097e04e5e570ceb379e23bdfcb3d1b76.ppt
- Количество слайдов: 98
 LYMPHOMAS
LYMPHOMAS
 INTRODUCTION • Lymphomas are cancers that begin by the malignant transformation of a lymphocyte in the lymphatic system. – the prefix "lymph-" indicates their origination in the malignant change of a lymphocyte and – the suffix "-oma" is derived from the Greek word meaning "tumor. "
INTRODUCTION • Lymphomas are cancers that begin by the malignant transformation of a lymphocyte in the lymphatic system. – the prefix "lymph-" indicates their origination in the malignant change of a lymphocyte and – the suffix "-oma" is derived from the Greek word meaning "tumor. "
 INTRODUCTION • Lymphoma encompasses 2 large groups of neoplasms, namely non-Hodgkin lymphoma (NHL) and Hodgkin disease. • NHL includes many clinicopathologic subtypes, each with distinct epidemiologies; etiologies; morphologic, immunophenotypic, genetic, and clinical features; and responses to therapy.
INTRODUCTION • Lymphoma encompasses 2 large groups of neoplasms, namely non-Hodgkin lymphoma (NHL) and Hodgkin disease. • NHL includes many clinicopathologic subtypes, each with distinct epidemiologies; etiologies; morphologic, immunophenotypic, genetic, and clinical features; and responses to therapy.
 HODGKIN DISEASE (HD)
HODGKIN DISEASE (HD)
 Hodgkin’s Disease • Hodgkin disease (HD) is a potentially curable malignant lymphoma with distinct histology, biologic behavior, and clinical characteristics. • Histologically, the picture is unique, with 1 -2% of neoplastic cells (Reed-Sternberg [RS] cells) in a background of a variety of reactive mixed inflammatory cells consisting of lymphocytes, plasma cells, neutrophils, eosinophils, and histiocytes
Hodgkin’s Disease • Hodgkin disease (HD) is a potentially curable malignant lymphoma with distinct histology, biologic behavior, and clinical characteristics. • Histologically, the picture is unique, with 1 -2% of neoplastic cells (Reed-Sternberg [RS] cells) in a background of a variety of reactive mixed inflammatory cells consisting of lymphocytes, plasma cells, neutrophils, eosinophils, and histiocytes
 Hodgkin’s Disease - In the Beginning First described in 1832 by Dr. Thomas Hodgkin Neoplasm of B lymphocytes – large pleomorphic prominent nucleolus in a halo - Hodgkin cells Reed-Sternberg cell – binucleate Hodgkin cell with owl eye appearance Classification: Classical Hodgkin’s Nodular sclerosis – low grade Mixed cellularity Lymphocyte rich classical Lymphocyte depleted. – high grade Nodular lymphocyte-rich Hodgkin’s 1798 -1866
Hodgkin’s Disease - In the Beginning First described in 1832 by Dr. Thomas Hodgkin Neoplasm of B lymphocytes – large pleomorphic prominent nucleolus in a halo - Hodgkin cells Reed-Sternberg cell – binucleate Hodgkin cell with owl eye appearance Classification: Classical Hodgkin’s Nodular sclerosis – low grade Mixed cellularity Lymphocyte rich classical Lymphocyte depleted. – high grade Nodular lymphocyte-rich Hodgkin’s 1798 -1866
 Hodgkin’s Disease - Epidemiology • Accounts for ~ 30% of all malignant lymphomas • Socioeconomic class is associated with a higher risk of HD. • Composed of two different disease entities: – Lymphocyte-predominant Hodgkin’s (LPHD), making up ~ 5% of cases – Classical HD, representing ~ 95% of all HDs. • A common factor of both HD types is that neoplastic cells constitute only a small minority of the cells in the affected tissue, often corresponding to < 2% of the total tumor
Hodgkin’s Disease - Epidemiology • Accounts for ~ 30% of all malignant lymphomas • Socioeconomic class is associated with a higher risk of HD. • Composed of two different disease entities: – Lymphocyte-predominant Hodgkin’s (LPHD), making up ~ 5% of cases – Classical HD, representing ~ 95% of all HDs. • A common factor of both HD types is that neoplastic cells constitute only a small minority of the cells in the affected tissue, often corresponding to < 2% of the total tumor
 Hodgkin’s Disease - Epidemiology • Bimodal age distribution – first peak between 2 nd - 3 rd decade of life – second peak between 5 th - 6 th decade of life • Male: Female 2: 1 in kids, adults almost equal M: F • Mixed cellularity (MC) Hodgkin’s Disease is more common at younger ages • More common in immune deficiency patients
Hodgkin’s Disease - Epidemiology • Bimodal age distribution – first peak between 2 nd - 3 rd decade of life – second peak between 5 th - 6 th decade of life • Male: Female 2: 1 in kids, adults almost equal M: F • Mixed cellularity (MC) Hodgkin’s Disease is more common at younger ages • More common in immune deficiency patients
 Hodgkin’s Disease - Epidemiology • Fatal disease with 90% of untreated patients dying within 2 to 3 years • With chemotherapy, >80% of patients suffering from HD are cured. • Pathogenesis of HD is still largely unknown. • HD nearly always arises and disseminates in lymph nodes
Hodgkin’s Disease - Epidemiology • Fatal disease with 90% of untreated patients dying within 2 to 3 years • With chemotherapy, >80% of patients suffering from HD are cured. • Pathogenesis of HD is still largely unknown. • HD nearly always arises and disseminates in lymph nodes
 H D – Etiology • The etiology of HD is unknown. – Infectious agents, especially the Epstein-Barr virus (EBV), may be involved in the pathogenesis of HD. – Patients with HIV infection have a higher incidence of HD compared to the population without HIV infection. – Genetic predisposition may play a role in the pathogenesis of HD. • Approximately 1% of patients with HD have a family history of the disease. • Siblings of an affected individual have a 3 - to 7 -fold increased risk for developing HD. • This risk is higher in monozygotic twins.
H D – Etiology • The etiology of HD is unknown. – Infectious agents, especially the Epstein-Barr virus (EBV), may be involved in the pathogenesis of HD. – Patients with HIV infection have a higher incidence of HD compared to the population without HIV infection. – Genetic predisposition may play a role in the pathogenesis of HD. • Approximately 1% of patients with HD have a family history of the disease. • Siblings of an affected individual have a 3 - to 7 -fold increased risk for developing HD. • This risk is higher in monozygotic twins.
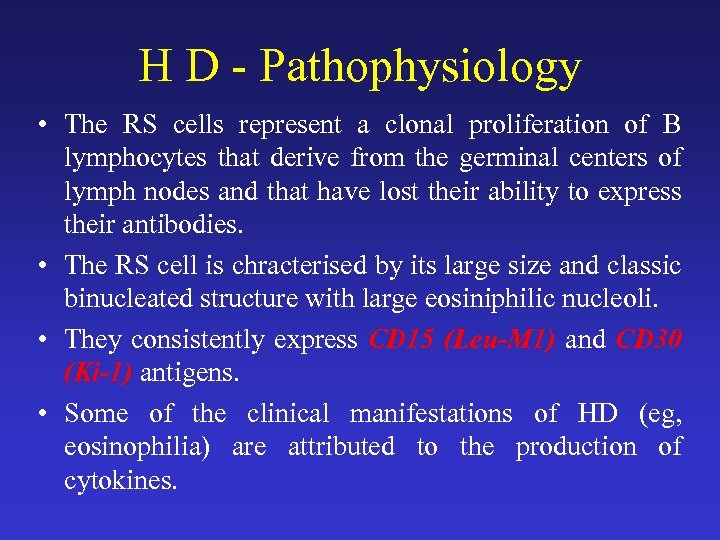 H D - Pathophysiology • The RS cells represent a clonal proliferation of B lymphocytes that derive from the germinal centers of lymph nodes and that have lost their ability to express their antibodies. • The RS cell is chracterised by its large size and classic binucleated structure with large eosiniphilic nucleoli. • They consistently express CD 15 (Leu-M 1) and CD 30 (Ki-1) antigens. • Some of the clinical manifestations of HD (eg, eosinophilia) are attributed to the production of cytokines.
H D - Pathophysiology • The RS cells represent a clonal proliferation of B lymphocytes that derive from the germinal centers of lymph nodes and that have lost their ability to express their antibodies. • The RS cell is chracterised by its large size and classic binucleated structure with large eosiniphilic nucleoli. • They consistently express CD 15 (Leu-M 1) and CD 30 (Ki-1) antigens. • Some of the clinical manifestations of HD (eg, eosinophilia) are attributed to the production of cytokines.
 H D - Pathophysiology
H D - Pathophysiology
 H D - History • Asymptomatic lymphadenopathy may be present (above the diaphragm in 80% of patients). • Constitutional symptoms (eg, unexplained weight loss, fever, night sweats) are present in 40% of patients. • Chest pain, cough, and/or shortness of breath may be present due to a large mediastinal mass or lung involvement. Rarely, hemoptysis is observed.
H D - History • Asymptomatic lymphadenopathy may be present (above the diaphragm in 80% of patients). • Constitutional symptoms (eg, unexplained weight loss, fever, night sweats) are present in 40% of patients. • Chest pain, cough, and/or shortness of breath may be present due to a large mediastinal mass or lung involvement. Rarely, hemoptysis is observed.
 H D - History • Patients may present with pruritus. • Alcohol-induced pain at sites of nodal disease is specific for HD and occurs in less than 10% of patients. • Intermittent fever is observed in approximately 25% of cases. Infrequently, the classic Pel-Ebstein fever (high fever for 1 -2 wk followed by an afebrile period of 1 -2 wk) is observed. • Back or bone pain occurs rarely.
H D - History • Patients may present with pruritus. • Alcohol-induced pain at sites of nodal disease is specific for HD and occurs in less than 10% of patients. • Intermittent fever is observed in approximately 25% of cases. Infrequently, the classic Pel-Ebstein fever (high fever for 1 -2 wk followed by an afebrile period of 1 -2 wk) is observed. • Back or bone pain occurs rarely.
 H D - Physical • Palpable painless lymphadenopathy occurs in the : – cervical area (60 -80%), – axilla (6 -20%), and, less commonly, – inguinal area (6 -20%). • It is described as : – – – asymmetrical, discrete painless, non-tender elastic character on palpation ( rubbery) not adherent to skin fluctuate in size • Involvement of the Waldeyer ring or occipital or epitrochlear areas is observed infrequently. • Splenomegaly may be present.
H D - Physical • Palpable painless lymphadenopathy occurs in the : – cervical area (60 -80%), – axilla (6 -20%), and, less commonly, – inguinal area (6 -20%). • It is described as : – – – asymmetrical, discrete painless, non-tender elastic character on palpation ( rubbery) not adherent to skin fluctuate in size • Involvement of the Waldeyer ring or occipital or epitrochlear areas is observed infrequently. • Splenomegaly may be present.
 H D - Physical • Patients may have hepatomegaly. • Superior vena cava syndrome resulting from massive mediastinal lymphadenopathy is observed rarely. • Central nervous system (CNS) symptoms or signs may be due to paraneoplastic syndromes, including cerebellar degeneration, neuropathy, Guillain-Barré syndrome, or multifocal leukoencephalopathy.
H D - Physical • Patients may have hepatomegaly. • Superior vena cava syndrome resulting from massive mediastinal lymphadenopathy is observed rarely. • Central nervous system (CNS) symptoms or signs may be due to paraneoplastic syndromes, including cerebellar degeneration, neuropathy, Guillain-Barré syndrome, or multifocal leukoencephalopathy.
 H D - Physical
H D - Physical
 H D - Physical
H D - Physical
 H D – Lab studies • CBP : Anemia (normochromic/normocytic), eosinophilia, neutrophilia, lymphopenia • ESR -raised • LFT- (liver infil / obs at porta hepatis) • RFT- prior to treatment • Urate , Ca, • LDH - adverse prognosis • CXR- mediastinal mass • CT thorax / abdomen / pelvis-for staging • Other: Gallium scan, PET, Lymphangiography , Laporotomy
H D – Lab studies • CBP : Anemia (normochromic/normocytic), eosinophilia, neutrophilia, lymphopenia • ESR -raised • LFT- (liver infil / obs at porta hepatis) • RFT- prior to treatment • Urate , Ca, • LDH - adverse prognosis • CXR- mediastinal mass • CT thorax / abdomen / pelvis-for staging • Other: Gallium scan, PET, Lymphangiography , Laporotomy
 H D – Lab studies • Erythrocyte sedimentation rate (ESR) may be elevated. • Complete blood count (CBC) should be performed. – anemia usually is due to anemia of chronic disease, but it may be due to bone marrow involvement or the presence of an autoantibody – Cytopenias are common in advanced disease. – Platelet counts can be increased or decreased.
H D – Lab studies • Erythrocyte sedimentation rate (ESR) may be elevated. • Complete blood count (CBC) should be performed. – anemia usually is due to anemia of chronic disease, but it may be due to bone marrow involvement or the presence of an autoantibody – Cytopenias are common in advanced disease. – Platelet counts can be increased or decreased.
