Munich_Nancy_Zaichenko-last2.ppt
- Количество слайдов: 78

Lviv Polytechnic National University Bioactive nanoscale assemblies of oligoelectrolytes and PEGylated oligomers with drugs and nucleic acids for targeted delivery systems A. Zaichenko zaichenk@polynet. lviv. ua 1 1

The aim of the study: synthesis and testing oligoperoxide based functional synthetic polymer carriers as well as nanosized drug (gene) delivery systems for medicine and biotechnology 2 2

Talk outline I. Introduction. II. Why we need novel carriers and drug (gene) delivery systems? II. Functional surface-active oligoelectrolytes and nonionic surfactants as carriers for drug and gene delivery. III. Conjugates of oligomer carriers with drugs and nucleic acids as well as nanoscale delivery systems. Structures and properties. IY. Cellular study and biomedical application of developed drug and gene delivery systems 3

I. Why we need novel smart carriers and drug (gene) delivery systems? 4 4

Novel carriers for DRUG DELIVERY 5

Anticancer drug delivery • Delivery of doxorubicin or other cytostatics to tumor cells by nanosized carriers to decrease efficient cytotoxic concentration of the drugs comparing with its concentration when acting in free form. 6

Application of novel drug delivery vehicles • to decrease potential negative side effects of delivered drugs in the treated organism. 7

Major problems in development of novel drugs • High price: Development and application of one novel drug of wide spread use cost up to 1 billion USD • Low efficiency due to non-addressed action: Only 0. 001 -0. 01% of drugs applied intravenously can reach their biological targets in the organism • Development of drug resistance: During one year approximately 50% of cancer patients gain resistance to applied chemotherapeutic drugs 8

Most drug delivery carriers are nanosized. Some of them possess: • Toxic actions towards cell structure and functions; • Immunogenic (allergic) action; • Teratogenic action; • Other negative actions. 9

Novel carriers for GENE DELIVERY 10

Novel carriers for Antisense RNA (DNA) Delivery Overcoming Blood-Brain Barrier When Injected Intravenously. 11

The developed carriers must be applied for: 1. delivery of any drug; 2. delivery of water-insoluble drugs; 3. delivery of nucleic acids (gene therapy and biotechnologies); 4. attachment of any biomarker molecule for cell recognition and addressed drug delivery; 5. imaging in diagnostics and monitoring treatment course; 6. overcome biological barriers; 7. lowering toxicity; 12 8. prolongation of the action in the body.

II. Functional surface-active oligoelectrolytes and nonionic polymeric surfactants as carriers for drug and gene delivery: Oligoperoxide based strategy of the synthesis 13

I. Functional surface-active oligoperoxides, oligoelectrolytes and nonionic surfactants of telechelic, block, and comb-like structures. The main approaches of synthesis of functional oligoperoxides and derived polymers 1. Copolymerization of unsaturated ditertiary peroxides with functional monomers in hydrocarbon media and aqueous medium. 2. Telomerization of functional monomers in the presence of peroxidecontaining telogen in hydrocarbon media and aqueous medium. 3. Polymer analogous transformations using carboxyl, amino, epoxy, isocyanate, anhydride and other reactive functional groups of peroxidecontaining oligoelectrolytes. 14 14
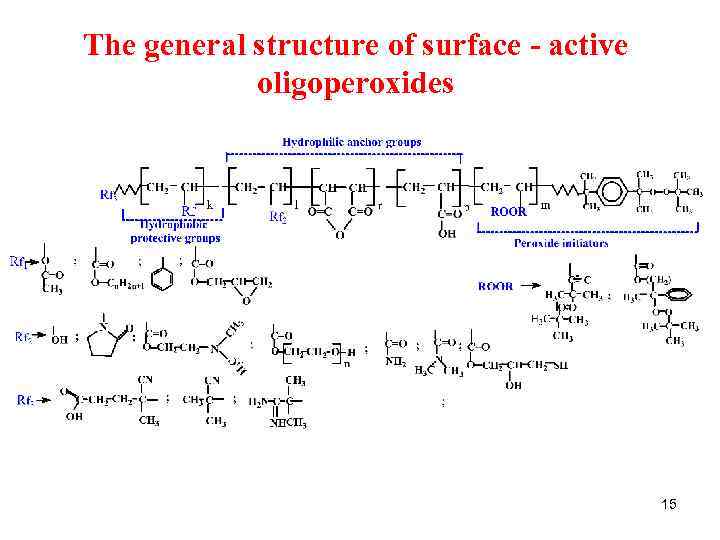
The general structure of surface - active oligoperoxides 15
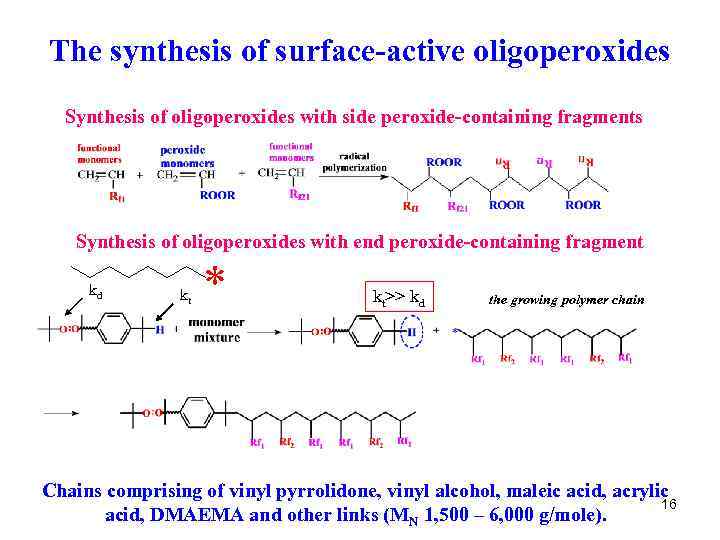
The synthesis of surface-active oligoperoxides Synthesis of oligoperoxides with side peroxide-containing fragments Synthesis of oligoperoxides with end peroxide-containing fragment kd kt * kt>> kd the growing polymer chain Chains comprising of vinyl pyrrolidone, vinyl alcohol, maleic acid, acrylic 16 acid, DMAEMA and other links (MN 1, 500 – 6, 000 g/mole).
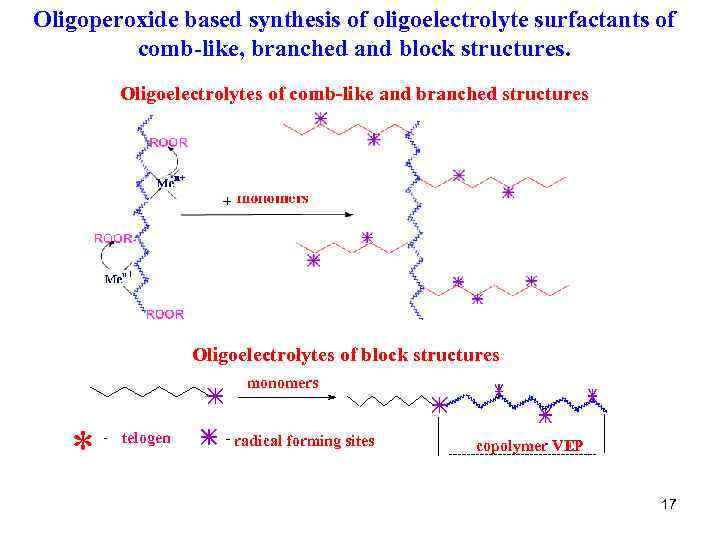
Oligoperoxide based synthesis of oligoelectrolyte surfactants of comb-like, branched and block structures. Oligoelectrolytes of comb-like and branched structures Oligoelectrolytes of block structures monomers * - telogen - radical forming sites copolymer VEP 17
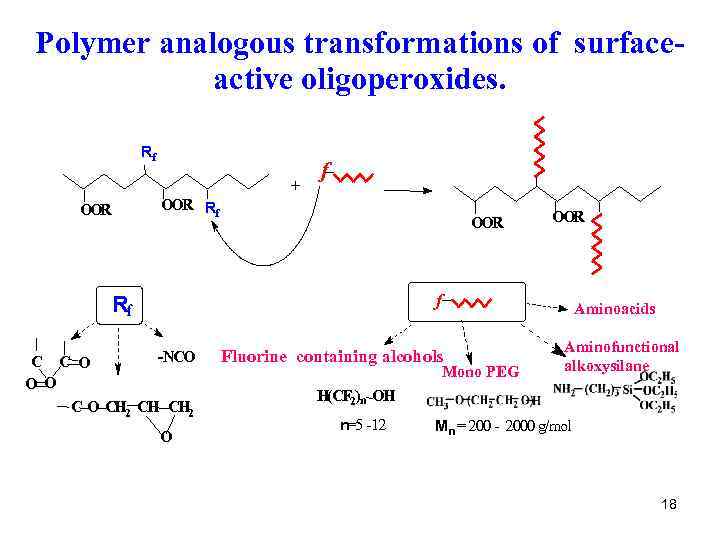
Polymer analogous transformations of surfaceactive oligoperoxides. Rf + OOR f OOR Rf OOR f Rf -NCO C C O O О C O CH 2 O Aminoacids Fluorine containing alcohols Mono PEG Aminofunctional alkoxysilane H(CF 2)n OH n=5 -12 Mn = 200 - 2000 g/mol 18
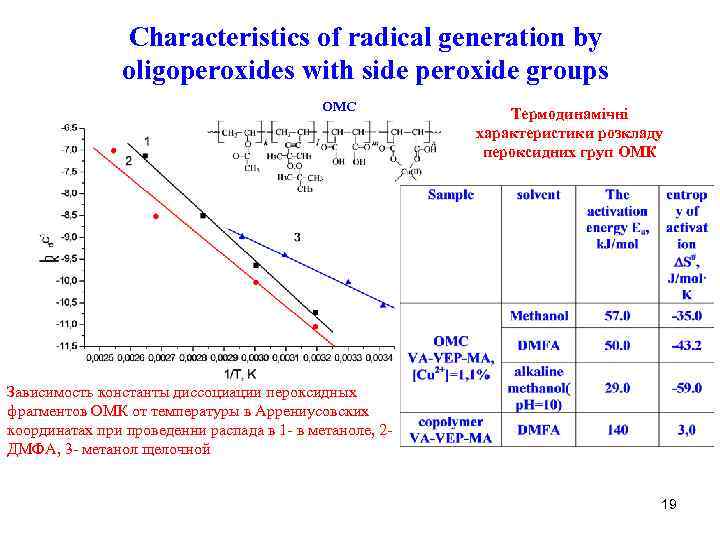
Characteristics of radical generation by oligoperoxides with side peroxide groups OMC Термодинамічні характеристики розкладу пероксидних груп ОМК Зависимость константы диссоциации пероксидных фрагментов ОМК от температуры в Аррениусовских координатах при проведенни распада в 1 - в метаноле, 2 ДМФА, 3 - метанол щелочной 19
Oligoperoxide synthesis of comb-like polymeric oligoelectrolytes 20

