Yx](CN)6 + K 2 Ten + (7 -n)Te 3 a series of mixed ligand cluster complexes are formed. 5 It was established by NMR study of water soluble cyano complexes that there is an equilibrium between similar mixed ligand complexes. It means that any solid with fixed composition is an averaged mixture not only geometrical isomers, but also clusters with different composition. For example, a compound with nominal composition of cluster core {Re 6 Te 6 Se 2} should be described as the compound containing a set of cluster core units: 6 {Re 6 Te 8}, {Re 6 Te 7 Se 1}, {Re 6 Te 6 Se 2 }, {Re 6 Te 5 Se 3}, {Re 6 Te 4 Se 4} … 4 7 8 y in [Re 6 Te 8 -y. Sey(CN)6]4 - Such compounds will have average properties: structural, vibrational, electronic, redox, etc. Y. V. Mironov, J. A. Cody, T. E. Albrecht-Schmitt, J. A. Ibers, JACS, 1997, v. 119, pp. 493 -498. V. E. Fedorov, S. V. Tkachev, N. G. Naumov, Y. V. Mironov, Y. I. Mironov, J. Inorg. Chem. , 1998, v. 43, pp. 1562 -1571. Russ.
x in {Re 6 Te 8 -x. Sex}Te 7 1 Mixed ligand compounds At high temperature substitution of inner ligands in the cluster core {Re 6 Te 8}Te 7 + x Y (Y=S, Se) {Re 6 Te 8 -x. Yx}Te 7 + x Te 2 and further transformation of solids {Re 6 Te 8 -x. Yx}Te 7 into cyano complexes [Re 6 Te 8 -x. Yx]Te 7 + 6 KCN (molten) K 4[Re 6 Te 8 -x. Yx](CN)6 + K 2 Ten + (7 -n)Te 3 a series of mixed ligand cluster complexes are formed. 5 It was established by NMR study of water soluble cyano complexes that there is an equilibrium between similar mixed ligand complexes. It means that any solid with fixed composition is an averaged mixture not only geometrical isomers, but also clusters with different composition. For example, a compound with nominal composition of cluster core {Re 6 Te 6 Se 2} should be described as the compound containing a set of cluster core units: 6 {Re 6 Te 8}, {Re 6 Te 7 Se 1}, {Re 6 Te 6 Se 