 H D – Lab studies • Lactate dehydrogenase (LDH) may be increased. LDH may correlate with the bulk of disease. • Rarely, HD is associated with nephrotic syndrome. • Alkaline phosphatase may be increased due to the presence of liver or bone involvement. • Other uncommon laboratory findings include hypercalcemia, hypernatremia, and hypoglycemia (due to the presence of insulin autoantibodies). • Beta-2 -microglobulin correlate with tumor burden, systemic symptoms, and prognosis.
H D – Lab studies • Lactate dehydrogenase (LDH) may be increased. LDH may correlate with the bulk of disease. • Rarely, HD is associated with nephrotic syndrome. • Alkaline phosphatase may be increased due to the presence of liver or bone involvement. • Other uncommon laboratory findings include hypercalcemia, hypernatremia, and hypoglycemia (due to the presence of insulin autoantibodies). • Beta-2 -microglobulin correlate with tumor burden, systemic symptoms, and prognosis.
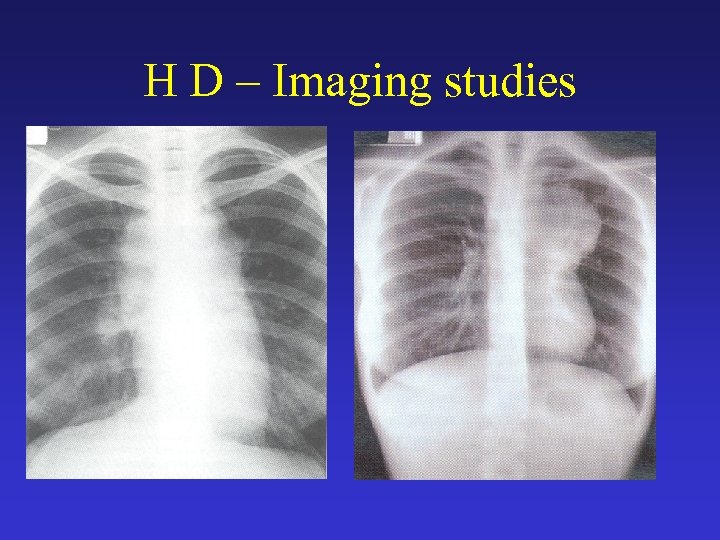
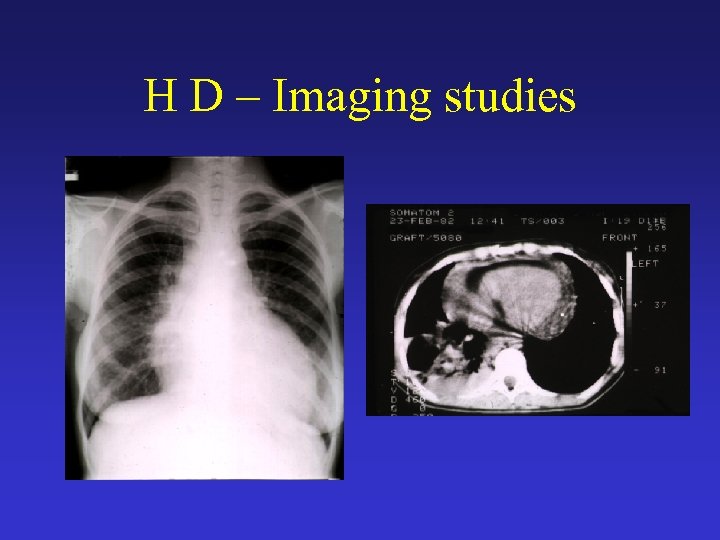
 H D – Imaging studies • Chest radiography • CT scans of the chest, abdomen, and pelvis o Possible abnormal findings include enlarged lymph nodes, hepatomegaly and/or splenomegaly with or without focal parenchymal abnormalities, lung nodules or infiltrates, and pleural effusions. o A mediastinal mass, representing mediastinal lymphadenopathy, is a very common finding. • Gallium-67 scan or positron emission tomography (PET) scan may be used as a baseline test to assess response to therapy.
H D – Imaging studies • Chest radiography • CT scans of the chest, abdomen, and pelvis o Possible abnormal findings include enlarged lymph nodes, hepatomegaly and/or splenomegaly with or without focal parenchymal abnormalities, lung nodules or infiltrates, and pleural effusions. o A mediastinal mass, representing mediastinal lymphadenopathy, is a very common finding. • Gallium-67 scan or positron emission tomography (PET) scan may be used as a baseline test to assess response to therapy.
 H D – Imaging studies
H D – Imaging studies
 H D – Imaging studies
H D – Imaging studies
 H D – Imaging studies
H D – Imaging studies
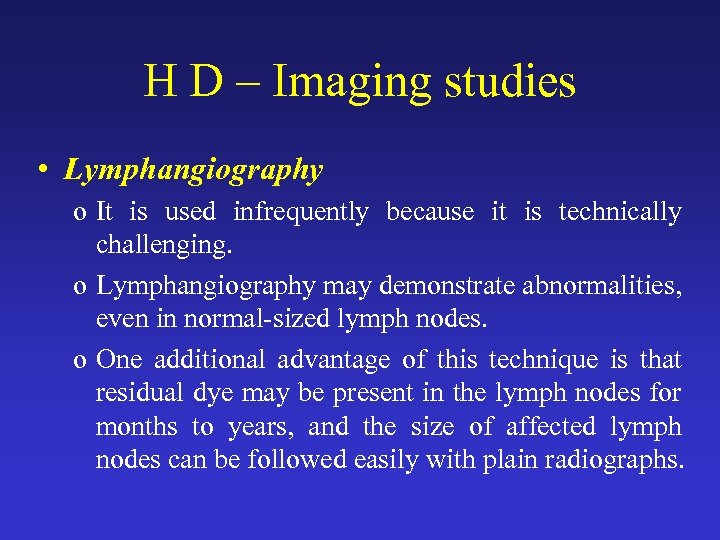 H D – Imaging studies • Lymphangiography o It is used infrequently because it is technically challenging. o Lymphangiography may demonstrate abnormalities, even in normal-sized lymph nodes. o One additional advantage of this technique is that residual dye may be present in the lymph nodes for months to years, and the size of affected lymph nodes can be followed easily with plain radiographs.
H D – Imaging studies • Lymphangiography o It is used infrequently because it is technically challenging. o Lymphangiography may demonstrate abnormalities, even in normal-sized lymph nodes. o One additional advantage of this technique is that residual dye may be present in the lymph nodes for months to years, and the size of affected lymph nodes can be followed easily with plain radiographs.
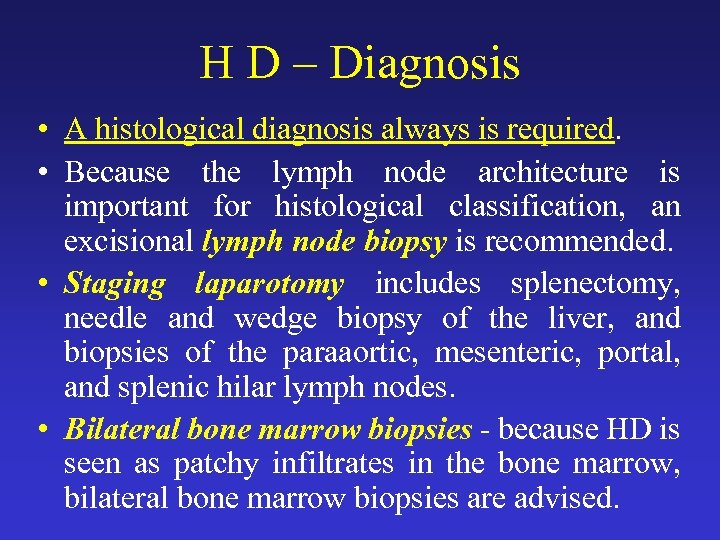 H D – Diagnosis • A histological diagnosis always is required. • Because the lymph node architecture is important for histological classification, an excisional lymph node biopsy is recommended. • Staging laparotomy includes splenectomy, needle and wedge biopsy of the liver, and biopsies of the paraaortic, mesenteric, portal, and splenic hilar lymph nodes. • Bilateral bone marrow biopsies - because HD is seen as patchy infiltrates in the bone marrow, bilateral bone marrow biopsies are advised.
H D – Diagnosis • A histological diagnosis always is required. • Because the lymph node architecture is important for histological classification, an excisional lymph node biopsy is recommended. • Staging laparotomy includes splenectomy, needle and wedge biopsy of the liver, and biopsies of the paraaortic, mesenteric, portal, and splenic hilar lymph nodes. • Bilateral bone marrow biopsies - because HD is seen as patchy infiltrates in the bone marrow, bilateral bone marrow biopsies are advised.
 H D – Histologic Findings • According to the recent World Health Organization (WHO) classification, the first 4 subtypes are referred to as classic HD. – Nodular sclerosis Hodgkin disease - 60 -80% – Mixed-cellularity Hodgkin disease - 15 -30% – Lymphocyte-depleted Hodgkin disease - Less than 1% – Lymphocyte-rich classic Hodgkin disease - 5%
H D – Histologic Findings • According to the recent World Health Organization (WHO) classification, the first 4 subtypes are referred to as classic HD. – Nodular sclerosis Hodgkin disease - 60 -80% – Mixed-cellularity Hodgkin disease - 15 -30% – Lymphocyte-depleted Hodgkin disease - Less than 1% – Lymphocyte-rich classic Hodgkin disease - 5%
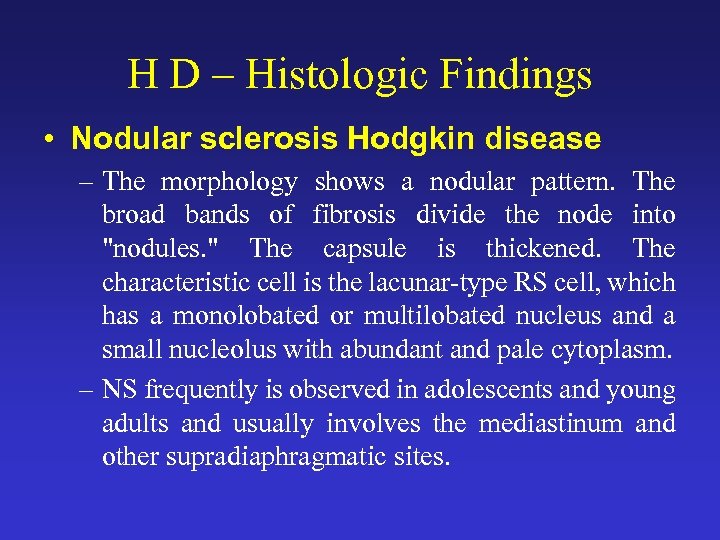 H D – Histologic Findings • Nodular sclerosis Hodgkin disease – The morphology shows a nodular pattern. The broad bands of fibrosis divide the node into "nodules. " The capsule is thickened. The characteristic cell is the lacunar-type RS cell, which has a monolobated or multilobated nucleus and a small nucleolus with abundant and pale cytoplasm. – NS frequently is observed in adolescents and young adults and usually involves the mediastinum and other supradiaphragmatic sites.
H D – Histologic Findings • Nodular sclerosis Hodgkin disease – The morphology shows a nodular pattern. The broad bands of fibrosis divide the node into "nodules. " The capsule is thickened. The characteristic cell is the lacunar-type RS cell, which has a monolobated or multilobated nucleus and a small nucleolus with abundant and pale cytoplasm. – NS frequently is observed in adolescents and young adults and usually involves the mediastinum and other supradiaphragmatic sites.
 H D – Histologic Findings • Mixed-cellularity Hodgkin disease – Histologically, the infiltrate usually is diffuse. RS cells are of the classic type (large, with bilobate, double or multiple nuclei, and a large eosinophilic inclusionlike nucleolus). – It commonly affects the abdominal lymph nodes and spleen. – Patients with this histology typically have advanced -stage disease with systemic symptoms and immunodeficiency.
H D – Histologic Findings • Mixed-cellularity Hodgkin disease – Histologically, the infiltrate usually is diffuse. RS cells are of the classic type (large, with bilobate, double or multiple nuclei, and a large eosinophilic inclusionlike nucleolus). – It commonly affects the abdominal lymph nodes and spleen. – Patients with this histology typically have advanced -stage disease with systemic symptoms and immunodeficiency.