Oligoperoxide synthesis of comb-like polymeric oligoelectrolytes PMR-spectrum of OMC (a) and resulting comb-like copolymer OMC-graft -oligo(VEP-DMAEMA) (b) 1 1 4 6 7 3 2 2 4 3 5 1 1. 17 – 1. 18 ppm (CH 3)3 COO (VEP) 2 1. 39 – 1. 43 ppm C C-C(CH 3)2(VEP) 3 3. 35 ppm –CH-CH- (MA) 4 2. 32 ppm –C(O)-CH 3(VA) 5 12. 45 ppm –C(O)-OH(MA) 6 2. 45 ppm CH 2 -C(O)- (DMAEM) 7 2. 65 ppm N-CH 2 -O- (DMAEM) 21
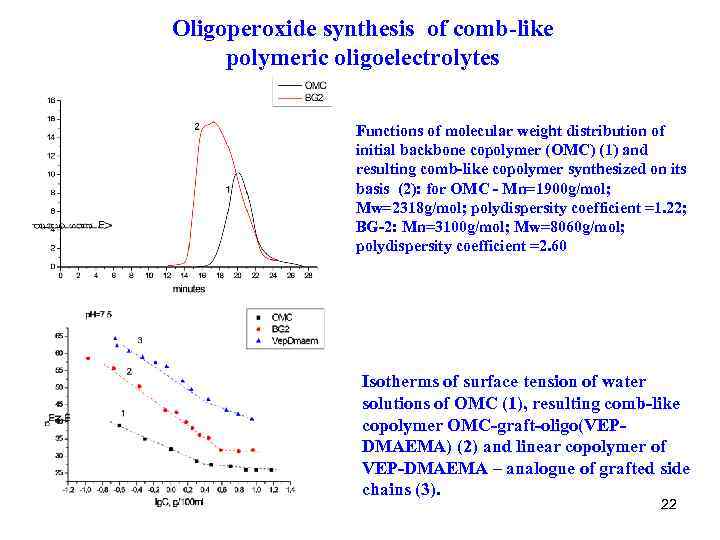
Oligoperoxide synthesis of comb-like polymeric oligoelectrolytes Functions of molecular weight distribution of initial backbone copolymer (OMC) (1) and resulting comb-like copolymer synthesized on its basis (2): for OMC - Mn=1900 g/mol; Mw=2318 g/mol; polydispersity coefficient =1. 22; BG-2: Mn=3100 g/mol; Mw=8060 g/mol; polydispersity coefficient =2. 60 Isotherms of surface tension of water solutions of OMC (1), resulting comb-like copolymer OMC-graft-oligo(VEPDMAEMA) (2) and linear copolymer of VEP-DMAEMA – analogue of grafted side chains (3). 22
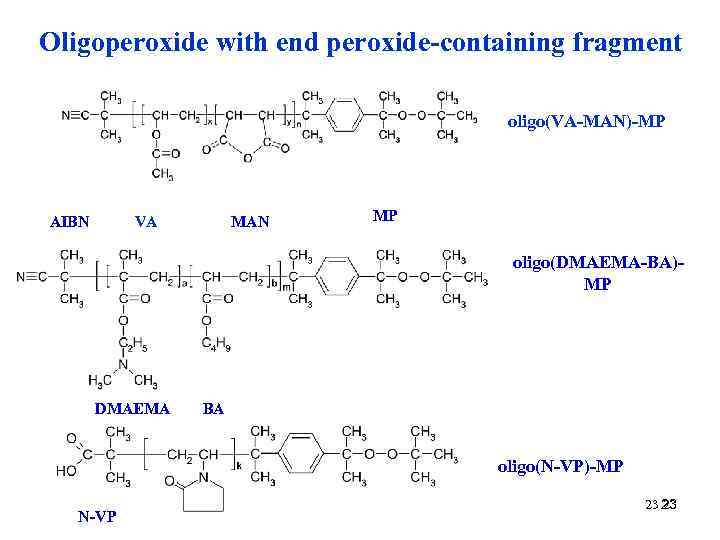
Oligoperoxide with end peroxide-containing fragment oligo(VA-MAN)-MP AIBN VA MAN MP oligo(DMAEMA-BA)MP DMAEMA BA oligo(N-VP)-MP N-VP 23 23 23
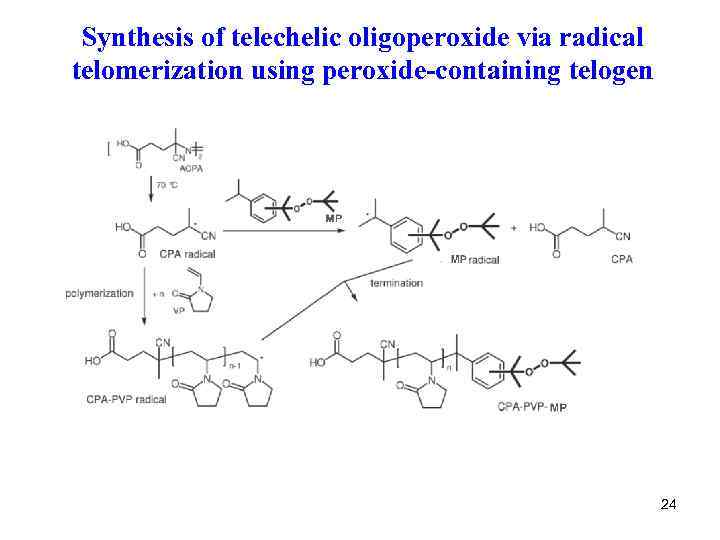
Synthesis of telechelic oligoperoxide via radical telomerization using peroxide-containing telogen 24
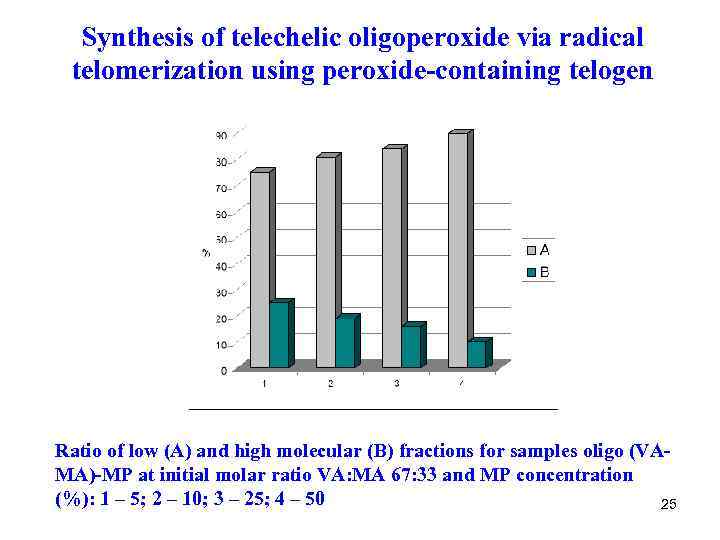
Synthesis of telechelic oligoperoxide via radical telomerization using peroxide-containing telogen Ratio of low (A) and high molecular (B) fractions for samples oligo (VAMA)-MP at initial molar ratio VA: MA 67: 33 and MP concentration (%): 1 – 5; 2 – 10; 3 – 25; 4 – 50 25
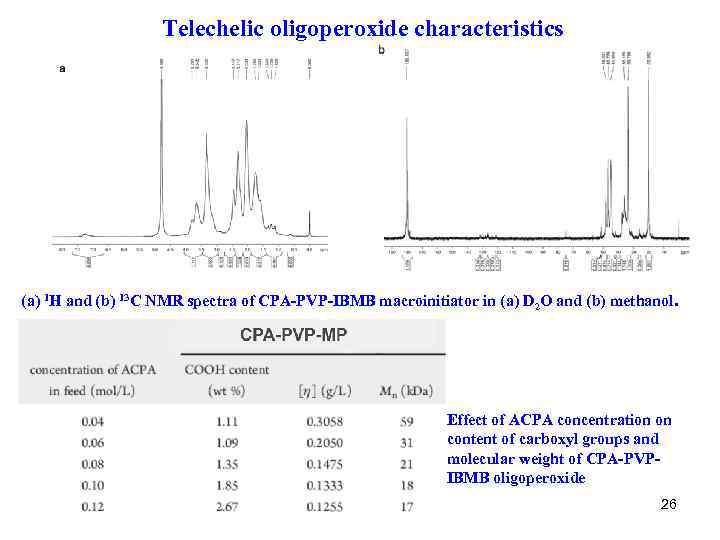
Telechelic oligoperoxide characteristics (a) 1 H and (b) 13 C NMR spectra of CPA-PVP-IBMB macroinitiator in (a) D 2 O and (b) methanol. Effect of ACPA concentration on content of carboxyl groups and molecular weight of CPA-PVPIBMB oligoperoxide 26
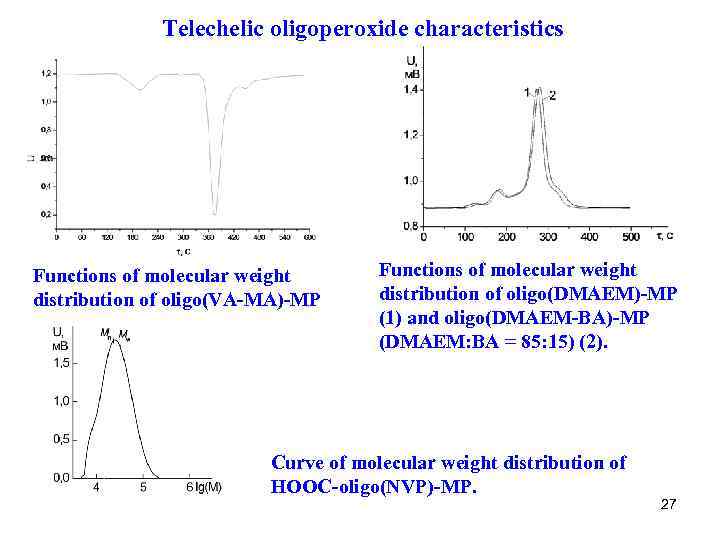
Telechelic oligoperoxide characteristics Functions of molecular weight distribution of oligo(VA-MA)-MP Functions of molecular weight distribution of oligo(DMAEM)-MP (1) and oligo(DMAEM-BA)-MP (DMAEM: BA = 85: 15) (2). Curve of molecular weight distribution of НООС-oligo(NVP)-MP. 27
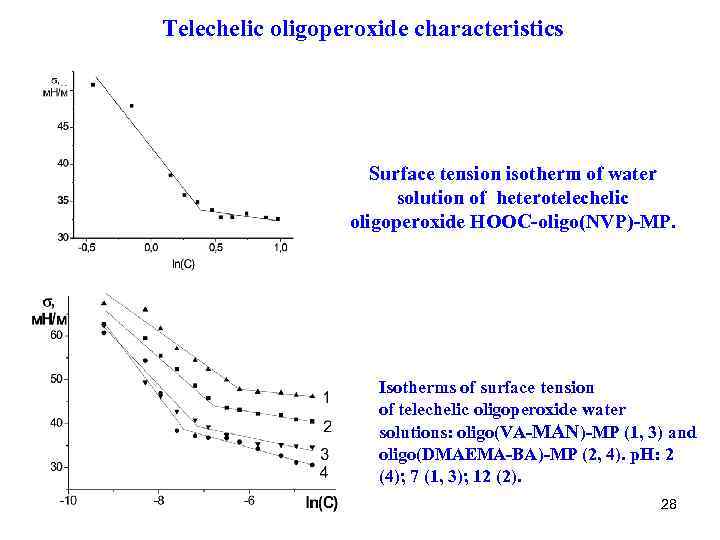
Telechelic oligoperoxide characteristics Surface tension isotherm of water solution of heterotelechelic oligoperoxide НООС-oligo(NVP)-MP. Isotherms of surface tension of telechelic oligoperoxide water solutions: oligo(VA-MAN)-MP (1, 3) and oligo(DMAEMA-BA)-MP (2, 4). p. H: 2 (4); 7 (1, 3); 12 (2). 28
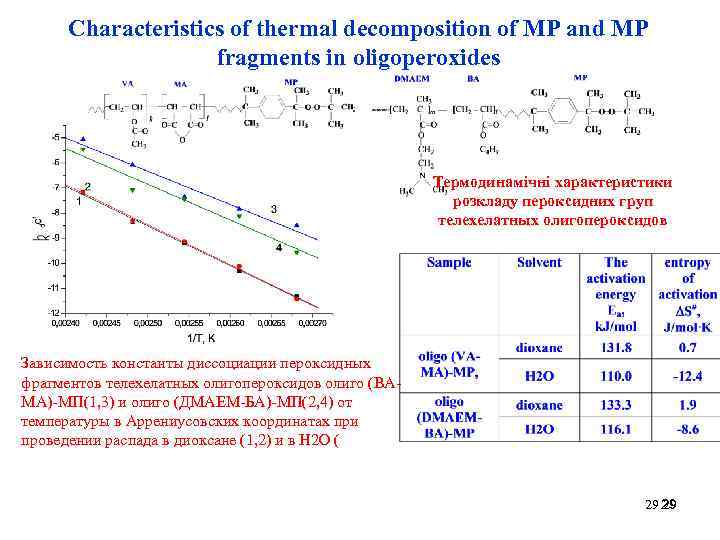
