144392b4226bd60da3899dfcab0a7903.ppt
- Количество слайдов: 90
 Lung Cancer Facts & Figures • Leading cause of cancer-related mortality in US • Accounts for more deaths than breast, prostate and colorectal cancers combined • Histologically and molecularly a very heterogeneous disease • Unfavorable stage distribution at the time of diagnosis • Smoking remains the major risk factor; med age ~ 70 • 25 -30, 000 never smoking Americans will develop lung cancer this year (more common than esophageal, gastric, ovarian, testicular, Hodgkin’s, myeloma, CML) Siegel, R. L. , Miller, K. D. , & Jemal, A. Cancer Statistics 2016. CA Cancer J Clin; 2016.
Lung Cancer Facts & Figures • Leading cause of cancer-related mortality in US • Accounts for more deaths than breast, prostate and colorectal cancers combined • Histologically and molecularly a very heterogeneous disease • Unfavorable stage distribution at the time of diagnosis • Smoking remains the major risk factor; med age ~ 70 • 25 -30, 000 never smoking Americans will develop lung cancer this year (more common than esophageal, gastric, ovarian, testicular, Hodgkin’s, myeloma, CML) Siegel, R. L. , Miller, K. D. , & Jemal, A. Cancer Statistics 2016. CA Cancer J Clin; 2016.
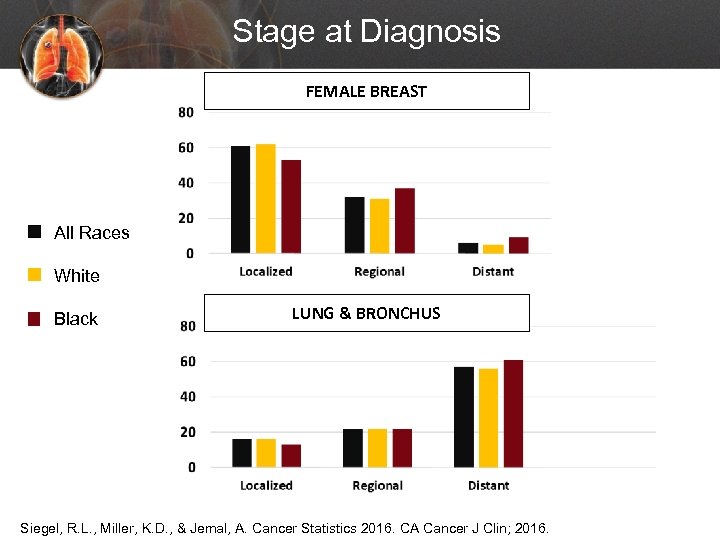 Stage at Diagnosis FEMALE BREAST All Races White Black LUNG & BRONCHUS Siegel, R. L. , Miller, K. D. , & Jemal, A. Cancer Statistics 2016. CA Cancer J Clin; 2016.
Stage at Diagnosis FEMALE BREAST All Races White Black LUNG & BRONCHUS Siegel, R. L. , Miller, K. D. , & Jemal, A. Cancer Statistics 2016. CA Cancer J Clin; 2016.
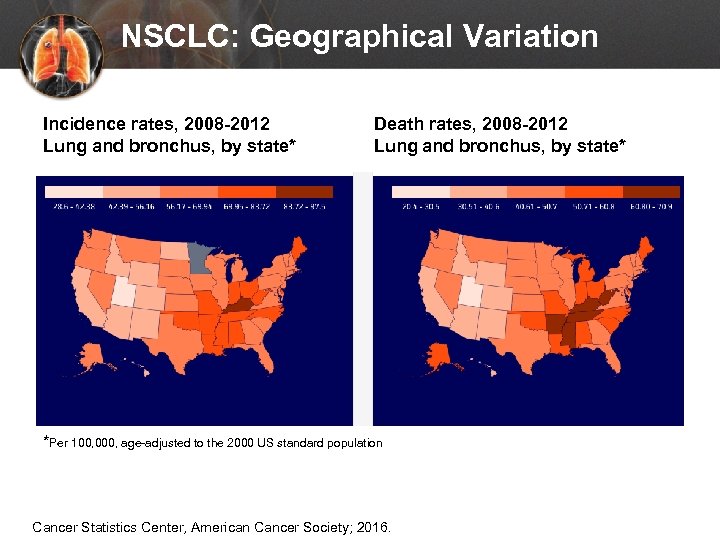 NSCLC: Geographical Variation Incidence rates, 2008 -2012 Lung and bronchus, by state* Death rates, 2008 -2012 Lung and bronchus, by state* *Per 100, 000, age-adjusted to the 2000 US standard population Cancer Statistics Center, American Cancer Society; 2016.
NSCLC: Geographical Variation Incidence rates, 2008 -2012 Lung and bronchus, by state* Death rates, 2008 -2012 Lung and bronchus, by state* *Per 100, 000, age-adjusted to the 2000 US standard population Cancer Statistics Center, American Cancer Society; 2016.
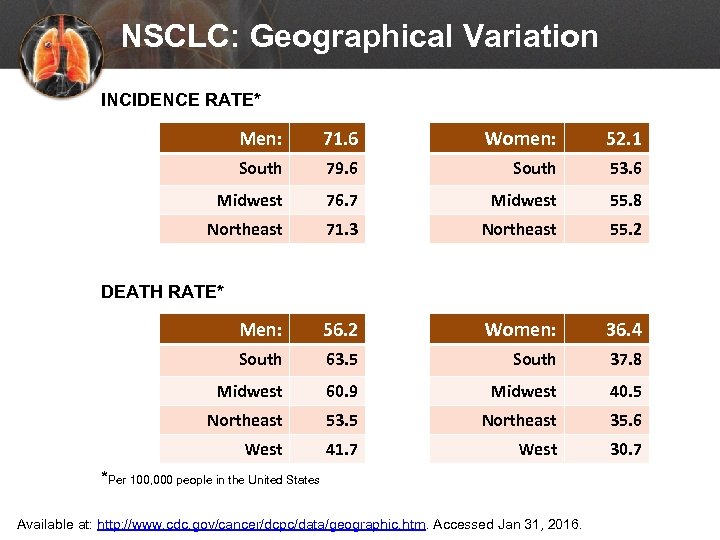 NSCLC: Geographical Variation INCIDENCE RATE* Men: 71. 6 Women: 52. 1 South 79. 6 South 53. 6 Midwest 76. 7 Midwest 55. 8 Northeast 71. 3 Northeast 55. 2 Men: 56. 2 Women: 36. 4 South 63. 5 South 37. 8 Midwest 60. 9 Midwest 40. 5 Northeast 53. 5 Northeast 35. 6 West 41. 7 West 30. 7 DEATH RATE* *Per 100, 000 people in the United States Available at: http: //www. cdc. gov/cancer/dcpc/data/geographic. htm. Accessed Jan 31, 2016.
NSCLC: Geographical Variation INCIDENCE RATE* Men: 71. 6 Women: 52. 1 South 79. 6 South 53. 6 Midwest 76. 7 Midwest 55. 8 Northeast 71. 3 Northeast 55. 2 Men: 56. 2 Women: 36. 4 South 63. 5 South 37. 8 Midwest 60. 9 Midwest 40. 5 Northeast 53. 5 Northeast 35. 6 West 41. 7 West 30. 7 DEATH RATE* *Per 100, 000 people in the United States Available at: http: //www. cdc. gov/cancer/dcpc/data/geographic. htm. Accessed Jan 31, 2016.
 Major Paradigm Shifts in Advanced NSCLC Past 2 Decades • Importance of histology to select therapy – Squamous vs. non-squamous • Addition of maintenance and second line chemotherapy • Introduction of anti-angiogenic therapy – VEGF • Discovery and targeting of oncogenic driver mutations – EGFR, ALK and others • Emergence of immunotherapy – Anti PD-1 and PD-L 1
Major Paradigm Shifts in Advanced NSCLC Past 2 Decades • Importance of histology to select therapy – Squamous vs. non-squamous • Addition of maintenance and second line chemotherapy • Introduction of anti-angiogenic therapy – VEGF • Discovery and targeting of oncogenic driver mutations – EGFR, ALK and others • Emergence of immunotherapy – Anti PD-1 and PD-L 1
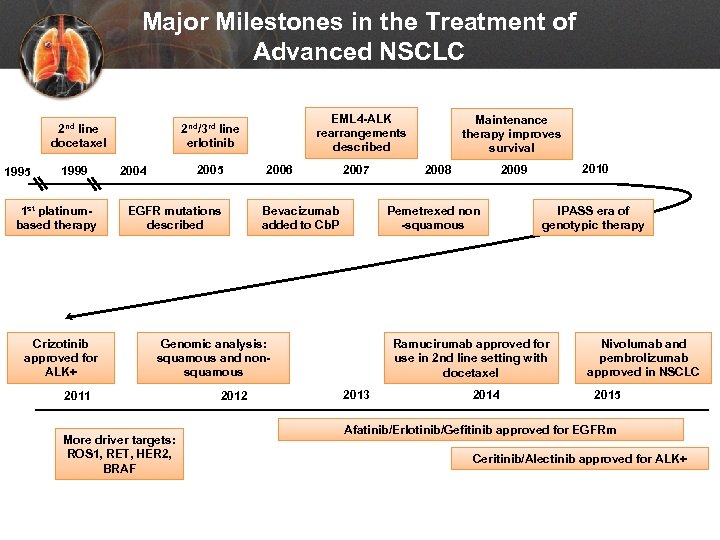 Major Milestones in the Treatment of Advanced NSCLC 2 nd line docetaxel 1995 1999 1 st platinumbased therapy Crizotinib approved for ALK+ EML 4 -ALK rearrangements described 2 nd/3 rd line erlotinib 2005 2004 EGFR mutations described 2006 2007 Bevacizumab added to Cb. P More driver targets: ROS 1, RET, HER 2, BRAF 2012 2008 2010 2009 Pemetrexed non -squamous Genomic analysis: squamous and nonsquamous 2011 Maintenance therapy improves survival IPASS era of genotypic therapy Ramucirumab approved for use in 2 nd line setting with docetaxel 2013 2014 Nivolumab and pembrolizumab approved in NSCLC 2015 Afatinib/Erlotinib/Gefitinib approved for EGFRm Ceritinib/Alectinib approved for ALK+
Major Milestones in the Treatment of Advanced NSCLC 2 nd line docetaxel 1995 1999 1 st platinumbased therapy Crizotinib approved for ALK+ EML 4 -ALK rearrangements described 2 nd/3 rd line erlotinib 2005 2004 EGFR mutations described 2006 2007 Bevacizumab added to Cb. P More driver targets: ROS 1, RET, HER 2, BRAF 2012 2008 2010 2009 Pemetrexed non -squamous Genomic analysis: squamous and nonsquamous 2011 Maintenance therapy improves survival IPASS era of genotypic therapy Ramucirumab approved for use in 2 nd line setting with docetaxel 2013 2014 Nivolumab and pembrolizumab approved in NSCLC 2015 Afatinib/Erlotinib/Gefitinib approved for EGFRm Ceritinib/Alectinib approved for ALK+
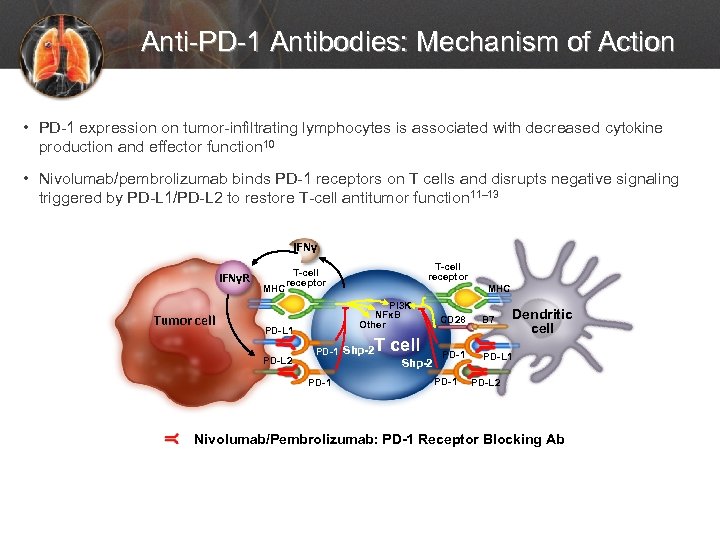 Anti-PD-1 Antibodies: Mechanism of Action • PD-1 expression on tumor-infiltrating lymphocytes is associated with decreased cytokine production and effector function 10 • Nivolumab/pembrolizumab binds PD-1 receptors on T cells and disrupts negative signaling triggered by PD-L 1/PD-L 2 to restore T-cell antitumor function 11– 13 IFNγR Tumor cell MHC T-cell receptor PI 3 K NFκB Other PD-L 1 PD-L 2 MHC T cell PD-1 Shp-2 PD-1 Dendritic cell CD 28 B 7 PD-1 PD-L 2 Nivolumab/Pembrolizumab: PD-1 Receptor Blocking Ab
Anti-PD-1 Antibodies: Mechanism of Action • PD-1 expression on tumor-infiltrating lymphocytes is associated with decreased cytokine production and effector function 10 • Nivolumab/pembrolizumab binds PD-1 receptors on T cells and disrupts negative signaling triggered by PD-L 1/PD-L 2 to restore T-cell antitumor function 11– 13 IFNγR Tumor cell MHC T-cell receptor PI 3 K NFκB Other PD-L 1 PD-L 2 MHC T cell PD-1 Shp-2 PD-1 Dendritic cell CD 28 B 7 PD-1 PD-L 2 Nivolumab/Pembrolizumab: PD-1 Receptor Blocking Ab
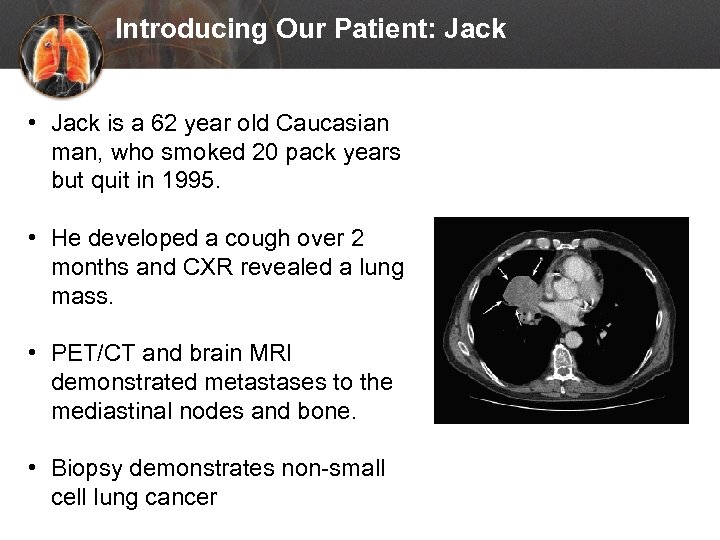 Introducing Our Patient: Jack • Jack is a 62 year old Caucasian man, who smoked 20 pack years but quit in 1995. • He developed a cough over 2 months and CXR revealed a lung mass. • PET/CT and brain MRI demonstrated metastases to the mediastinal nodes and bone. • Biopsy demonstrates non-small cell lung cancer
Introducing Our Patient: Jack • Jack is a 62 year old Caucasian man, who smoked 20 pack years but quit in 1995. • He developed a cough over 2 months and CXR revealed a lung mass. • PET/CT and brain MRI demonstrated metastases to the mediastinal nodes and bone. • Biopsy demonstrates non-small cell lung cancer
 Results of First-line Testing on Jack • Adenocarcinoma (non-squamous) • No EGFR mutation or ALK rearrangement • PD-L 1 testing pending
Results of First-line Testing on Jack • Adenocarcinoma (non-squamous) • No EGFR mutation or ALK rearrangement • PD-L 1 testing pending
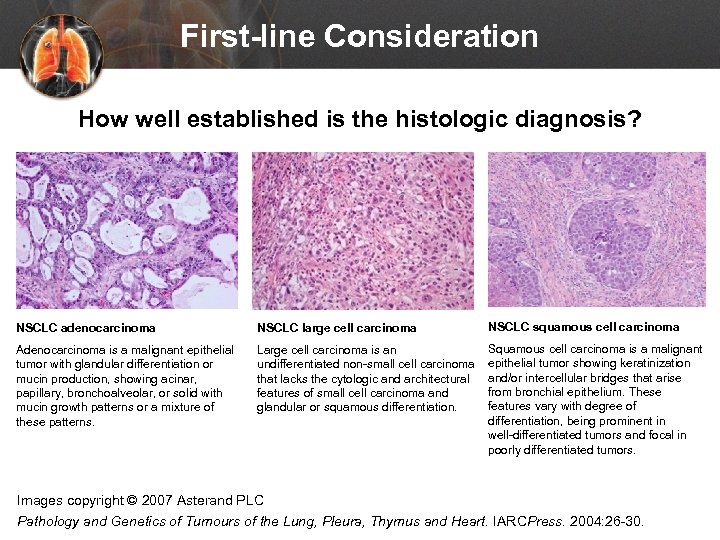 First-line Consideration How well established is the histologic diagnosis? NSCLC adenocarcinoma NSCLC large cell carcinoma NSCLC squamous cell carcinoma Adenocarcinoma is a malignant epithelial tumor with glandular differentiation or mucin production, showing acinar, papillary, bronchoalveolar, or solid with mucin growth patterns or a mixture of these patterns. Large cell carcinoma is an undifferentiated non-small cell carcinoma that lacks the cytologic and architectural features of small cell carcinoma and glandular or squamous differentiation. Squamous cell carcinoma is a malignant epithelial tumor showing keratinization and/or intercellular bridges that arise from bronchial epithelium. These features vary with degree of differentiation, being prominent in well-differentiated tumors and focal in poorly differentiated tumors. Images copyright © 2007 Asterand PLC Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. IARCPress. 2004: 26 -30.
First-line Consideration How well established is the histologic diagnosis? NSCLC adenocarcinoma NSCLC large cell carcinoma NSCLC squamous cell carcinoma Adenocarcinoma is a malignant epithelial tumor with glandular differentiation or mucin production, showing acinar, papillary, bronchoalveolar, or solid with mucin growth patterns or a mixture of these patterns. Large cell carcinoma is an undifferentiated non-small cell carcinoma that lacks the cytologic and architectural features of small cell carcinoma and glandular or squamous differentiation. Squamous cell carcinoma is a malignant epithelial tumor showing keratinization and/or intercellular bridges that arise from bronchial epithelium. These features vary with degree of differentiation, being prominent in well-differentiated tumors and focal in poorly differentiated tumors. Images copyright © 2007 Asterand PLC Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. IARCPress. 2004: 26 -30.