2 }, {Re 6 Te 5 Se 3}, {Re 6 Te 4 Se 4} … 4 7 8 y in [Re 6 Te 8 -y. Sey(CN)6]4 - Such compounds will have average properties: structural, vibrational, electronic, redox, etc. Y. V. Mironov, J. A. Cody, T. E. Albrecht-Schmitt, J. A. Ibers, JACS, 1997, v. 119, pp. 493 -498. V. E. Fedorov, S. V. Tkachev, N. G. Naumov, Y. V. Mironov, Y. I. Mironov, J. Inorg. Chem. , 1998, v. 43, pp. 1562 -1571. Russ.
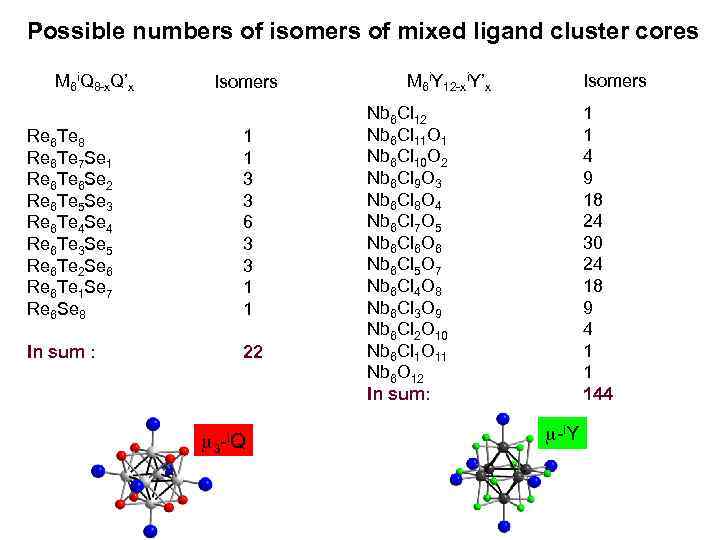 Possible numbers of isomers of mixed ligand cluster cores M 6 i. Q 8 -x. Q’x Isomers Re 6 Te 8 Re 6 Te 7 Se 1 Re 6 Te 6 Se 2 Re 6 Te 5 Se 3 Re 6 Te 4 Se 4 Re 6 Te 3 Se 5 Re 6 Te 2 Se 6 Re 6 Te 1 Se 7 Re 6 Se 8 1 1 3 3 6 3 3 1 1 In sum : 22 m 3 -i. Q M 6 i. Y 12 -xi. Y’x Isomers Nb 6 Cl 12 Nb 6 Cl 11 O 1 Nb 6 Cl 10 O 2 Nb 6 Cl 9 O 3 Nb 6 Cl 8 O 4 Nb 6 Cl 7 O 5 Nb 6 Cl 6 O 6 Nb 6 Cl 5 O 7 Nb 6 Cl 4 O 8 Nb 6 Cl 3 O 9 Nb 6 Cl 2 O 10 Nb 6 Cl 1 O 11 Nb 6 O 12 In sum: 1 1 4 9 18 24 30 24 18 9 4 1 1 144 m-i. Y
Possible numbers of isomers of mixed ligand cluster cores M 6 i. Q 8 -x. Q’x Isomers Re 6 Te 8 Re 6 Te 7 Se 1 Re 6 Te 6 Se 2 Re 6 Te 5 Se 3 Re 6 Te 4 Se 4 Re 6 Te 3 Se 5 Re 6 Te 2 Se 6 Re 6 Te 1 Se 7 Re 6 Se 8 1 1 3 3 6 3 3 1 1 In sum : 22 m 3 -i. Q M 6 i. Y 12 -xi. Y’x Isomers Nb 6 Cl 12 Nb 6 Cl 11 O 1 Nb 6 Cl 10 O 2 Nb 6 Cl 9 O 3 Nb 6 Cl 8 O 4 Nb 6 Cl 7 O 5 Nb 6 Cl 6 O 6 Nb 6 Cl 5 O 7 Nb 6 Cl 4 O 8 Nb 6 Cl 3 O 9 Nb 6 Cl 2 O 10 Nb 6 Cl 1 O 11 Nb 6 O 12 In sum: 1 1 4 9 18 24 30 24 18 9 4 1 1 144 m-i. Y
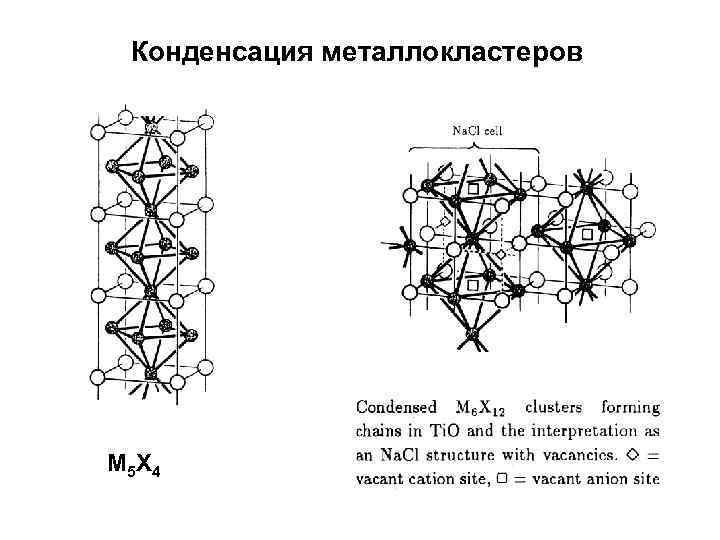 Конденсация металлокластеров M 5 X 4
Конденсация металлокластеров M 5 X 4
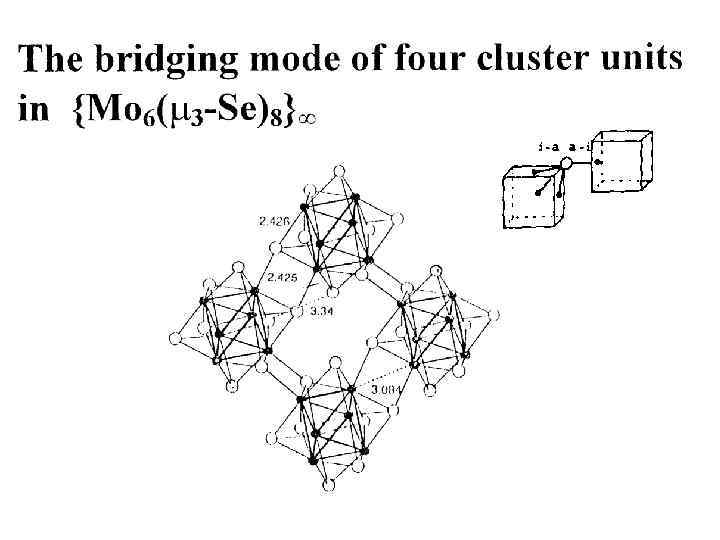
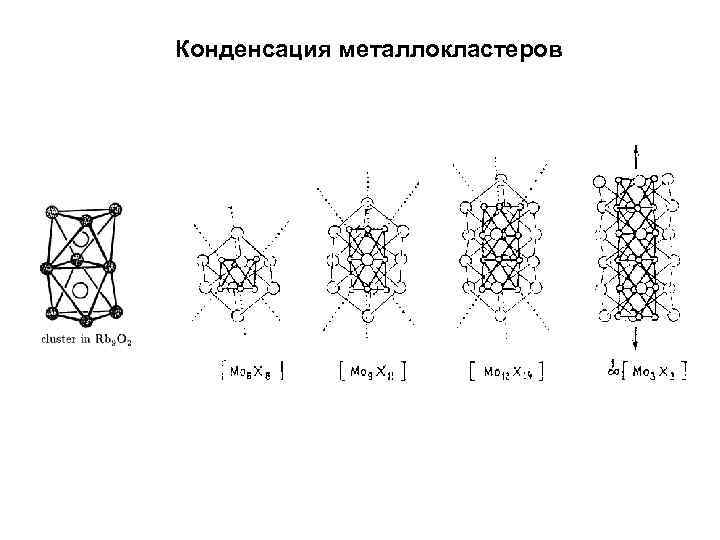 Конденсация металлокластеров
Конденсация металлокластеров
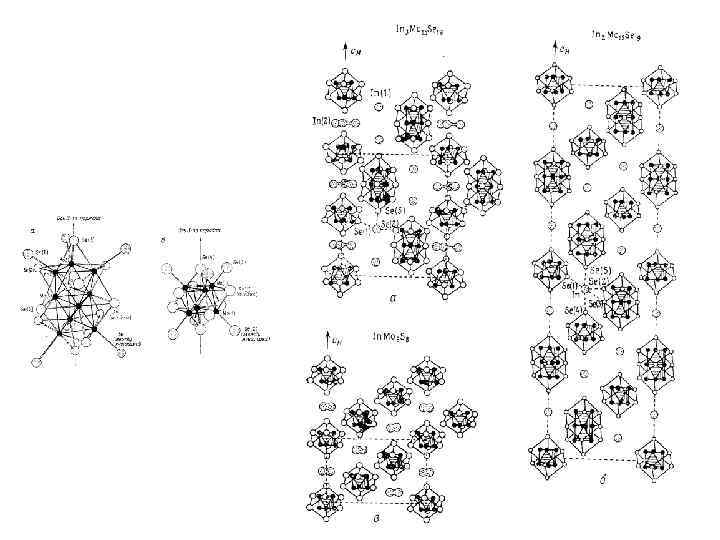
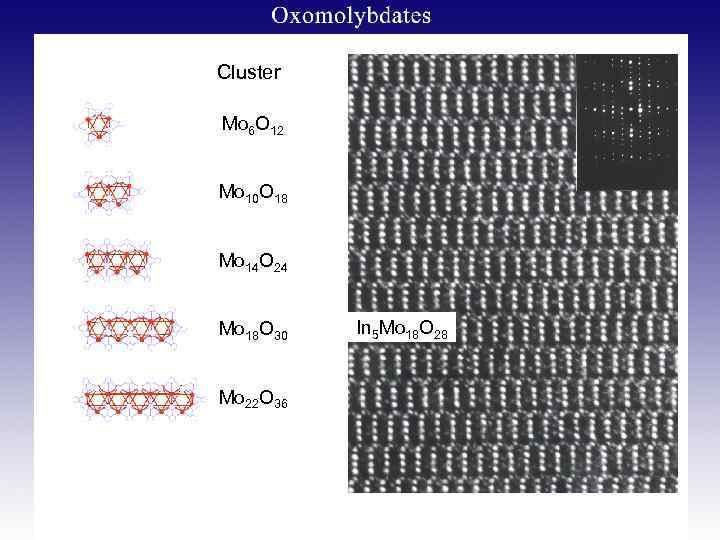 Cluster Mo 6 O 12 Mo 10 O 18 Mo 14 O 24 Mo 18 O 30 Mo 22 O 36 In 5 Mo 18 O 28