Characteristics of thermal decomposition of MP and MP fragments in oligoperoxides Термодинамічні характеристики розкладу пероксидних груп телехелатных олигопероксидов Зависимость константы диссоциации пероксидных фрагментов телехелатных олигопероксидов олиго (ВАМА)-МП(1, 3) и олиго (ДМАЕМ-БА)-МП(2, 4) от температуры в Аррениусовских координатах при проведении распада в диоксане (1, 2) и в Н 2 О ( 29 29 29
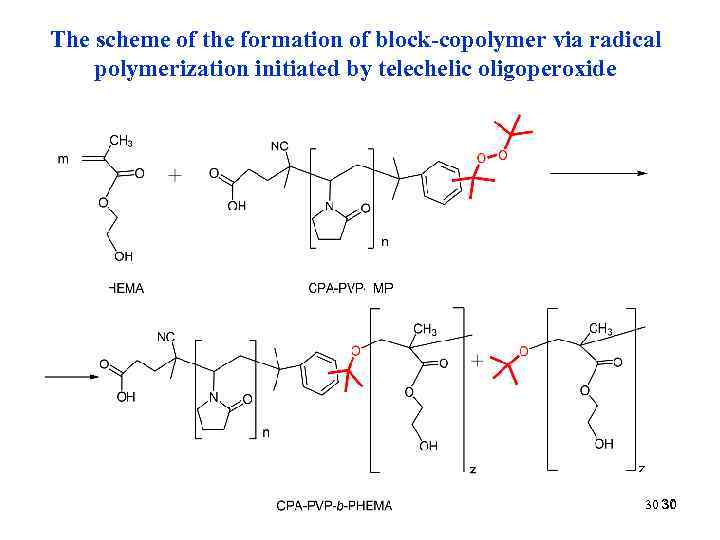
The scheme of the formation of block-copolymer via radical polymerization initiated by telechelic oligoperoxide MP 30 30 30
![Synthesis of block-copolymer via radical polymerization initiated by telechelic oligoperoxide [DMAEM]-links =95. 5%, [MP]-fragments Synthesis of block-copolymer via radical polymerization initiated by telechelic oligoperoxide [DMAEM]-links =95. 5%, [MP]-fragments](https://present5.com/presentation/11583608_173929459/image-31.jpg)
Synthesis of block-copolymer via radical polymerization initiated by telechelic oligoperoxide [DMAEM]-links =95. 5%, [MP]-fragments =5. 5%, Mn= 4500 g/mol (polydisper. =1. 03) Monomer mixture 2 -aminoethyl 2 methylacrylate + N-vinyl pyrrolidone Butyl acrylate Characterization of the block copolymer: [DMAEM]=12. 6, [MP]=0. 6%, [NVP]=70. 2%, [BA]=12. 4%, [AEM]=4. 1%; Mn= 6500 g/mol; 31 (polydispersity=1. 29)
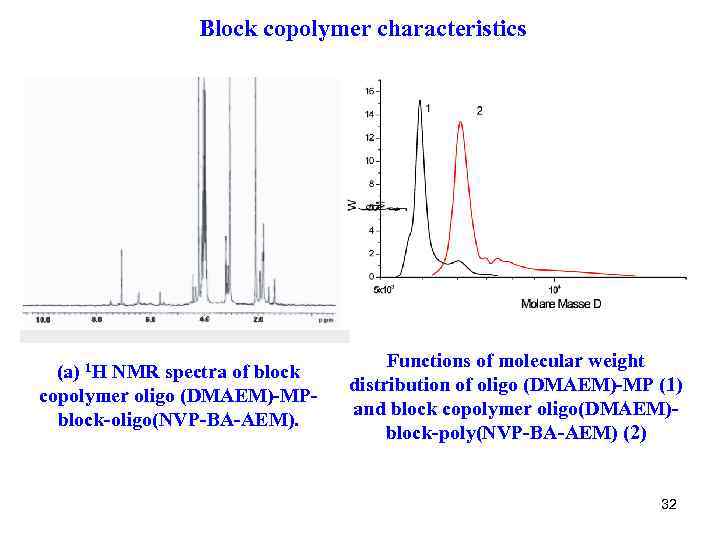
Block copolymer characteristics (a) 1 H NMR spectra of block copolymer oligo (DMAEM)-MP- block-oligo(NVP-BA-AEM). Functions of molecular weight distribution of oligo (DMAEM)-MP (1) and block copolymer oligo(DMAEM)- block-poly(NVP-BA-AEM) (2) 32
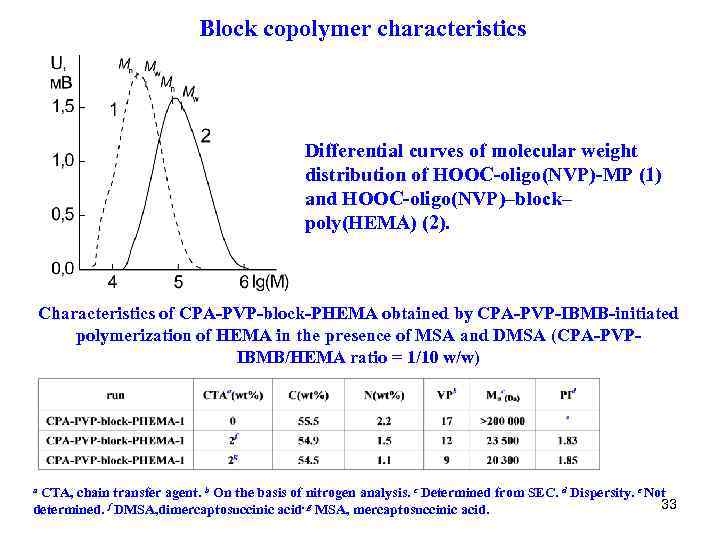
Block copolymer characteristics Differential curves of molecular weight distribution of НООС-oligo(NVP)-MP (1) and HOOC-oligo(NVP)–block– poly(HEMA) (2). Characteristics of CPA-PVP-block-PHEMA obtained by CPA-PVP-IBMB-initiated polymerization of HEMA in the presence of MSA and DMSA (CPA-PVPIBMB/HEMA ratio = 1/10 w/w) a CTA, chain transfer agent. b On the basis of nitrogen analysis. c Determined from SEC. d Dispersity. e Not 33 determined. f DMSA, dimercaptosuccinic acid. g MSA, mercaptosuccinic acid.
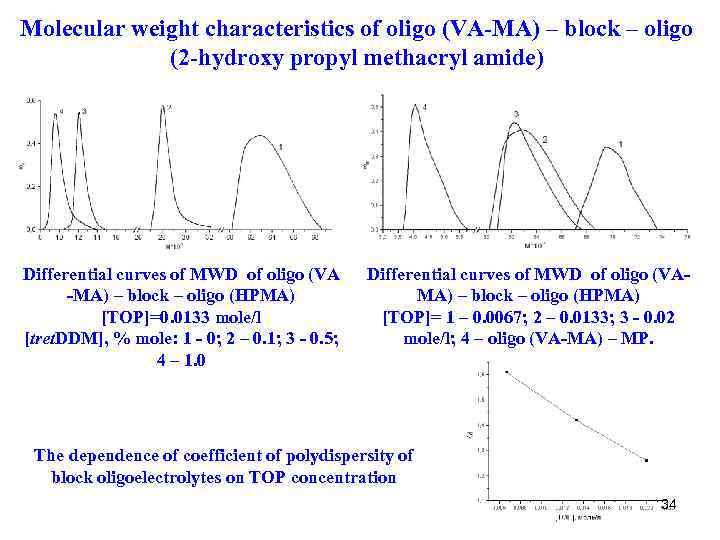
Molecular weight characteristics of oligo (VA-MA) – block – oligo (2 -hydroxy propyl methacryl amide) Differential curves of MWD of oligo (VA -MA) – block – oligo (HPMA) [TOP]=0. 0133 mole/l [tret. DDM], % mole: 1 - 0; 2 – 0. 1; 3 - 0. 5; 4 – 1. 0 Differential curves of MWD of oligo (VAMA) – block – oligo (HPMA) [TOP]= 1 – 0. 0067; 2 – 0. 0133; 3 - 0. 02 mole/l; 4 – oligo (VA-MA) – MP. The dependence of coefficient of polydispersity of block oligoelectrolytes on TOP concentration 34