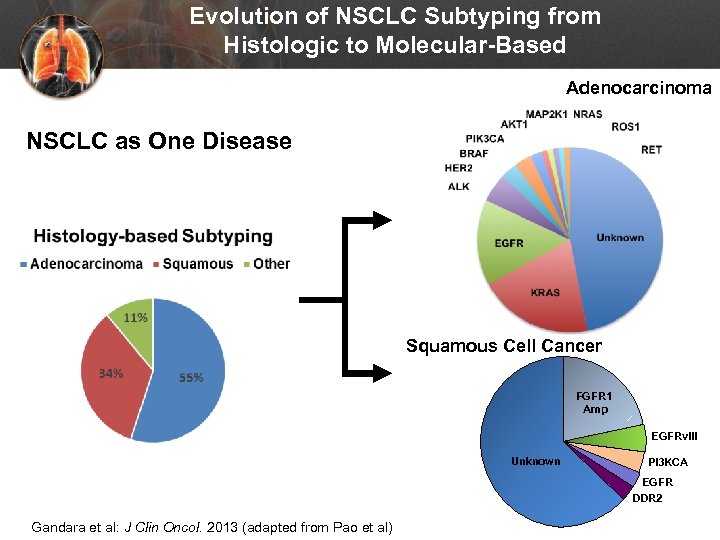 Evolution of NSCLC Subtyping from Histologic to Molecular-Based Adenocarcinoma NSCLC as One Disease Squamous Cell Cancer FGFR 1 Amp EGFRv. III Unknown PI 3 KCA EGFR DDR 2 Gandara et al: J Clin Oncol. 2013 (adapted from Pao et al)
Evolution of NSCLC Subtyping from Histologic to Molecular-Based Adenocarcinoma NSCLC as One Disease Squamous Cell Cancer FGFR 1 Amp EGFRv. III Unknown PI 3 KCA EGFR DDR 2 Gandara et al: J Clin Oncol. 2013 (adapted from Pao et al)
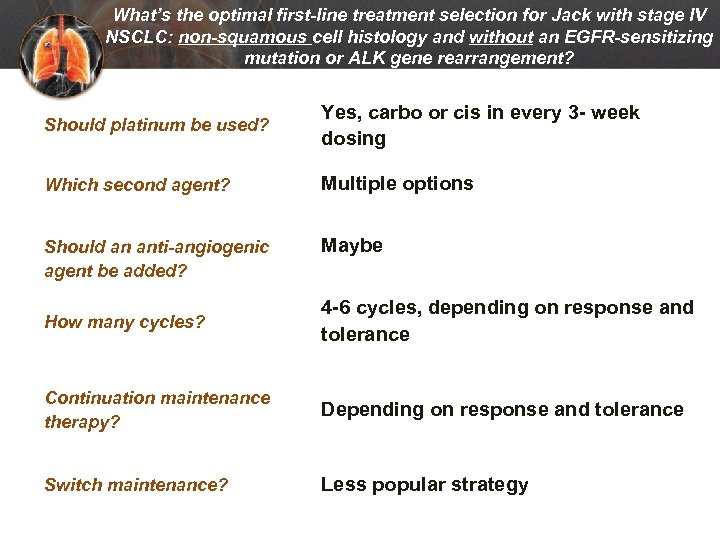 What’s the optimal first-line treatment selection for Jack with stage IV NSCLC: non-squamous cell histology and without an EGFR-sensitizing mutation or ALK gene rearrangement? Should platinum be used? Yes, carbo or cis in every 3 - week dosing Which second agent? Multiple options Should an anti-angiogenic agent be added? Maybe How many cycles? 4 -6 cycles, depending on response and tolerance Continuation maintenance therapy? Depending on response and tolerance Switch maintenance? Less popular strategy
What’s the optimal first-line treatment selection for Jack with stage IV NSCLC: non-squamous cell histology and without an EGFR-sensitizing mutation or ALK gene rearrangement? Should platinum be used? Yes, carbo or cis in every 3 - week dosing Which second agent? Multiple options Should an anti-angiogenic agent be added? Maybe How many cycles? 4 -6 cycles, depending on response and tolerance Continuation maintenance therapy? Depending on response and tolerance Switch maintenance? Less popular strategy
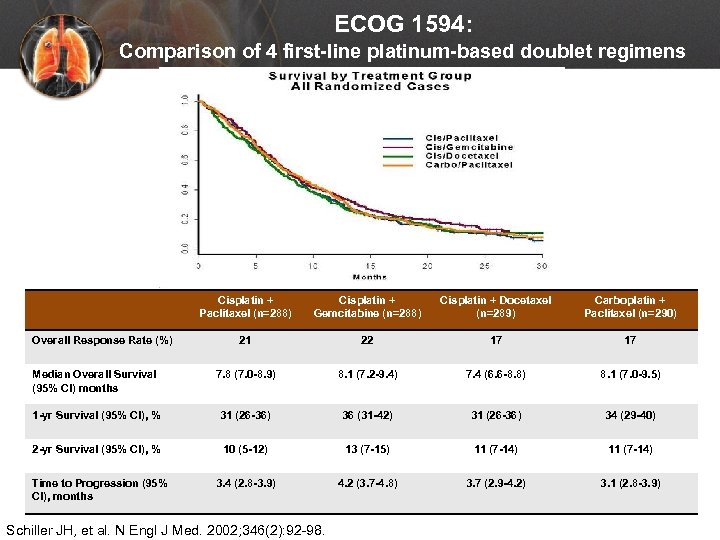 ECOG 1594: Comparison of 4 first-line platinum-based doublet regimens Cisplatin + Paclitaxel (n=288) Cisplatin + Gemcitabine (n=288) Cisplatin + Docetaxel (n=289) Carboplatin + Paclitaxel (n=290) 21 22 17 17 Median Overall Survival (95% CI) months 7. 8 (7. 0 -8. 9) 8. 1 (7. 2 -9. 4) 7. 4 (6. 6 -8. 8) 8. 1 (7. 0 -9. 5) 1 -yr Survival (95% CI), % 31 (26 -36) 36 (31 -42) 31 (26 -36) 34 (29 -40) 2 -yr Survival (95% CI), % 10 (5 -12) 13 (7 -15) 11 (7 -14) 3. 4 (2. 8 -3. 9) 4. 2 (3. 7 -4. 8) 3. 7 (2. 9 -4. 2) 3. 1 (2. 8 -3. 9) Overall Response Rate (%) Time to Progression (95% CI), months Schiller JH, et al. N Engl J Med. 2002; 346(2): 92 -98.
ECOG 1594: Comparison of 4 first-line platinum-based doublet regimens Cisplatin + Paclitaxel (n=288) Cisplatin + Gemcitabine (n=288) Cisplatin + Docetaxel (n=289) Carboplatin + Paclitaxel (n=290) 21 22 17 17 Median Overall Survival (95% CI) months 7. 8 (7. 0 -8. 9) 8. 1 (7. 2 -9. 4) 7. 4 (6. 6 -8. 8) 8. 1 (7. 0 -9. 5) 1 -yr Survival (95% CI), % 31 (26 -36) 36 (31 -42) 31 (26 -36) 34 (29 -40) 2 -yr Survival (95% CI), % 10 (5 -12) 13 (7 -15) 11 (7 -14) 3. 4 (2. 8 -3. 9) 4. 2 (3. 7 -4. 8) 3. 7 (2. 9 -4. 2) 3. 1 (2. 8 -3. 9) Overall Response Rate (%) Time to Progression (95% CI), months Schiller JH, et al. N Engl J Med. 2002; 346(2): 92 -98.
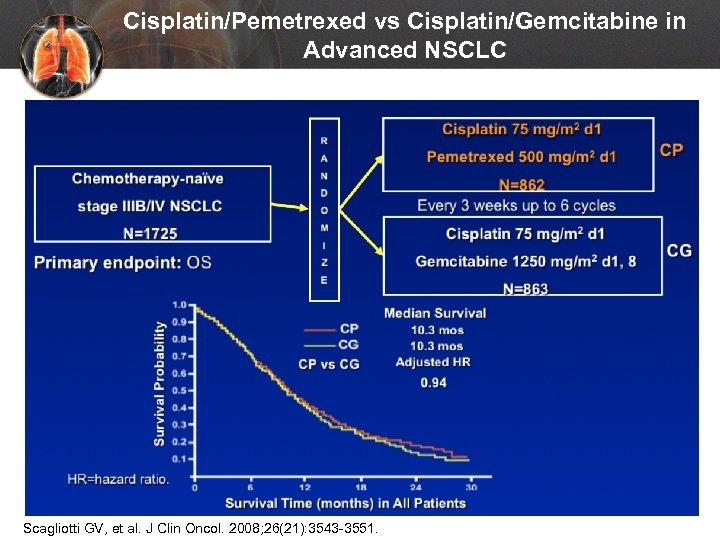 Cisplatin/Pemetrexed vs Cisplatin/Gemcitabine in Advanced NSCLC Scagliotti GV, et al. J Clin Oncol. 2008; 26(21): 3543 -3551.
Cisplatin/Pemetrexed vs Cisplatin/Gemcitabine in Advanced NSCLC Scagliotti GV, et al. J Clin Oncol. 2008; 26(21): 3543 -3551.
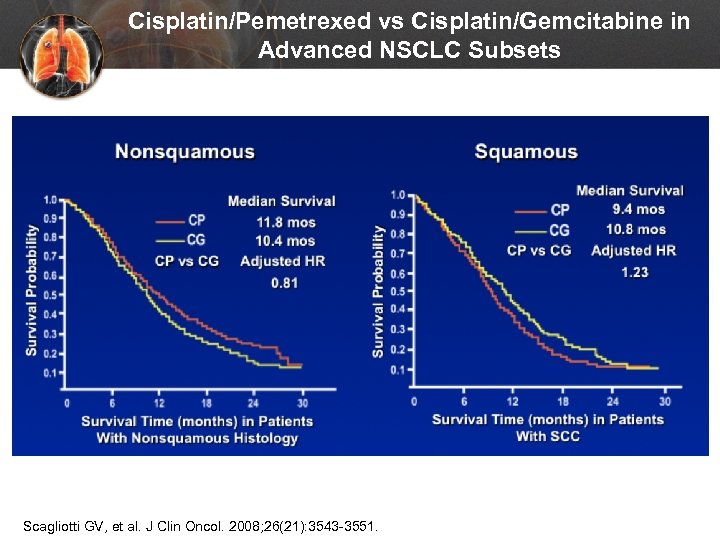 Cisplatin/Pemetrexed vs Cisplatin/Gemcitabine in Advanced NSCLC Subsets Scagliotti GV, et al. J Clin Oncol. 2008; 26(21): 3543 -3551.
Cisplatin/Pemetrexed vs Cisplatin/Gemcitabine in Advanced NSCLC Subsets Scagliotti GV, et al. J Clin Oncol. 2008; 26(21): 3543 -3551.
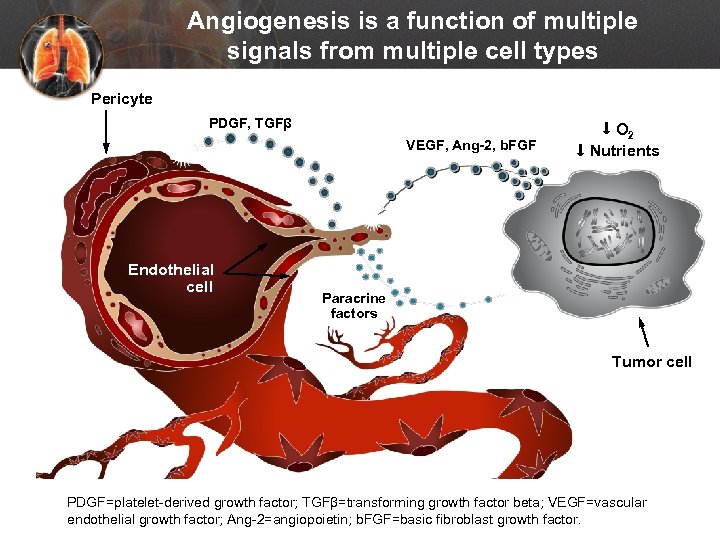 Angiogenesis is a function of multiple signals from multiple cell types Pericyte PDGF, TGFβ VEGF, Ang-2, b. FGF Endothelial cell O 2 Nutrients Paracrine factors Tumor cell PDGF=platelet-derived growth factor; TGFβ=transforming growth factor beta; VEGF=vascular endothelial growth factor; Ang-2=angiopoietin; b. FGF=basic fibroblast growth factor.
Angiogenesis is a function of multiple signals from multiple cell types Pericyte PDGF, TGFβ VEGF, Ang-2, b. FGF Endothelial cell O 2 Nutrients Paracrine factors Tumor cell PDGF=platelet-derived growth factor; TGFβ=transforming growth factor beta; VEGF=vascular endothelial growth factor; Ang-2=angiopoietin; b. FGF=basic fibroblast growth factor.
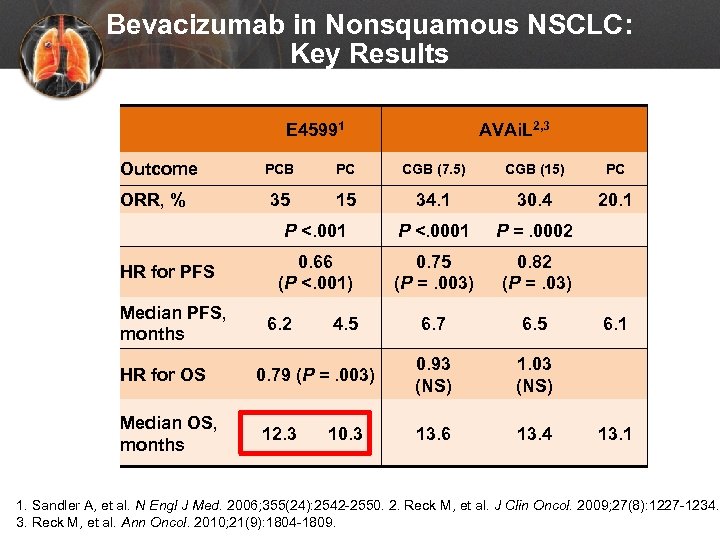 Bevacizumab in Nonsquamous NSCLC: Key Results E 45991 Outcome AVAi. L 2, 3 HR for PFS Median PFS, months HR for OS Median OS, months PC CGB (7. 5) CGB (15) PC 35 15 34. 1 30. 4 20. 1 P <. 001 ORR, % PCB P <. 0001 P =. 0002 0. 66 (P <. 001) 0. 75 (P =. 003) 0. 82 (P =. 03) 6. 7 6. 5 0. 93 (NS) 1. 03 (NS) 13. 6 13. 4 6. 2 4. 5 0. 79 (P =. 003) 12. 3 10. 3 6. 1 13. 1 1. Sandler A, et al. N Engl J Med. 2006; 355(24): 2542 -2550. 2. Reck M, et al. J Clin Oncol. 2009; 27(8): 1227 -1234. 3. Reck M, et al. Ann Oncol. 2010; 21(9): 1804 -1809.
Bevacizumab in Nonsquamous NSCLC: Key Results E 45991 Outcome AVAi. L 2, 3 HR for PFS Median PFS, months HR for OS Median OS, months PC CGB (7. 5) CGB (15) PC 35 15 34. 1 30. 4 20. 1 P <. 001 ORR, % PCB P <. 0001 P =. 0002 0. 66 (P <. 001) 0. 75 (P =. 003) 0. 82 (P =. 03) 6. 7 6. 5 0. 93 (NS) 1. 03 (NS) 13. 6 13. 4 6. 2 4. 5 0. 79 (P =. 003) 12. 3 10. 3 6. 1 13. 1 1. Sandler A, et al. N Engl J Med. 2006; 355(24): 2542 -2550. 2. Reck M, et al. J Clin Oncol. 2009; 27(8): 1227 -1234. 3. Reck M, et al. Ann Oncol. 2010; 21(9): 1804 -1809.
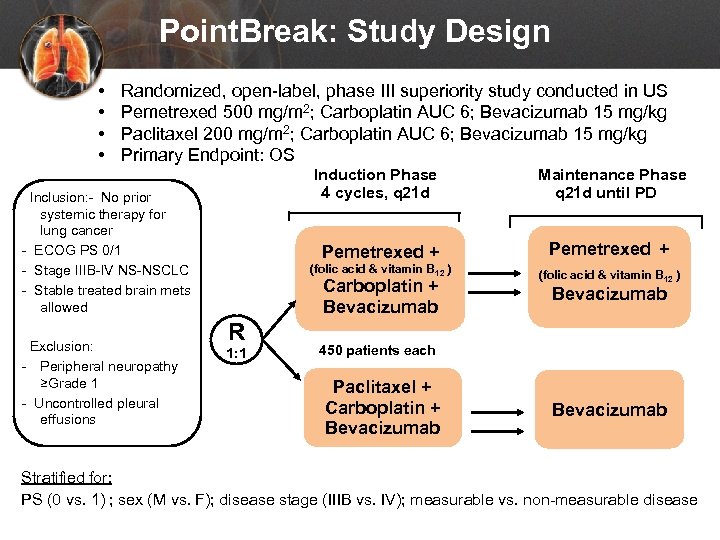 Point. Break: Study Design • • Randomized, open-label, phase III superiority study conducted in US Pemetrexed 500 mg/m 2; Carboplatin AUC 6; Bevacizumab 15 mg/kg Paclitaxel 200 mg/m 2; Carboplatin AUC 6; Bevacizumab 15 mg/kg Primary Endpoint: OS Induction Phase 4 cycles, q 21 d Pemetrexed + Exclusion: - Peripheral neuropathy ≥Grade 1 - Uncontrolled pleural effusions Pemetrexed + (folic acid & vitamin B 12 ) Inclusion: - No prior systemic therapy for lung cancer - ECOG PS 0/1 - Stage IIIB-IV NS-NSCLC - Stable treated brain mets allowed Maintenance Phase q 21 d until PD (folic acid & vitamin B 12 ) Carboplatin + Bevacizumab R 1: 1 Bevacizumab 450 patients each Paclitaxel + Carboplatin + Bevacizumab Stratified for: PS (0 vs. 1) ; sex (M vs. F); disease stage (IIIB vs. IV); measurable vs. non-measurable disease
Point. Break: Study Design • • Randomized, open-label, phase III superiority study conducted in US Pemetrexed 500 mg/m 2; Carboplatin AUC 6; Bevacizumab 15 mg/kg Paclitaxel 200 mg/m 2; Carboplatin AUC 6; Bevacizumab 15 mg/kg Primary Endpoint: OS Induction Phase 4 cycles, q 21 d Pemetrexed + Exclusion: - Peripheral neuropathy ≥Grade 1 - Uncontrolled pleural effusions Pemetrexed + (folic acid & vitamin B 12 ) Inclusion: - No prior systemic therapy for lung cancer - ECOG PS 0/1 - Stage IIIB-IV NS-NSCLC - Stable treated brain mets allowed Maintenance Phase q 21 d until PD (folic acid & vitamin B 12 ) Carboplatin + Bevacizumab R 1: 1 Bevacizumab 450 patients each Paclitaxel + Carboplatin + Bevacizumab Stratified for: PS (0 vs. 1) ; sex (M vs. F); disease stage (IIIB vs. IV); measurable vs. non-measurable disease
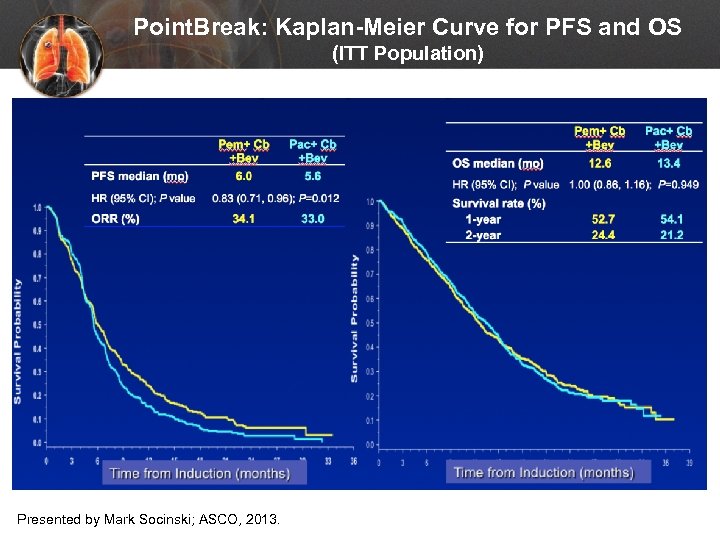 Point. Break: Kaplan-Meier Curve for PFS and OS (ITT Population) Presented by Mark Socinski; ASCO, 2013.