Cluster Mo 6 O 12 Mo 10 O 18 Mo 14 O 24 Mo 18 O 30 Mo 22 O 36 In 5 Mo 18 O 28



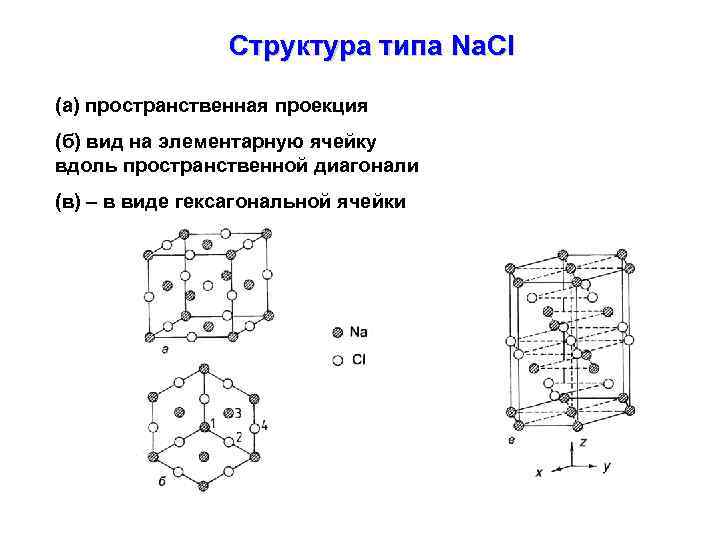 Структура типа Na. Cl (а) пространственная проекция (б) вид на элементарную ячейку вдоль пространственной диагонали (в) – в виде гексагональной ячейки
Структура типа Na. Cl (а) пространственная проекция (б) вид на элементарную ячейку вдоль пространственной диагонали (в) – в виде гексагональной ячейки
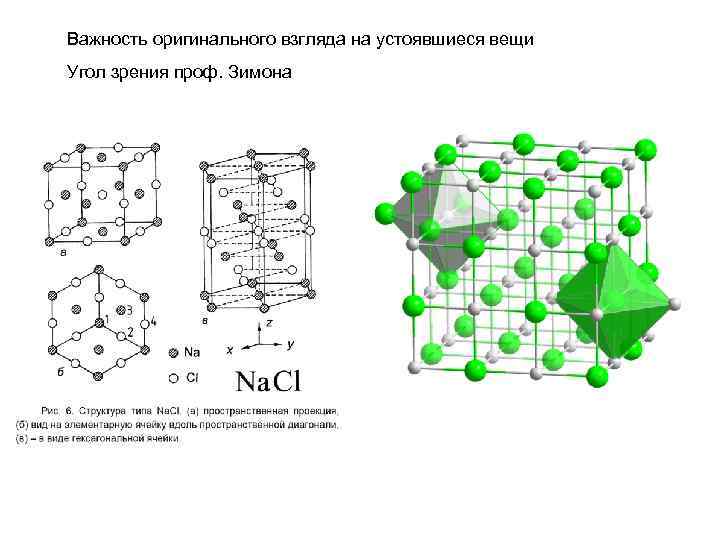 Важность оригинального взгляда на устоявшиеся вещи Угол зрения проф. Зимона
Важность оригинального взгляда на устоявшиеся вещи Угол зрения проф. Зимона

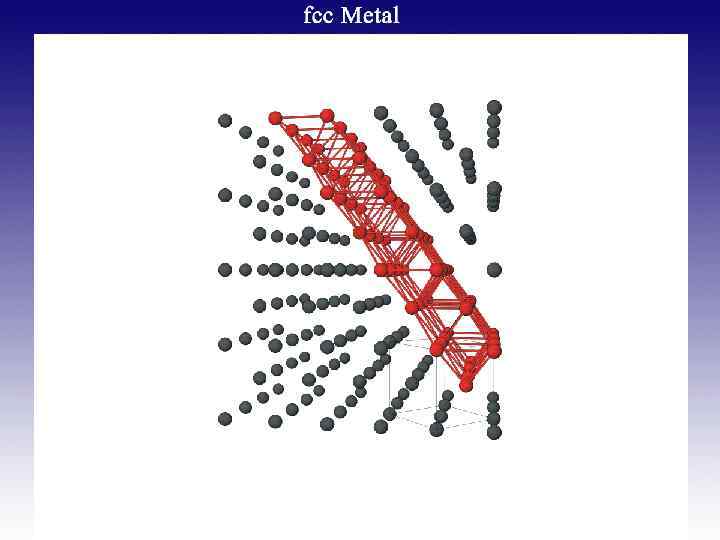
![Octahedral [Re 6(m 3 -Q)8]2+ Cluster Well-defined geometry Expanded dimension Unique properties Gabriel, Boubekeur, Octahedral [Re 6(m 3 -Q)8]2+ Cluster Well-defined geometry Expanded dimension Unique properties Gabriel, Boubekeur,](https://present5.com/presentation/5652994_182163158/image-53.jpg) Octahedral [Re 6(m 3 -Q)8]2+ Cluster Well-defined geometry Expanded dimension Unique properties Gabriel, Boubekeur, Uriel, and Batail, Chem. Rev. 2001, 101, 2037.
Octahedral [Re 6(m 3 -Q)8]2+ Cluster Well-defined geometry Expanded dimension Unique properties Gabriel, Boubekeur, Uriel, and Batail, Chem. Rev. 2001, 101, 2037.
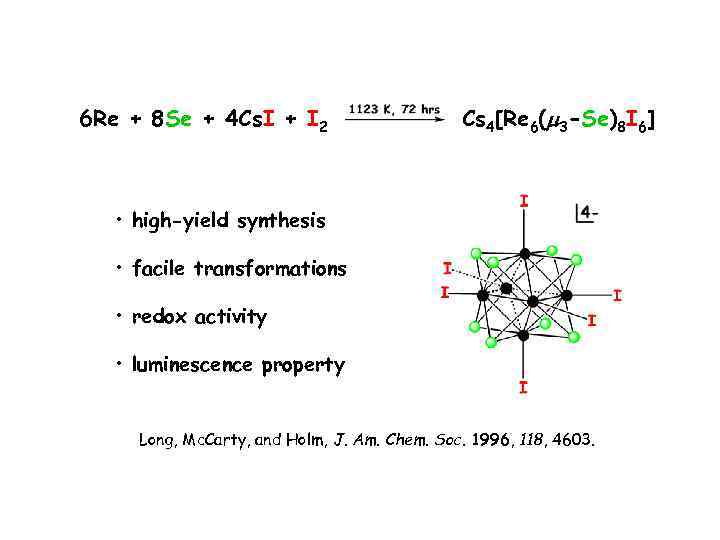 6 Re + 8 Se + 4 Cs. I + I 2 Cs 4[Re 6(m 3 -Se)8 I 6] • high-yield synthesis • facile transformations • redox activity • luminescence property Long, Mc. Carty, and Holm, J. Am. Chem. Soc. 1996, 118, 4603.
6 Re + 8 Se + 4 Cs. I + I 2 Cs 4[Re 6(m 3 -Se)8 I 6] • high-yield synthesis • facile transformations • redox activity • luminescence property Long, Mc. Carty, and Holm, J. Am. Chem. Soc. 1996, 118, 4603.
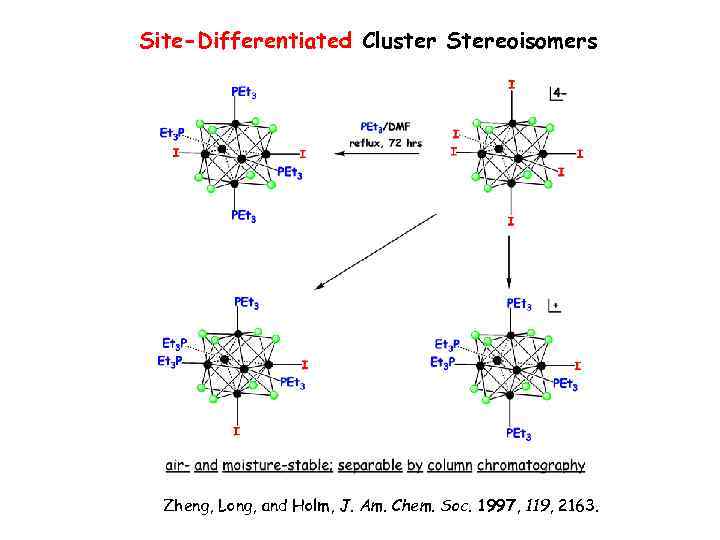 Site-Differentiated Cluster Stereoisomers Zheng, Long, and Holm, J. Am. Chem. Soc. 1997, 119, 2163.