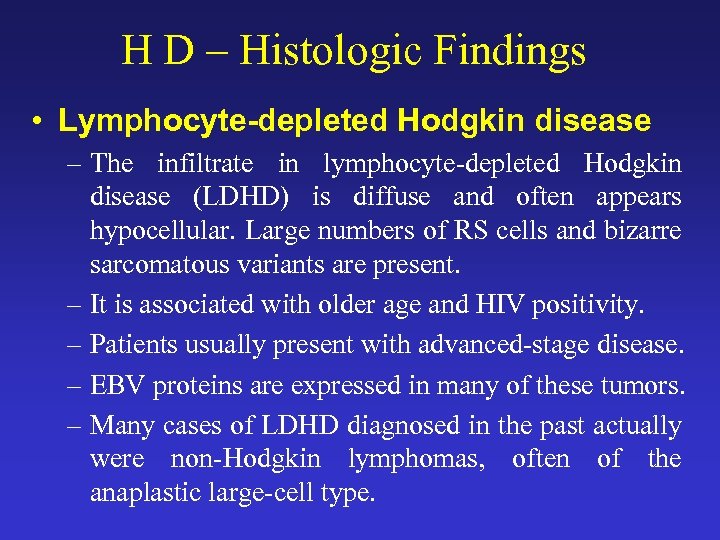 H D – Histologic Findings • Lymphocyte-depleted Hodgkin disease – The infiltrate in lymphocyte-depleted Hodgkin disease (LDHD) is diffuse and often appears hypocellular. Large numbers of RS cells and bizarre sarcomatous variants are present. – It is associated with older age and HIV positivity. – Patients usually present with advanced-stage disease. – EBV proteins are expressed in many of these tumors. – Many cases of LDHD diagnosed in the past actually were non-Hodgkin lymphomas, often of the anaplastic large-cell type.
H D – Histologic Findings • Lymphocyte-depleted Hodgkin disease – The infiltrate in lymphocyte-depleted Hodgkin disease (LDHD) is diffuse and often appears hypocellular. Large numbers of RS cells and bizarre sarcomatous variants are present. – It is associated with older age and HIV positivity. – Patients usually present with advanced-stage disease. – EBV proteins are expressed in many of these tumors. – Many cases of LDHD diagnosed in the past actually were non-Hodgkin lymphomas, often of the anaplastic large-cell type.
 H D – Histologic Findings • Lymphocyte-rich classic Hodgkin disease – In this type of HD, RS cells of the classic or lacunar type are observed, with a background infiltrate of lymphocytes. – It requires immunohistochemical diagnosis. – Some cases may have a nodular pattern. – Clinically, the presentation and survival patterns are similar to those for MCHD.
H D – Histologic Findings • Lymphocyte-rich classic Hodgkin disease – In this type of HD, RS cells of the classic or lacunar type are observed, with a background infiltrate of lymphocytes. – It requires immunohistochemical diagnosis. – Some cases may have a nodular pattern. – Clinically, the presentation and survival patterns are similar to those for MCHD.
 H D – Histologic Findings
H D – Histologic Findings
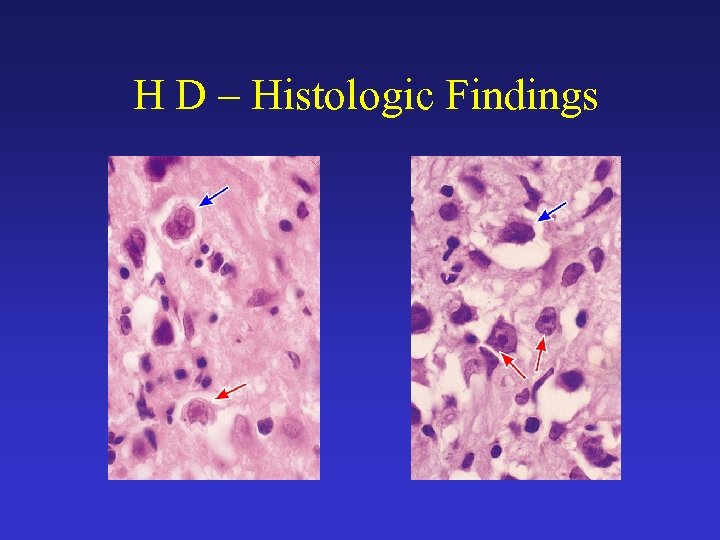 H D – Histologic Findings
H D – Histologic Findings
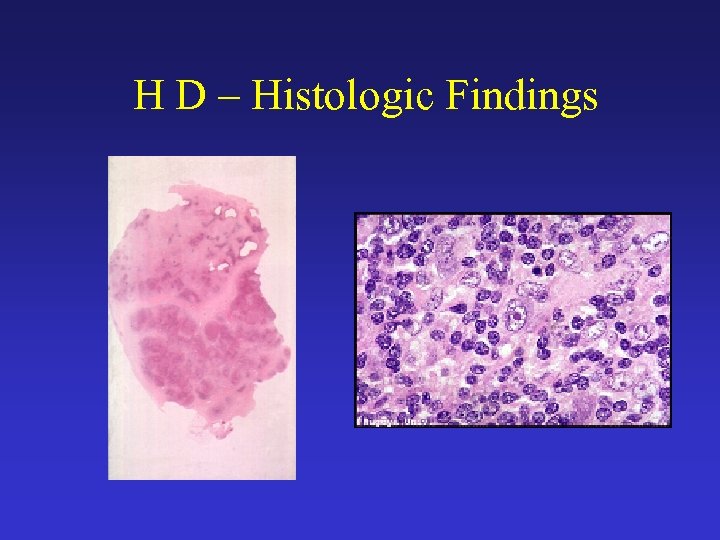 H D – Histologic Findings
H D – Histologic Findings
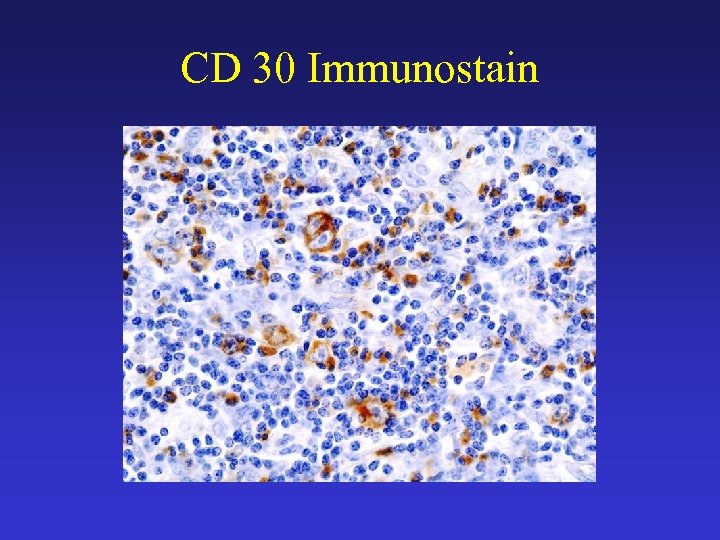 CD 30 Immunostain
CD 30 Immunostain
 H D – Staging • The Ann Arbor classification (1971) is used most commonly. • Clinical staging involves assessment of disease extent by clinical examination and imaging techniques. • When staging laparotomies are used as part of staging, disease extent is designated pathologic staging.
H D – Staging • The Ann Arbor classification (1971) is used most commonly. • Clinical staging involves assessment of disease extent by clinical examination and imaging techniques. • When staging laparotomies are used as part of staging, disease extent is designated pathologic staging.
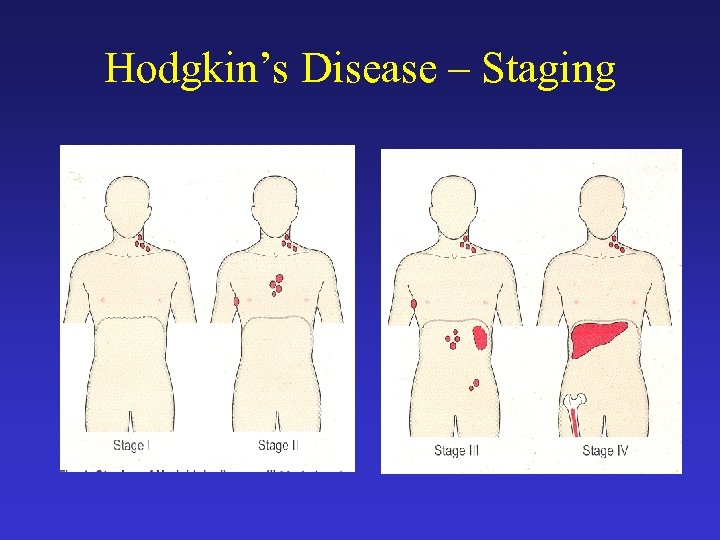
 Hodgkin’s Disease – Staging Stage Definition I (IE) Involvement of a single lymph node region (I) or of a single extralymphatic organ or site II Involvement of two or more lymph node regions on the same side of the diaphragm (II) or localized involvement of an extralymphatic organ or site and one or more lymph node regions on the same side of the diaphragm (IIE) III Involvement of lymph node regions on both sides of the diaphragm (III) which may be accompanied by involvement of the spleen (IIIS) or by localized involvement of an extralymphatic organ or site (IIIE) or both (IIISE) IV Diffuse or disseminated involvement of one or more extra lymphatic organs or tissues with or without associated lymph node involvement B symptoms: fever > 38ºC for three consecutive days, drenching night sweats or unexplained loss 10% or more of weight the preceding 6 months
Hodgkin’s Disease – Staging Stage Definition I (IE) Involvement of a single lymph node region (I) or of a single extralymphatic organ or site II Involvement of two or more lymph node regions on the same side of the diaphragm (II) or localized involvement of an extralymphatic organ or site and one or more lymph node regions on the same side of the diaphragm (IIE) III Involvement of lymph node regions on both sides of the diaphragm (III) which may be accompanied by involvement of the spleen (IIIS) or by localized involvement of an extralymphatic organ or site (IIIE) or both (IIISE) IV Diffuse or disseminated involvement of one or more extra lymphatic organs or tissues with or without associated lymph node involvement B symptoms: fever > 38ºC for three consecutive days, drenching night sweats or unexplained loss 10% or more of weight the preceding 6 months
 Hodgkin’s Disease – Staging
Hodgkin’s Disease – Staging
 Hodgkin’s Disease – Staging • The stage of HD correlates with prognosis. • Also, the bulk of nodal disease and the extent of subdiaphragmatic involvement have been found to correlate with prognosis. • Approximately one third of new patients have splenic involvement based on laparotomy data. • Spread in HD takes place via the lymphatics, hematogenous routes, and direct extension. • Contiguous involvement of extranodal sites, eg, involvement of the lung parenchyma due to direct extension of large mediastinal lymphadenopathy, is not considered stage IV disease.
Hodgkin’s Disease – Staging • The stage of HD correlates with prognosis. • Also, the bulk of nodal disease and the extent of subdiaphragmatic involvement have been found to correlate with prognosis. • Approximately one third of new patients have splenic involvement based on laparotomy data. • Spread in HD takes place via the lymphatics, hematogenous routes, and direct extension. • Contiguous involvement of extranodal sites, eg, involvement of the lung parenchyma due to direct extension of large mediastinal lymphadenopathy, is not considered stage IV disease.
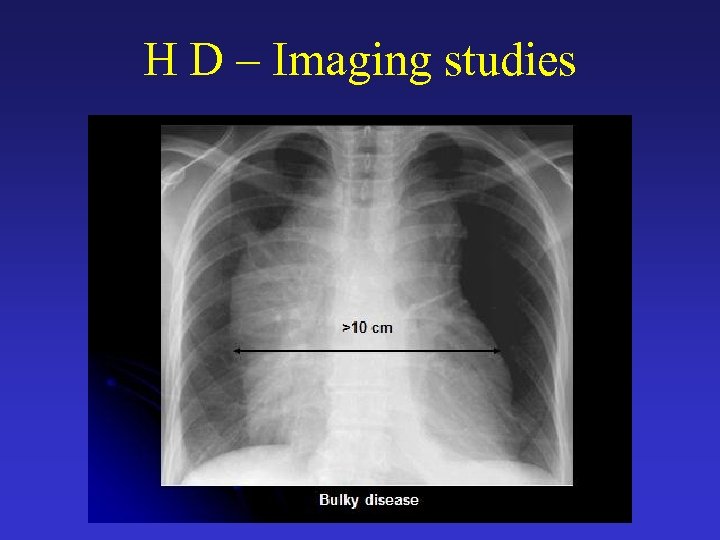
 H D – Prognosis • Early stages (I-IIA) - Early-stage disease is considered unfavorable when at least 1 of the following poor prognostic factors is present: o Bulky disease (defined as a mass >5 -10 cm or a mediastinal mass larger than one third of the chest diameter) o Elevated ESR o B-symptoms o Multiple lymph node sites (>3) or extranodal disease
H D – Prognosis • Early stages (I-IIA) - Early-stage disease is considered unfavorable when at least 1 of the following poor prognostic factors is present: o Bulky disease (defined as a mass >5 -10 cm or a mediastinal mass larger than one third of the chest diameter) o Elevated ESR o B-symptoms o Multiple lymph node sites (>3) or extranodal disease
 H D – Prognosis • Advanced stages (IIB-IV) – The International Prognostic Factors Project on Advanced Hodgkin's Disease has developed a prognostic score based on 7 adverse factors in advanced HD. These factors are • • (1) an albumin level of less than 4 g/d. L, (2) a hemoglobin level of less than 10. 5 g/d. L, (3) male sex, (4) age older than 45 years, (5) stage IV disease, (6) a WBC count of higher than 15, 000/mm 3, and (7) an absolute lymphocyte count of less than 600/mm 3 or a lymphocyte count of less than 8% of the total WBC count.