Point. Break: Kaplan-Meier Curve for PFS and OS (ITT Population) Presented by Mark Socinski; ASCO, 2013.
 What if… If Jack was 81 years old, should different first-line regimens be considered?
What if… If Jack was 81 years old, should different first-line regimens be considered?
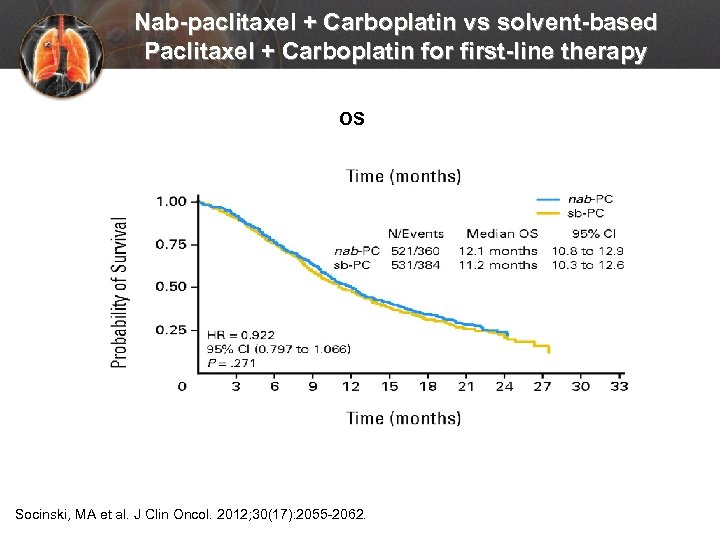 Nab-paclitaxel + Carboplatin vs solvent-based Paclitaxel + Carboplatin for first-line therapy OS Socinski, MA et al. J Clin Oncol. 2012; 30(17): 2055 -2062.
Nab-paclitaxel + Carboplatin vs solvent-based Paclitaxel + Carboplatin for first-line therapy OS Socinski, MA et al. J Clin Oncol. 2012; 30(17): 2055 -2062.
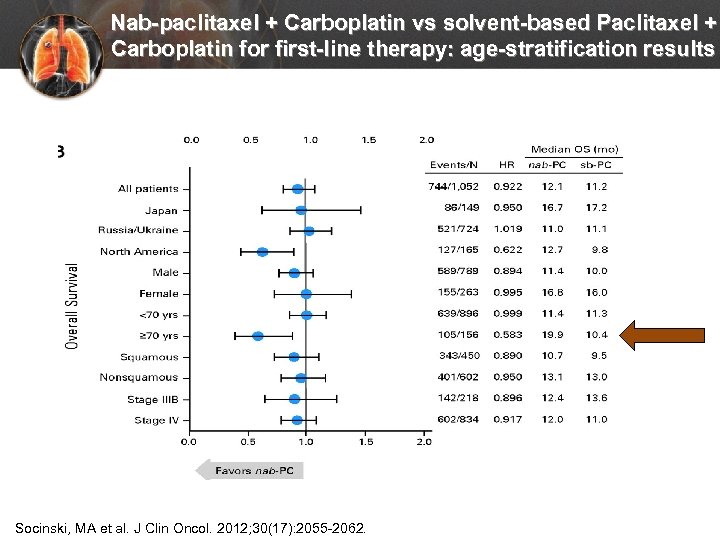 Nab-paclitaxel + Carboplatin vs solvent-based Paclitaxel + Carboplatin for first-line therapy: age-stratification results Socinski, MA et al. J Clin Oncol. 2012; 30(17): 2055 -2062.
Nab-paclitaxel + Carboplatin vs solvent-based Paclitaxel + Carboplatin for first-line therapy: age-stratification results Socinski, MA et al. J Clin Oncol. 2012; 30(17): 2055 -2062.
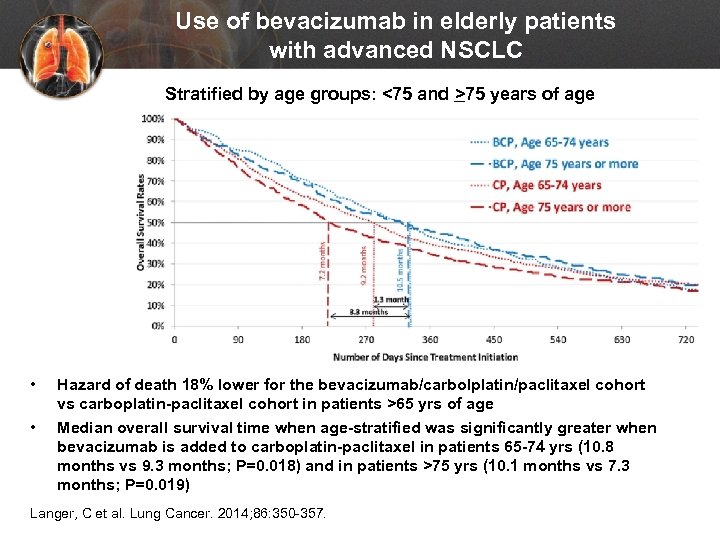 Use of bevacizumab in elderly patients with advanced NSCLC Stratified by age groups: <75 and >75 years of age • • Hazard of death 18% lower for the bevacizumab/carbolplatin/paclitaxel cohort vs carboplatin-paclitaxel cohort in patients >65 yrs of age Median overall survival time when age-stratified was significantly greater when bevacizumab is added to carboplatin-paclitaxel in patients 65 -74 yrs (10. 8 months vs 9. 3 months; P=0. 018) and in patients >75 yrs (10. 1 months vs 7. 3 months; P=0. 019) Langer, C et al. Lung Cancer. 2014; 86: 350 -357.
Use of bevacizumab in elderly patients with advanced NSCLC Stratified by age groups: <75 and >75 years of age • • Hazard of death 18% lower for the bevacizumab/carbolplatin/paclitaxel cohort vs carboplatin-paclitaxel cohort in patients >65 yrs of age Median overall survival time when age-stratified was significantly greater when bevacizumab is added to carboplatin-paclitaxel in patients 65 -74 yrs (10. 8 months vs 9. 3 months; P=0. 018) and in patients >75 yrs (10. 1 months vs 7. 3 months; P=0. 019) Langer, C et al. Lung Cancer. 2014; 86: 350 -357.
 What if… Jack had a good response to first-line platinum chemotherapy. Should continuation or switch maintenance therapy be used after chemotherapy?
What if… Jack had a good response to first-line platinum chemotherapy. Should continuation or switch maintenance therapy be used after chemotherapy?
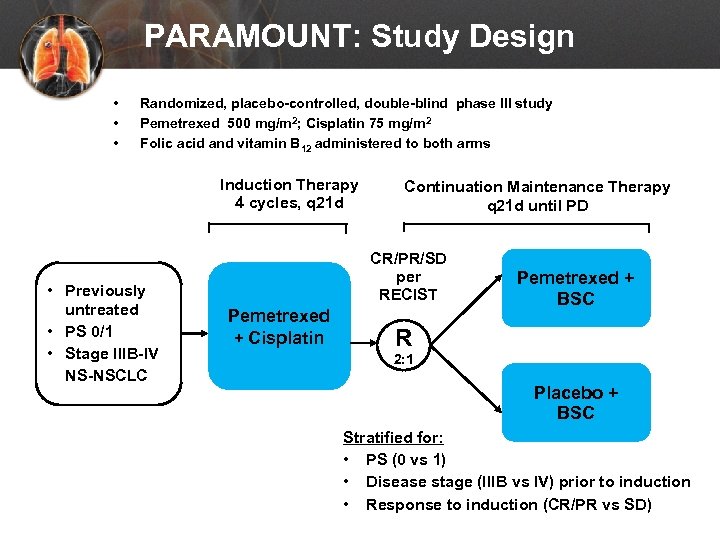 PARAMOUNT: Study Design • • • Randomized, placebo-controlled, double-blind phase III study Pemetrexed 500 mg/m 2; Cisplatin 75 mg/m 2 Folic acid and vitamin B 12 administered to both arms Induction Therapy 4 cycles, q 21 d • Previously untreated • PS 0/1 • Stage IIIB-IV NS-NSCLC Continuation Maintenance Therapy q 21 d until PD CR/PR/SD per RECIST Pemetrexed + Cisplatin Pemetrexed + BSC R 2: 1 Placebo + BSC Stratified for: • PS (0 vs 1) • Disease stage (IIIB vs IV) prior to induction • Response to induction (CR/PR vs SD)
PARAMOUNT: Study Design • • • Randomized, placebo-controlled, double-blind phase III study Pemetrexed 500 mg/m 2; Cisplatin 75 mg/m 2 Folic acid and vitamin B 12 administered to both arms Induction Therapy 4 cycles, q 21 d • Previously untreated • PS 0/1 • Stage IIIB-IV NS-NSCLC Continuation Maintenance Therapy q 21 d until PD CR/PR/SD per RECIST Pemetrexed + Cisplatin Pemetrexed + BSC R 2: 1 Placebo + BSC Stratified for: • PS (0 vs 1) • Disease stage (IIIB vs IV) prior to induction • Response to induction (CR/PR vs SD)
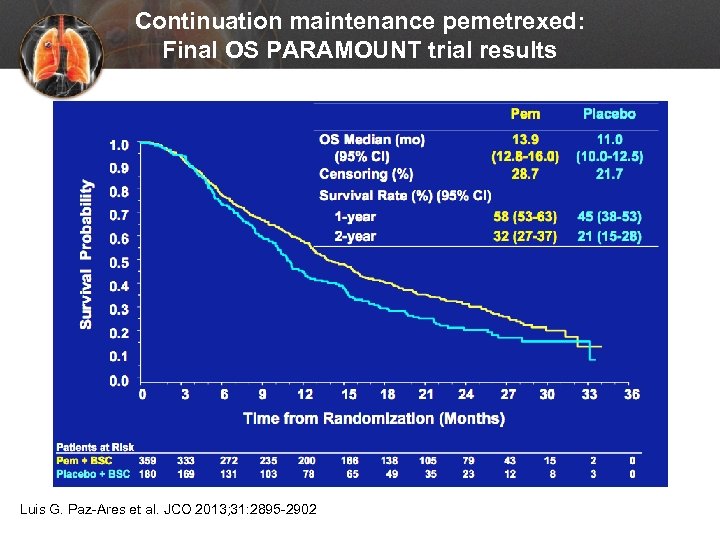 Continuation maintenance pemetrexed: Final OS PARAMOUNT trial results Luis G. Paz-Ares et al. JCO 2013; 31: 2895 -2902
Continuation maintenance pemetrexed: Final OS PARAMOUNT trial results Luis G. Paz-Ares et al. JCO 2013; 31: 2895 -2902
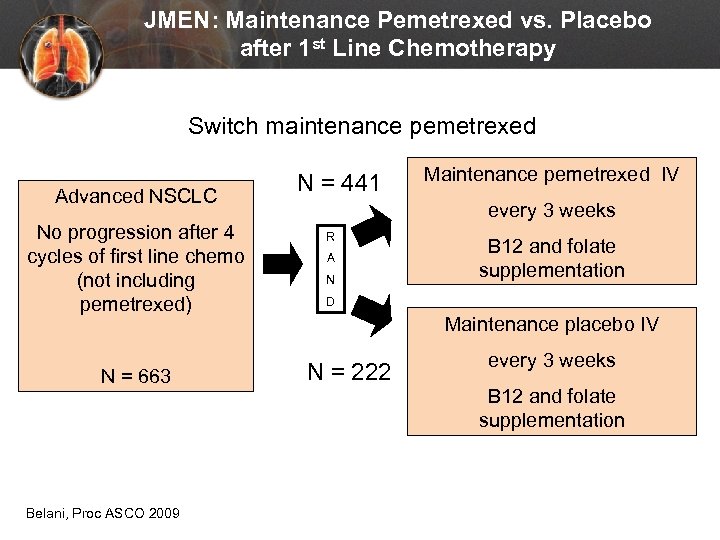 JMEN: Maintenance Pemetrexed vs. Placebo after 1 st Line Chemotherapy Switch maintenance pemetrexed Advanced NSCLC No progression after 4 cycles of first line chemo (not including pemetrexed) N = 663 Belani, Proc ASCO 2009 N = 441 Maintenance pemetrexed IV every 3 weeks R A N B 12 and folate supplementation D Maintenance placebo IV N = 222 every 3 weeks B 12 and folate supplementation
JMEN: Maintenance Pemetrexed vs. Placebo after 1 st Line Chemotherapy Switch maintenance pemetrexed Advanced NSCLC No progression after 4 cycles of first line chemo (not including pemetrexed) N = 663 Belani, Proc ASCO 2009 N = 441 Maintenance pemetrexed IV every 3 weeks R A N B 12 and folate supplementation D Maintenance placebo IV N = 222 every 3 weeks B 12 and folate supplementation
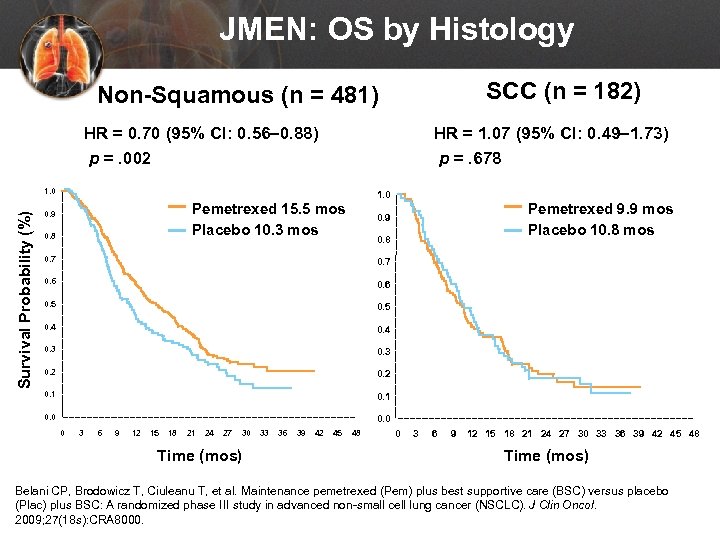 JMEN: OS by Histology SCC (n = 182) Non-Squamous (n = 481) HR = 0. 70 (95% CI: 0. 56– 0. 88) p =. 002 HR = 1. 07 (95% CI: 0. 49– 1. 73) p =. 678 Survival Probability (%) 1. 0 Pemetrexed 15. 5 mos Placebo 10. 3 mos 0. 9 0. 8 Pemetrexed 9. 9 mos Placebo 10. 8 mos 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0 3 6 9 12 15 18 21 24 27 30 Time (mos) 33 36 39 42 45 48 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 Time (mos) Belani CP, Brodowicz T, Ciuleanu T, et al. Maintenance pemetrexed (Pem) plus best supportive care (BSC) versus placebo (Plac) plus BSC: A randomized phase III study in advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2009; 27(18 s): CRA 8000.
JMEN: OS by Histology SCC (n = 182) Non-Squamous (n = 481) HR = 0. 70 (95% CI: 0. 56– 0. 88) p =. 002 HR = 1. 07 (95% CI: 0. 49– 1. 73) p =. 678 Survival Probability (%) 1. 0 Pemetrexed 15. 5 mos Placebo 10. 3 mos 0. 9 0. 8 Pemetrexed 9. 9 mos Placebo 10. 8 mos 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0 3 6 9 12 15 18 21 24 27 30 Time (mos) 33 36 39 42 45 48 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 Time (mos) Belani CP, Brodowicz T, Ciuleanu T, et al. Maintenance pemetrexed (Pem) plus best supportive care (BSC) versus placebo (Plac) plus BSC: A randomized phase III study in advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2009; 27(18 s): CRA 8000.
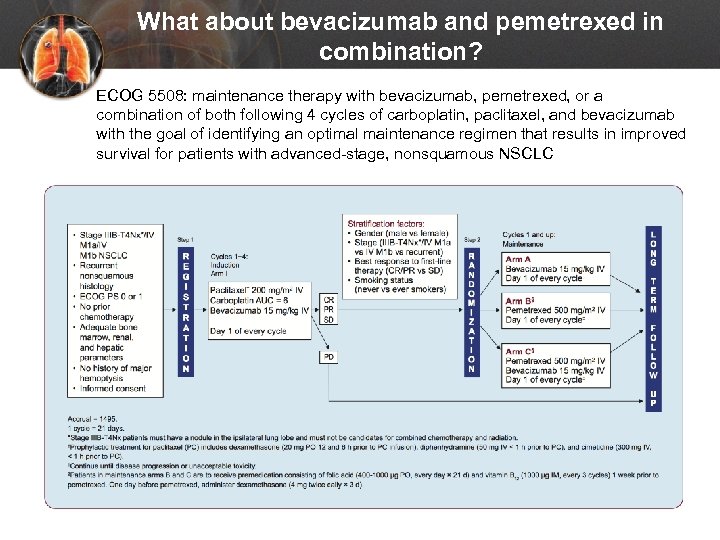 What about bevacizumab and pemetrexed in combination? ECOG 5508: maintenance therapy with bevacizumab, pemetrexed, or a combination of both following 4 cycles of carboplatin, paclitaxel, and bevacizumab with the goal of identifying an optimal maintenance regimen that results in improved survival for patients with advanced-stage, nonsquamous NSCLC
What about bevacizumab and pemetrexed in combination? ECOG 5508: maintenance therapy with bevacizumab, pemetrexed, or a combination of both following 4 cycles of carboplatin, paclitaxel, and bevacizumab with the goal of identifying an optimal maintenance regimen that results in improved survival for patients with advanced-stage, nonsquamous NSCLC
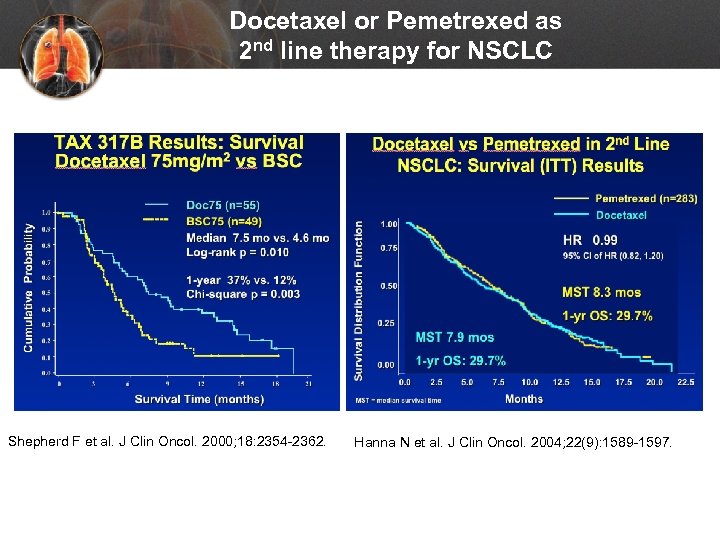 Docetaxel or Pemetrexed as 2 nd line therapy for NSCLC Shepherd F et al. J Clin Oncol. 2000; 18: 2354 -2362. Hanna N et al. J Clin Oncol. 2004; 22(9): 1589 -1597.