Site-Differentiated Cluster Stereoisomers Zheng, Long, and Holm, J. Am. Chem. Soc. 1997, 119, 2163.
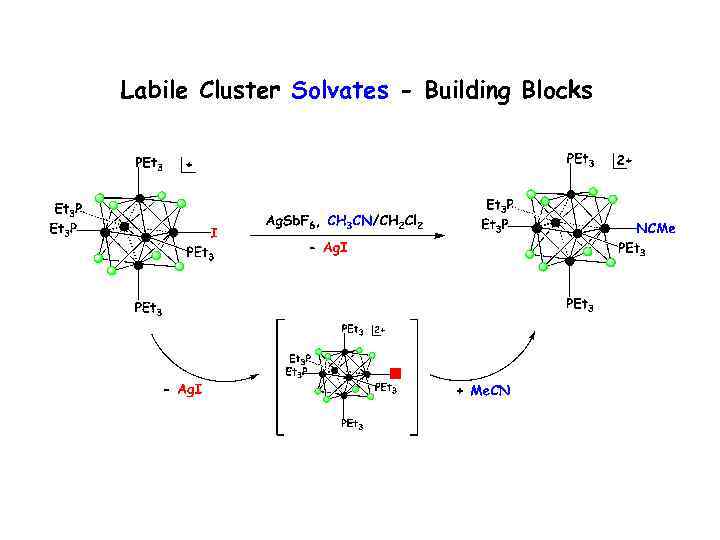 Labile Cluster Solvates - Building Blocks
Labile Cluster Solvates - Building Blocks
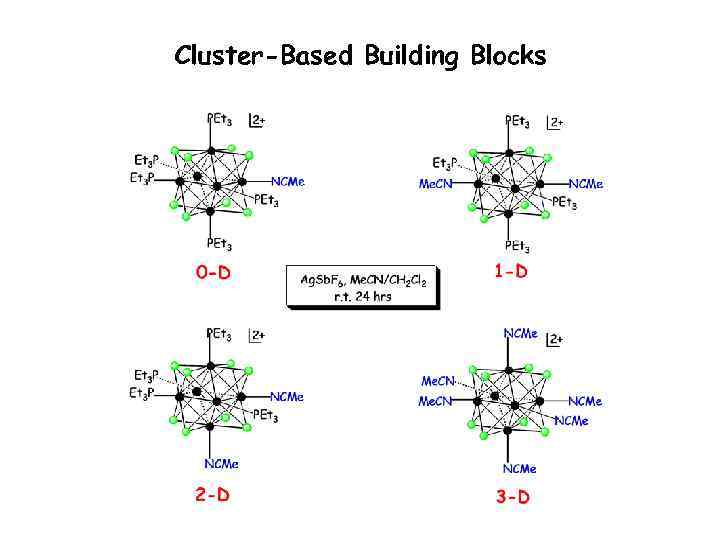 Cluster-Based Building Blocks
Cluster-Based Building Blocks

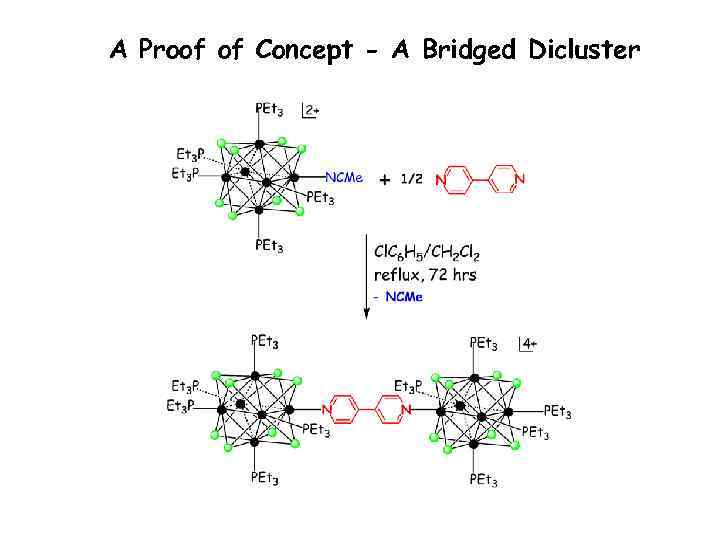 A Proof of Concept - A Bridged Dicluster
A Proof of Concept - A Bridged Dicluster
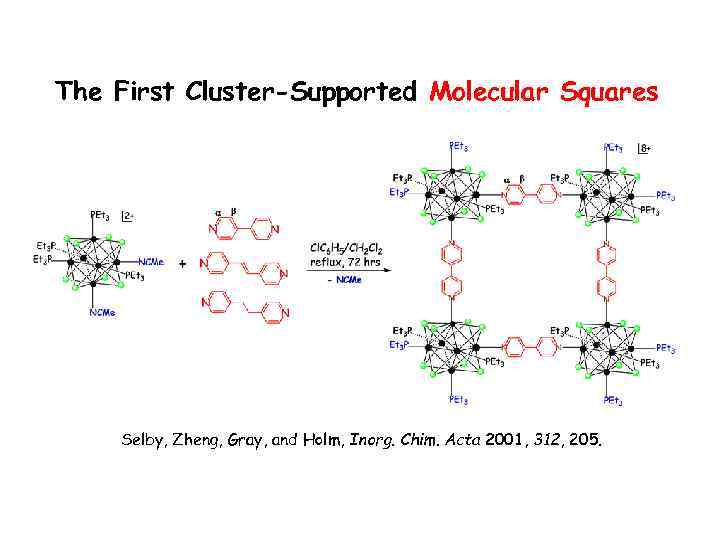 The First Cluster-Supported Molecular Squares Selby, Zheng, Gray, and Holm, Inorg. Chim. Acta 2001, 312, 205.
The First Cluster-Supported Molecular Squares Selby, Zheng, Gray, and Holm, Inorg. Chim. Acta 2001, 312, 205.