H D – Prognosis • Advanced stages (IIB-IV) – The International Prognostic Factors Project on Advanced Hodgkin's Disease has developed a prognostic score based on 7 adverse factors in advanced HD. These factors are • • (1) an albumin level of less than 4 g/d. L, (2) a hemoglobin level of less than 10. 5 g/d. L, (3) male sex, (4) age older than 45 years, (5) stage IV disease, (6) a WBC count of higher than 15, 000/mm 3, and (7) an absolute lymphocyte count of less than 600/mm 3 or a lymphocyte count of less than 8% of the total WBC count.
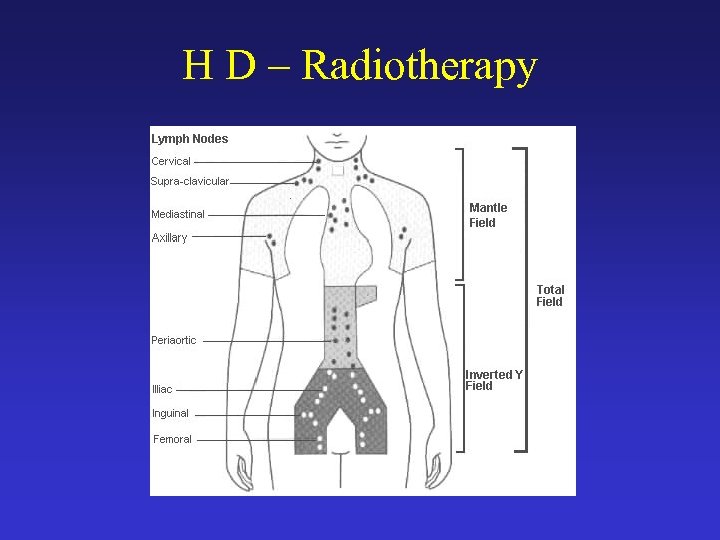
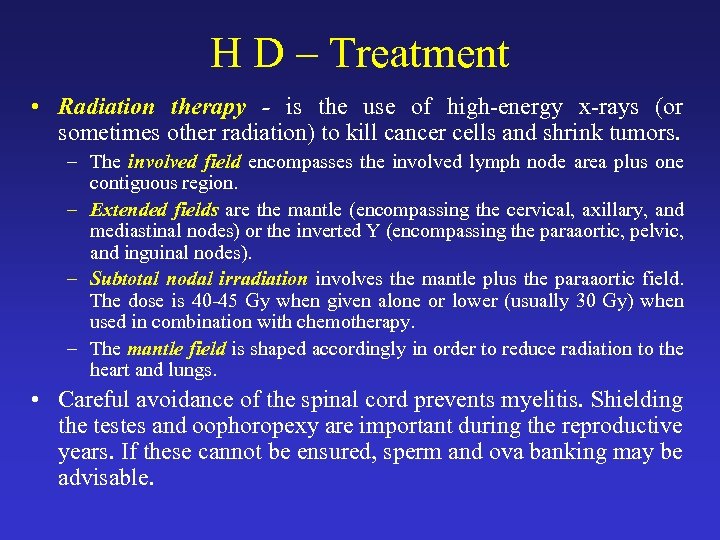 H D – Treatment • Radiation therapy - is the use of high-energy x-rays (or sometimes other radiation) to kill cancer cells and shrink tumors. – The involved field encompasses the involved lymph node area plus one contiguous region. – Extended fields are the mantle (encompassing the cervical, axillary, and mediastinal nodes) or the inverted Y (encompassing the paraaortic, pelvic, and inguinal nodes). – Subtotal nodal irradiation involves the mantle plus the paraaortic field. The dose is 40 -45 Gy when given alone or lower (usually 30 Gy) when used in combination with chemotherapy. – The mantle field is shaped accordingly in order to reduce radiation to the heart and lungs. • Careful avoidance of the spinal cord prevents myelitis. Shielding the testes and oophoropexy are important during the reproductive years. If these cannot be ensured, sperm and ova banking may be advisable.
H D – Treatment • Radiation therapy - is the use of high-energy x-rays (or sometimes other radiation) to kill cancer cells and shrink tumors. – The involved field encompasses the involved lymph node area plus one contiguous region. – Extended fields are the mantle (encompassing the cervical, axillary, and mediastinal nodes) or the inverted Y (encompassing the paraaortic, pelvic, and inguinal nodes). – Subtotal nodal irradiation involves the mantle plus the paraaortic field. The dose is 40 -45 Gy when given alone or lower (usually 30 Gy) when used in combination with chemotherapy. – The mantle field is shaped accordingly in order to reduce radiation to the heart and lungs. • Careful avoidance of the spinal cord prevents myelitis. Shielding the testes and oophoropexy are important during the reproductive years. If these cannot be ensured, sperm and ova banking may be advisable.
 H D – Radiotherapy
H D – Radiotherapy
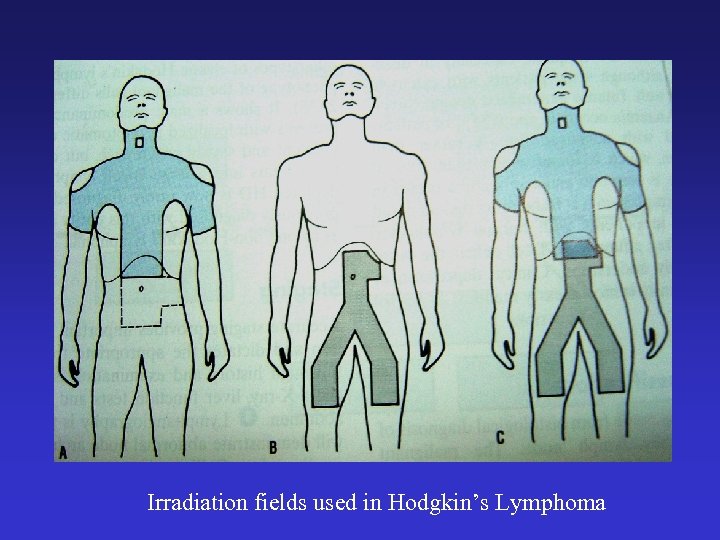 Irradiation fields used in Hodgkin’s Lymphoma
Irradiation fields used in Hodgkin’s Lymphoma
 H D – Chemotherapy • Conventional chemotherapy : – – – MOPP ABVD Alternating regimens – MOPP/ABVD Hybrid regimens – MOPP/ABV Stanford V BEACOPP • High-dose chemotherapy with transplantation – High-dose chemotherapy (HDC) at doses that ablate the bone marrow is feasible with reinfusion of the patient's previously collected hematopoietic stem cells (autologous transplantation) or infusion of stem cells from a donor source (allogeneic transplantation).
H D – Chemotherapy • Conventional chemotherapy : – – – MOPP ABVD Alternating regimens – MOPP/ABVD Hybrid regimens – MOPP/ABV Stanford V BEACOPP • High-dose chemotherapy with transplantation – High-dose chemotherapy (HDC) at doses that ablate the bone marrow is feasible with reinfusion of the patient's previously collected hematopoietic stem cells (autologous transplantation) or infusion of stem cells from a donor source (allogeneic transplantation).
 H D – Response Assesment • The goal of treatment is to induce a complete response (CR). • Any other outcome is considered a treatment failure. • In assessing response, note that residual masses do not always represent active disease. • Gallium and PET scans are useful in this setting because they can differentiate active and inactive residual masses.
H D – Response Assesment • The goal of treatment is to induce a complete response (CR). • Any other outcome is considered a treatment failure. • In assessing response, note that residual masses do not always represent active disease. • Gallium and PET scans are useful in this setting because they can differentiate active and inactive residual masses.
 H D – Treatment Guidelines o The treatment of early-stage favorable - options include the following: o Extended-field radiotherapy, if pathologically staged (20% will be upstaged upon laparotomy) o Subtotal nodal irradiation, if clinically staged (approximately 20% will relapse, usually at nonirradiated sites) o Combined modality therapy - usually represents chemotherapy (ABVD) for 2 -4 cycles, followed by radiotherapy of the involved field o ABVD alone without radiation, which may be adequate therapy for early-stage disease (under investigation).
H D – Treatment Guidelines o The treatment of early-stage favorable - options include the following: o Extended-field radiotherapy, if pathologically staged (20% will be upstaged upon laparotomy) o Subtotal nodal irradiation, if clinically staged (approximately 20% will relapse, usually at nonirradiated sites) o Combined modality therapy - usually represents chemotherapy (ABVD) for 2 -4 cycles, followed by radiotherapy of the involved field o ABVD alone without radiation, which may be adequate therapy for early-stage disease (under investigation).
 H D – Treatment Guidelines o Treatment for early-stage unfavorable disease is as follows: o Combined modality therapy, which is chemotherapy (ABVD) for 4 -6 cycles followed by involved-field radiotherapy, is used. o Approximately 80% of patients are cured, and treatment fails in 20%. Of these, 5% progress during treatment and 15% relapse. – The use of more aggressive chemotherapy for the treatment of unfavorable early-stage HD is being explored.
H D – Treatment Guidelines o Treatment for early-stage unfavorable disease is as follows: o Combined modality therapy, which is chemotherapy (ABVD) for 4 -6 cycles followed by involved-field radiotherapy, is used. o Approximately 80% of patients are cured, and treatment fails in 20%. Of these, 5% progress during treatment and 15% relapse. – The use of more aggressive chemotherapy for the treatment of unfavorable early-stage HD is being explored.
 H D – Treatment Guidelines o Treatment for special presentations includes the following: o For patients with clinical stage IA disease with neck involvement and NLPHD histology, radiotherapy alone is adequate therapy. o For patients with clinical stage IA disease that is nonbulky and involves the mediastinum with NS histology, mantle irradiation alone may be adequate therapy.
H D – Treatment Guidelines o Treatment for special presentations includes the following: o For patients with clinical stage IA disease with neck involvement and NLPHD histology, radiotherapy alone is adequate therapy. o For patients with clinical stage IA disease that is nonbulky and involves the mediastinum with NS histology, mantle irradiation alone may be adequate therapy.
 H D – Treatment Guidelines • Advanced stages (IIB-IV) o Chemotherapy is used, followed by involved-field radiotherapy in selected cases (eg, bulky disease). o The overall cure rate is 60 -70%. o ABVD is the regimen of choice, but other combinations are under investigation. o The optimal duration of treatment is uncertain. In most cases, treatment continues for 2 cycles post CR. Usually, a total of 6 cycles is administered. o Two new regimens (Stanford V and BEACOPP) are being evaluated in advanced HD and have shown promising results
H D – Treatment Guidelines • Advanced stages (IIB-IV) o Chemotherapy is used, followed by involved-field radiotherapy in selected cases (eg, bulky disease). o The overall cure rate is 60 -70%. o ABVD is the regimen of choice, but other combinations are under investigation. o The optimal duration of treatment is uncertain. In most cases, treatment continues for 2 cycles post CR. Usually, a total of 6 cycles is administered. o Two new regimens (Stanford V and BEACOPP) are being evaluated in advanced HD and have shown promising results
 H D – Treatment Guidelines • Relapsed or resistant HD o Relapse after radiotherapy: o Approximately 25% of patients treated with radiation therapy alone relapse. o These patients are successfully salvaged with conventional combination chemotherapy (ABVD). More than 60% of patients are cured.
H D – Treatment Guidelines • Relapsed or resistant HD o Relapse after radiotherapy: o Approximately 25% of patients treated with radiation therapy alone relapse. o These patients are successfully salvaged with conventional combination chemotherapy (ABVD). More than 60% of patients are cured.
 H D – Treatment Guidelines o Relapses after conventional chemotherapy can be divided into 3 groups: o Induction failure includes patients who never achieve a complete response or those who relapse very early (within 2 -3 mo) following completion of therapy HDC with autologous hematopoietic stem cell transplantation is the treatment of choice. Second-line (salvage) conventional-dose chemotherapy regimens often are used before HDC in an attempt to achieve minimal disease. o Early relapse represents relapse within 12 months after chemotherapy completion HDC with autologous transplantation is the treatment of choice. o Late relapse represents relapse after 12 or more months postchemotherapy completion.