Design of comb-like polymeric carriers combining PEG and oligoelectrolyte side branches 35
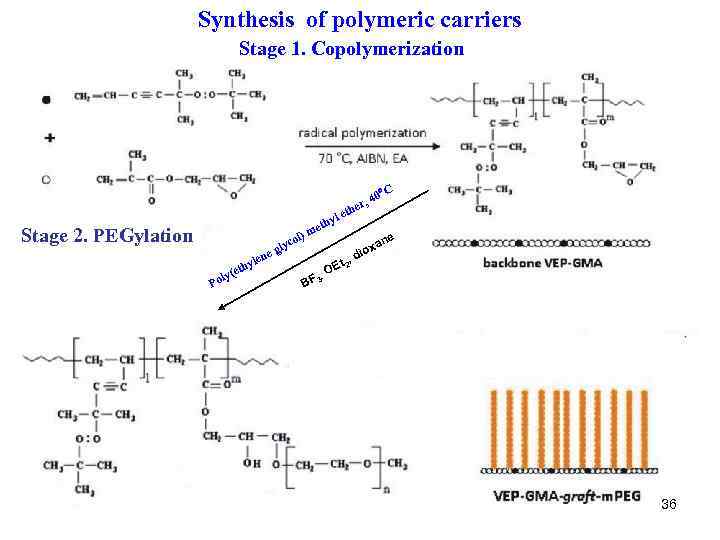
Synthesis of polymeric carriers Stage 1. Copolymerization 40 er , C th Stage 2. PEGylation m ol) ene ly ( Po yl eth c gly yl e eth t 2, OE · e an x dio BF 3 36
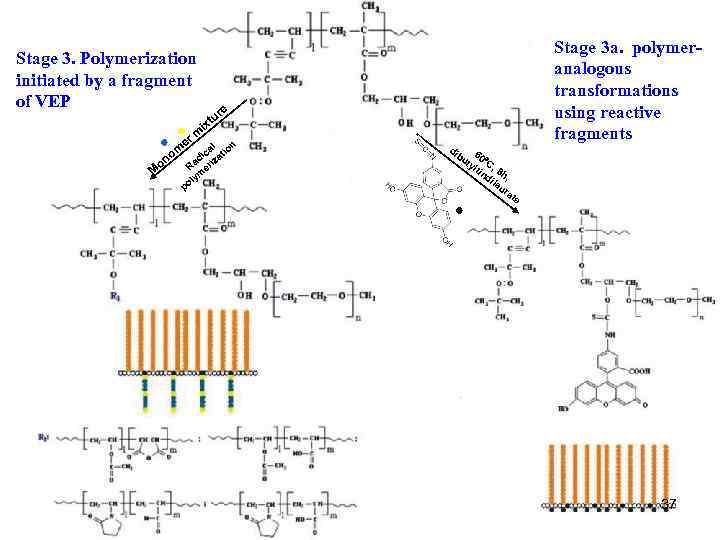
Stage 3. Polymerization initiated by a fragment of VEP er M o re tu ix m n l ca tio di iza Ra er m ly po m no Stage 3 a. polymeranalogous transformations using reactive fragments di bu 60 ty C, lti nd 8 h, ila ur at e 37
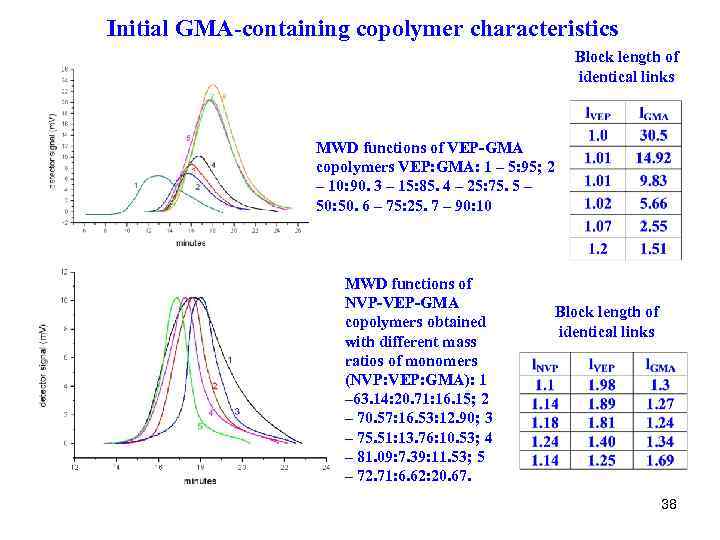
Initial GMA-containing copolymer characteristics Block length of identical links MWD functions of VEP-GMA copolymers VEP: GMA: 1 – 5: 95; 2 – 10: 90. 3 – 15: 85. 4 – 25: 75. 5 – 50: 50. 6 – 75: 25. 7 – 90: 10 MWD functions of NVP-VEP-GMA copolymers obtained with different mass ratios of monomers (NVP: VEP: GMA): 1 – 63. 14: 20. 71: 16. 15; 2 – 70. 57: 16. 53: 12. 90; 3 – 75. 51: 13. 76: 10. 53; 4 – 81. 09: 7. 39: 11. 53; 5 – 72. 71: 6. 62: 20. 67. Block length of identical links 38
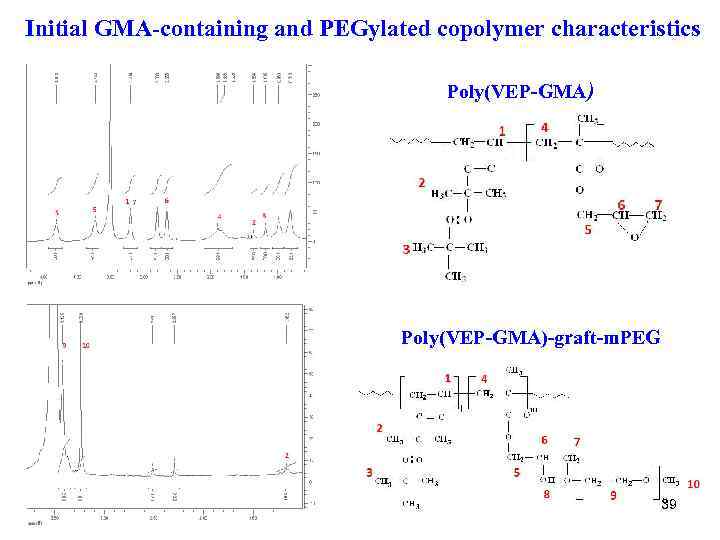
Initial GMA-containing and PEGylated copolymer characteristics Poly(VEP-GMA)-graft-m. PEG 39
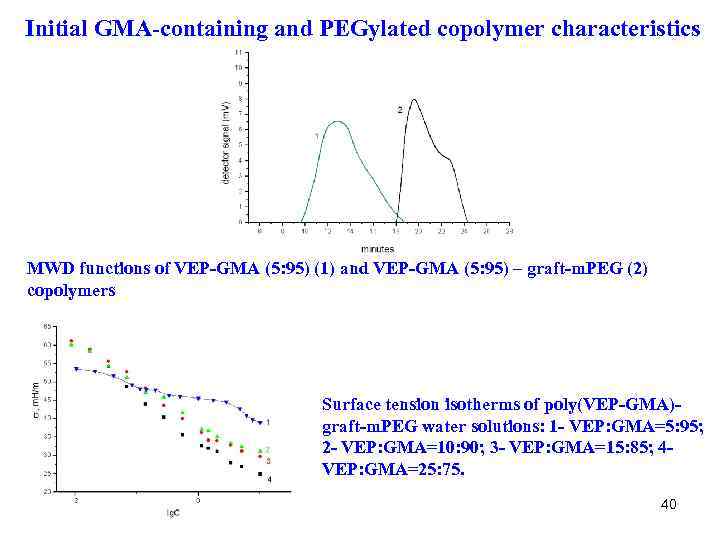
Initial GMA-containing and PEGylated copolymer characteristics MWD functions of VEP-GMA (5: 95) (1) and VEP-GMA (5: 95) – graft-m. PEG (2) copolymers Surface tension isotherms of poly(VEP-GMA)graft-m. PEG water solutions: 1 - VEP: GMA=5: 95; 2 - VEP: GMA=10: 90; 3 - VEP: GMA=15: 85; 4 VEP: GMA=25: 75. 40
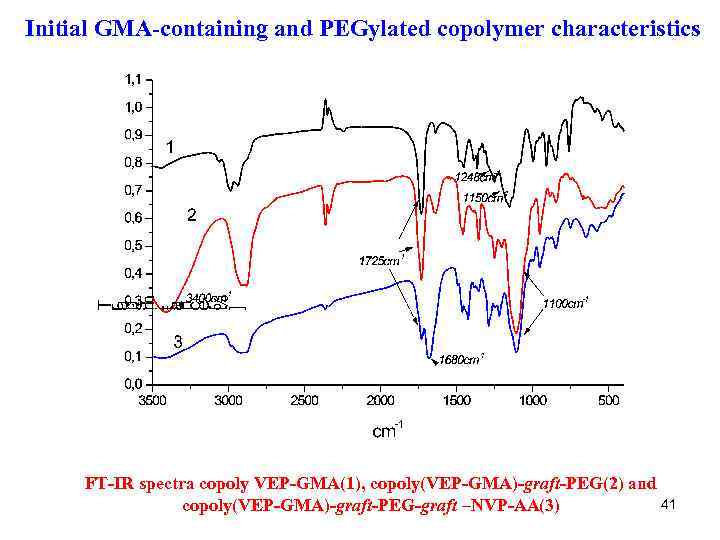
Initial GMA-containing and PEGylated copolymer characteristics FT-IR spectra copoly VEP-GMA(1), copoly(VEP-GMA)-graft-PEG(2) and 41 copoly(VEP-GMA)-graft-PEG-graft –NVP-AA(3)