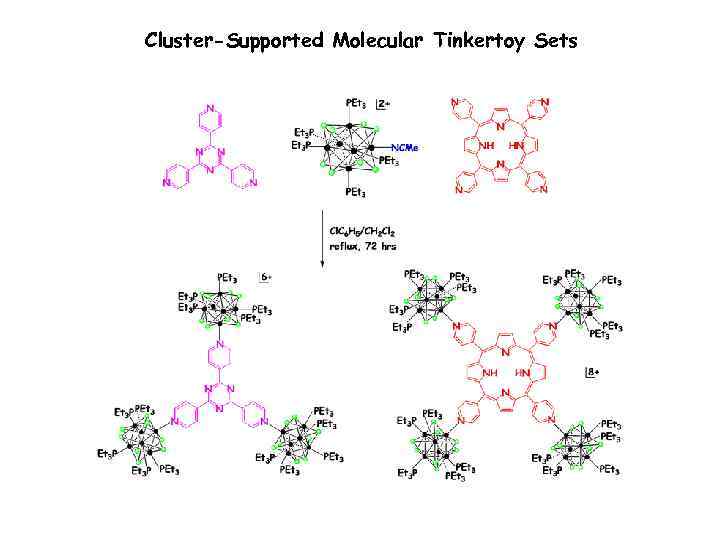 Cluster-Supported Molecular Tinkertoy Sets
Cluster-Supported Molecular Tinkertoy Sets
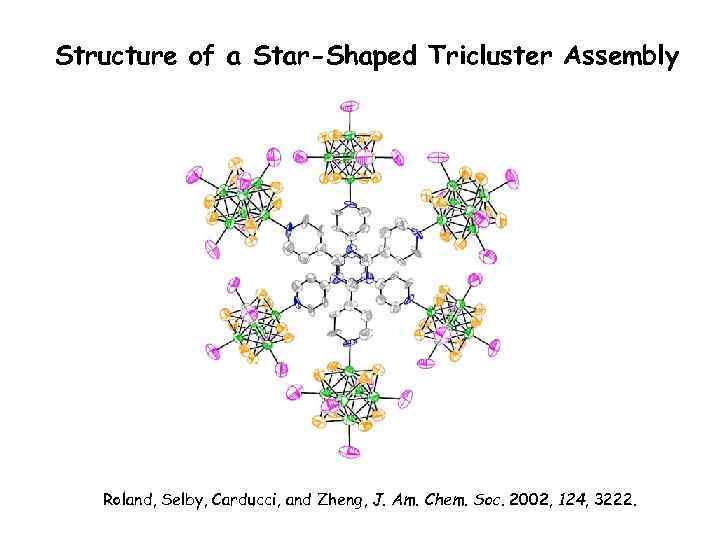 Structure of a Star-Shaped Tricluster Assembly Roland, Selby, Carducci, and Zheng, J. Am. Chem. Soc. 2002, 124, 3222.
Structure of a Star-Shaped Tricluster Assembly Roland, Selby, Carducci, and Zheng, J. Am. Chem. Soc. 2002, 124, 3222.
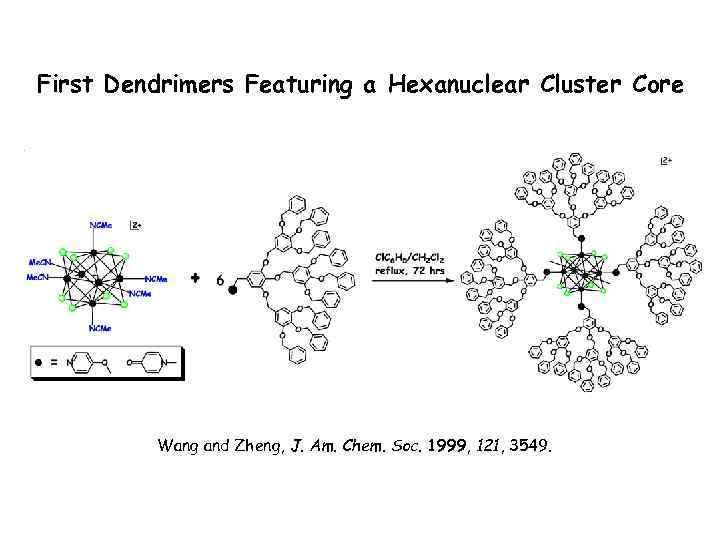 First Dendrimers Featuring a Hexanuclear Cluster Core Wang and Zheng, J. Am. Chem. Soc. 1999, 121, 3549.
First Dendrimers Featuring a Hexanuclear Cluster Core Wang and Zheng, J. Am. Chem. Soc. 1999, 121, 3549.
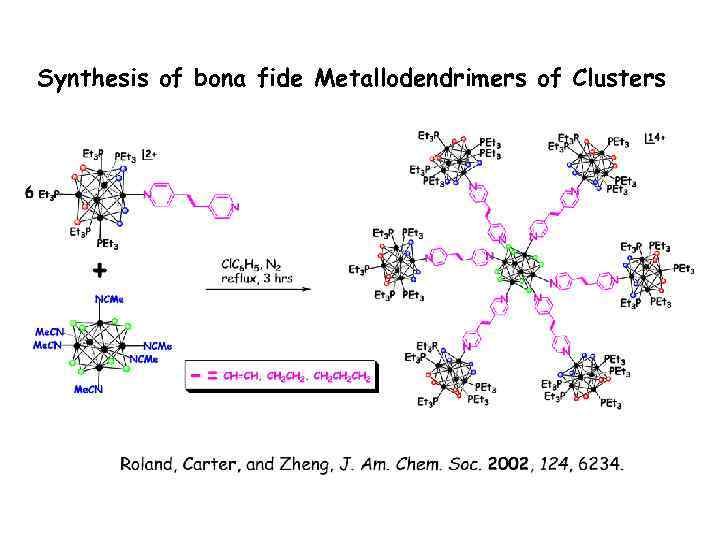 Synthesis of bona fide Metallodendrimers of Clusters
Synthesis of bona fide Metallodendrimers of Clusters
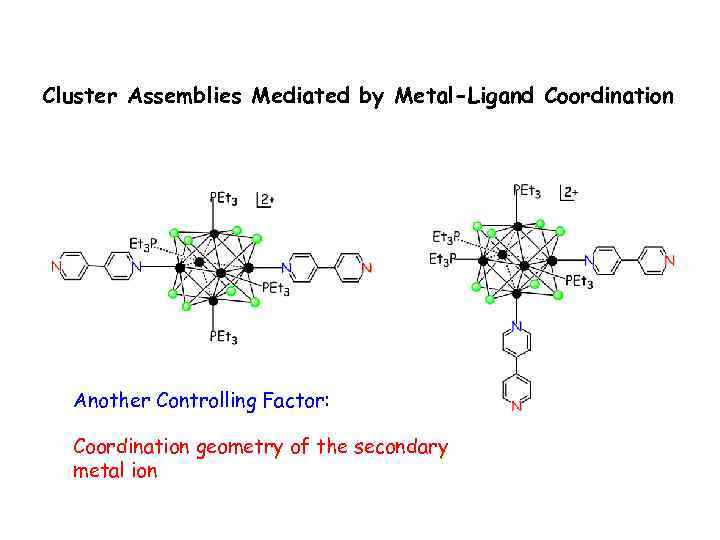 Cluster Assemblies Mediated by Metal-Ligand Coordination Another Controlling Factor: Coordination geometry of the secondary metal ion
Cluster Assemblies Mediated by Metal-Ligand Coordination Another Controlling Factor: Coordination geometry of the secondary metal ion
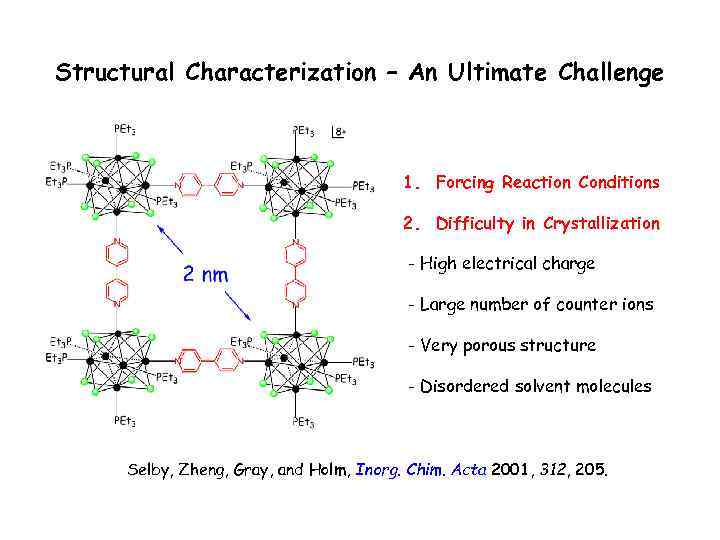 Structural Characterization – An Ultimate Challenge 1. Forcing Reaction Conditions 2. Difficulty in Crystallization - High electrical charge - Large number of counter ions - Very porous structure - Disordered solvent molecules Selby, Zheng, Gray, and Holm, Inorg. Chim. Acta 2001, 312, 205.