H D – Treatment Guidelines o Relapses after conventional chemotherapy can be divided into 3 groups: o Induction failure includes patients who never achieve a complete response or those who relapse very early (within 2 -3 mo) following completion of therapy HDC with autologous hematopoietic stem cell transplantation is the treatment of choice. Second-line (salvage) conventional-dose chemotherapy regimens often are used before HDC in an attempt to achieve minimal disease. o Early relapse represents relapse within 12 months after chemotherapy completion HDC with autologous transplantation is the treatment of choice. o Late relapse represents relapse after 12 or more months postchemotherapy completion.
 H D – Complications • • • Cardiac disease Pulmonary disease Myelodysplasia/leukemia Infertility Breast cancer Non-Hodgkin lymphoma Other solid tumors Infectious complications Hypothyroidism after neck/mediastinal radiotherapy Immunodeficiency after chemotherapy and/or radiation therapy
H D – Complications • • • Cardiac disease Pulmonary disease Myelodysplasia/leukemia Infertility Breast cancer Non-Hodgkin lymphoma Other solid tumors Infectious complications Hypothyroidism after neck/mediastinal radiotherapy Immunodeficiency after chemotherapy and/or radiation therapy
 NON-HODGKIN LYMPHOMAS (NHL)
NON-HODGKIN LYMPHOMAS (NHL)
 NHL - Epidemiology • Since the early 1970 s, the incidence rates of NHL have nearly doubled. • NHL is the most prevalent hematopoietic neoplasm - 4% of all cancer diagnoses and seventh in frequency among all cancers. • NHL is more than 5 times as common as Hodgkin disease. • Incidence varies with race; white people have a higher risk than black and Asian American people.
NHL - Epidemiology • Since the early 1970 s, the incidence rates of NHL have nearly doubled. • NHL is the most prevalent hematopoietic neoplasm - 4% of all cancer diagnoses and seventh in frequency among all cancers. • NHL is more than 5 times as common as Hodgkin disease. • Incidence varies with race; white people have a higher risk than black and Asian American people.
 NHL - Epidemiology • In general, incidence is slightly higher in men than in women, with a male-to-female ratio of approximately 1. 4: 1. • The median age at presentation for all subtypes of NHL is older than 50 years, except for patients with high-grade lymphoblastic and small noncleaved lymphomas, which are the most common types of NHL observed in children and young adults.
NHL - Epidemiology • In general, incidence is slightly higher in men than in women, with a male-to-female ratio of approximately 1. 4: 1. • The median age at presentation for all subtypes of NHL is older than 50 years, except for patients with high-grade lymphoblastic and small noncleaved lymphomas, which are the most common types of NHL observed in children and young adults.
 NHL - Etiology • Chromosomal translocations and molecular rearrangements play an important role in the pathogenesis of many lymphomas and correlate with histology and immunophenotype. • Some viruses are implicated in the pathogenesis of NHL – EBV, HTLV-1, HCV, KSHV • Environmental factors linked to the development of NHL include chemicals, chemotherapy, and radiation exposure • Congenital, acquired , and induced (eg, immunosuppression) immunodeficiency states are associated with increased incidence of NHL • The chronic inflammation
NHL - Etiology • Chromosomal translocations and molecular rearrangements play an important role in the pathogenesis of many lymphomas and correlate with histology and immunophenotype. • Some viruses are implicated in the pathogenesis of NHL – EBV, HTLV-1, HCV, KSHV • Environmental factors linked to the development of NHL include chemicals, chemotherapy, and radiation exposure • Congenital, acquired , and induced (eg, immunosuppression) immunodeficiency states are associated with increased incidence of NHL • The chronic inflammation
 NHL - Patophysiology • NHL represents a progressive clonal expansion of B cells or T cells and/or natural killer (NK) cells, arising from the accumulation of genetic lesions that affect proto-oncogenes or tumor suppressor genes, resulting in the uncontrolled and excessive growth and cell immortalization. • The accumulation of these dividing cells results in the tumor masses in lymph nodes and other sites
NHL - Patophysiology • NHL represents a progressive clonal expansion of B cells or T cells and/or natural killer (NK) cells, arising from the accumulation of genetic lesions that affect proto-oncogenes or tumor suppressor genes, resulting in the uncontrolled and excessive growth and cell immortalization. • The accumulation of these dividing cells results in the tumor masses in lymph nodes and other sites
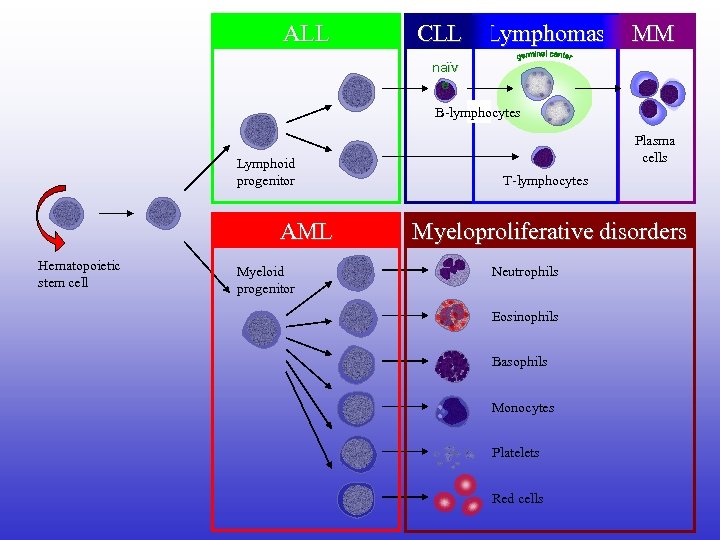 ALL CLL Lymphomas MM naïv e B-lymphocytes Lymphoid progenitor AML Hematopoietic stem cell Myeloid progenitor Plasma cells T-lymphocytes Myeloproliferative disorders Neutrophils Eosinophils Basophils Monocytes Platelets Red cells
ALL CLL Lymphomas MM naïv e B-lymphocytes Lymphoid progenitor AML Hematopoietic stem cell Myeloid progenitor Plasma cells T-lymphocytes Myeloproliferative disorders Neutrophils Eosinophils Basophils Monocytes Platelets Red cells
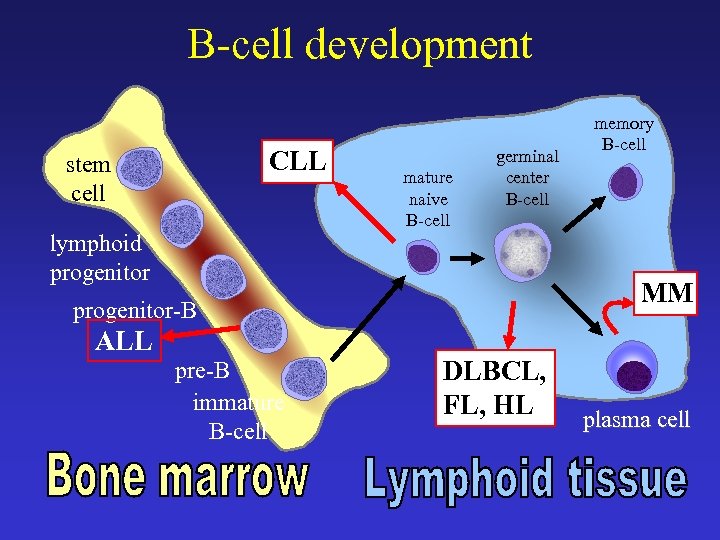 B-cell development CLL stem cell lymphoid progenitor mature naive B-cell germinal center B-cell MM progenitor-B ALL pre-B immature B-cell memory B-cell DLBCL, FL, HL plasma cell
B-cell development CLL stem cell lymphoid progenitor mature naive B-cell germinal center B-cell MM progenitor-B ALL pre-B immature B-cell memory B-cell DLBCL, FL, HL plasma cell
 NHL - Patophysiology • Most NHLs are of B-cell origin (almost 85%); only 15% are derived from T/NK cells, and the small remainder stem from macrophages. • NHLs are tumors originating from lymphoid tissues, mainly of lymph nodes. Various neoplastic tumor cell lines correspond to each of the cellular components of antigen-stimulated lymphoid follicles.
NHL - Patophysiology • Most NHLs are of B-cell origin (almost 85%); only 15% are derived from T/NK cells, and the small remainder stem from macrophages. • NHLs are tumors originating from lymphoid tissues, mainly of lymph nodes. Various neoplastic tumor cell lines correspond to each of the cellular components of antigen-stimulated lymphoid follicles.
 NHL - Classification • NHL are characterized by the level of differentiation, the size of the cell of origin, the origin cell's rate of proliferation, and the histologic pattern of growth a heterogenous group with a great diversity of subtypes several NHL classification schemas exist, reflecting the growing understanding of the complex diversity of the NHL subtypes.
NHL - Classification • NHL are characterized by the level of differentiation, the size of the cell of origin, the origin cell's rate of proliferation, and the histologic pattern of growth a heterogenous group with a great diversity of subtypes several NHL classification schemas exist, reflecting the growing understanding of the complex diversity of the NHL subtypes.
 NHL - Classifications • The Working Formulation, originally proposed in 1982, classified and grouped lymphomas by morphology and clinical behavior (ie low, intermediate, or high grade). • In the 1990 s, the Revised European-American Lymphoma (REAL) classification attempted to apply immunophenotypic and genetic features in identifying distinct clinicopathologic NHL entities. • The recently proposed World Health Organization (WHO) classification further elaborates upon the REAL approach.
NHL - Classifications • The Working Formulation, originally proposed in 1982, classified and grouped lymphomas by morphology and clinical behavior (ie low, intermediate, or high grade). • In the 1990 s, the Revised European-American Lymphoma (REAL) classification attempted to apply immunophenotypic and genetic features in identifying distinct clinicopathologic NHL entities. • The recently proposed World Health Organization (WHO) classification further elaborates upon the REAL approach.