Why these oligoperoxide based oligoelectrolytes? • controlled design of a structure • controlled molecular weight (1, 000 – 30, 000 g/mole) • narrowed molecular weight distribution • controlled macro and microstructure • controlled functionality and reactivity • controlled solubility, surface activity • biocompatibility and non toxicity • Capability to form free radicals and initiate radical reactions 42 42

2. Study of drug and nucleic acid conjugation with oligomer carriers as well as water based nanosized delivery systems. Structural and colloidal-chemical characteristics of the conjugates. 43
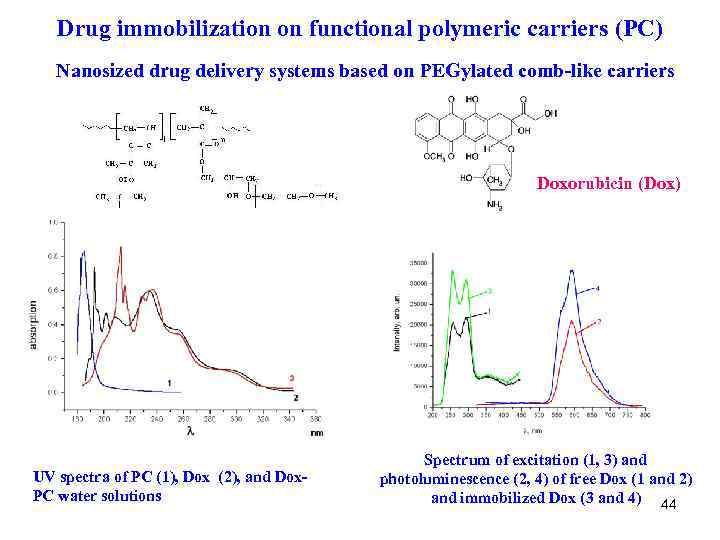
Drug immobilization on functional polymeric carriers (PC) Nanosized drug delivery systems based on PEGylated comb-like carriers Doxorubicin (Dox) UV spectra of PC (1), Dox (2), and Dox. PC water solutions Spectrum of excitation (1, 3) and photoluminescence (2, 4) of free Dox (1 and 2) and immobilized Dox (3 and 4) 44
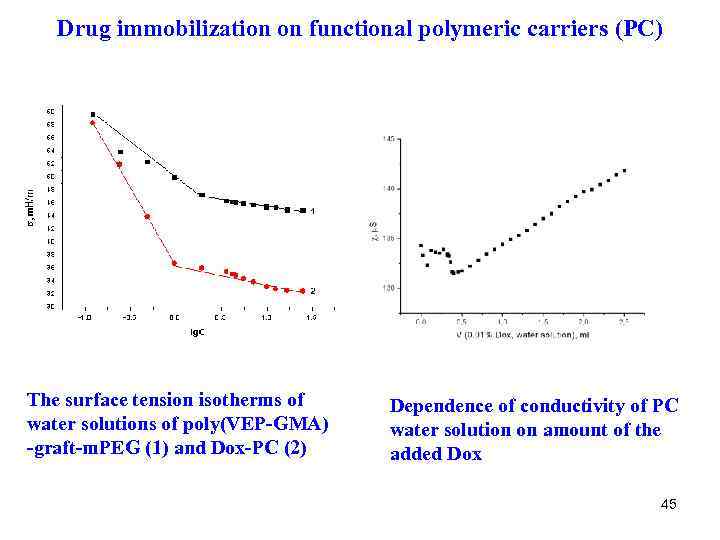
Drug immobilization on functional polymeric carriers (PC) The surface tension isotherms of water solutions of poly(VEP-GMA) -graft-m. PEG (1) and Dox-PC (2) Dependence of conductivity of PC water solution on amount of the added Dox 45
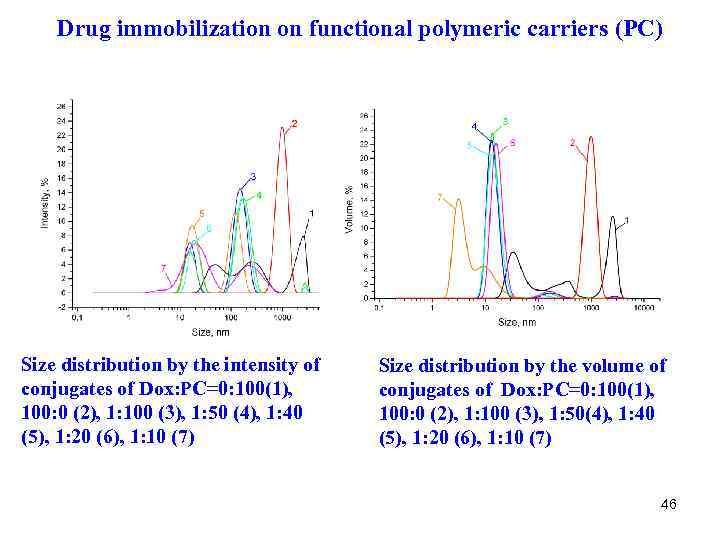
Drug immobilization on functional polymeric carriers (PC) Size distribution by the intensity of conjugates of Dox: PC=0: 100(1), 100: 0 (2), 1: 100 (3), 1: 50 (4), 1: 40 (5), 1: 20 (6), 1: 10 (7) Size distribution by the volume of conjugates of Dox: PC=0: 100(1), 100: 0 (2), 1: 100 (3), 1: 50(4), 1: 40 (5), 1: 20 (6), 1: 10 (7) 46
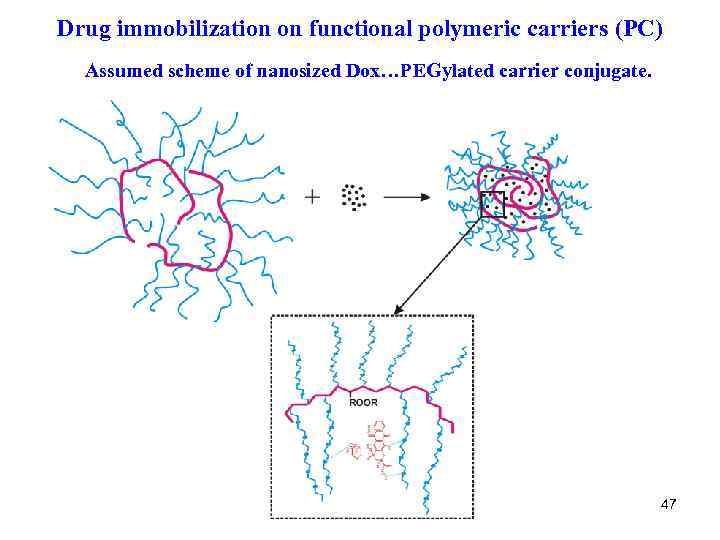
Drug immobilization on functional polymeric carriers (PC) Assumed scheme of nanosized Dox…PEGylated carrier conjugate. 47