Docetaxel or Pemetrexed as 2 nd line therapy for NSCLC Shepherd F et al. J Clin Oncol. 2000; 18: 2354 -2362. Hanna N et al. J Clin Oncol. 2004; 22(9): 1589 -1597.
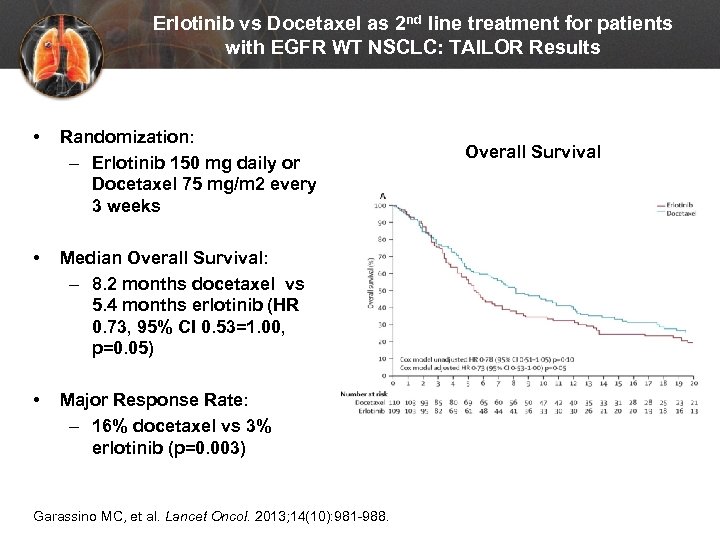 Erlotinib vs Docetaxel as 2 nd line treatment for patients with EGFR WT NSCLC: TAILOR Results • Randomization: – Erlotinib 150 mg daily or Docetaxel 75 mg/m 2 every 3 weeks • Median Overall Survival: – 8. 2 months docetaxel vs 5. 4 months erlotinib (HR 0. 73, 95% CI 0. 53=1. 00, p=0. 05) • Major Response Rate: – 16% docetaxel vs 3% erlotinib (p=0. 003) Garassino MC, et al. Lancet Oncol. 2013; 14(10): 981 -988. Overall Survival
Erlotinib vs Docetaxel as 2 nd line treatment for patients with EGFR WT NSCLC: TAILOR Results • Randomization: – Erlotinib 150 mg daily or Docetaxel 75 mg/m 2 every 3 weeks • Median Overall Survival: – 8. 2 months docetaxel vs 5. 4 months erlotinib (HR 0. 73, 95% CI 0. 53=1. 00, p=0. 05) • Major Response Rate: – 16% docetaxel vs 3% erlotinib (p=0. 003) Garassino MC, et al. Lancet Oncol. 2013; 14(10): 981 -988. Overall Survival
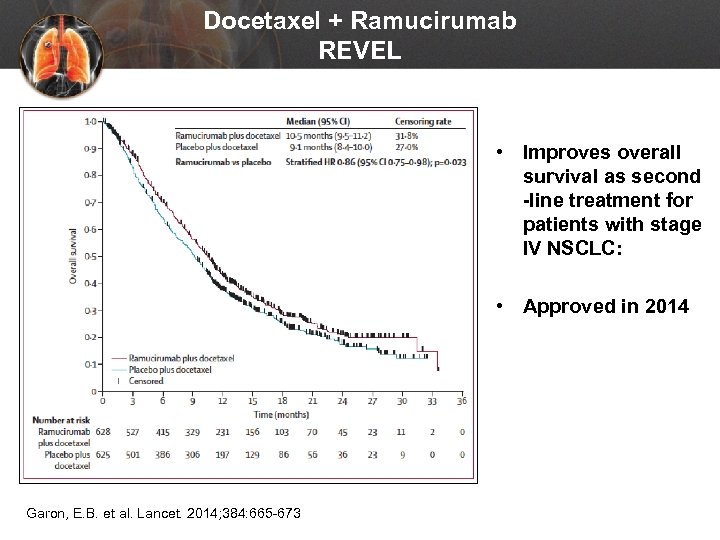 Docetaxel + Ramucirumab REVEL • Improves overall survival as second -line treatment for patients with stage IV NSCLC: • Approved in 2014 Garon, E. B. et al. Lancet. 2014; 384: 665 -673
Docetaxel + Ramucirumab REVEL • Improves overall survival as second -line treatment for patients with stage IV NSCLC: • Approved in 2014 Garon, E. B. et al. Lancet. 2014; 384: 665 -673
 What if… Jack is offered chemotherapy second line, but asks about these immunotherapy drugs for non-small cell lung cancer he’s been seeing in the media?
What if… Jack is offered chemotherapy second line, but asks about these immunotherapy drugs for non-small cell lung cancer he’s been seeing in the media?
 Immunotherapy in NSCLC – 2015 in Review • March 2015 – Nivolumab approved in squamous cancers following platinum-based therapy - Check. Mate 017 • October 2015 – Pembrolizumab approved in PD-L 1+ NSCLC following platinum-based therapy (required 22 C 3 IHC companion diagnostic) – KEYNOTE-001 • October 2015 – Nivolumab indication expanded to non-squamous NSCLC (optional 28 -8 IHC complementary diagnostic) – Check. Mate 057 • December 2015 – Pembrolizumab - positive results presented from KEYNOTE-010 study (pembrolizumab vs docetaxel in PD-L 1 + previously treated NSCLC patients)
Immunotherapy in NSCLC – 2015 in Review • March 2015 – Nivolumab approved in squamous cancers following platinum-based therapy - Check. Mate 017 • October 2015 – Pembrolizumab approved in PD-L 1+ NSCLC following platinum-based therapy (required 22 C 3 IHC companion diagnostic) – KEYNOTE-001 • October 2015 – Nivolumab indication expanded to non-squamous NSCLC (optional 28 -8 IHC complementary diagnostic) – Check. Mate 057 • December 2015 – Pembrolizumab - positive results presented from KEYNOTE-010 study (pembrolizumab vs docetaxel in PD-L 1 + previously treated NSCLC patients)
 Immunotherapy in NSCLC – 2016 in Review • June 2016 -- KEYNOTE-0242: Pembrolizumab monotherapy provides long-term OS benefit for treatment-naïve and previously treated patients with advanced NSCLC. These data support PD-L 1 as a predictive biomarker for pembrolizumab response. • August 2016– Check. Mate-0263: Nivolumab monotherapy in a broad previously untreated advanced NSCLC patient population did not meet it’s primary endpoint of PFS in patients whose tumors expressed PD-L 1 at ≥ 5%. • August 2016– OAK 4: Increased OS with atezolizumab vs docetaxel in locally advanced or metastatic NSCLC whose disease progressed on platinum based chemotherapy. Based on these positive results from this phase 3 OAK trial, the FDA has recently granted breakthrough therapy designation (BTD) for atezolizumab for the treatment of PD-L 1 positive NSCLC patients whose disease has progressed during or after platinum based chemotherapy (and appropriate targeted therapy for those with EGFR-positive or ALK-positive tumors).
Immunotherapy in NSCLC – 2016 in Review • June 2016 -- KEYNOTE-0242: Pembrolizumab monotherapy provides long-term OS benefit for treatment-naïve and previously treated patients with advanced NSCLC. These data support PD-L 1 as a predictive biomarker for pembrolizumab response. • August 2016– Check. Mate-0263: Nivolumab monotherapy in a broad previously untreated advanced NSCLC patient population did not meet it’s primary endpoint of PFS in patients whose tumors expressed PD-L 1 at ≥ 5%. • August 2016– OAK 4: Increased OS with atezolizumab vs docetaxel in locally advanced or metastatic NSCLC whose disease progressed on platinum based chemotherapy. Based on these positive results from this phase 3 OAK trial, the FDA has recently granted breakthrough therapy designation (BTD) for atezolizumab for the treatment of PD-L 1 positive NSCLC patients whose disease has progressed during or after platinum based chemotherapy (and appropriate targeted therapy for those with EGFR-positive or ALK-positive tumors).
 Immunotherapy in NSCLC – 2016 in Review Ongoing combination therapies: • Check. Mate-012 and -2275 study on combination therapies: Nivolumab plus ipilimumab in PD-L 1 positive patients and nivolumab plus ipilimumab vs nivolumab plus chemotherapy in PD-L 1 negative patients. • MYSTIC 6 is a global phase 3 study of 675 treatment-naïve advanced NSCLC pts (wild type EGFR and ALK) randomized 1: 1: 1 to durvalumab + tremelimumab, durvalumab monotherapy, or standard-of-care chemotherapy. 1. http: //meetinglibrary. asco. org/content/167384 -176 (Hui R et al, J Clin Oncol 34; suppl: abstr 9026; 2016) 2. http: //www. businesswire. com/news/home/20160616005393/en/Merck’s-KEYTRUDA®%C 2%A 0 -pembrolizumab-Demonstrates-Superior. Progression-Free-Survival 3. http: //investor. bms. com/investors/news-and-events/press-release-details/2016/Bristol-Myers-Squibb-Announces-Top-Line-Resultsfrom-Check. Mate--026 -a-Phase-3 -Study-of-Opdivo-nivolumab-in-Treatment-Nave-Patients-with-Advanced-Non-Small-Cell-Lung. Cancer/default. aspx 4. http: //www. gene. com/media/press-releases/14634/2016 -08 -31/phase-iii-study-showed-genentechs-cancer 5. http: //theoncologist. alphamedpress. org/site/conference/asco/2016/files/checkpoint_inhibitors. html 6. http: //oncologypro. esmo. org/Meeting-Resources/ELCC-2016/MYSTIC-a-global-phase-3 -study-of-durvalumab-MEDI 4736 -plus-tremelimumabcombination-therapy-or-durvalumab-monotherapy-versus-platinum-based-chemotherapy-CT-in-the-first-line-treatment-of-patients-pts-withadvanced-stage-IV-NSCLC
Immunotherapy in NSCLC – 2016 in Review Ongoing combination therapies: • Check. Mate-012 and -2275 study on combination therapies: Nivolumab plus ipilimumab in PD-L 1 positive patients and nivolumab plus ipilimumab vs nivolumab plus chemotherapy in PD-L 1 negative patients. • MYSTIC 6 is a global phase 3 study of 675 treatment-naïve advanced NSCLC pts (wild type EGFR and ALK) randomized 1: 1: 1 to durvalumab + tremelimumab, durvalumab monotherapy, or standard-of-care chemotherapy. 1. http: //meetinglibrary. asco. org/content/167384 -176 (Hui R et al, J Clin Oncol 34; suppl: abstr 9026; 2016) 2. http: //www. businesswire. com/news/home/20160616005393/en/Merck’s-KEYTRUDA®%C 2%A 0 -pembrolizumab-Demonstrates-Superior. Progression-Free-Survival 3. http: //investor. bms. com/investors/news-and-events/press-release-details/2016/Bristol-Myers-Squibb-Announces-Top-Line-Resultsfrom-Check. Mate--026 -a-Phase-3 -Study-of-Opdivo-nivolumab-in-Treatment-Nave-Patients-with-Advanced-Non-Small-Cell-Lung. Cancer/default. aspx 4. http: //www. gene. com/media/press-releases/14634/2016 -08 -31/phase-iii-study-showed-genentechs-cancer 5. http: //theoncologist. alphamedpress. org/site/conference/asco/2016/files/checkpoint_inhibitors. html 6. http: //oncologypro. esmo. org/Meeting-Resources/ELCC-2016/MYSTIC-a-global-phase-3 -study-of-durvalumab-MEDI 4736 -plus-tremelimumabcombination-therapy-or-durvalumab-monotherapy-versus-platinum-based-chemotherapy-CT-in-the-first-line-treatment-of-patients-pts-withadvanced-stage-IV-NSCLC
 NCCN update on immunotherapeutic agents The 2016 NCCN update includes: • Immune checkpoint inhibitors as preferred agents (in the absence of contraindications) for second-line and beyond (subsequent) therapy in patients with metastatic NSCLC (both squamous and nonsquamous histologies). • Nivolumab and pembrolizumab upgraded to Category 1 from category 2 A and are preferred based on improved OS rates, higher response rates, longer duration of response, and fewer adverse events when compared with docetaxel therapy.
NCCN update on immunotherapeutic agents The 2016 NCCN update includes: • Immune checkpoint inhibitors as preferred agents (in the absence of contraindications) for second-line and beyond (subsequent) therapy in patients with metastatic NSCLC (both squamous and nonsquamous histologies). • Nivolumab and pembrolizumab upgraded to Category 1 from category 2 A and are preferred based on improved OS rates, higher response rates, longer duration of response, and fewer adverse events when compared with docetaxel therapy.
 Changes in the Therapeutic Landscape of Stage IV Lung Cancer PD-L 1+ HER 2 EGFR mutants ALK ROS/RET/BRAF/Others KRAS Adeno LCC-NOS Sq. CC SCLC
Changes in the Therapeutic Landscape of Stage IV Lung Cancer PD-L 1+ HER 2 EGFR mutants ALK ROS/RET/BRAF/Others KRAS Adeno LCC-NOS Sq. CC SCLC
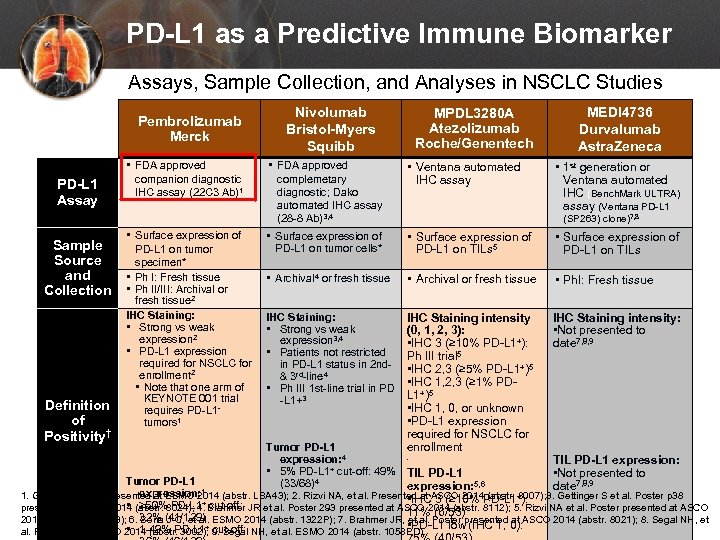 PD-L 1 as a Predictive Immune Biomarker Assays, Sample Collection, and Analyses in NSCLC Studies Pembrolizumab Merck PD-L 1 Assay Sample Source and Collection Definition of Positivity† • FDA approved companion diagnostic IHC assay (22 C 3 Ab)1 • Surface expression of PD-L 1 on tumor specimen* • Ph I: Fresh tissue • Ph II/III: Archival or fresh tissue 2 IHC Staining: • Strong vs weak expression 2 • PD-L 1 expression required for NSCLC for enrollment 2 • Note that one arm of KEYNOTE 001 trial requires PD-L 1 - tumors 1 Nivolumab Bristol-Myers Squibb MPDL 3280 A Atezolizumab Roche/Genentech • FDA approved complemetary diagnostic; Dako automated IHC assay (28 -8 Ab)3, 4 • Ventana automated IHC assay • Surface expression of PD-L 1 on tumor cells* • Surface expression of PD-L 1 on TILs 5 • Surface expression of PD-L 1 on TILs • Archival 4 or fresh tissue • Archival or fresh tissue • Ph. I: Fresh tissue IHC Staining: • Strong vs weak expression 3, 4 • Patients not restricted in PD-L 1 status in 2 nd- & 3 rd-line 4 • Ph III 1 st-line trial in PD -L 1+3 IHC Staining intensity (0, 1, 2, 3): • IHC 3 (≥ 10% PD-L 1+): Ph III trial 5 • IHC 2, 3 (≥ 5% PD-L 1+)5 • IHC 1, 2, 3 (≥ 1% PDL 1+)5 • IHC 1, 0, or unknown • PD-L 1 expression required for NSCLC for enrollment IHC Staining intensity: • Not presented to date 7, 8, 9 • 1 st generation or Ventana automated IHC (Bench. Mark ULTRA) assay (Ventana PD-L 1 (SP 263) clone)7, 8 Tumor PD-L 1 expression: 4 • 5% PD-L 1+ cut-off: 49% TIL PD-L 1 (33/68)4 expression: 5, 6 • x Tumor PD-L 1 MEDI 4736 Durvalumab Astra. Zeneca TIL PD-L 1 expression: • Not presented to date 7, 8, 9 expression: 1. Garon EB, et al. Presented at ESMO 2014 (abstr. LBA 43); 2. Rizvi NA, et al. Presented at ASCO 2014 (abstr. 8007); 3. Gettinger S et al. Poster p 38 • IHC 3 (≥ 10% PD-L 1+): • ≥ 50% PD-L 1+ cut-off: presented at ASCO 2014 (abstr. 8024); 4. Brahmer JR et al. Poster 293 presented at ASCO 2014 (abstr. 8112); 5. Rizvi NA et al. Poster presented at ASCO 11% (6/53) 32% (41/129) 2014 (abstr. TPS 8123); 6. Soria J-C, et al. ESMO 2014 (abstr. 1322 P); 7. Brahmer JR, et al. Poster presented at ASCO 2014 (abstr. 8021); 8. Segal NH, et • PD-L 1 low (IHC 1, 0): + cut-off: • 1 -49% PD-L 1 al. Presented at ASCO 2014 (abstr. 3002); 9. Segal NH, et al. ESMO 2014 (abstr. 1058 PD). 1
PD-L 1 as a Predictive Immune Biomarker Assays, Sample Collection, and Analyses in NSCLC Studies Pembrolizumab Merck PD-L 1 Assay Sample Source and Collection Definition of Positivity† • FDA approved companion diagnostic IHC assay (22 C 3 Ab)1 • Surface expression of PD-L 1 on tumor specimen* • Ph I: Fresh tissue • Ph II/III: Archival or fresh tissue 2 IHC Staining: • Strong vs weak expression 2 • PD-L 1 expression required for NSCLC for enrollment 2 • Note that one arm of KEYNOTE 001 trial requires PD-L 1 - tumors 1 Nivolumab Bristol-Myers Squibb MPDL 3280 A Atezolizumab Roche/Genentech • FDA approved complemetary diagnostic; Dako automated IHC assay (28 -8 Ab)3, 4 • Ventana automated IHC assay • Surface expression of PD-L 1 on tumor cells* • Surface expression of PD-L 1 on TILs 5 • Surface expression of PD-L 1 on TILs • Archival 4 or fresh tissue • Archival or fresh tissue • Ph. I: Fresh tissue IHC Staining: • Strong vs weak expression 3, 4 • Patients not restricted in PD-L 1 status in 2 nd- & 3 rd-line 4 • Ph III 1 st-line trial in PD -L 1+3 IHC Staining intensity (0, 1, 2, 3): • IHC 3 (≥ 10% PD-L 1+): Ph III trial 5 • IHC 2, 3 (≥ 5% PD-L 1+)5 • IHC 1, 2, 3 (≥ 1% PDL 1+)5 • IHC 1, 0, or unknown • PD-L 1 expression required for NSCLC for enrollment IHC Staining intensity: • Not presented to date 7, 8, 9 • 1 st generation or Ventana automated IHC (Bench. Mark ULTRA) assay (Ventana PD-L 1 (SP 263) clone)7, 8 Tumor PD-L 1 expression: 4 • 5% PD-L 1+ cut-off: 49% TIL PD-L 1 (33/68)4 expression: 5, 6 • x Tumor PD-L 1 MEDI 4736 Durvalumab Astra. Zeneca TIL PD-L 1 expression: • Not presented to date 7, 8, 9 expression: 1. Garon EB, et al. Presented at ESMO 2014 (abstr. LBA 43); 2. Rizvi NA, et al. Presented at ASCO 2014 (abstr. 8007); 3. Gettinger S et al. Poster p 38 • IHC 3 (≥ 10% PD-L 1+): • ≥ 50% PD-L 1+ cut-off: presented at ASCO 2014 (abstr. 8024); 4. Brahmer JR et al. Poster 293 presented at ASCO 2014 (abstr. 8112); 5. Rizvi NA et al. Poster presented at ASCO 11% (6/53) 32% (41/129) 2014 (abstr. TPS 8123); 6. Soria J-C, et al. ESMO 2014 (abstr. 1322 P); 7. Brahmer JR, et al. Poster presented at ASCO 2014 (abstr. 8021); 8. Segal NH, et • PD-L 1 low (IHC 1, 0): + cut-off: • 1 -49% PD-L 1 al. Presented at ASCO 2014 (abstr. 3002); 9. Segal NH, et al. ESMO 2014 (abstr. 1058 PD). 1
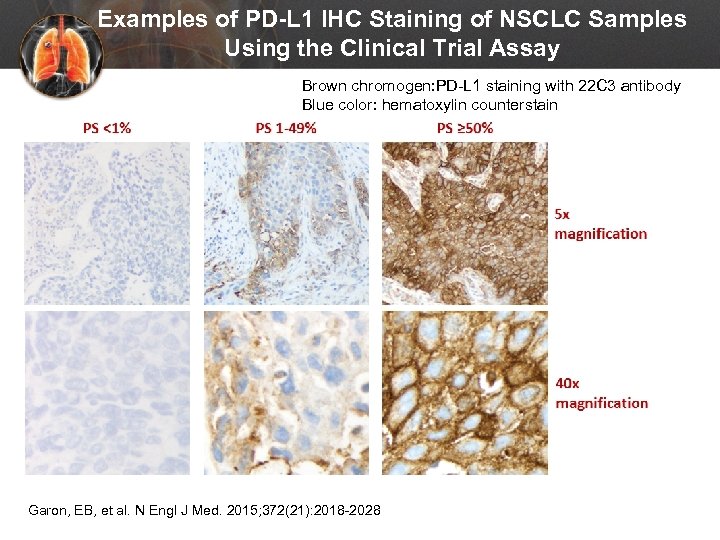 Examples of PD-L 1 IHC Staining of NSCLC Samples Using the Clinical Trial Assay Brown chromogen: PD-L 1 staining with 22 C 3 antibody Blue color: hematoxylin counterstain Garon, EB, et al. N Engl J Med. 2015; 372(21): 2018 -2028
Examples of PD-L 1 IHC Staining of NSCLC Samples Using the Clinical Trial Assay Brown chromogen: PD-L 1 staining with 22 C 3 antibody Blue color: hematoxylin counterstain Garon, EB, et al. N Engl J Med. 2015; 372(21): 2018 -2028
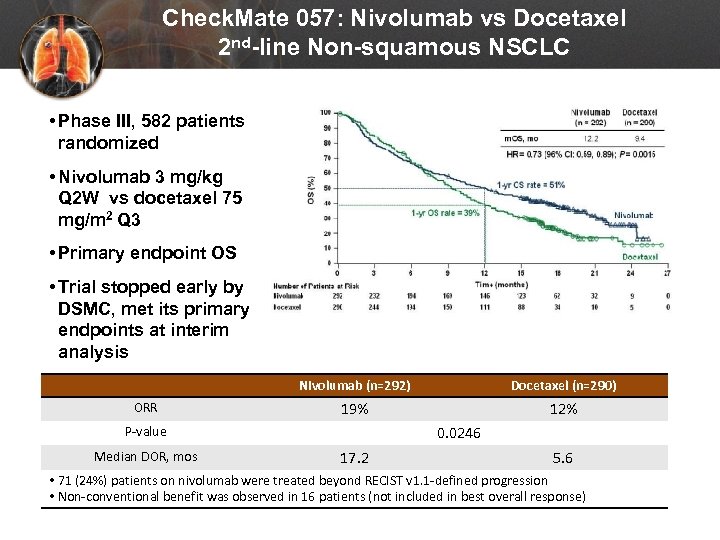 Check. Mate 057: Nivolumab vs Docetaxel 2 nd-line Non-squamous NSCLC • Phase III, 582 patients randomized • Nivolumab 3 mg/kg Q 2 W vs docetaxel 75 mg/m 2 Q 3 • Primary endpoint OS • Trial stopped early by DSMC, met its primary endpoints at interim analysis Nivolumab (n=292) ORR Docetaxel (n=290) 19% 12% 0. 0246 P-value Median DOR, mos 17. 2 5. 6 • 71 (24%) patients on nivolumab were treated beyond RECIST v 1. 1 -defined progression • Non-conventional benefit was observed in 16 patients (not included in best overall response) Paz-Ares L et al. Presented at: American Society of Clinical Oncology, 2015 Annual Meeting; (Abstract LBA 109).