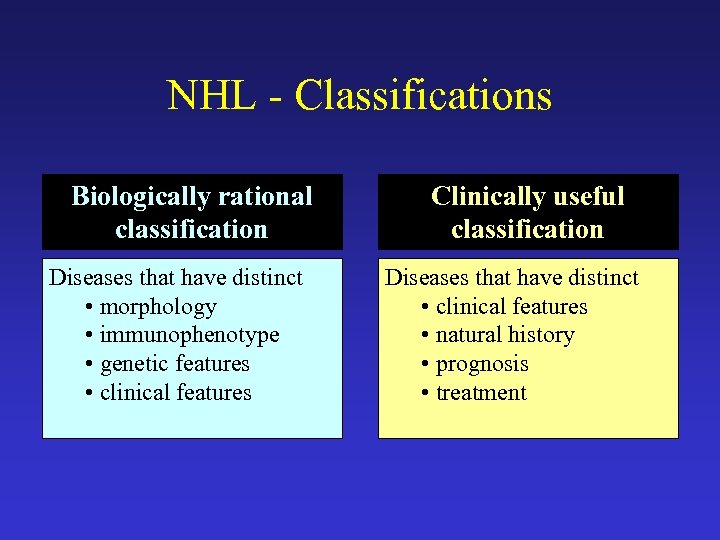 NHL - Classifications Biologically rational classification Diseases that have distinct • morphology • immunophenotype • genetic features • clinical features Clinically useful classification Diseases that have distinct • clinical features • natural history • prognosis • treatment
NHL - Classifications Biologically rational classification Diseases that have distinct • morphology • immunophenotype • genetic features • clinical features Clinically useful classification Diseases that have distinct • clinical features • natural history • prognosis • treatment
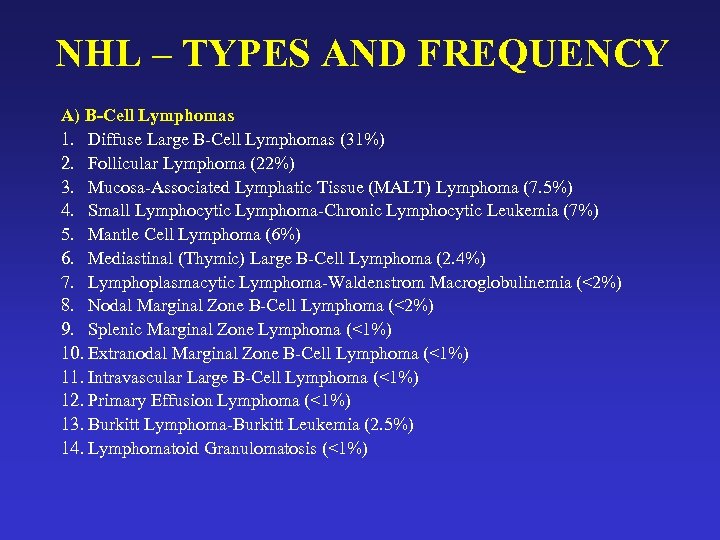 NHL – TYPES AND FREQUENCY A) B-Cell Lymphomas 1. Diffuse Large B-Cell Lymphomas (31%) 2. Follicular Lymphoma (22%) 3. Mucosa-Associated Lymphatic Tissue (MALT) Lymphoma (7. 5%) 4. Small Lymphocytic Lymphoma-Chronic Lymphocytic Leukemia (7%) 5. Mantle Cell Lymphoma (6%) 6. Mediastinal (Thymic) Large B-Cell Lymphoma (2. 4%) 7. Lymphoplasmacytic Lymphoma-Waldenstrom Macroglobulinemia (<2%) 8. Nodal Marginal Zone B-Cell Lymphoma (<2%) 9. Splenic Marginal Zone Lymphoma (<1%) 10. Extranodal Marginal Zone B-Cell Lymphoma (<1%) 11. Intravascular Large B-Cell Lymphoma (<1%) 12. Primary Effusion Lymphoma (<1%) 13. Burkitt Lymphoma-Burkitt Leukemia (2. 5%) 14. Lymphomatoid Granulomatosis (<1%)
NHL – TYPES AND FREQUENCY A) B-Cell Lymphomas 1. Diffuse Large B-Cell Lymphomas (31%) 2. Follicular Lymphoma (22%) 3. Mucosa-Associated Lymphatic Tissue (MALT) Lymphoma (7. 5%) 4. Small Lymphocytic Lymphoma-Chronic Lymphocytic Leukemia (7%) 5. Mantle Cell Lymphoma (6%) 6. Mediastinal (Thymic) Large B-Cell Lymphoma (2. 4%) 7. Lymphoplasmacytic Lymphoma-Waldenstrom Macroglobulinemia (<2%) 8. Nodal Marginal Zone B-Cell Lymphoma (<2%) 9. Splenic Marginal Zone Lymphoma (<1%) 10. Extranodal Marginal Zone B-Cell Lymphoma (<1%) 11. Intravascular Large B-Cell Lymphoma (<1%) 12. Primary Effusion Lymphoma (<1%) 13. Burkitt Lymphoma-Burkitt Leukemia (2. 5%) 14. Lymphomatoid Granulomatosis (<1%)
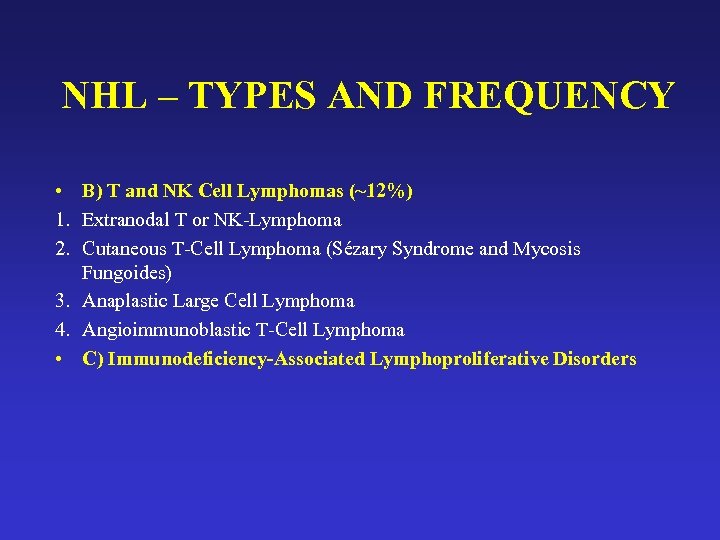 NHL – TYPES AND FREQUENCY • B) T and NK Cell Lymphomas (~12%) 1. Extranodal T or NK-Lymphoma 2. Cutaneous T-Cell Lymphoma (Sézary Syndrome and Mycosis Fungoides) 3. Anaplastic Large Cell Lymphoma 4. Angioimmunoblastic T-Cell Lymphoma • C) Immunodeficiency-Associated Lymphoproliferative Disorders
NHL – TYPES AND FREQUENCY • B) T and NK Cell Lymphomas (~12%) 1. Extranodal T or NK-Lymphoma 2. Cutaneous T-Cell Lymphoma (Sézary Syndrome and Mycosis Fungoides) 3. Anaplastic Large Cell Lymphoma 4. Angioimmunoblastic T-Cell Lymphoma • C) Immunodeficiency-Associated Lymphoproliferative Disorders
 NHL – History A. Low-grade lymphomas • Peripheral adenopathy that is painless and slowly progressive is the most common clinical presentation in these patients. • Spontaneous regression of enlarged lymph nodes can occur in low-grade lymphoma. • Primary extranodal involvement and B symptoms (ie, temperature >38°C, night sweats, weight loss >10% from baseline within 6 mo) are not common at presentation, but both are common in patients with advanced or end-stage disease. • Bone marrow is frequently involved and may be associated with cytopenia. • Fatigue and weakness are more common in patients with advanced-stage disease.
NHL – History A. Low-grade lymphomas • Peripheral adenopathy that is painless and slowly progressive is the most common clinical presentation in these patients. • Spontaneous regression of enlarged lymph nodes can occur in low-grade lymphoma. • Primary extranodal involvement and B symptoms (ie, temperature >38°C, night sweats, weight loss >10% from baseline within 6 mo) are not common at presentation, but both are common in patients with advanced or end-stage disease. • Bone marrow is frequently involved and may be associated with cytopenia. • Fatigue and weakness are more common in patients with advanced-stage disease.
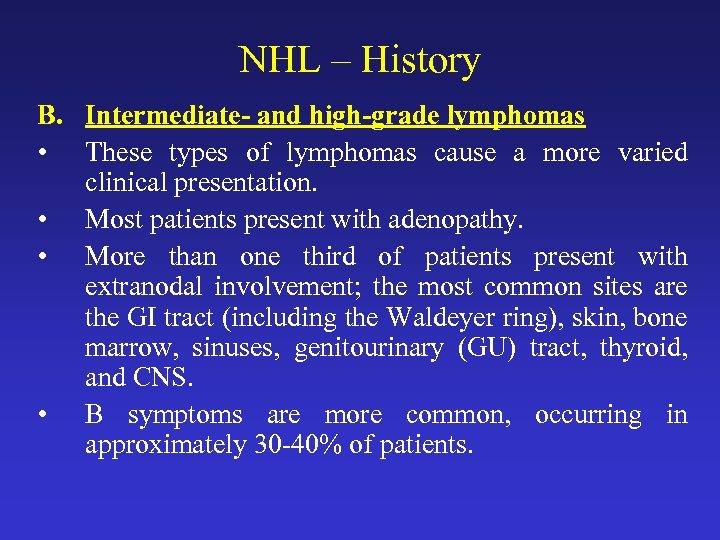 NHL – History B. Intermediate- and high-grade lymphomas • These types of lymphomas cause a more varied clinical presentation. • Most patients present with adenopathy. • More than one third of patients present with extranodal involvement; the most common sites are the GI tract (including the Waldeyer ring), skin, bone marrow, sinuses, genitourinary (GU) tract, thyroid, and CNS. • B symptoms are more common, occurring in approximately 30 -40% of patients.
NHL – History B. Intermediate- and high-grade lymphomas • These types of lymphomas cause a more varied clinical presentation. • Most patients present with adenopathy. • More than one third of patients present with extranodal involvement; the most common sites are the GI tract (including the Waldeyer ring), skin, bone marrow, sinuses, genitourinary (GU) tract, thyroid, and CNS. • B symptoms are more common, occurring in approximately 30 -40% of patients.
 NHL – History B. Intermediate- and high-grade lymphomas • • Lymphoblastic lymphoma, often manifests with an anteriorsuperior mediastinal mass, superior vena cava (SVC) syndrome, and leptomeningeal disease with cranial nerve palsies. Patients with Burkitt lymphoma (occurring in the United States) often present with a large abdominal mass and symptoms of bowel obstruction. Obstructive hydronephrosis secondary to bulky retroperitoneal lymphadenopathy obstructing the ureters can also be observed in these patients. Primary CNS lymphomas are high-grade neoplasms of B-cell origin. These lymphomas are more commonly observed in patients who are immunodeficient.
NHL – History B. Intermediate- and high-grade lymphomas • • Lymphoblastic lymphoma, often manifests with an anteriorsuperior mediastinal mass, superior vena cava (SVC) syndrome, and leptomeningeal disease with cranial nerve palsies. Patients with Burkitt lymphoma (occurring in the United States) often present with a large abdominal mass and symptoms of bowel obstruction. Obstructive hydronephrosis secondary to bulky retroperitoneal lymphadenopathy obstructing the ureters can also be observed in these patients. Primary CNS lymphomas are high-grade neoplasms of B-cell origin. These lymphomas are more commonly observed in patients who are immunodeficient.
 NHL – Clinical A. Low-grade lymphomas • Peripheral adenopathy • Splenomegaly: Splenomegaly is observed in approximately 40% of patients; the spleen rarely is the only involved site at presentation. • Hepatomegaly
NHL – Clinical A. Low-grade lymphomas • Peripheral adenopathy • Splenomegaly: Splenomegaly is observed in approximately 40% of patients; the spleen rarely is the only involved site at presentation. • Hepatomegaly
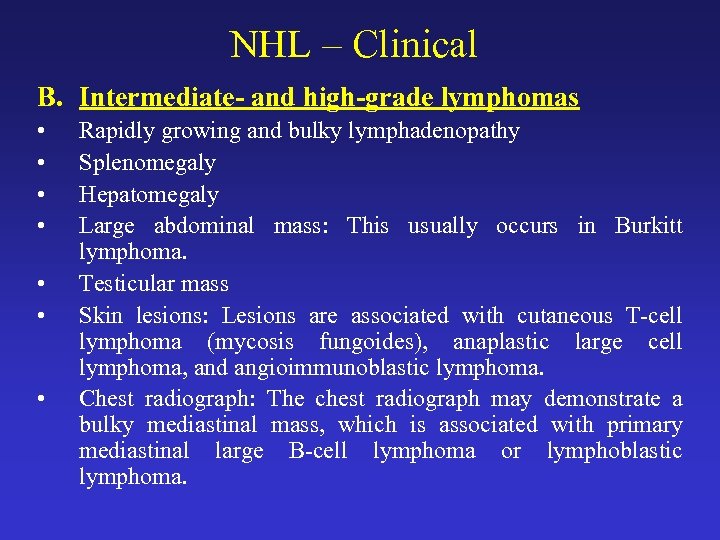 NHL – Clinical B. Intermediate- and high-grade lymphomas • • Rapidly growing and bulky lymphadenopathy Splenomegaly Hepatomegaly Large abdominal mass: This usually occurs in Burkitt lymphoma. Testicular mass Skin lesions: Lesions are associated with cutaneous T-cell lymphoma (mycosis fungoides), anaplastic large cell lymphoma, and angioimmunoblastic lymphoma. Chest radiograph: The chest radiograph may demonstrate a bulky mediastinal mass, which is associated with primary mediastinal large B-cell lymphoma or lymphoblastic lymphoma.
NHL – Clinical B. Intermediate- and high-grade lymphomas • • Rapidly growing and bulky lymphadenopathy Splenomegaly Hepatomegaly Large abdominal mass: This usually occurs in Burkitt lymphoma. Testicular mass Skin lesions: Lesions are associated with cutaneous T-cell lymphoma (mycosis fungoides), anaplastic large cell lymphoma, and angioimmunoblastic lymphoma. Chest radiograph: The chest radiograph may demonstrate a bulky mediastinal mass, which is associated with primary mediastinal large B-cell lymphoma or lymphoblastic lymphoma.