Other Applications – Delivery of WATER-INSOLUBLE DRUGS 48
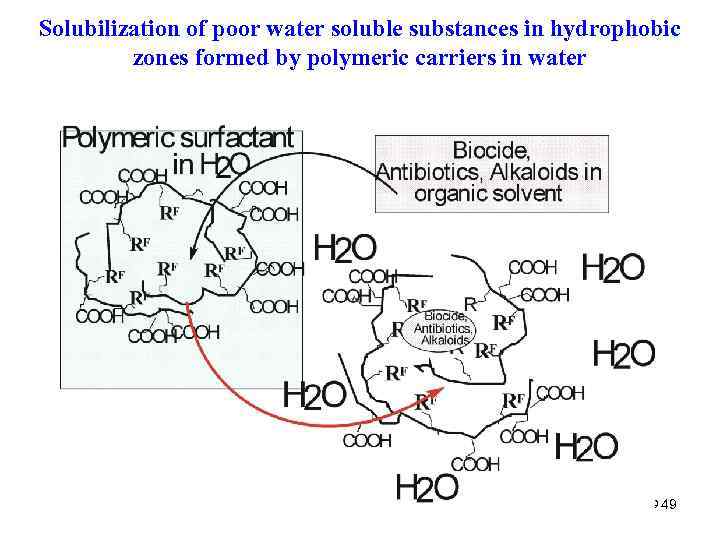
Solubilization of poor water soluble substances in hydrophobic zones formed by polymeric carriers in water 49 49
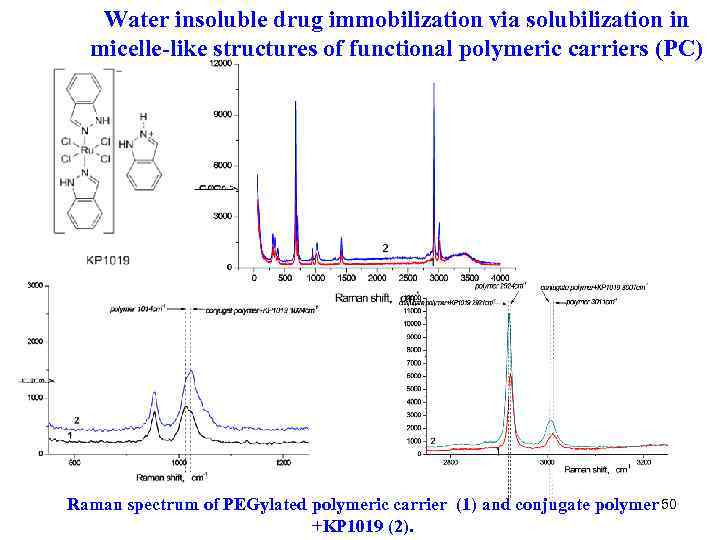
Water insoluble drug immobilization via solubilization in micelle-like structures of functional polymeric carriers (PC) Raman spectrum of PEGylated polymeric carrier (1) and conjugate polymer 50 +KP 1019 (2).
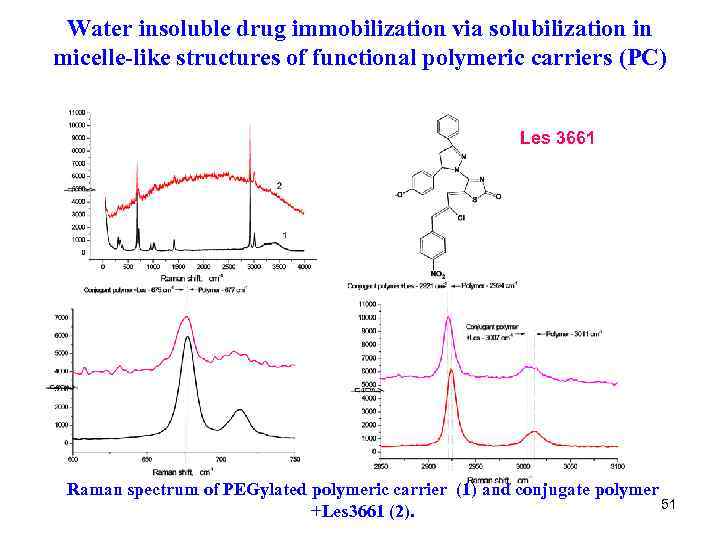
Water insoluble drug immobilization via solubilization in micelle-like structures of functional polymeric carriers (PC) Les 3661 Raman spectrum of PEGylated polymeric carrier (1) and conjugate polymer 51 +Les 3661 (2).
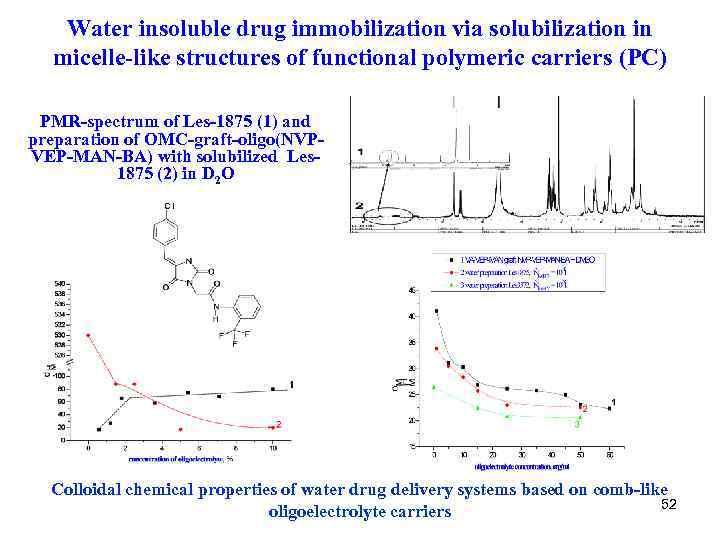
Water insoluble drug immobilization via solubilization in micelle-like structures of functional polymeric carriers (PC) PMR-spectrum of Les-1875 (1) and preparation of OMC-graft-oligo(NVPVEP-MAN-BA) with solubilized Les 1875 (2) in D 2 O Colloidal chemical properties of water drug delivery systems based on comb-like 52 oligoelectrolyte carriers
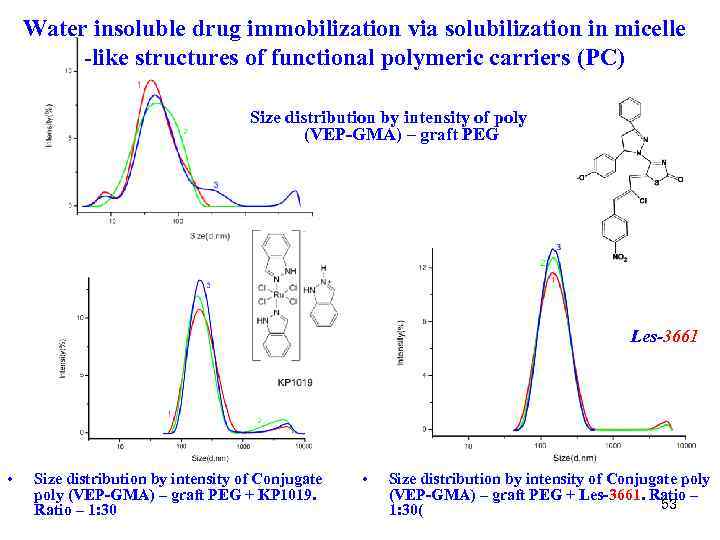
Water insoluble drug immobilization via solubilization in micelle -like structures of functional polymeric carriers (PC) Size distribution by intensity of poly (VEP-GMA) – graft PEG Les-3661 • Size distribution by intensity of Conjugate poly (VEP-GMA) – graft PEG + KP 1019. Ratio – 1: 30 • Size distribution by intensity of Conjugate poly (VEP-GMA) – graft PEG + Les-3661. Ratio – 53 1: 30(
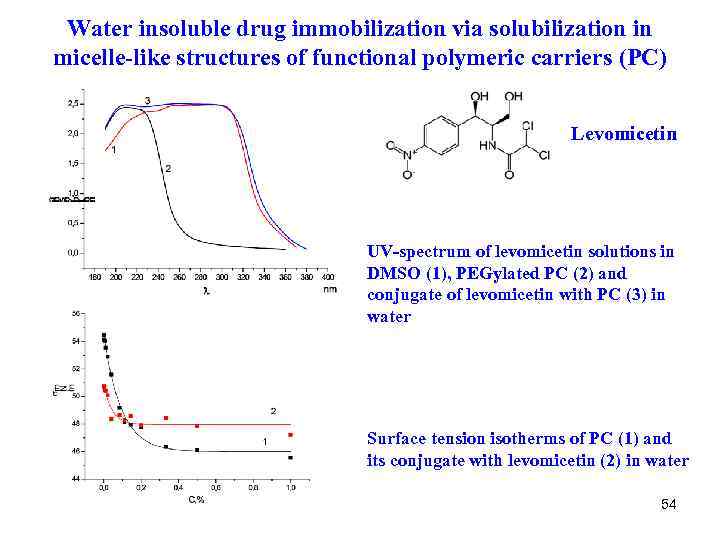
Water insoluble drug immobilization via solubilization in micelle-like structures of functional polymeric carriers (PC) Levomicetin UV-spectrum of levomicetin solutions in DMSO (1), PEGylated PC (2) and conjugate of levomicetin with PC (3) in water Surface tension isotherms of PC (1) and its conjugate with levomicetin (2) in water 54
Nucleic acid immobilization on functional polymeric carriers BG-2 55
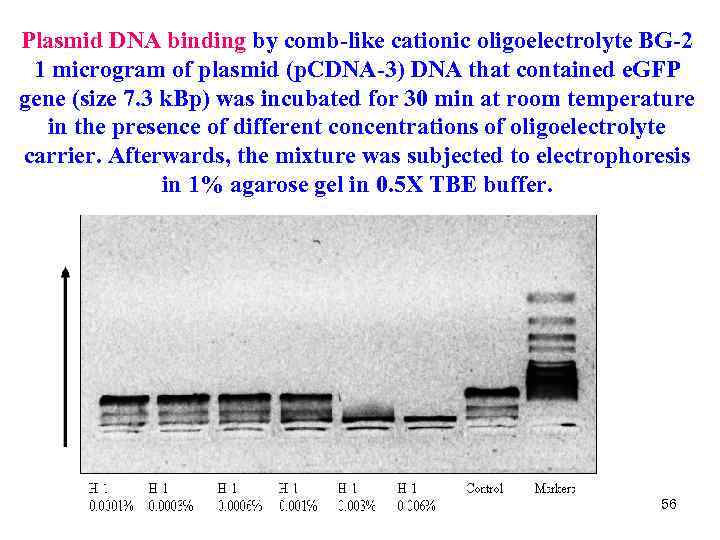
Plasmid DNA binding by comb-like cationic oligoelectrolyte BG-2 1 microgram of plasmid (p. CDNA-3) DNA that contained e. GFP gene (size 7. 3 k. Bp) was incubated for 30 min at room temperature in the presence of different concentrations of oligoelectrolyte carrier. Afterwards, the mixture was subjected to electrophoresis in 1% agarose gel in 0. 5 Х TBE buffer. 56
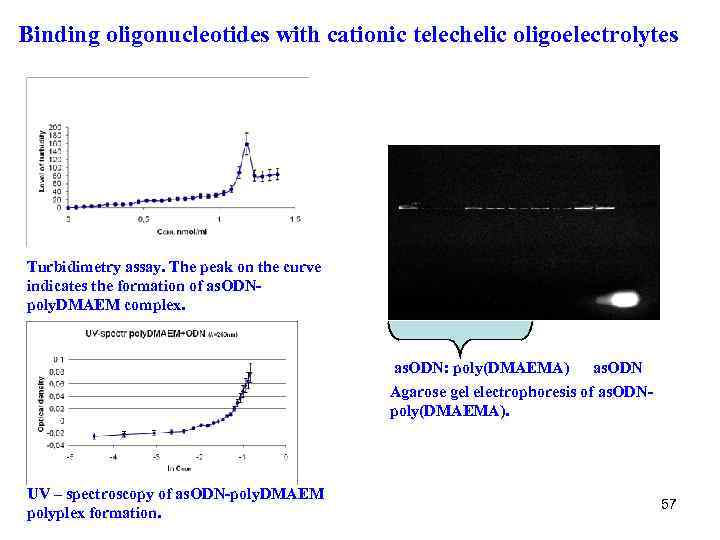
Binding oligonucleotides with cationic telechelic oligoelectrolytes Turbidimetry assay. The peak on the curve indicates the formation of as. ODNpoly. DMAEM complex. as. ODN: poly(DMAEMA) as. ODN Agarose gel electrophoresis of as. ODNpoly(DMAEMA). UV – sрectroscopy of as. ODN-poly. DMAEM polyplex formation. 57
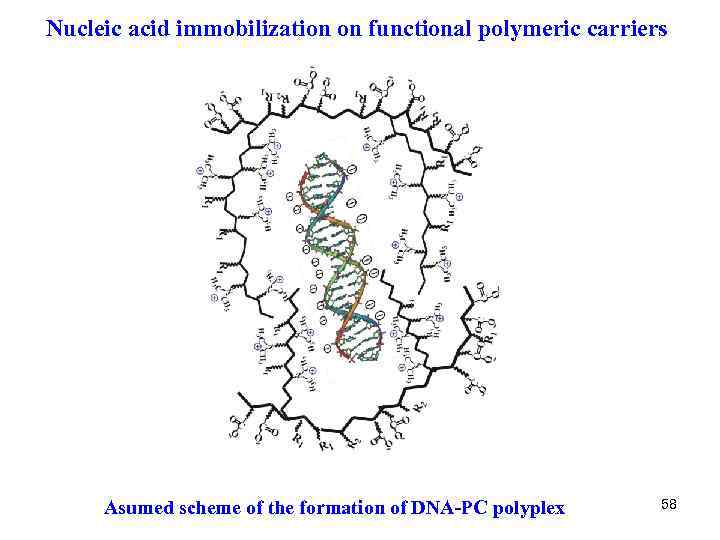
Nucleic acid immobilization on functional polymeric carriers Asumed scheme of the formation of DNA-PC polyplex 58