Check. Mate 057: Nivolumab vs Docetaxel 2 nd-line Non-squamous NSCLC • Phase III, 582 patients randomized • Nivolumab 3 mg/kg Q 2 W vs docetaxel 75 mg/m 2 Q 3 • Primary endpoint OS • Trial stopped early by DSMC, met its primary endpoints at interim analysis Nivolumab (n=292) ORR Docetaxel (n=290) 19% 12% 0. 0246 P-value Median DOR, mos 17. 2 5. 6 • 71 (24%) patients on nivolumab were treated beyond RECIST v 1. 1 -defined progression • Non-conventional benefit was observed in 16 patients (not included in best overall response) Paz-Ares L et al. Presented at: American Society of Clinical Oncology, 2015 Annual Meeting; (Abstract LBA 109).
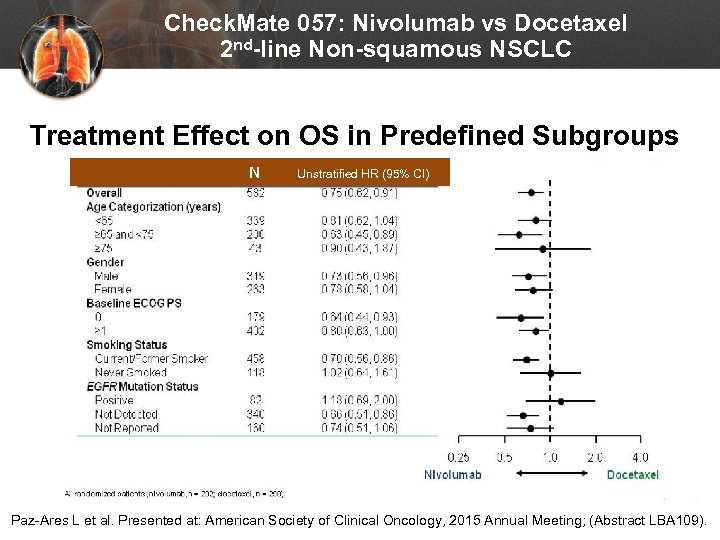 Check. Mate 057: Nivolumab vs Docetaxel 2 nd-line Non-squamous NSCLC Treatment Effect on OS in Predefined Subgroups N Unstratified HR (95% CI) Paz-Ares L et al. Presented at: American Society of Clinical Oncology, 2015 Annual Meeting; (Abstract LBA 109).
Check. Mate 057: Nivolumab vs Docetaxel 2 nd-line Non-squamous NSCLC Treatment Effect on OS in Predefined Subgroups N Unstratified HR (95% CI) Paz-Ares L et al. Presented at: American Society of Clinical Oncology, 2015 Annual Meeting; (Abstract LBA 109).
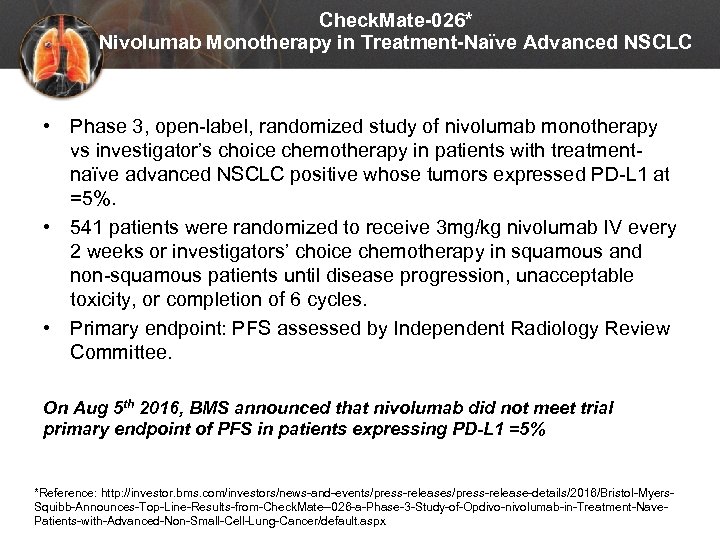 Check. Mate-026* Nivolumab Monotherapy in Treatment-Naïve Advanced NSCLC • Phase 3, open-label, randomized study of nivolumab monotherapy vs investigator’s choice chemotherapy in patients with treatmentnaïve advanced NSCLC positive whose tumors expressed PD-L 1 at =5%. • 541 patients were randomized to receive 3 mg/kg nivolumab IV every 2 weeks or investigators’ choice chemotherapy in squamous and non-squamous patients until disease progression, unacceptable toxicity, or completion of 6 cycles. • Primary endpoint: PFS assessed by Independent Radiology Review Committee. On Aug 5 th 2016, BMS announced that nivolumab did not meet trial primary endpoint of PFS in patients expressing PD-L 1 =5% *Reference: http: //investor. bms. com/investors/news-and-events/press-release-details/2016/Bristol-Myers. Squibb-Announces-Top-Line-Results-from-Check. Mate--026 -a-Phase-3 -Study-of-Opdivo-nivolumab-in-Treatment-Nave. Patients-with-Advanced-Non-Small-Cell-Lung-Cancer/default. aspx
Check. Mate-026* Nivolumab Monotherapy in Treatment-Naïve Advanced NSCLC • Phase 3, open-label, randomized study of nivolumab monotherapy vs investigator’s choice chemotherapy in patients with treatmentnaïve advanced NSCLC positive whose tumors expressed PD-L 1 at =5%. • 541 patients were randomized to receive 3 mg/kg nivolumab IV every 2 weeks or investigators’ choice chemotherapy in squamous and non-squamous patients until disease progression, unacceptable toxicity, or completion of 6 cycles. • Primary endpoint: PFS assessed by Independent Radiology Review Committee. On Aug 5 th 2016, BMS announced that nivolumab did not meet trial primary endpoint of PFS in patients expressing PD-L 1 =5% *Reference: http: //investor. bms. com/investors/news-and-events/press-release-details/2016/Bristol-Myers. Squibb-Announces-Top-Line-Results-from-Check. Mate--026 -a-Phase-3 -Study-of-Opdivo-nivolumab-in-Treatment-Nave. Patients-with-Advanced-Non-Small-Cell-Lung-Cancer/default. aspx
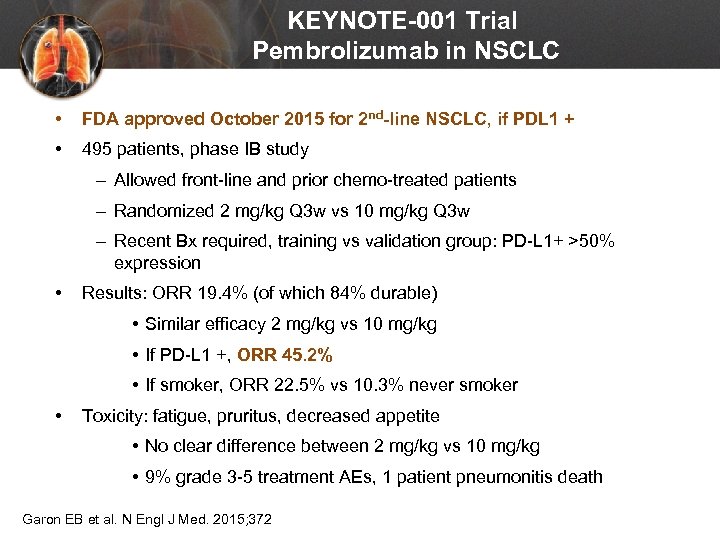 KEYNOTE-001 Trial Pembrolizumab in NSCLC • FDA approved October 2015 for 2 nd-line NSCLC, if PDL 1 + • 495 patients, phase IB study – Allowed front-line and prior chemo-treated patients – Randomized 2 mg/kg Q 3 w vs 10 mg/kg Q 3 w – Recent Bx required, training vs validation group: PD-L 1+ >50% expression • Results: ORR 19. 4% (of which 84% durable) • Similar efficacy 2 mg/kg vs 10 mg/kg • If PD-L 1 +, ORR 45. 2% • If smoker, ORR 22. 5% vs 10. 3% never smoker • Toxicity: fatigue, pruritus, decreased appetite • No clear difference between 2 mg/kg vs 10 mg/kg • 9% grade 3 -5 treatment AEs, 1 patient pneumonitis death Garon EB et al. N Engl J Med. 2015; 372: 2018 -2028.
KEYNOTE-001 Trial Pembrolizumab in NSCLC • FDA approved October 2015 for 2 nd-line NSCLC, if PDL 1 + • 495 patients, phase IB study – Allowed front-line and prior chemo-treated patients – Randomized 2 mg/kg Q 3 w vs 10 mg/kg Q 3 w – Recent Bx required, training vs validation group: PD-L 1+ >50% expression • Results: ORR 19. 4% (of which 84% durable) • Similar efficacy 2 mg/kg vs 10 mg/kg • If PD-L 1 +, ORR 45. 2% • If smoker, ORR 22. 5% vs 10. 3% never smoker • Toxicity: fatigue, pruritus, decreased appetite • No clear difference between 2 mg/kg vs 10 mg/kg • 9% grade 3 -5 treatment AEs, 1 patient pneumonitis death Garon EB et al. N Engl J Med. 2015; 372: 2018 -2028.
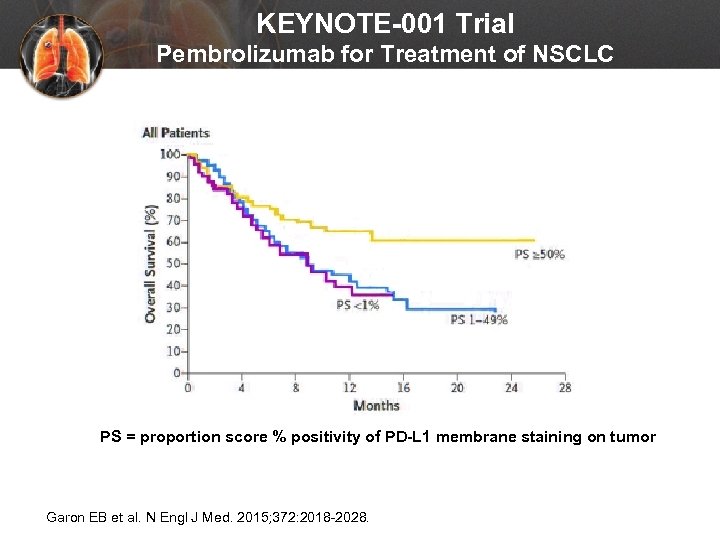 KEYNOTE-001 Trial Pembrolizumab for Treatment of NSCLC PS = proportion score % positivity of PD-L 1 membrane staining on tumor Garon EB et al. N Engl J Med. 2015; 372: 2018 -2028.
KEYNOTE-001 Trial Pembrolizumab for Treatment of NSCLC PS = proportion score % positivity of PD-L 1 membrane staining on tumor Garon EB et al. N Engl J Med. 2015; 372: 2018 -2028.
 KEYNOTE-001 Trial Updated Long-Term Results • 550 treatment-naïve and previously treated patients received pembrolizumab 2 or 10 mg/kg Q 3 W or 10 mg/kg Q 2 W until intolerable toxicity or disease progression • PD-L 1 expression was assessed by IHC using the 22 C 3 antibody • At a median follow up of 23 months, pembrolizumab demonstrated long-term OS benefit for PD-L 1 -positive NSCLC patients: median OS was 22 months for treatment-naïve patients (similar to standr of care chemotherapy outcomes) and 10. 6 months for previously treated patients. • OS increased with increasing PD-L 1 tumor proportion score. Hui R et al, J Clin Oncol 34; suppl: abstr 9026; 2016
KEYNOTE-001 Trial Updated Long-Term Results • 550 treatment-naïve and previously treated patients received pembrolizumab 2 or 10 mg/kg Q 3 W or 10 mg/kg Q 2 W until intolerable toxicity or disease progression • PD-L 1 expression was assessed by IHC using the 22 C 3 antibody • At a median follow up of 23 months, pembrolizumab demonstrated long-term OS benefit for PD-L 1 -positive NSCLC patients: median OS was 22 months for treatment-naïve patients (similar to standr of care chemotherapy outcomes) and 10. 6 months for previously treated patients. • OS increased with increasing PD-L 1 tumor proportion score. Hui R et al, J Clin Oncol 34; suppl: abstr 9026; 2016
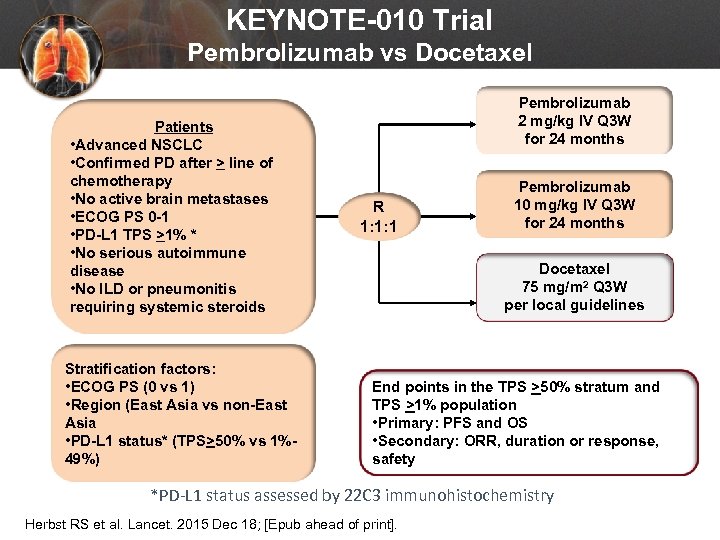 KEYNOTE-010 Trial Pembrolizumab vs Docetaxel Patients • Advanced NSCLC • Confirmed PD after > line of chemotherapy • No active brain metastases • ECOG PS 0 -1 • PD-L 1 TPS >1% * • No serious autoimmune disease • No ILD or pneumonitis requiring systemic steroids Stratification factors: • ECOG PS (0 vs 1) • Region (East Asia vs non-East Asia • PD-L 1 status* (TPS>50% vs 1%49%) Pembrolizumab 2 mg/kg IV Q 3 W for 24 months R 1: 1: 1 Pembrolizumab 10 mg/kg IV Q 3 W for 24 months Docetaxel 75 mg/m 2 Q 3 W per local guidelines End points in the TPS >50% stratum and TPS >1% population • Primary: PFS and OS • Secondary: ORR, duration or response, safety *PD-L 1 status assessed by 22 C 3 immunohistochemistry Herbst RS et al. Lancet. 2015 Dec 18; [Epub ahead of print].