Structural Characterization – An Ultimate Challenge 1. Forcing Reaction Conditions 2. Difficulty in Crystallization - High electrical charge - Large number of counter ions - Very porous structure - Disordered solvent molecules Selby, Zheng, Gray, and Holm, Inorg. Chim. Acta 2001, 312, 205.
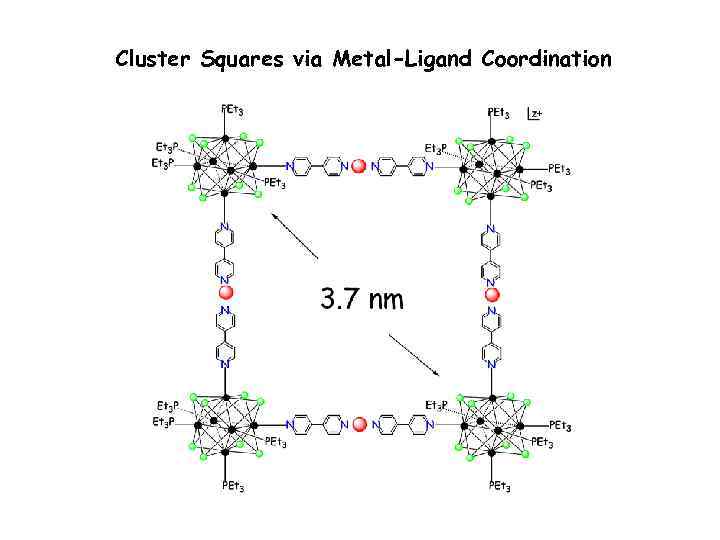 Cluster Squares via Metal-Ligand Coordination
Cluster Squares via Metal-Ligand Coordination
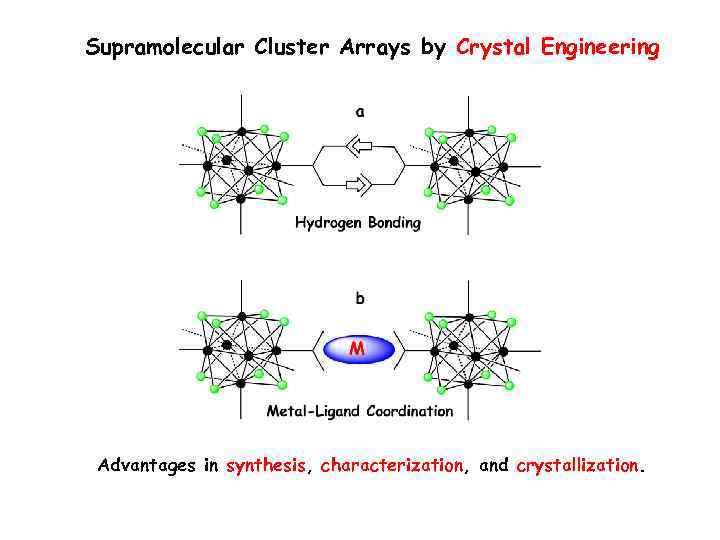 Supramolecular Cluster Arrays by Crystal Engineering Advantages in synthesis, characterization, and crystallization.
Supramolecular Cluster Arrays by Crystal Engineering Advantages in synthesis, characterization, and crystallization.
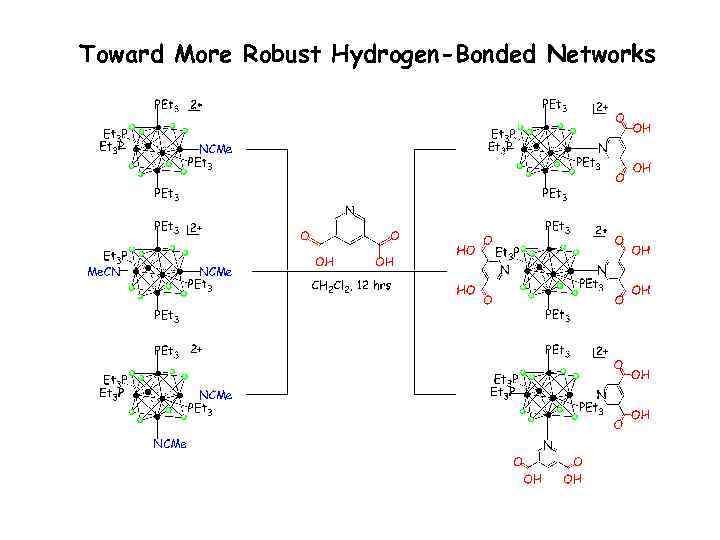 Toward More Robust Hydrogen-Bonded Networks
Toward More Robust Hydrogen-Bonded Networks
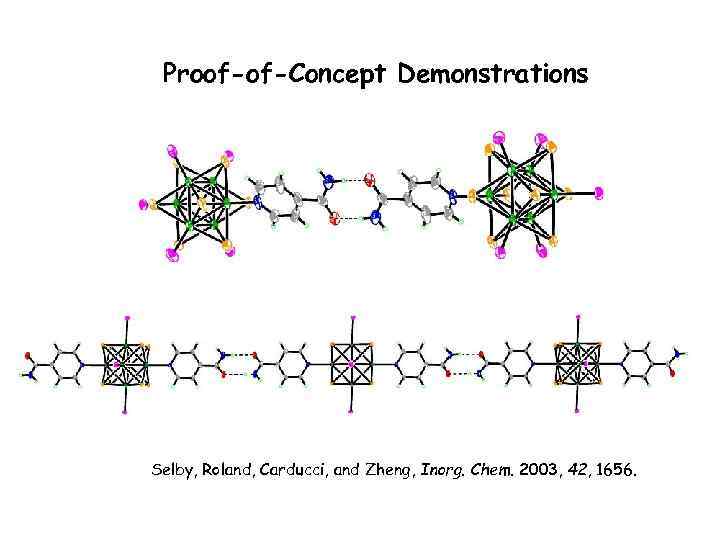 Proof-of-Concept Demonstrations Selby, Roland, Carducci, and Zheng, Inorg. Chem. 2003, 42, 1656.
Proof-of-Concept Demonstrations Selby, Roland, Carducci, and Zheng, Inorg. Chem. 2003, 42, 1656.