4. Cellular study and biomedical application of developed drug and gene delivery systems *study was fulfilled by team of Institute of Cell Biology of NASU under guidance of professor R. Stoika 59
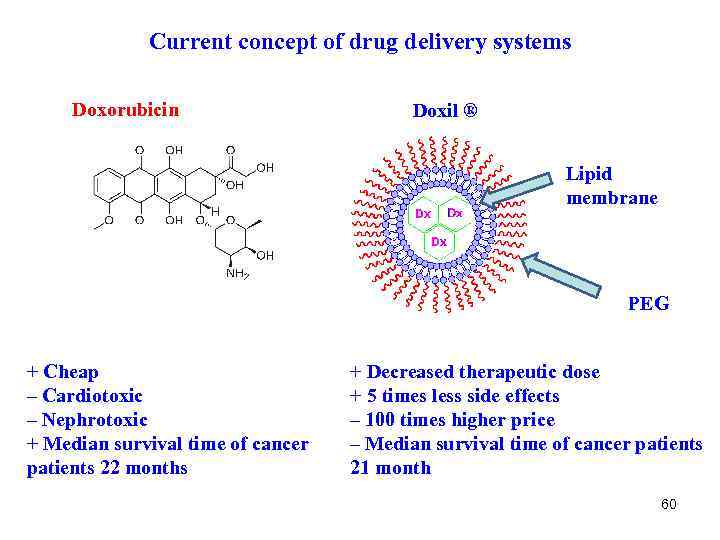
Current concept of drug delivery systems Doxorubicin Doxil ® Lipid membrane PEG + Cheap – Cardiotoxic – Nephrotoxic + Median survival time of cancer patients 22 months + Decreased therapeutic dose + 5 times less side effects – 100 times higher price – Median survival time of cancer patients 21 month 60

How to enhance therapeutic efficiency of drug delivery systems? 1) Increase of maximum tolerated dose of drug for more successful killing of non-proliferating tumor cells 2) Usage of cytoprotector to protect normal tissues from toxic side effects of Dx 3) Stable and effective platform for drug delivery polymeric carrier containing monoperoxine, vinylacetate and maleic anhydride residues 61

In vitro study of cellular and molecular mechanisms of anticancer activity of novel Dx nanocomposites, functionalized with NAE 62
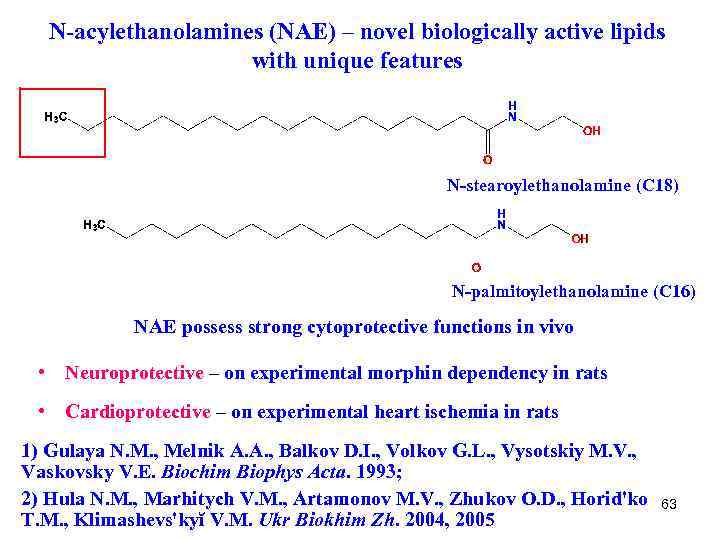
N-acylethanolamines (NAE) – novel biologically active lipids with unique features N-stearoylethanolamine (C 18) N-palmitoylethanolamine (C 16) NAE possess strong cytoprotective functions in vivo • Neuroprotective – on experimental morphin dependency in rats • Cardioprotective – on experimental heart ischemia in rats 1) Gulaya N. M. , Melnik A. A. , Balkov D. I. , Volkov G. L. , Vysotskiy M. V. , Vaskovsky V. E. Biochim Biophys Acta. 1993; 2) Hula N. M. , Marhitych V. M. , Artamonov M. V. , Zhukov O. D. , Horid'ko T. M. , Klimashevs'kyĭ V. M. Ukr Biokhim Zh. 2004, 2005 63
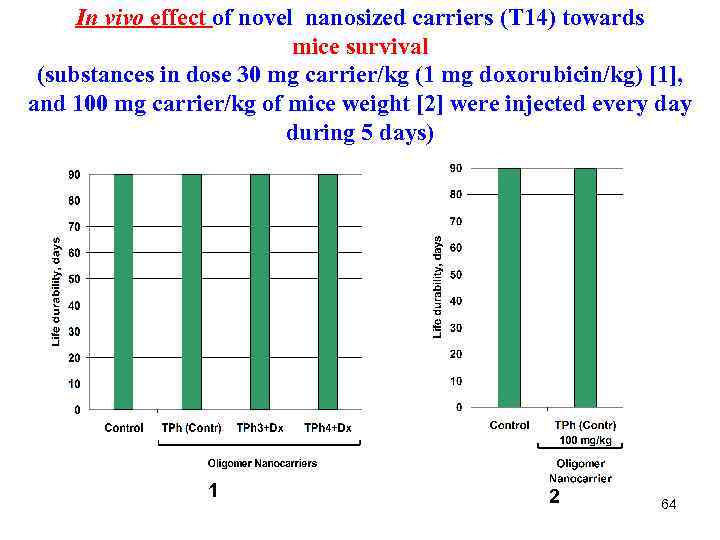
In vivo effect of novel nanosized carriers (Т 14) towards mice survival (substances in dose 30 mg carrier/kg (1 mg doxorubicin/kg) [1], and 100 mg carrier/kg of mice weight [2] were injected every day during 5 days) 1 2 64
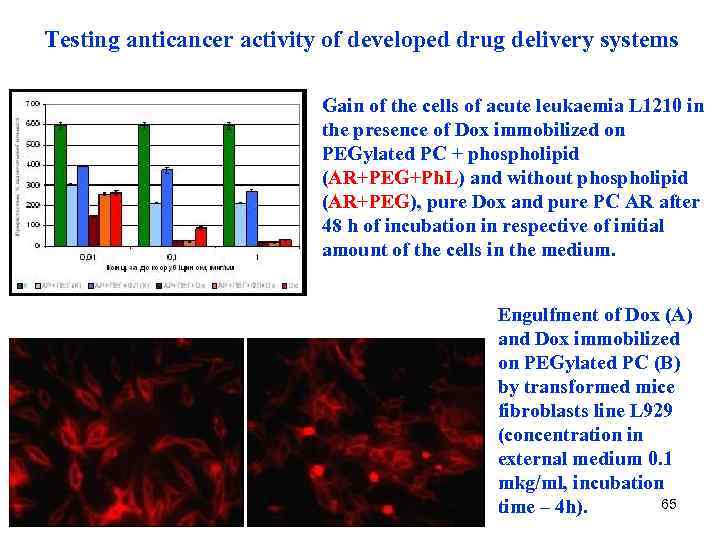
Testing anticancer activity of developed drug delivery systems Gain of the cells of acute leukaemia L 1210 in the presence of Dox immobilized on PEGylated PC + phospholipid (AR+PEG+Ph. L) and without phospholipid (AR+PEG), pure Dox and pure PC AR after 48 h of incubation in respective of initial amount of the cells in the medium. Engulfment of Dox (A) and Dox immobilized on PEGylated PC (B) by transformed mice fibroblasts line L 929 (concentration in external medium 0. 1 mkg/ml, incubation 65 time – 4 h).
66
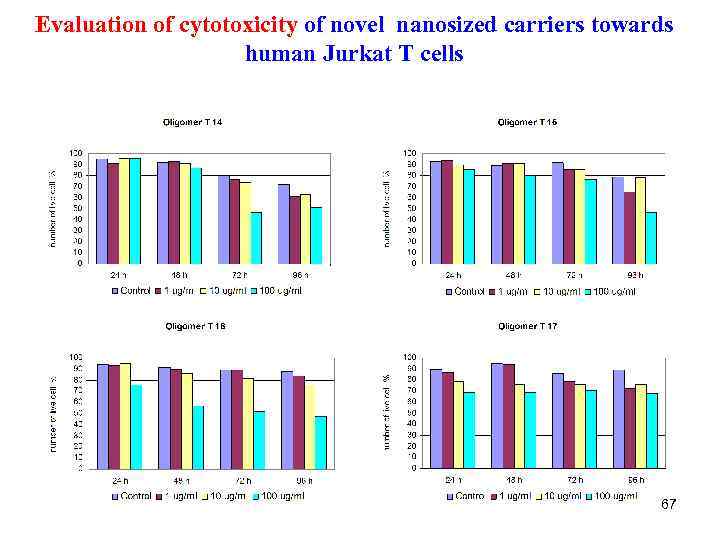
Evaluation of cytotoxicity of novel nanosized carriers towards human Jurkat T cells 67
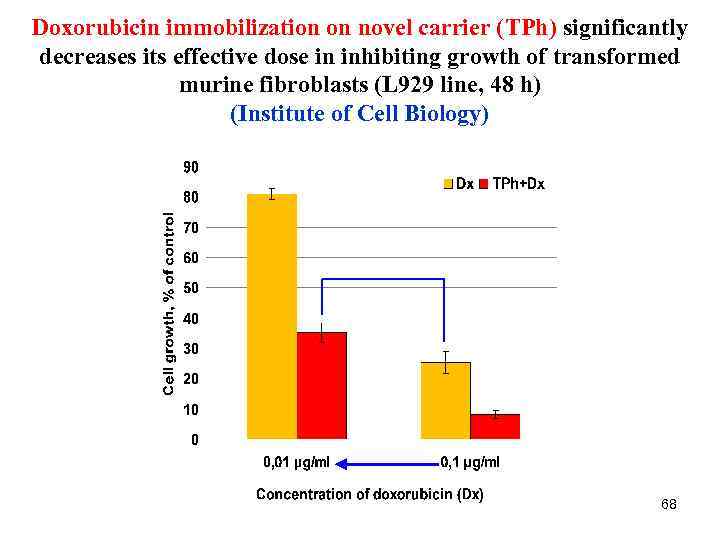
Doxorubicin immobilization on novel carrier (TPh) significantly decreases its effective dose in inhibiting growth of transformed murine fibroblasts (L 929 line, 48 h) (Institute of Cell Biology) 68
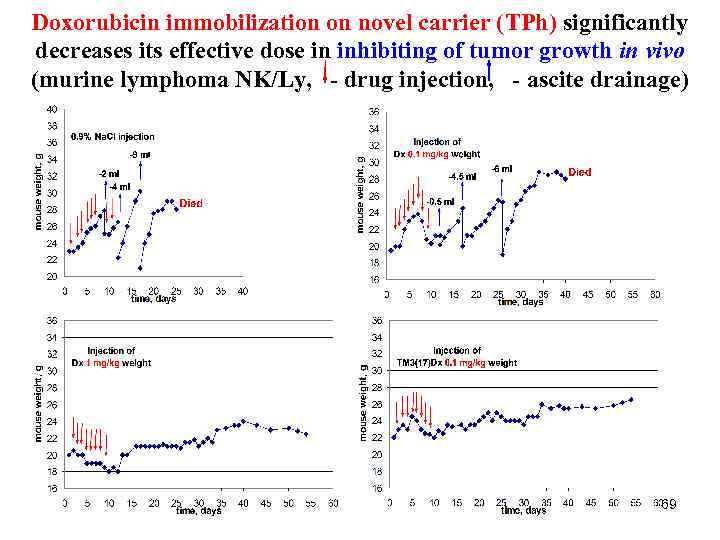
Doxorubicin immobilization on novel carrier (TPh) significantly decreases its effective dose in inhibiting of tumor growth in vivo (murine lymphoma NK/Ly, - drug injection, - ascite drainage) 69
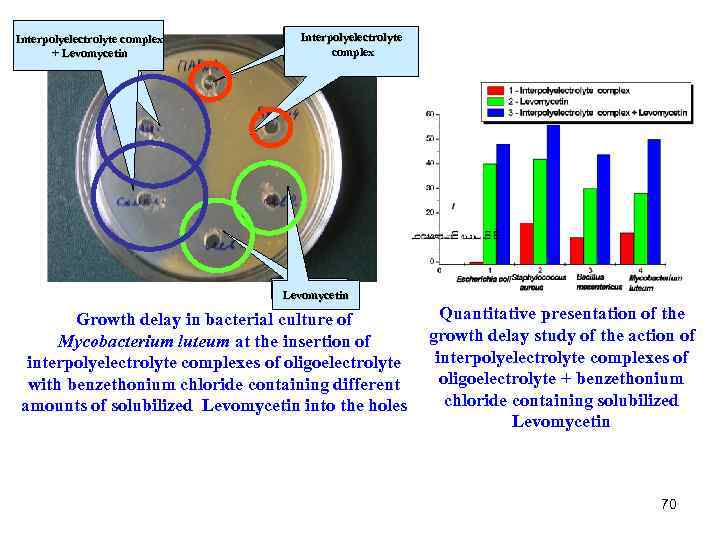
Interpolyelectrolyte complex + Levomycetin Interpolyelectrolyte complex Levomycetin Quantitative presentation of the Growth delay in bacterial culture of growth delay study of the action of Mycobacterium luteum at the insertion of interpolyelectrolyte complexes of oligoelectrolyte + benzethonium with benzethonium chloride containing different chloride containing solubilized amounts of solubilized Levomycetin into the holes Levomycetin 70