KEYNOTE-010 Trial Pembrolizumab vs Docetaxel Patients • Advanced NSCLC • Confirmed PD after > line of chemotherapy • No active brain metastases • ECOG PS 0 -1 • PD-L 1 TPS >1% * • No serious autoimmune disease • No ILD or pneumonitis requiring systemic steroids Stratification factors: • ECOG PS (0 vs 1) • Region (East Asia vs non-East Asia • PD-L 1 status* (TPS>50% vs 1%49%) Pembrolizumab 2 mg/kg IV Q 3 W for 24 months R 1: 1: 1 Pembrolizumab 10 mg/kg IV Q 3 W for 24 months Docetaxel 75 mg/m 2 Q 3 W per local guidelines End points in the TPS >50% stratum and TPS >1% population • Primary: PFS and OS • Secondary: ORR, duration or response, safety *PD-L 1 status assessed by 22 C 3 immunohistochemistry Herbst RS et al. Lancet. 2015 Dec 18; [Epub ahead of print].
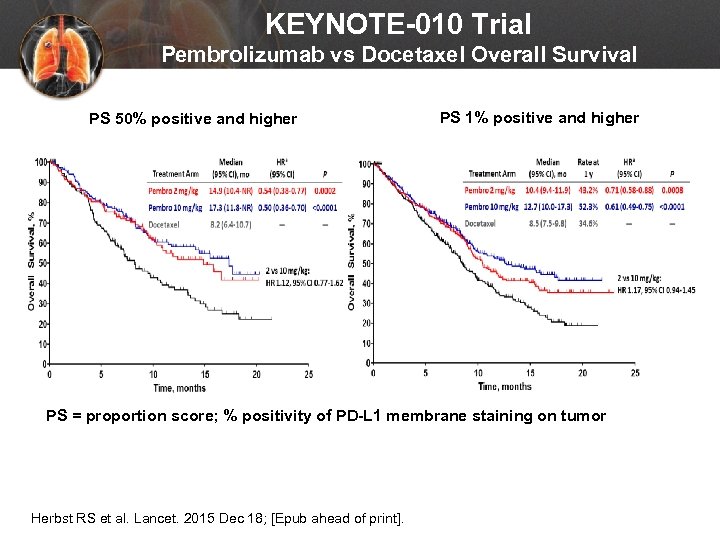 KEYNOTE-010 Trial Pembrolizumab vs Docetaxel Overall Survival PS 1% positive and higher PS 50% positive and higher PS 1% positive and higher PS = proportion score; % positivity of PD-L 1 membrane staining on tumor Herbst RS et al. Lancet. 2015 Dec 18; [Epub ahead of print].
KEYNOTE-010 Trial Pembrolizumab vs Docetaxel Overall Survival PS 1% positive and higher PS 50% positive and higher PS 1% positive and higher PS = proportion score; % positivity of PD-L 1 membrane staining on tumor Herbst RS et al. Lancet. 2015 Dec 18; [Epub ahead of print].
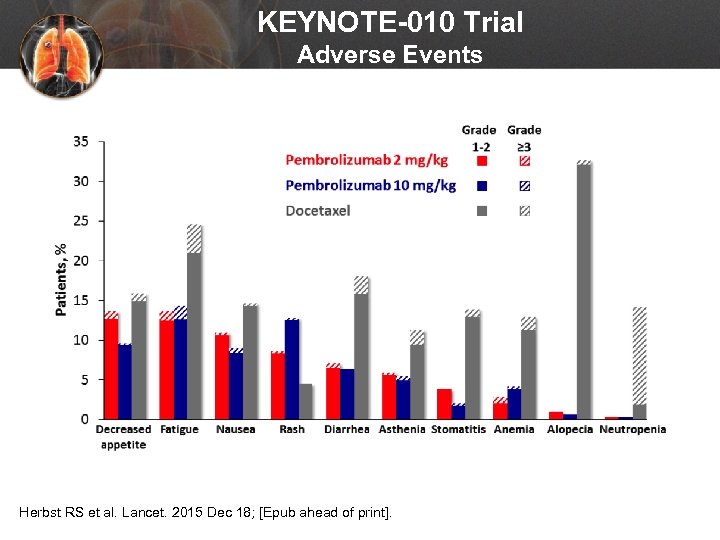 KEYNOTE-010 Trial Adverse Events Herbst RS et al. Lancet. 2015 Dec 18; [Epub ahead of print].
KEYNOTE-010 Trial Adverse Events Herbst RS et al. Lancet. 2015 Dec 18; [Epub ahead of print].
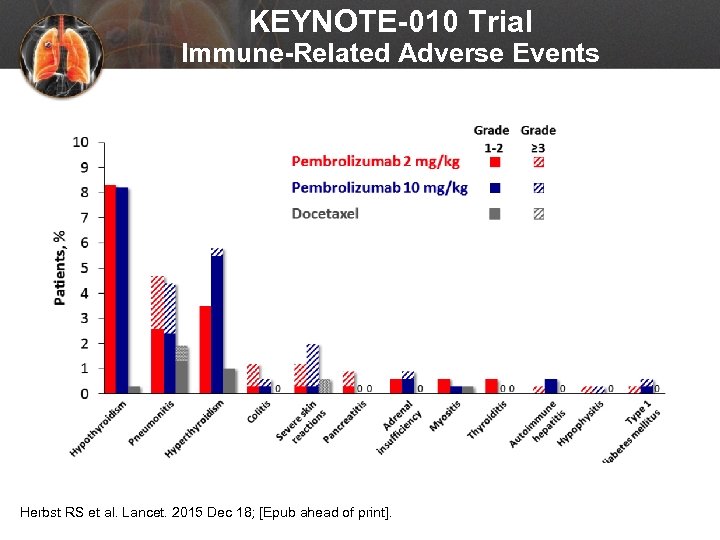 KEYNOTE-010 Trial Immune-Related Adverse Events Herbst RS et al. Lancet. 2015 Dec 18; [Epub ahead of print].
KEYNOTE-010 Trial Immune-Related Adverse Events Herbst RS et al. Lancet. 2015 Dec 18; [Epub ahead of print].
 Immune Adverse Events • Onset: – Average is 6 -12 weeks after initiation of therapy – Can occur within days of the first dose, after several • Patient complaints are autoimmune and drug-related until proven otherwise – Rule out infections, metabolic causes, tumor effects, etc. • Early recognition, evaluation, and treatment are critical
Immune Adverse Events • Onset: – Average is 6 -12 weeks after initiation of therapy – Can occur within days of the first dose, after several • Patient complaints are autoimmune and drug-related until proven otherwise – Rule out infections, metabolic causes, tumor effects, etc. • Early recognition, evaluation, and treatment are critical
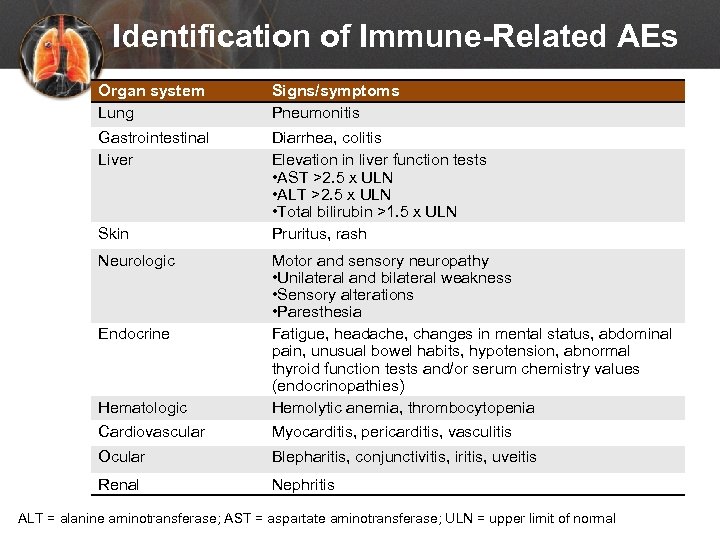 Identification of Immune-Related AEs Organ system Lung Signs/symptoms Pneumonitis Gastrointestinal Liver Diarrhea, colitis Elevation in liver function tests • AST >2. 5 x ULN • ALT >2. 5 x ULN • Total bilirubin >1. 5 x ULN Pruritus, rash Skin Neurologic Hematologic Cardiovascular Motor and sensory neuropathy • Unilateral and bilateral weakness • Sensory alterations • Paresthesia Fatigue, headache, changes in mental status, abdominal pain, unusual bowel habits, hypotension, abnormal thyroid function tests and/or serum chemistry values (endocrinopathies) Hemolytic anemia, thrombocytopenia Myocarditis, pericarditis, vasculitis Ocular Blepharitis, conjunctivitis, iritis, uveitis Renal Nephritis Endocrine ALT = alanine aminotransferase; AST = aspartate aminotransferase; ULN = upper limit of normal
Identification of Immune-Related AEs Organ system Lung Signs/symptoms Pneumonitis Gastrointestinal Liver Diarrhea, colitis Elevation in liver function tests • AST >2. 5 x ULN • ALT >2. 5 x ULN • Total bilirubin >1. 5 x ULN Pruritus, rash Skin Neurologic Hematologic Cardiovascular Motor and sensory neuropathy • Unilateral and bilateral weakness • Sensory alterations • Paresthesia Fatigue, headache, changes in mental status, abdominal pain, unusual bowel habits, hypotension, abnormal thyroid function tests and/or serum chemistry values (endocrinopathies) Hemolytic anemia, thrombocytopenia Myocarditis, pericarditis, vasculitis Ocular Blepharitis, conjunctivitis, iritis, uveitis Renal Nephritis Endocrine ALT = alanine aminotransferase; AST = aspartate aminotransferase; ULN = upper limit of normal
 General Principles of Immune-related Toxicity Management • Generally based on severity of symptoms • Grade 1: – Supportive care; +/- withhold drug • Grade 2: – Withhold drug, consider re-dosing if toxicity resolves to ≤ grade 1; low-dose corticosteroids (prednisone 0. 5 mg/kg/day or equivalent) if symptoms do not resolve within a week • Grade 3 -4: – Discontinue drug; high-dose corticosteroids (prednisone 1 -2 mg/kg/day or equivalent) tapered over ≥ 1 month once toxicity resolves to ≤ grade 1
General Principles of Immune-related Toxicity Management • Generally based on severity of symptoms • Grade 1: – Supportive care; +/- withhold drug • Grade 2: – Withhold drug, consider re-dosing if toxicity resolves to ≤ grade 1; low-dose corticosteroids (prednisone 0. 5 mg/kg/day or equivalent) if symptoms do not resolve within a week • Grade 3 -4: – Discontinue drug; high-dose corticosteroids (prednisone 1 -2 mg/kg/day or equivalent) tapered over ≥ 1 month once toxicity resolves to ≤ grade 1
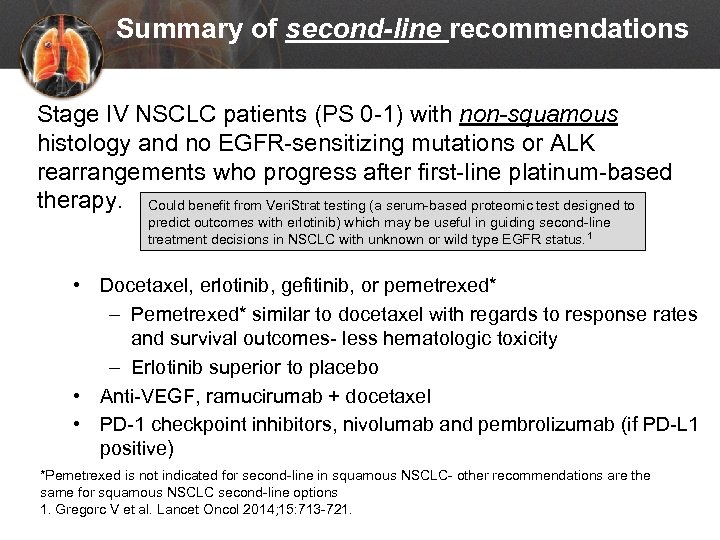 Summary of second-line recommendations Stage IV NSCLC patients (PS 0 -1) with non-squamous histology and no EGFR-sensitizing mutations or ALK rearrangements who progress after first-line platinum-based therapy. Could benefit from Veri. Strat testing (a serum-based proteomic test designed to predict outcomes with erlotinib) which may be useful in guiding second-line treatment decisions in NSCLC with unknown or wild type EGFR status. 1 • Docetaxel, erlotinib, gefitinib, or pemetrexed* – Pemetrexed* similar to docetaxel with regards to response rates and survival outcomes- less hematologic toxicity – Erlotinib superior to placebo • Anti-VEGF, ramucirumab + docetaxel • PD-1 checkpoint inhibitors, nivolumab and pembrolizumab (if PD-L 1 positive) *Pemetrexed is not indicated for second-line in squamous NSCLC- other recommendations are the same for squamous NSCLC second-line options 1. Gregorc V et al. Lancet Oncol 2014; 15: 713 -721.
Summary of second-line recommendations Stage IV NSCLC patients (PS 0 -1) with non-squamous histology and no EGFR-sensitizing mutations or ALK rearrangements who progress after first-line platinum-based therapy. Could benefit from Veri. Strat testing (a serum-based proteomic test designed to predict outcomes with erlotinib) which may be useful in guiding second-line treatment decisions in NSCLC with unknown or wild type EGFR status. 1 • Docetaxel, erlotinib, gefitinib, or pemetrexed* – Pemetrexed* similar to docetaxel with regards to response rates and survival outcomes- less hematologic toxicity – Erlotinib superior to placebo • Anti-VEGF, ramucirumab + docetaxel • PD-1 checkpoint inhibitors, nivolumab and pembrolizumab (if PD-L 1 positive) *Pemetrexed is not indicated for second-line in squamous NSCLC- other recommendations are the same for squamous NSCLC second-line options 1. Gregorc V et al. Lancet Oncol 2014; 15: 713 -721.
 What if… Jack presented with squamous cell histology – what first line treatments would you consider?
What if… Jack presented with squamous cell histology – what first line treatments would you consider?