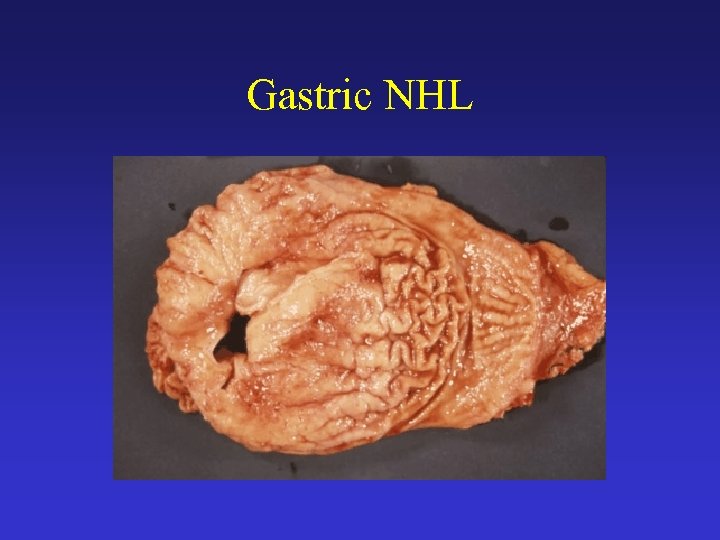 Gastric NHL
Gastric NHL
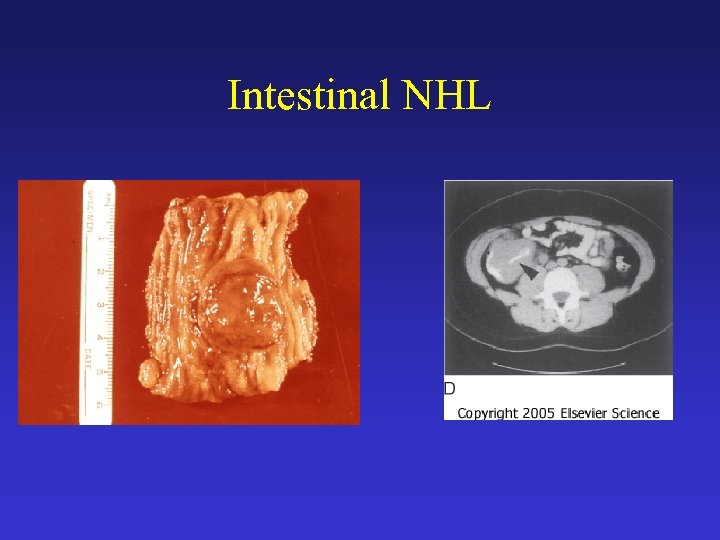 Intestinal NHL
Intestinal NHL
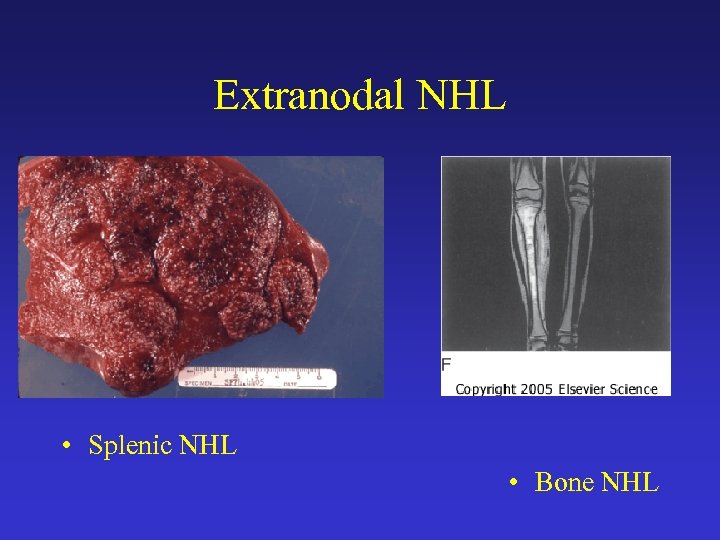 Extranodal NHL • Splenic NHL • Bone NHL
Extranodal NHL • Splenic NHL • Bone NHL
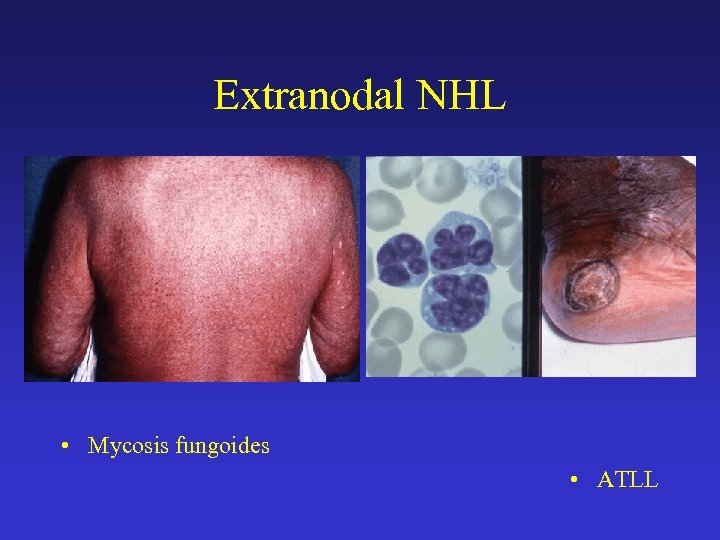 Extranodal NHL • Mycosis fungoides • ATLL
Extranodal NHL • Mycosis fungoides • ATLL
 NHL – Diagnosis v. Biopsy of peripheral (or most accessible) lymphadenopathy – Excisional lymph node biopsy is required because lymphoma diagnosis relies heavily on careful assessment of altered nodal architecture accompanying lymphomatous infiltrates. – Fine-needle aspiration (FNA) is insufficient for establishing a diagnosis; needle-core biopsies have a limited role in establishing a diagnosis of NHL. – A well-processed hematoxylin and eosin (H&E)– stained section of an excised lymph node is the mainstay of pathologic diagnosis.
NHL – Diagnosis v. Biopsy of peripheral (or most accessible) lymphadenopathy – Excisional lymph node biopsy is required because lymphoma diagnosis relies heavily on careful assessment of altered nodal architecture accompanying lymphomatous infiltrates. – Fine-needle aspiration (FNA) is insufficient for establishing a diagnosis; needle-core biopsies have a limited role in establishing a diagnosis of NHL. – A well-processed hematoxylin and eosin (H&E)– stained section of an excised lymph node is the mainstay of pathologic diagnosis.
 NHL – Diagnosis v. Bone marrow aspirate and biopsy – Perform this procedure for staging rather than diagnostic purposes. – Bilateral bone marrow aspirate and biopsy should be performed because bone marrow involvement is usually patchy. – In bone marrow sections, the neoplastic cells may infiltrate in a focal (ie, paratrabecular or nonparatrabecular, depending on the type of lymphoma), interstitial, or diffuse pattern.
NHL – Diagnosis v. Bone marrow aspirate and biopsy – Perform this procedure for staging rather than diagnostic purposes. – Bilateral bone marrow aspirate and biopsy should be performed because bone marrow involvement is usually patchy. – In bone marrow sections, the neoplastic cells may infiltrate in a focal (ie, paratrabecular or nonparatrabecular, depending on the type of lymphoma), interstitial, or diffuse pattern.
 NHL – Diagnosis v. Biopsy of extranodal sites – In approximately 30 -35% of adult patients with NHL, the extranodal sites are the primary presenting sites, and the most common site is the GI tract. – Processing extranodal biopsy material for lymphoma protocol studies is important whenever suspicion of a hematolymphoid neoplasm exists. – Lumbar puncture for cerebrospinal fluid (CSF) examination
NHL – Diagnosis v. Biopsy of extranodal sites – In approximately 30 -35% of adult patients with NHL, the extranodal sites are the primary presenting sites, and the most common site is the GI tract. – Processing extranodal biopsy material for lymphoma protocol studies is important whenever suspicion of a hematolymphoid neoplasm exists. – Lumbar puncture for cerebrospinal fluid (CSF) examination
 NHL – Diagnosis • Histologic Findings: – NHLs are a heterogenous group of lymphoproliferative malignancies with varying morphologic features depending on the specific type of this disorder. – The abnormal lymphocytes in the lymph node, bone marrow, or extranodal sites can be small cleaved or noncleaved, intermediate, or large cell and can have a follicular or diffuse pattern. – In contrast with reactive follicular hyperplasia, lymphomas usually alter the lymph node architecture, and the capsule is usually involved.
NHL – Diagnosis • Histologic Findings: – NHLs are a heterogenous group of lymphoproliferative malignancies with varying morphologic features depending on the specific type of this disorder. – The abnormal lymphocytes in the lymph node, bone marrow, or extranodal sites can be small cleaved or noncleaved, intermediate, or large cell and can have a follicular or diffuse pattern. – In contrast with reactive follicular hyperplasia, lymphomas usually alter the lymph node architecture, and the capsule is usually involved.
 NHL – Diagnosis v Immunophenotypic analysis of lymph node, bone marrow, and/or peripheral blood – Compliments and confirms the results of routine tissue section and may be useful in resolving a diagnostic dilemma in patients with an atypical morphology. – Helps to distinguish reactive from neoplastic lymphoid infiltrates, lymphoid from nonlymphoid malignancies, and specific lymphoid neoplasms. – Provides information about lineage and clonality, which are complimentary to the histology of a given case. – Useful for subclassifying certain lymphoma subtypes, which has therapeutic and prognostic importance.
NHL – Diagnosis v Immunophenotypic analysis of lymph node, bone marrow, and/or peripheral blood – Compliments and confirms the results of routine tissue section and may be useful in resolving a diagnostic dilemma in patients with an atypical morphology. – Helps to distinguish reactive from neoplastic lymphoid infiltrates, lymphoid from nonlymphoid malignancies, and specific lymphoid neoplasms. – Provides information about lineage and clonality, which are complimentary to the histology of a given case. – Useful for subclassifying certain lymphoma subtypes, which has therapeutic and prognostic importance.
 NHL – Diagnosis v. Cytogenetic studies • Have contributed to the understanding of the biology and prognosis of lymphoma. • Are critical to the discovery of oncogene abnormalities that now are known to be intimately involved in the pathogenesis of NHL.
NHL – Diagnosis v. Cytogenetic studies • Have contributed to the understanding of the biology and prognosis of lymphoma. • Are critical to the discovery of oncogene abnormalities that now are known to be intimately involved in the pathogenesis of NHL.
 NHL – Staging • Staging is important in selecting a treatment and also for prognosis – – – Careful history Physical examination Biopsy of lymphadenopaty Chest X-ray CT-scan 0 f chest, abdomen and pelvis Bilateral bone biopsy CBC with differential count General chemistry panel (beta-2 -microglobulin and LDH included) HIV, HTLV, HCV serology CSF study Upper GI series Ultrasound of testis
NHL – Staging • Staging is important in selecting a treatment and also for prognosis – – – Careful history Physical examination Biopsy of lymphadenopaty Chest X-ray CT-scan 0 f chest, abdomen and pelvis Bilateral bone biopsy CBC with differential count General chemistry panel (beta-2 -microglobulin and LDH included) HIV, HTLV, HCV serology CSF study Upper GI series Ultrasound of testis
 NHL – Staging ð Stage I involves a single lymph node region (I) or localized involvement of a single extralymphatic organ or site (IE). ð Stage II involves 2 or more lymph node regions on the same side of the diaphragm (II) or localized involvement of a single associated extralymphatic organ in addition to criteria for stage II (IIE). ð Stage III involves lymph node regions on both sides of the diaphragm (III) that also may be accompanied by localized involvement of an extralymphatic organ or site (IIIE), spleen (IIIS), or both (IIISE). ð Stage IV represents disseminated or multifocal involvement of one or more extralymphatic sites with or without associated lymph node involvement or isolated extralymphatic organ involvement with distant (nonregional) nodal involvement.
NHL – Staging ð Stage I involves a single lymph node region (I) or localized involvement of a single extralymphatic organ or site (IE). ð Stage II involves 2 or more lymph node regions on the same side of the diaphragm (II) or localized involvement of a single associated extralymphatic organ in addition to criteria for stage II (IIE). ð Stage III involves lymph node regions on both sides of the diaphragm (III) that also may be accompanied by localized involvement of an extralymphatic organ or site (IIIE), spleen (IIIS), or both (IIISE). ð Stage IV represents disseminated or multifocal involvement of one or more extralymphatic sites with or without associated lymph node involvement or isolated extralymphatic organ involvement with distant (nonregional) nodal involvement.
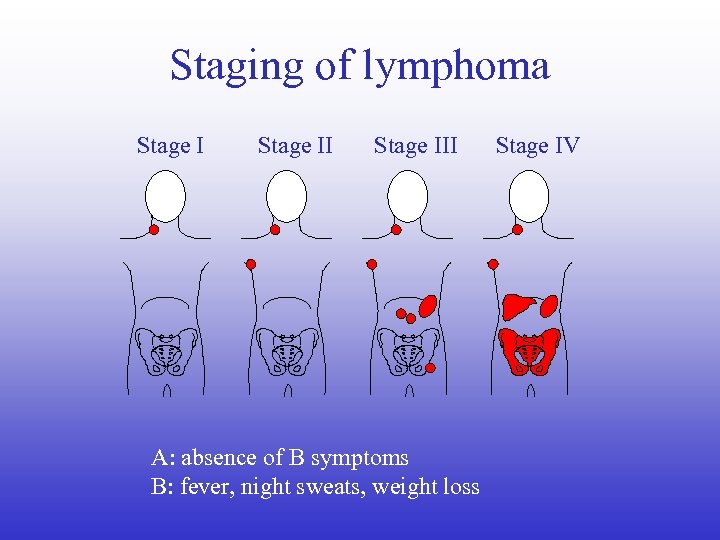 Staging of lymphoma Stage III A: absence of B symptoms B: fever, night sweats, weight loss Stage IV
Staging of lymphoma Stage III A: absence of B symptoms B: fever, night sweats, weight loss Stage IV
 NHL – Staging • Subscript letters designate involvement of extralymphatic organs, as follows: L, lung; H, liver; P, pleura; O, bone; M, bone marrow; and D, skin. • The designation E is used when extranodal lymphoid malignancies arise in tissues that are separate from but near the major lymphatic aggregates. • In this system, stages I-IV can be appended by A or B designations. The B designation is applied in patients with any of the following symptoms: unexplained loss of more than 10% of body weight in the preceding 6 months before diagnosis, unexplained fever with temperature above 38°C, and drenching night sweats.