Conclusions coming from drug delivery experiments 71

Application of the developed polymeric drug delivery platforms permitted: • to decrease acting concentrations of highly toxic anticancer drugs (ex. Doxorubicin) from 50% to 100 times (in vitro and in vivo, depending on specific target tumor cells and drugs); • this result predicts the corresponding decrease of potential negative side effects of these drugs in the treated organism. 72
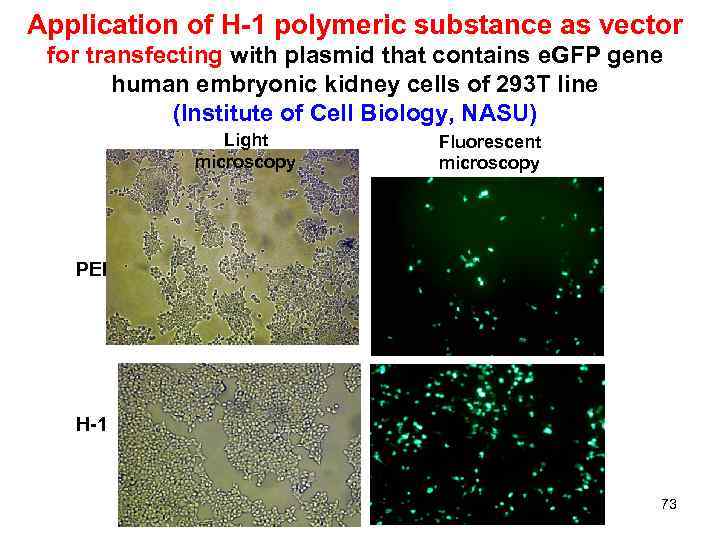
Application of H-1 polymeric substance as vector for transfecting with plasmid that contains e. GFP gene human embryonic kidney cells of 293 T line (Institute of Cell Biology, NASU) Light microscopy Fluorescent microscopy РЕІ H-1 73
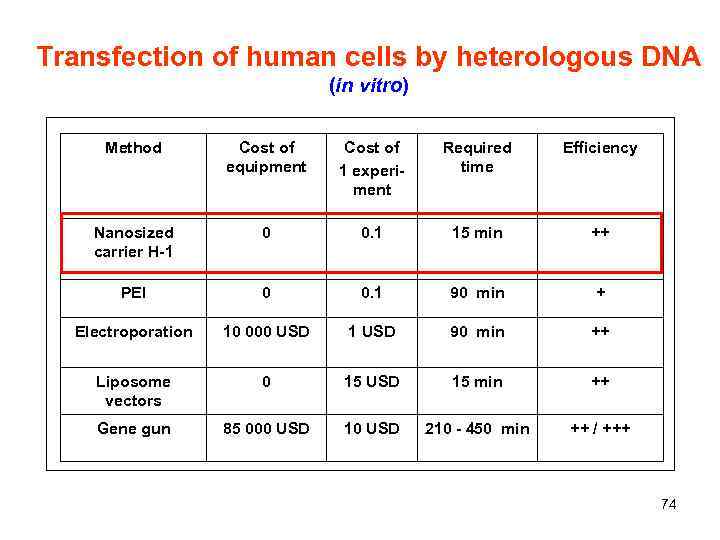
Transfection of human cells by heterologous DNA (in vitro) Method Cost of equipment Cost of 1 experiment Required time Efficiency Nanosized carrier H-1 0 0. 1 15 min ++ PEI 0 0. 1 90 min + Electroporation 10 000 USD 1 USD 90 min ++ Liposome vectors 0 15 USD 15 min ++ Gene gun 85 000 USD 10 USD 210 - 450 min ++ / +++ 74

Intelectual Property PCT Application No IB 2010/001538 USA Provisional Patent Highly efficient systems for delivery of nucleic acids Rostyslav Stoika, Yevhen Filyak, Oleksandr Zaichenko, Nataliya Mitina 75
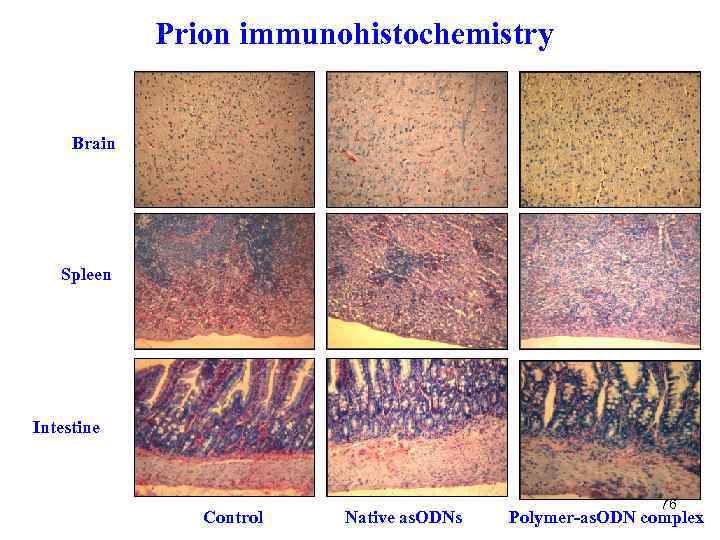
Prion immunohistochemistry Brain Spleen Intestine Control Native as. ODNs 76 Polymer-as. ODN complex
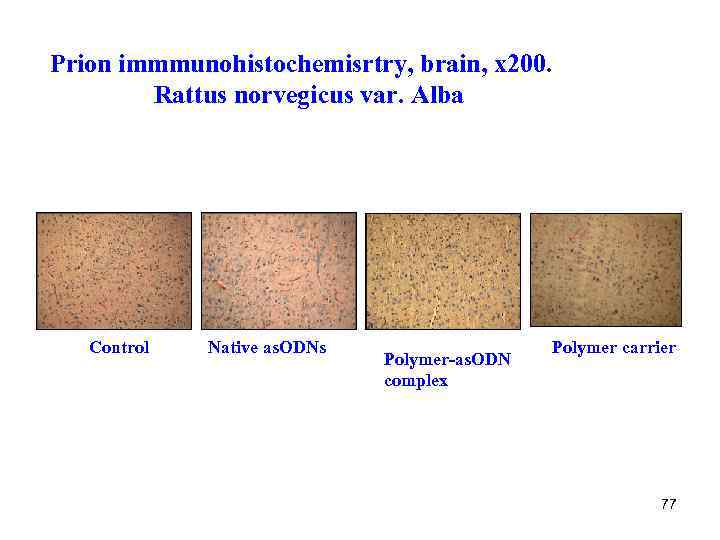
Prion immmunohistochemisrtry, brain, x 200. Rattus norvegicus var. Alba Control Native as. ODNs Polymer-as. ODN complex Polymer carrier 77

Acknowledgements Many thanks to my team and partners Dr. N. Mitina, Dr. K. Rayevska, Dr. T. Skorokhoda, Ph. D student A. Riabtseva; • Professor R. Stoika, Dr. N. Boiko, Dr. E. Filyak and team from Cell Biology Institute of NASU, Ph. D students L. Ivanitska, N. Finyuk; for the experimental work as well as for the collaboration, ideas and discussion 78 78
Munich_Nancy_Zaichenko-last2.ppt