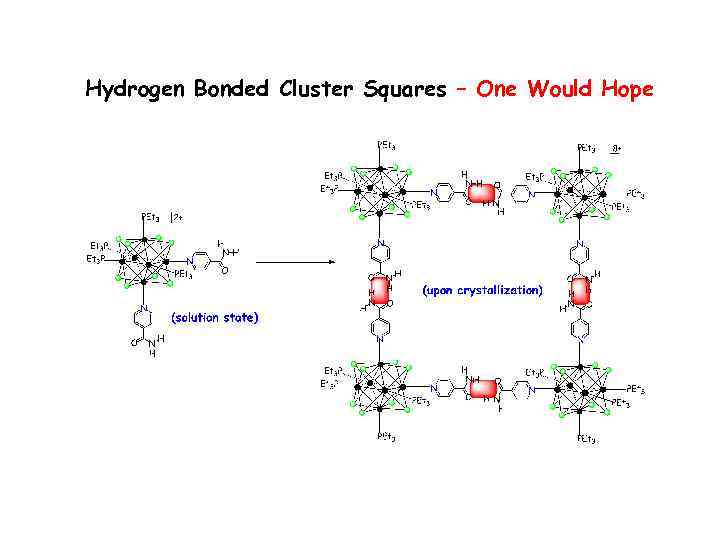 Hydrogen Bonded Cluster Squares – One Would Hope
Hydrogen Bonded Cluster Squares – One Would Hope
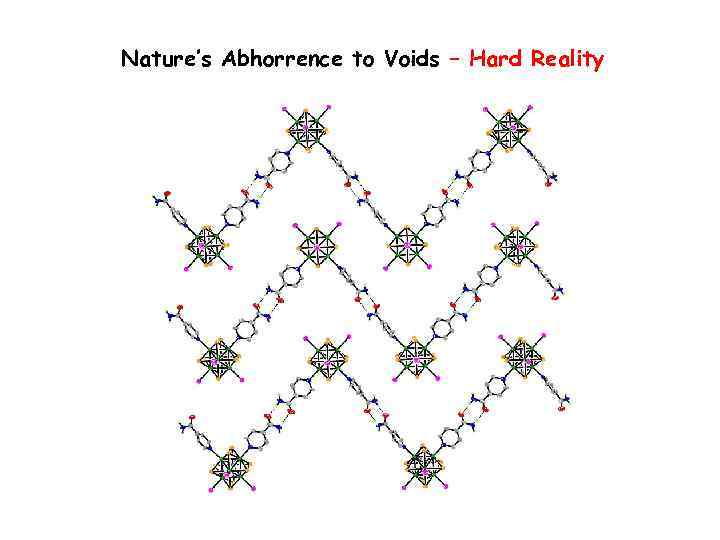 Nature’s Abhorrence to Voids – Hard Reality
Nature’s Abhorrence to Voids – Hard Reality
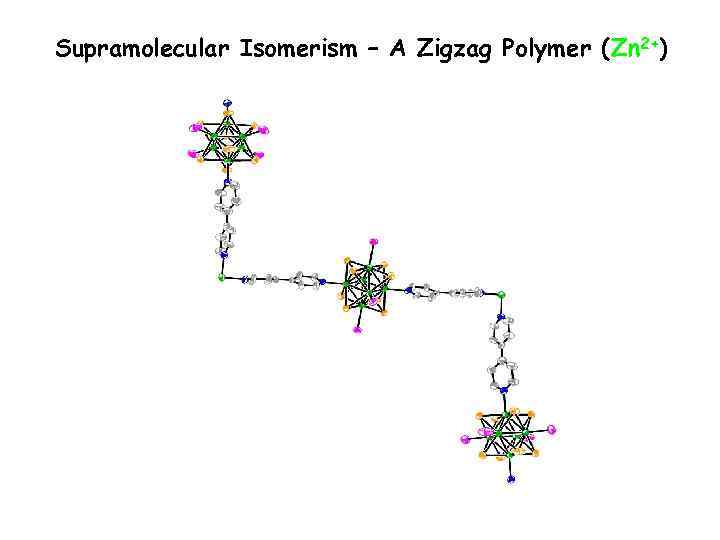 Supramolecular Isomerism – A Zigzag Polymer (Zn 2+)
Supramolecular Isomerism – A Zigzag Polymer (Zn 2+)
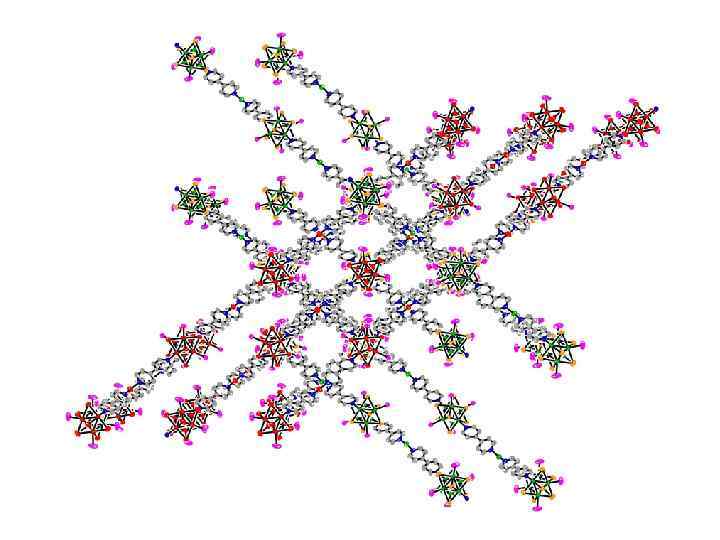
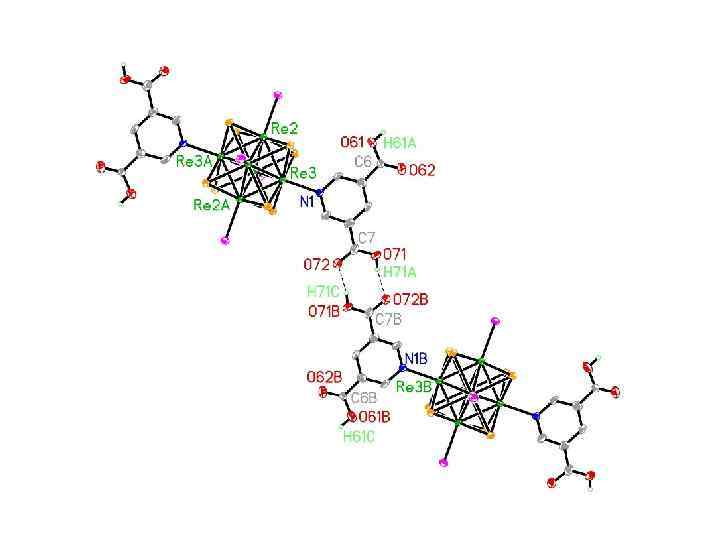
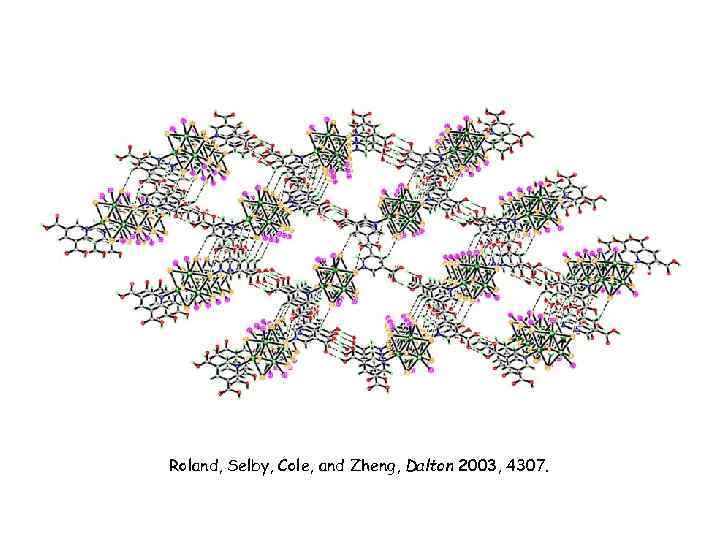 Roland, Selby, Cole, and Zheng, Dalton 2003, 4307.