NHL – Staging • Subscript letters designate involvement of extralymphatic organs, as follows: L, lung; H, liver; P, pleura; O, bone; M, bone marrow; and D, skin. • The designation E is used when extranodal lymphoid malignancies arise in tissues that are separate from but near the major lymphatic aggregates. • In this system, stages I-IV can be appended by A or B designations. The B designation is applied in patients with any of the following symptoms: unexplained loss of more than 10% of body weight in the preceding 6 months before diagnosis, unexplained fever with temperature above 38°C, and drenching night sweats.
 NHL – Prognosis • The International Prognostic Index (IPI) : o o o Age - Younger than 60 years versus older than 60 years LDH level - Within the reference range versus elevated Performance status - ECOG 0 -1 versus 2 -4 Ann Arbor stage - Stage I-II versus III-IV Number of extranodal sites - 0 -1 versus more than 1 o Patients with 0 -1, 2 -3, and 4 -5 risk factors have 75%, 50%, and 25% chance, respectively, of having a relapse-free and OS at 5 years.
NHL – Prognosis • The International Prognostic Index (IPI) : o o o Age - Younger than 60 years versus older than 60 years LDH level - Within the reference range versus elevated Performance status - ECOG 0 -1 versus 2 -4 Ann Arbor stage - Stage I-II versus III-IV Number of extranodal sites - 0 -1 versus more than 1 o Patients with 0 -1, 2 -3, and 4 -5 risk factors have 75%, 50%, and 25% chance, respectively, of having a relapse-free and OS at 5 years.
 NHL – Medical care • A. Indolent stage I and contiguous stage II NHL – Involved-field radiation therapy may be the treatment of choice in patients with localized low-grade NHL, especially of the head and neck. For these patients, radiation therapy (2500 -4000 c. Gy) produces a 10 -year failure-free survival (FFS) rate of 50 -60%, with an overall survival (OS) rate of 60 -80%. – Offer adjuvant chemotherapy to selected patients with stage I-II NHL who have unfavorable prognostic factors (eg, B symptoms, >2 nodal sites) and to those with follicular mixed histology.
NHL – Medical care • A. Indolent stage I and contiguous stage II NHL – Involved-field radiation therapy may be the treatment of choice in patients with localized low-grade NHL, especially of the head and neck. For these patients, radiation therapy (2500 -4000 c. Gy) produces a 10 -year failure-free survival (FFS) rate of 50 -60%, with an overall survival (OS) rate of 60 -80%. – Offer adjuvant chemotherapy to selected patients with stage I-II NHL who have unfavorable prognostic factors (eg, B symptoms, >2 nodal sites) and to those with follicular mixed histology.
 NHL – Medical care • B. Indolent noncontiguous stage II, III, and IV NHL – Treatment for symptomatic patients includes • (1) purine nucleoside analogues (ie, fludarabine, 2 -CDA), which have significant antitumor activity in low-grade NHL; • (2) oral alkylating agents with or without steroids (ie, cyclophosphamide, chlorambucil); • (3) combination chemotherapy using cyclophosphamide, vincristine, and prednisone (CVP) or cyclophosphamide, hydroxydaunomycin, Oncovin (vincristine), and prednisone (CHOP). • (4) intensive therapy with chemotherapy and total body irradiation (TBI) followed by autologous or allogeneic bone marrow or peripheral stem cell transplantation, especially for younger patients with poor prognostic factors, is under clinical investigation. • (5) For patients who are unable to tolerate other options, anti-CD 20 monoclonal antibody (rituximab) may be considered as the first-line therapy, either alone or with combination chemotherapy.
NHL – Medical care • B. Indolent noncontiguous stage II, III, and IV NHL – Treatment for symptomatic patients includes • (1) purine nucleoside analogues (ie, fludarabine, 2 -CDA), which have significant antitumor activity in low-grade NHL; • (2) oral alkylating agents with or without steroids (ie, cyclophosphamide, chlorambucil); • (3) combination chemotherapy using cyclophosphamide, vincristine, and prednisone (CVP) or cyclophosphamide, hydroxydaunomycin, Oncovin (vincristine), and prednisone (CHOP). • (4) intensive therapy with chemotherapy and total body irradiation (TBI) followed by autologous or allogeneic bone marrow or peripheral stem cell transplantation, especially for younger patients with poor prognostic factors, is under clinical investigation. • (5) For patients who are unable to tolerate other options, anti-CD 20 monoclonal antibody (rituximab) may be considered as the first-line therapy, either alone or with combination chemotherapy.
 NHL – Medical care • A. Aggressive stage I and contiguous stage II (nonbulky or <10 cm) NHL – Patients with intermediate-grade NHL - combination chemotherapy (3 cycles of CHOP) plus involved field radiation therapy. – Patients with high-grade disease should be strongly considered for treatment with more aggressive regimens beyond CHOP.
NHL – Medical care • A. Aggressive stage I and contiguous stage II (nonbulky or <10 cm) NHL – Patients with intermediate-grade NHL - combination chemotherapy (3 cycles of CHOP) plus involved field radiation therapy. – Patients with high-grade disease should be strongly considered for treatment with more aggressive regimens beyond CHOP.
 NHL – Medical care • B. Aggressive noncontiguous stage II, III, and IV NHL – The treatment of choice for these patients is combination chemotherapy, either alone or supplemented by involved field irradiation. – Doxorubicin-based combination chemotherapy produces long-term disease-free survival (DFS) in 35 -45% of patients. – For intermediate-grade lymphomas, CHOP chemotherapy remains the standard of care at this time.
NHL – Medical care • B. Aggressive noncontiguous stage II, III, and IV NHL – The treatment of choice for these patients is combination chemotherapy, either alone or supplemented by involved field irradiation. – Doxorubicin-based combination chemotherapy produces long-term disease-free survival (DFS) in 35 -45% of patients. – For intermediate-grade lymphomas, CHOP chemotherapy remains the standard of care at this time.
 NHL – Medical care • B. Aggressive noncontiguous stage II, III, and IV NHL – Autologous and allogeneic bone marrow or peripheral stem cell transplantation for patients at high risk of relapse are under clinical investigation. – Innovative approaches to improve the results of CHOP for patients at high risk of relapse, such as monoclonal antibody therapy, are under clinical investigation. – CNS prophylaxis, usually with 4 -6 injections of methotrexate intrathecally, is recommended for patients with paranasal sinus or testicular involvement, diffuse small noncleaved cell or Burkitt lymphoma, or lymphoblastic lymphoma.
NHL – Medical care • B. Aggressive noncontiguous stage II, III, and IV NHL – Autologous and allogeneic bone marrow or peripheral stem cell transplantation for patients at high risk of relapse are under clinical investigation. – Innovative approaches to improve the results of CHOP for patients at high risk of relapse, such as monoclonal antibody therapy, are under clinical investigation. – CNS prophylaxis, usually with 4 -6 injections of methotrexate intrathecally, is recommended for patients with paranasal sinus or testicular involvement, diffuse small noncleaved cell or Burkitt lymphoma, or lymphoblastic lymphoma.
 NHL – Medical care • B. Aggressive noncontiguous stage II, III, and IV NHL – Treatment of acute lymphoblastic lymphoma, a very aggressive form of NHL, is usually patterned after acute lymphoblastic leukemia (ALL) therapy. Intensive combination chemotherapy with CNS prophylaxis is the standard treatment of this aggressive histologic type of NHL. – Other subtypes of high-grade lymphomas are usually treated with more aggressive variations of CHOP chemotherapy, including the addition of high-dose methotrexate or other chemotherapy drugs and higher doses of cyclophosphamide
NHL – Medical care • B. Aggressive noncontiguous stage II, III, and IV NHL – Treatment of acute lymphoblastic lymphoma, a very aggressive form of NHL, is usually patterned after acute lymphoblastic leukemia (ALL) therapy. Intensive combination chemotherapy with CNS prophylaxis is the standard treatment of this aggressive histologic type of NHL. – Other subtypes of high-grade lymphomas are usually treated with more aggressive variations of CHOP chemotherapy, including the addition of high-dose methotrexate or other chemotherapy drugs and higher doses of cyclophosphamide
 NHL – Medical care • Further Inpatient Care: – Further inpatient care depends on the patient's active problem, tumor type and stage, and overall prognosis. – Admit patients for complications of disease progression (eg, pain control for intractable pain) or adverse effects from chemotherapy (eg, neutropenic fever, dehydration secondary to diarrhea, vomiting requiring IV hydration, severe mucositis). – Admit patients for infusional chemotherapy or highdose chemotherapy followed by stem cell transplantation.
NHL – Medical care • Further Inpatient Care: – Further inpatient care depends on the patient's active problem, tumor type and stage, and overall prognosis. – Admit patients for complications of disease progression (eg, pain control for intractable pain) or adverse effects from chemotherapy (eg, neutropenic fever, dehydration secondary to diarrhea, vomiting requiring IV hydration, severe mucositis). – Admit patients for infusional chemotherapy or highdose chemotherapy followed by stem cell transplantation.
 NHL – Medical care • Further Outpatient Care: – Treatment and follow-up care of patients with NHL are usually performed on an outpatient basis. – Monitoring the patient's blood cell count while receiving chemotherapy (eg, prior to each treatment cycle and 10 -14 d after each treatment cycle) is important. – Monitor adverse effects of chemotherapy with a detailed patient history, an examination, a CBC count, and chemistries (especially LFTs, electrolytes, LDH, BUN/creatinine). – Treat symptomatic adverse effects such as nausea, vomiting, diarrhea, mucositis, anorexia, pain, and fatigue.
NHL – Medical care • Further Outpatient Care: – Treatment and follow-up care of patients with NHL are usually performed on an outpatient basis. – Monitoring the patient's blood cell count while receiving chemotherapy (eg, prior to each treatment cycle and 10 -14 d after each treatment cycle) is important. – Monitor adverse effects of chemotherapy with a detailed patient history, an examination, a CBC count, and chemistries (especially LFTs, electrolytes, LDH, BUN/creatinine). – Treat symptomatic adverse effects such as nausea, vomiting, diarrhea, mucositis, anorexia, pain, and fatigue.
 NHL – Medical care • Further Outpatient Care: – Administer packed red blood cell (PRBC) transfusions for patients with symptomatic anemia and provide platelet transfusions for patients with a platelet count less than 10 -20 k/CU mm. – Provide growth factor (eg, GCSF, GM-CSF, erythropoietin) support as necessary. – Perform a disease and response to treatment evaluation by obtaining patient history, physical examination (at intervals q 2 -3 mo), and imaging studies (eg, CT scans at intervals q 4 -12 mo). – Provide psychosocial support for the patient and family.
NHL – Medical care • Further Outpatient Care: – Administer packed red blood cell (PRBC) transfusions for patients with symptomatic anemia and provide platelet transfusions for patients with a platelet count less than 10 -20 k/CU mm. – Provide growth factor (eg, GCSF, GM-CSF, erythropoietin) support as necessary. – Perform a disease and response to treatment evaluation by obtaining patient history, physical examination (at intervals q 2 -3 mo), and imaging studies (eg, CT scans at intervals q 4 -12 mo). – Provide psychosocial support for the patient and family.
 NHL – Complications • Disease-related complications o o o o Cytopenias. Bleeding Infection Cardiac problems Respiratory problems SVC syndrome secondary to a large mediastinal tumor Spinal cord compression secondary to vertebral metastases Neurologic problems secondary to primary CNS lymphoma or lymphomatous meningitis o GI obstruction, perforation, and bleeding o Pain secondary to tumor invasion o Leukocytosis (lymphocytosis) in leukemic phase of disease
NHL – Complications • Disease-related complications o o o o Cytopenias. Bleeding Infection Cardiac problems Respiratory problems SVC syndrome secondary to a large mediastinal tumor Spinal cord compression secondary to vertebral metastases Neurologic problems secondary to primary CNS lymphoma or lymphomatous meningitis o GI obstruction, perforation, and bleeding o Pain secondary to tumor invasion o Leukocytosis (lymphocytosis) in leukemic phase of disease
 NHL – COMPLICATIONS • Chemotherapy complications o o o and other treatment-related Cytopenias (ie, neutropenia, anemia, thrombocytopenia) Nausea or vomiting Infection Fatigue Neuropathy Dehydration after diarrhea or vomiting Cardiac toxicity from doxorubicin Catheter-related sepsis Catheter-related thrombosis Secondary malignancies Tumor lysis syndrome
NHL – COMPLICATIONS • Chemotherapy complications o o o and other treatment-related Cytopenias (ie, neutropenia, anemia, thrombocytopenia) Nausea or vomiting Infection Fatigue Neuropathy Dehydration after diarrhea or vomiting Cardiac toxicity from doxorubicin Catheter-related sepsis Catheter-related thrombosis Secondary malignancies Tumor lysis syndrome