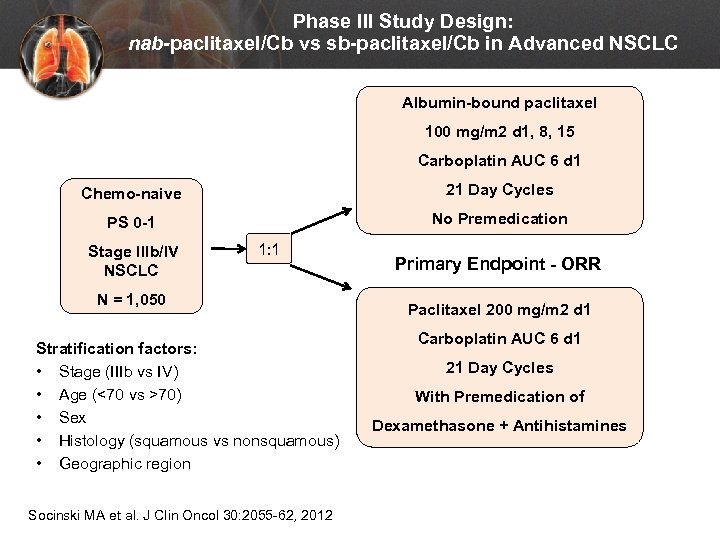 Phase III Study Design: nab-paclitaxel/Cb vs sb-paclitaxel/Cb in Advanced NSCLC Albumin-bound paclitaxel 100 mg/m 2 d 1, 8, 15 Carboplatin AUC 6 d 1 Chemo-naive 21 Day Cycles PS 0 -1 No Premedication Stage IIIb/IV NSCLC 1: 1 N = 1, 050 Stratification factors: • Stage (IIIb vs IV) • Age (<70 vs >70) • Sex • Histology (squamous vs nonsquamous) • Geographic region Socinski MA et al. J Clin Oncol 30: 2055 -62, 2012 Primary Endpoint - ORR Paclitaxel 200 mg/m 2 d 1 Carboplatin AUC 6 d 1 21 Day Cycles With Premedication of Dexamethasone + Antihistamines
Phase III Study Design: nab-paclitaxel/Cb vs sb-paclitaxel/Cb in Advanced NSCLC Albumin-bound paclitaxel 100 mg/m 2 d 1, 8, 15 Carboplatin AUC 6 d 1 Chemo-naive 21 Day Cycles PS 0 -1 No Premedication Stage IIIb/IV NSCLC 1: 1 N = 1, 050 Stratification factors: • Stage (IIIb vs IV) • Age (<70 vs >70) • Sex • Histology (squamous vs nonsquamous) • Geographic region Socinski MA et al. J Clin Oncol 30: 2055 -62, 2012 Primary Endpoint - ORR Paclitaxel 200 mg/m 2 d 1 Carboplatin AUC 6 d 1 21 Day Cycles With Premedication of Dexamethasone + Antihistamines
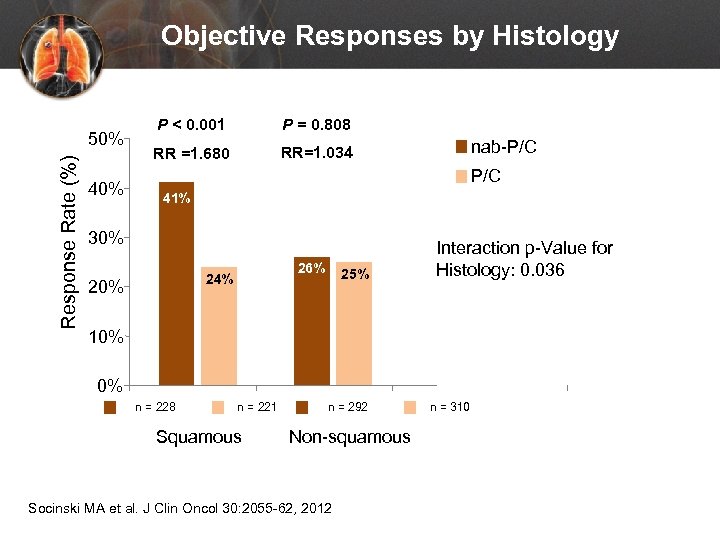 Objective Responses by Histology Response Rate (%) 50% 40% P < 0. 001 P = 0. 808 RR =1. 680 RR=1. 034 nab-P/C 41% 30% 26% 24% 20% 25% Interaction p-Value for Histology: 0. 036 10% 0% n = 228 n = 221 Squamous n = 292 Non-squamous Socinski MA et al. J Clin Oncol 30: 2055 -62, 2012 n = 310
Objective Responses by Histology Response Rate (%) 50% 40% P < 0. 001 P = 0. 808 RR =1. 680 RR=1. 034 nab-P/C 41% 30% 26% 24% 20% 25% Interaction p-Value for Histology: 0. 036 10% 0% n = 228 n = 221 Squamous n = 292 Non-squamous Socinski MA et al. J Clin Oncol 30: 2055 -62, 2012 n = 310
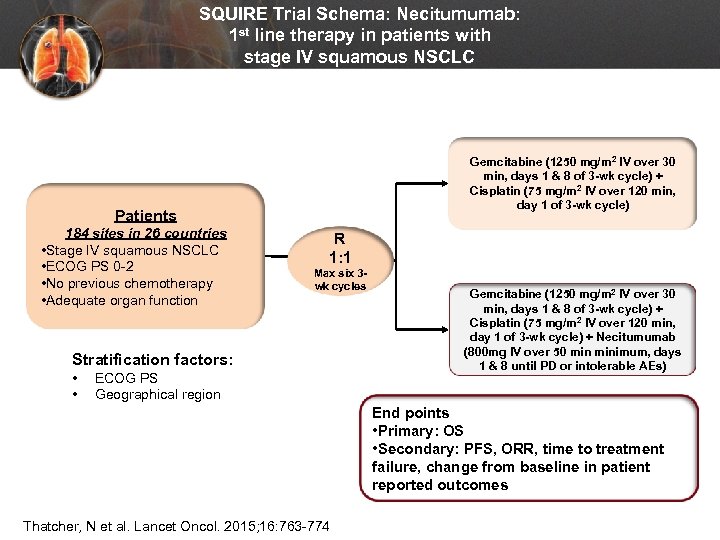 SQUIRE Trial Schema: Necitumumab: 1 st line therapy in patients with stage IV squamous NSCLC Gemcitabine (1250 mg/m 2 IV over 30 min, days 1 & 8 of 3 -wk cycle) + Cisplatin (75 mg/m 2 IV over 120 min, day 1 of 3 -wk cycle) Patients 184 sites in 26 countries • Stage IV squamous NSCLC • ECOG PS 0 -2 • No previous chemotherapy • Adequate organ function R 1: 1 Max six 3 wk cycles Stratification factors: • • ECOG PS Geographical region Gemcitabine (1250 mg/m 2 IV over 30 min, days 1 & 8 of 3 -wk cycle) + Cisplatin (75 mg/m 2 IV over 120 min, day 1 of 3 -wk cycle) + Necitumumab (800 mg IV over 50 minimum, days 1 & 8 until PD or intolerable AEs) End points • Primary: OS • Secondary: PFS, ORR, time to treatment failure, change from baseline in patient reported outcomes Thatcher, N et al. Lancet Oncol. 2015; 16: 763 -774
SQUIRE Trial Schema: Necitumumab: 1 st line therapy in patients with stage IV squamous NSCLC Gemcitabine (1250 mg/m 2 IV over 30 min, days 1 & 8 of 3 -wk cycle) + Cisplatin (75 mg/m 2 IV over 120 min, day 1 of 3 -wk cycle) Patients 184 sites in 26 countries • Stage IV squamous NSCLC • ECOG PS 0 -2 • No previous chemotherapy • Adequate organ function R 1: 1 Max six 3 wk cycles Stratification factors: • • ECOG PS Geographical region Gemcitabine (1250 mg/m 2 IV over 30 min, days 1 & 8 of 3 -wk cycle) + Cisplatin (75 mg/m 2 IV over 120 min, day 1 of 3 -wk cycle) + Necitumumab (800 mg IV over 50 minimum, days 1 & 8 until PD or intolerable AEs) End points • Primary: OS • Secondary: PFS, ORR, time to treatment failure, change from baseline in patient reported outcomes Thatcher, N et al. Lancet Oncol. 2015; 16: 763 -774
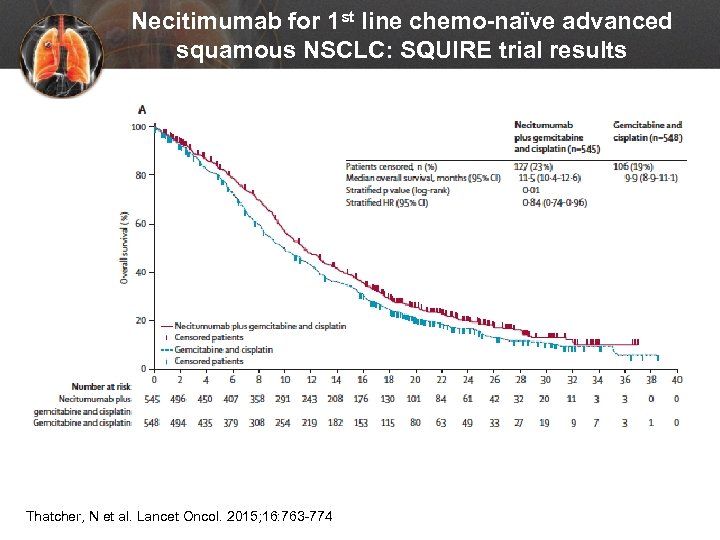 Necitimumab for 1 st line chemo-naïve advanced squamous NSCLC: SQUIRE trial results Thatcher, N et al. Lancet Oncol. 2015; 16: 763 -774
Necitimumab for 1 st line chemo-naïve advanced squamous NSCLC: SQUIRE trial results Thatcher, N et al. Lancet Oncol. 2015; 16: 763 -774
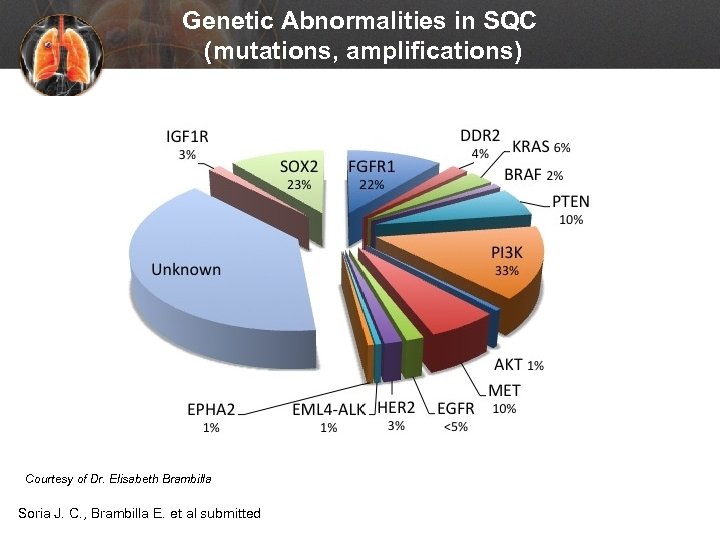 Genetic Abnormalities in SQC (mutations, amplifications) Courtesy of Dr. Elisabeth Brambilla Soria J. C. , Brambilla E. et al submitted
Genetic Abnormalities in SQC (mutations, amplifications) Courtesy of Dr. Elisabeth Brambilla Soria J. C. , Brambilla E. et al submitted
 What if… What if Jack progressed after platinum with squamous NSCLC – what are the second-line treatment options? Pemetrexed is not approved for this subset
What if… What if Jack progressed after platinum with squamous NSCLC – what are the second-line treatment options? Pemetrexed is not approved for this subset
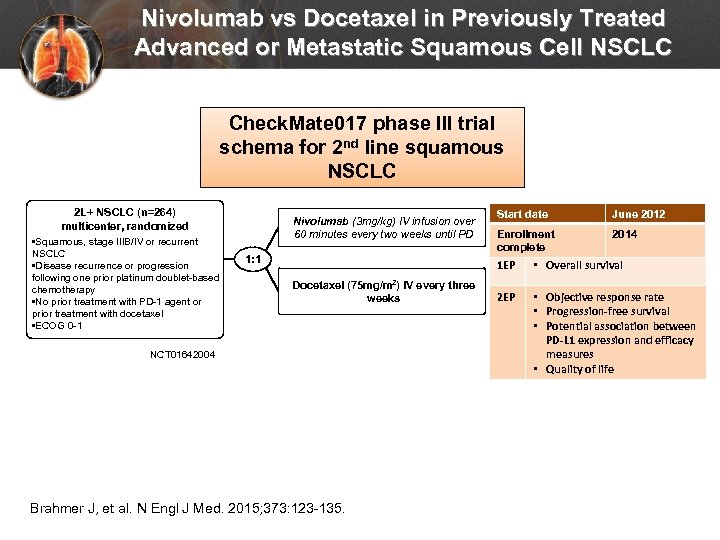 Nivolumab vs Docetaxel in Previously Treated Advanced or Metastatic Squamous Cell NSCLC Check. Mate 017 phase III trial schema for 2 nd line squamous NSCLC 2 L+ NSCLC (n=264) multicenter, randomized • Squamous, stage IIIB/IV or recurrent NSCLC • Disease recurrence or progression following one prior platinum doublet-based chemotherapy • No prior treatment with PD-1 agent or prior treatment with docetaxel • ECOG 0 -1 Nivolumab (3 mg/kg) IV infusion over 60 minutes every two weeks until PD 1: 1 Start date June 2012 Enrollment complete 2014 1 EP Docetaxel (75 mg/m 2) IV every three weeks NCT 01642004 Brahmer J, et al. N Engl J Med. 2015; 373: 123 -135. • Overall survival 2 EP • Objective response rate • Progression-free survival • Potential association between PD-L 1 expression and efficacy measures • Quality of life
Nivolumab vs Docetaxel in Previously Treated Advanced or Metastatic Squamous Cell NSCLC Check. Mate 017 phase III trial schema for 2 nd line squamous NSCLC 2 L+ NSCLC (n=264) multicenter, randomized • Squamous, stage IIIB/IV or recurrent NSCLC • Disease recurrence or progression following one prior platinum doublet-based chemotherapy • No prior treatment with PD-1 agent or prior treatment with docetaxel • ECOG 0 -1 Nivolumab (3 mg/kg) IV infusion over 60 minutes every two weeks until PD 1: 1 Start date June 2012 Enrollment complete 2014 1 EP Docetaxel (75 mg/m 2) IV every three weeks NCT 01642004 Brahmer J, et al. N Engl J Med. 2015; 373: 123 -135. • Overall survival 2 EP • Objective response rate • Progression-free survival • Potential association between PD-L 1 expression and efficacy measures • Quality of life
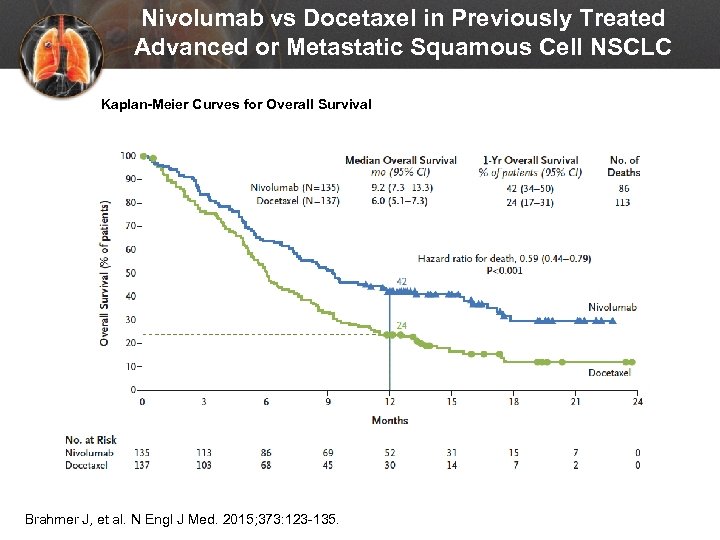 Nivolumab vs Docetaxel in Previously Treated Advanced or Metastatic Squamous Cell NSCLC Kaplan-Meier Curves for Overall Survival Brahmer J, et al. N Engl J Med. 2015; 373: 123 -135.
Nivolumab vs Docetaxel in Previously Treated Advanced or Metastatic Squamous Cell NSCLC Kaplan-Meier Curves for Overall Survival Brahmer J, et al. N Engl J Med. 2015; 373: 123 -135.
 First-line treatment of squamous NSCLC First Line: Platinum doublets remain standard of care – Gemcitabine- or taxane-based regimens including nab-paclitaxel • Consider cisplatin/gemcitabine/necitumumab Bevacizumab contraindicated due to bleeding complications Pemetrexed not appropriate for squamous NSCLC Second Line: - Nivolumab (PD-L 1 +/-/unknown) or pembrolizumab (PD-L 1 +) - Docetaxel +/- ramucirumab, other single agent chemotherapy
First-line treatment of squamous NSCLC First Line: Platinum doublets remain standard of care – Gemcitabine- or taxane-based regimens including nab-paclitaxel • Consider cisplatin/gemcitabine/necitumumab Bevacizumab contraindicated due to bleeding complications Pemetrexed not appropriate for squamous NSCLC Second Line: - Nivolumab (PD-L 1 +/-/unknown) or pembrolizumab (PD-L 1 +) - Docetaxel +/- ramucirumab, other single agent chemotherapy
 What if… What if Jack has stage IV non-squamous NSCLC with an EGFR-sensitizing mutation? What are evidence-based treatment recommendations for Jack?
What if… What if Jack has stage IV non-squamous NSCLC with an EGFR-sensitizing mutation? What are evidence-based treatment recommendations for Jack?
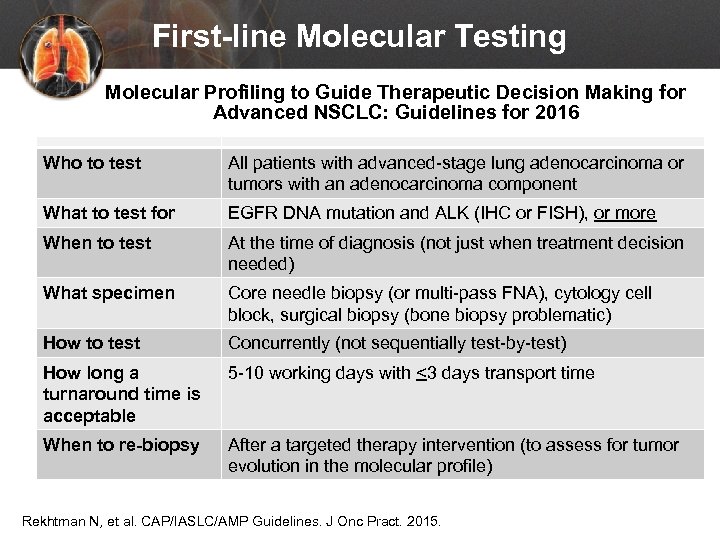 First-line Molecular Testing Molecular Profiling to Guide Therapeutic Decision Making for Advanced NSCLC: Guidelines for 2016 Who to test All patients with advanced-stage lung adenocarcinoma or tumors with an adenocarcinoma component What to test for EGFR DNA mutation and ALK (IHC or FISH), or more When to test At the time of diagnosis (not just when treatment decision needed) What specimen Core needle biopsy (or multi-pass FNA), cytology cell block, surgical biopsy (bone biopsy problematic) How to test Concurrently (not sequentially test-by-test) How long a turnaround time is acceptable 5 -10 working days with <3 days transport time When to re-biopsy After a targeted therapy intervention (to assess for tumor evolution in the molecular profile) Rekhtman N, et al. CAP/IASLC/AMP Guidelines. J Onc Pract. 2015.
First-line Molecular Testing Molecular Profiling to Guide Therapeutic Decision Making for Advanced NSCLC: Guidelines for 2016 Who to test All patients with advanced-stage lung adenocarcinoma or tumors with an adenocarcinoma component What to test for EGFR DNA mutation and ALK (IHC or FISH), or more When to test At the time of diagnosis (not just when treatment decision needed) What specimen Core needle biopsy (or multi-pass FNA), cytology cell block, surgical biopsy (bone biopsy problematic) How to test Concurrently (not sequentially test-by-test) How long a turnaround time is acceptable 5 -10 working days with <3 days transport time When to re-biopsy After a targeted therapy intervention (to assess for tumor evolution in the molecular profile) Rekhtman N, et al. CAP/IASLC/AMP Guidelines. J Onc Pract. 2015.
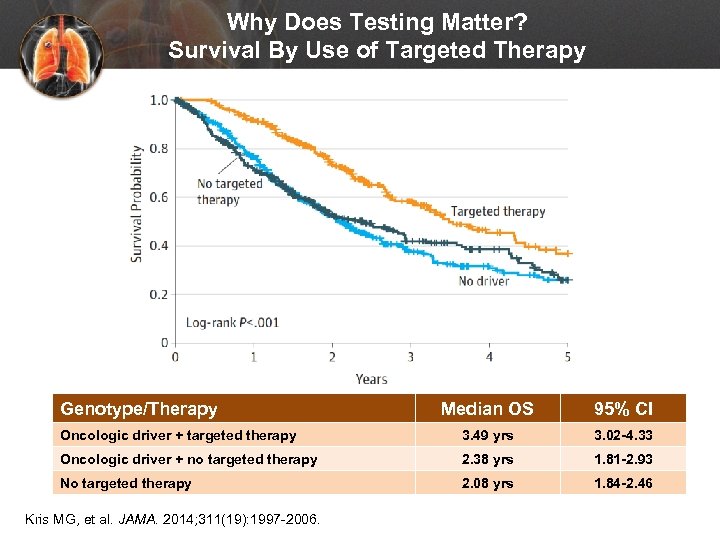 Why Does Testing Matter? Survival By Use of Targeted Therapy Genotype/Therapy Median OS 95% CI Oncologic driver + targeted therapy 3. 49 yrs 3. 02 -4. 33 Oncologic driver + no targeted therapy 2. 38 yrs 1. 81 -2. 93 No targeted therapy 2. 08 yrs 1. 84 -2. 46 Kris MG, et al. JAMA. 2014; 311(19): 1997 -2006.
Why Does Testing Matter? Survival By Use of Targeted Therapy Genotype/Therapy Median OS 95% CI Oncologic driver + targeted therapy 3. 49 yrs 3. 02 -4. 33 Oncologic driver + no targeted therapy 2. 38 yrs 1. 81 -2. 93 No targeted therapy 2. 08 yrs 1. 84 -2. 46 Kris MG, et al. JAMA. 2014; 311(19): 1997 -2006.
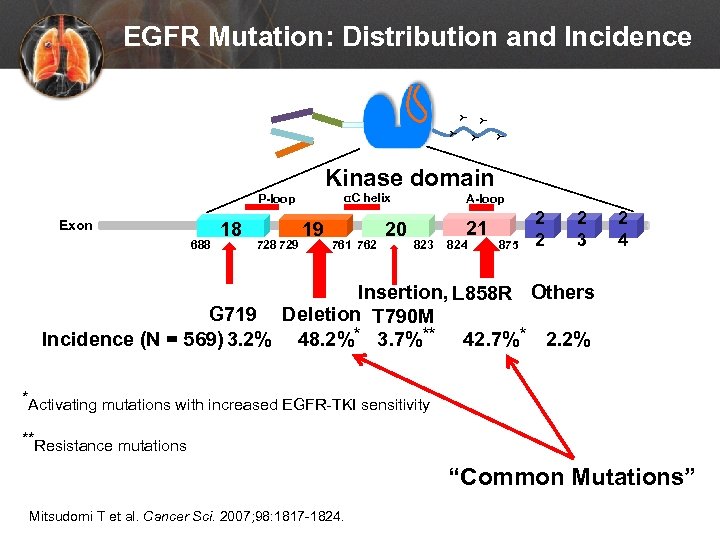 Y Y Y EGFR Mutation: Distribution and Incidence Kinase domain αC helix P-loop Exon 688 18 729 19 761 762 20 A-loop 823 21 824 875 2 2 2 3 2 4 Insertion, L 858 R Others G 719 Deletion T 790 M Incidence (N = 569) 3. 2% 48. 2%* 3. 7%** 42. 7%* 2. 2% *Activating mutations with increased EGFR-TKI sensitivity **Resistance mutations “Common Mutations” Mitsudomi T et al. Cancer Sci. 2007; 98: 1817 -1824.