Roland, Selby, Cole, and Zheng, Dalton 2003, 4307.
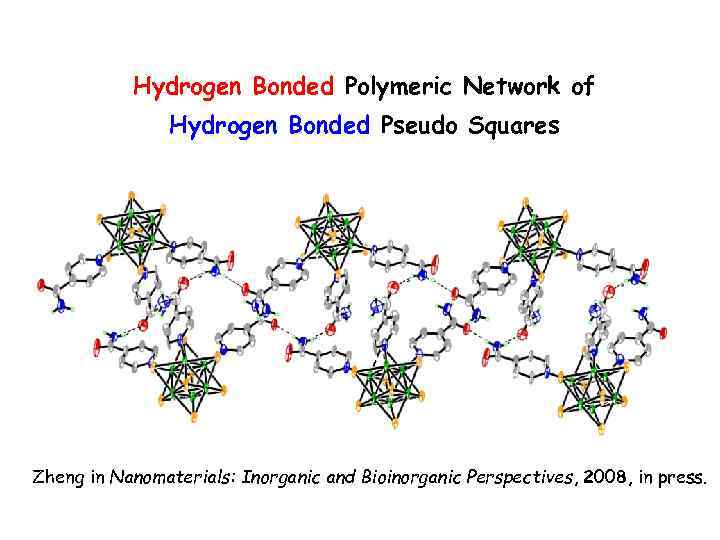 Hydrogen Bonded Polymeric Network of Hydrogen Bonded Pseudo Squares Zheng in Nanomaterials: Inorganic and Bioinorganic Perspectives, 2008, in press.
Hydrogen Bonded Polymeric Network of Hydrogen Bonded Pseudo Squares Zheng in Nanomaterials: Inorganic and Bioinorganic Perspectives, 2008, in press.

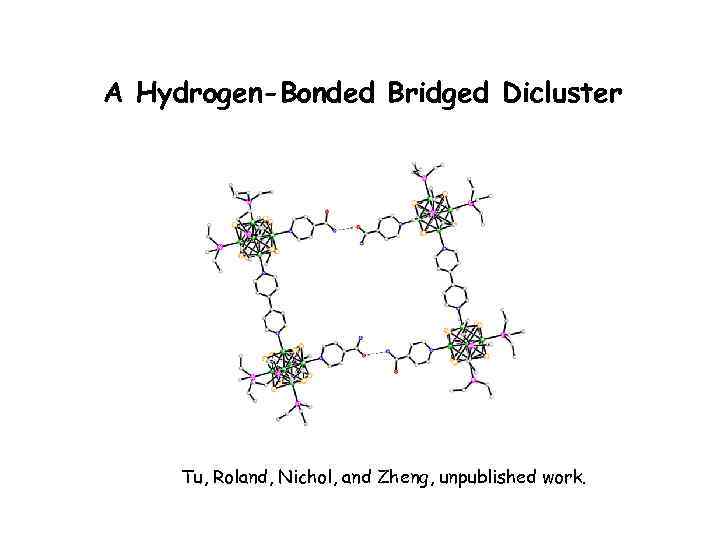 A Hydrogen-Bonded Bridged Dicluster Tu, Roland, Nichol, and Zheng, unpublished work.
A Hydrogen-Bonded Bridged Dicluster Tu, Roland, Nichol, and Zheng, unpublished work.
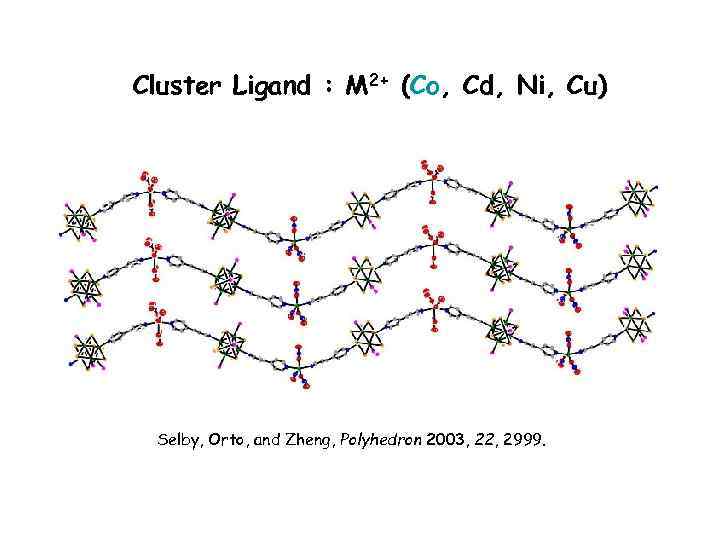 Cluster Ligand : M 2+ (Co, Cd, Ni, Cu) Selby, Orto, and Zheng, Polyhedron 2003, 22, 2999.
Cluster Ligand : M 2+ (Co, Cd, Ni, Cu) Selby, Orto, and Zheng, Polyhedron 2003, 22, 2999.
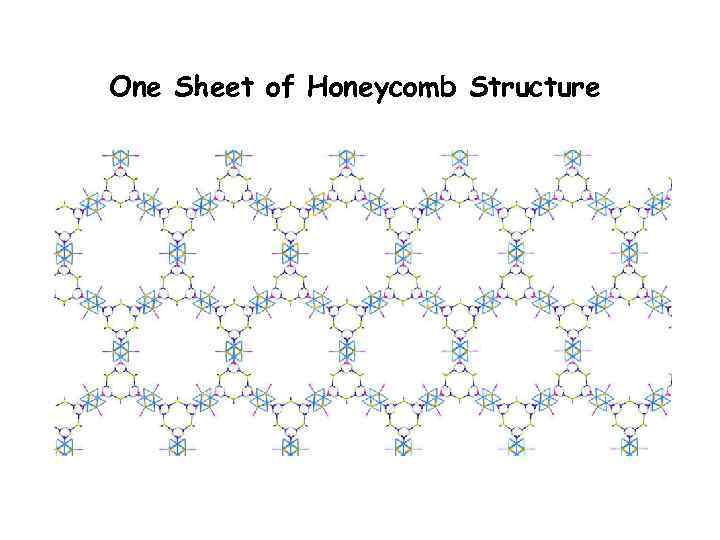 One Sheet of Honeycomb Structure
One Sheet of Honeycomb Structure