Y Y Y EGFR Mutation: Distribution and Incidence Kinase domain αC helix P-loop Exon 688 18 729 19 761 762 20 A-loop 823 21 824 875 2 2 2 3 2 4 Insertion, L 858 R Others G 719 Deletion T 790 M Incidence (N = 569) 3. 2% 48. 2%* 3. 7%** 42. 7%* 2. 2% *Activating mutations with increased EGFR-TKI sensitivity **Resistance mutations “Common Mutations” Mitsudomi T et al. Cancer Sci. 2007; 98: 1817 -1824.
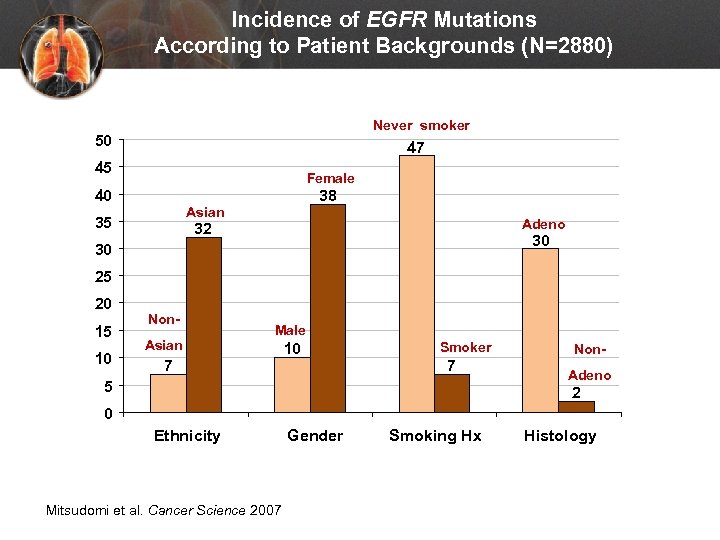 Incidence of EGFR Mutations According to Patient Backgrounds (N=2880) Never smoker 50 47 45 Female 40 38 Asian 35 Adeno 32 30 30 25 20 15 10 Non. Asian Male 7 10 Smoker 7 5 Non. Adeno 2 0 Ethnicity Mitsudomi et al. Cancer Science 2007 Gender Smoking Hx Histology
Incidence of EGFR Mutations According to Patient Backgrounds (N=2880) Never smoker 50 47 45 Female 40 38 Asian 35 Adeno 32 30 30 25 20 15 10 Non. Asian Male 7 10 Smoker 7 5 Non. Adeno 2 0 Ethnicity Mitsudomi et al. Cancer Science 2007 Gender Smoking Hx Histology
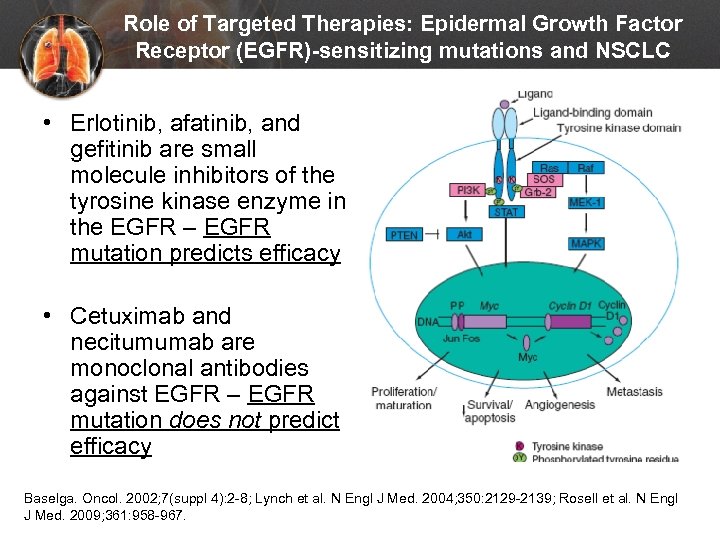 Role of Targeted Therapies: Epidermal Growth Factor Receptor (EGFR)-sensitizing mutations and NSCLC • Erlotinib, afatinib, and gefitinib are small molecule inhibitors of the tyrosine kinase enzyme in the EGFR – EGFR mutation predicts efficacy • Cetuximab and necitumumab are monoclonal antibodies against EGFR – EGFR mutation does not predict efficacy Baselga. Oncol. 2002; 7(suppl 4): 2 -8; Lynch et al. N Engl J Med. 2004; 350: 2129 -2139; Rosell et al. N Engl J Med. 2009; 361: 958 -967.
Role of Targeted Therapies: Epidermal Growth Factor Receptor (EGFR)-sensitizing mutations and NSCLC • Erlotinib, afatinib, and gefitinib are small molecule inhibitors of the tyrosine kinase enzyme in the EGFR – EGFR mutation predicts efficacy • Cetuximab and necitumumab are monoclonal antibodies against EGFR – EGFR mutation does not predict efficacy Baselga. Oncol. 2002; 7(suppl 4): 2 -8; Lynch et al. N Engl J Med. 2004; 350: 2129 -2139; Rosell et al. N Engl J Med. 2009; 361: 958 -967.
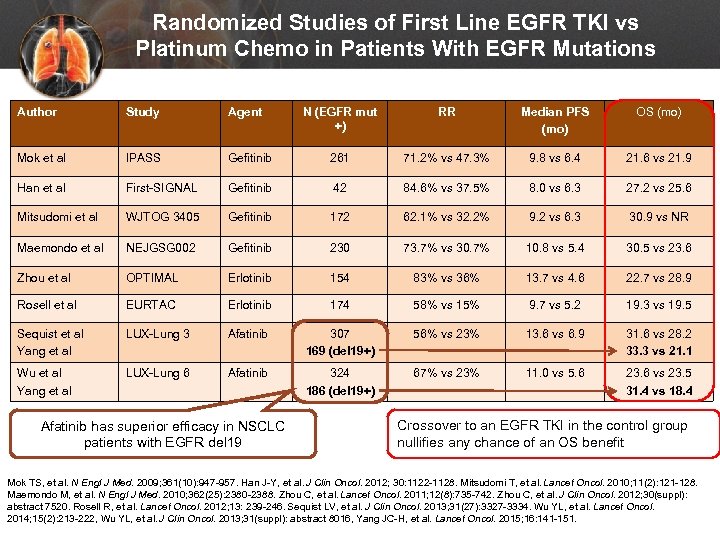 Randomized Studies of First Line EGFR TKI vs Platinum Chemo in Patients With EGFR Mutations Author Study Agent N (EGFR mut +) RR Median PFS (mo) OS (mo) Mok et al IPASS Gefitinib 261 71. 2% vs 47. 3% 9. 8 vs 6. 4 21. 6 vs 21. 9 Han et al First-SIGNAL Gefitinib 42 84. 6% vs 37. 5% 8. 0 vs 6. 3 27. 2 vs 25. 6 Mitsudomi et al WJTOG 3405 Gefitinib 172 62. 1% vs 32. 2% 9. 2 vs 6. 3 30. 9 vs NR Maemondo et al NEJGSG 002 Gefitinib 230 73. 7% vs 30. 7% 10. 8 vs 5. 4 30. 5 vs 23. 6 Zhou et al OPTIMAL Erlotinib 154 83% vs 36% 13. 7 vs 4. 6 22. 7 vs 28. 9 Rosell et al EURTAC Erlotinib 174 58% vs 15% 9. 7 vs 5. 2 19. 3 vs 19. 5 Sequist et al Yang et al LUX-Lung 3 Afatinib 307 169 (del 19+) 56% vs 23% 13. 6 vs 6. 9 31. 6 vs 28. 2 33. 3 vs 21. 1 Wu et al Yang et al LUX-Lung 6 Afatinib 324 186 (del 19+) 67% vs 23% 11. 0 vs 5. 6 23. 6 vs 23. 5 31. 4 vs 18. 4 Afatinib has superior efficacy in NSCLC patients with EGFR del 19 Crossover to an EGFR TKI in the control group nullifies any chance of an OS benefit Mok TS, et al. N Engl J Med. 2009; 361(10): 947 -957. Han J-Y, et al. J Clin Oncol. 2012; 30: 1122 -1128. Mitsudomi T, et al. Lancet Oncol. 2010; 11(2): 121 -128. Maemondo M, et al. N Engl J Med. 2010; 362(25): 2380 -2388. Zhou C, et al. Lancet Oncol. 2011; 12(8): 735 -742. Zhou C, et al. J Clin Oncol. 2012; 30(suppl): abstract 7520. Rosell R, et al. Lancet Oncol. 2012; 13: 239 -246. Sequist LV, et al. J Clin Oncol. 2013; 31(27): 3327 -3334. Wu YL, et al. Lancet Oncol. 2014; 15(2): 213 -222, Wu YL, et al. J Clin Oncol. 2013; 31(suppl): abstract 8016, Yang JC-H, et al. Lancet Oncol. 2015; 16: 141 -151.
Randomized Studies of First Line EGFR TKI vs Platinum Chemo in Patients With EGFR Mutations Author Study Agent N (EGFR mut +) RR Median PFS (mo) OS (mo) Mok et al IPASS Gefitinib 261 71. 2% vs 47. 3% 9. 8 vs 6. 4 21. 6 vs 21. 9 Han et al First-SIGNAL Gefitinib 42 84. 6% vs 37. 5% 8. 0 vs 6. 3 27. 2 vs 25. 6 Mitsudomi et al WJTOG 3405 Gefitinib 172 62. 1% vs 32. 2% 9. 2 vs 6. 3 30. 9 vs NR Maemondo et al NEJGSG 002 Gefitinib 230 73. 7% vs 30. 7% 10. 8 vs 5. 4 30. 5 vs 23. 6 Zhou et al OPTIMAL Erlotinib 154 83% vs 36% 13. 7 vs 4. 6 22. 7 vs 28. 9 Rosell et al EURTAC Erlotinib 174 58% vs 15% 9. 7 vs 5. 2 19. 3 vs 19. 5 Sequist et al Yang et al LUX-Lung 3 Afatinib 307 169 (del 19+) 56% vs 23% 13. 6 vs 6. 9 31. 6 vs 28. 2 33. 3 vs 21. 1 Wu et al Yang et al LUX-Lung 6 Afatinib 324 186 (del 19+) 67% vs 23% 11. 0 vs 5. 6 23. 6 vs 23. 5 31. 4 vs 18. 4 Afatinib has superior efficacy in NSCLC patients with EGFR del 19 Crossover to an EGFR TKI in the control group nullifies any chance of an OS benefit Mok TS, et al. N Engl J Med. 2009; 361(10): 947 -957. Han J-Y, et al. J Clin Oncol. 2012; 30: 1122 -1128. Mitsudomi T, et al. Lancet Oncol. 2010; 11(2): 121 -128. Maemondo M, et al. N Engl J Med. 2010; 362(25): 2380 -2388. Zhou C, et al. Lancet Oncol. 2011; 12(8): 735 -742. Zhou C, et al. J Clin Oncol. 2012; 30(suppl): abstract 7520. Rosell R, et al. Lancet Oncol. 2012; 13: 239 -246. Sequist LV, et al. J Clin Oncol. 2013; 31(27): 3327 -3334. Wu YL, et al. Lancet Oncol. 2014; 15(2): 213 -222, Wu YL, et al. J Clin Oncol. 2013; 31(suppl): abstract 8016, Yang JC-H, et al. Lancet Oncol. 2015; 16: 141 -151.
 What’s the optimal first-line treatment selection if Jack presented with non-squamous cell histology and with an EGFR-sensitizing mutation? First-line • Erlotinib, afatinib, and gefitinib are FDA approved • Afatinib appears more effective, but has more diarrhea, stomatits, and paronychia Second-line (after progression on first-line TKI): • Combination chemotherapy • Switch to another EGFR TKI Masters 2015; NCCN guidelines
What’s the optimal first-line treatment selection if Jack presented with non-squamous cell histology and with an EGFR-sensitizing mutation? First-line • Erlotinib, afatinib, and gefitinib are FDA approved • Afatinib appears more effective, but has more diarrhea, stomatits, and paronychia Second-line (after progression on first-line TKI): • Combination chemotherapy • Switch to another EGFR TKI Masters 2015; NCCN guidelines
 What if… Jack’s EGFR mutant tumor grows on afatinib - what are the treatment options?
What if… Jack’s EGFR mutant tumor grows on afatinib - what are the treatment options?
 Jack acquires resistance to targeted TKI. What are the treatment options? • Switch to another 1 st-generation TKI – usually futile • Local Rx given to limited site of progression while continuing TKI - reasonable • Chemotherapy (either single agent or combination) alone – reasonable • Anti-PD-1 – reasonable, but “average” chance of response • EGFR targeted therapy: – T 790 M small molecule inhibitor osimertinib, others in development – Afatinib + cetuximab – can be challenging to get insurance approval
Jack acquires resistance to targeted TKI. What are the treatment options? • Switch to another 1 st-generation TKI – usually futile • Local Rx given to limited site of progression while continuing TKI - reasonable • Chemotherapy (either single agent or combination) alone – reasonable • Anti-PD-1 – reasonable, but “average” chance of response • EGFR targeted therapy: – T 790 M small molecule inhibitor osimertinib, others in development – Afatinib + cetuximab – can be challenging to get insurance approval
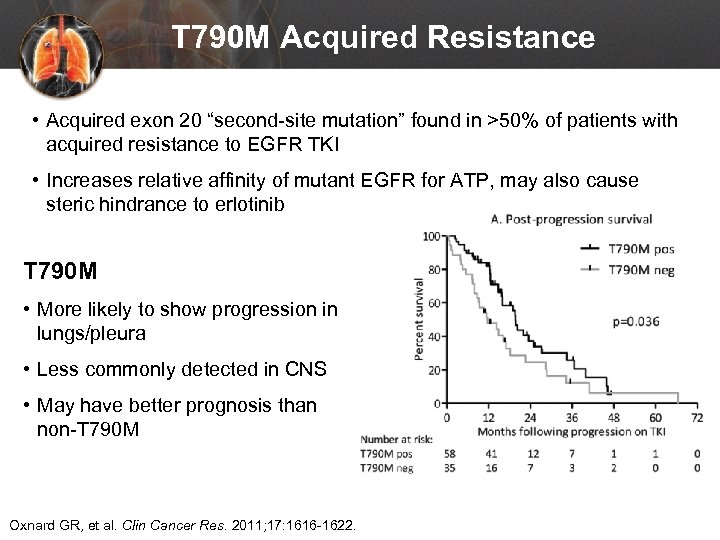 T 790 M Acquired Resistance • Acquired exon 20 “second-site mutation” found in >50% of patients with acquired resistance to EGFR TKI • Increases relative affinity of mutant EGFR for ATP, may also cause steric hindrance to erlotinib T 790 M • More likely to show progression in lungs/pleura • Less commonly detected in CNS • May have better prognosis than non-T 790 M Oxnard GR, et al. Clin Cancer Res. 2011; 17: 1616 -1622.
T 790 M Acquired Resistance • Acquired exon 20 “second-site mutation” found in >50% of patients with acquired resistance to EGFR TKI • Increases relative affinity of mutant EGFR for ATP, may also cause steric hindrance to erlotinib T 790 M • More likely to show progression in lungs/pleura • Less commonly detected in CNS • May have better prognosis than non-T 790 M Oxnard GR, et al. Clin Cancer Res. 2011; 17: 1616 -1622.
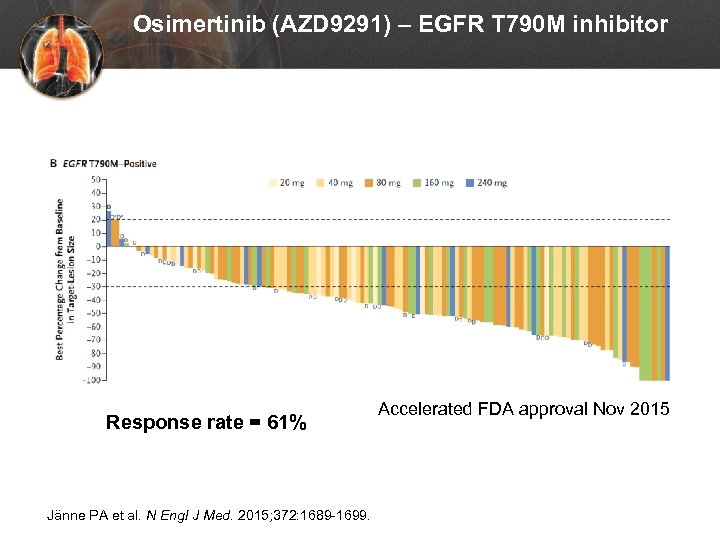 Osimertinib (AZD 9291) – EGFR T 790 M inhibitor Response rate = 61% Jänne PA et al. N Engl J Med. 2015; 372: 1689 -1699. Accelerated FDA approval Nov 2015
Osimertinib (AZD 9291) – EGFR T 790 M inhibitor Response rate = 61% Jänne PA et al. N Engl J Med. 2015; 372: 1689 -1699. Accelerated FDA approval Nov 2015
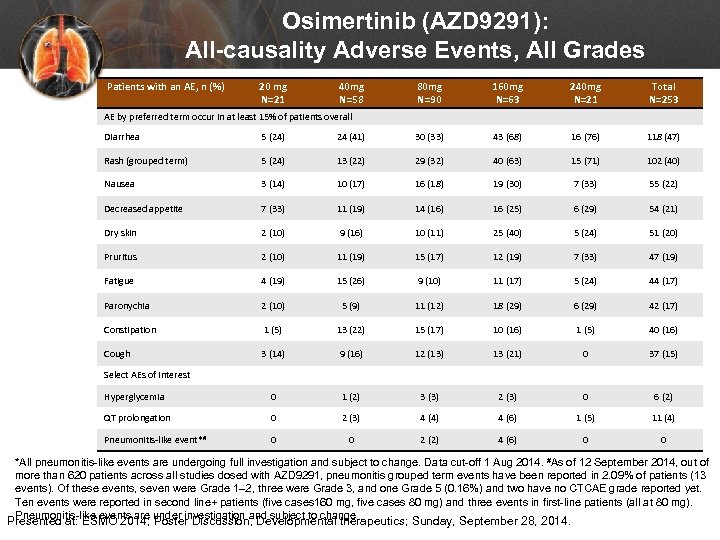 Osimertinib (AZD 9291): All-causality Adverse Events, All Grades Patients with an AE, n (%) 20 mg N=21 40 mg N=58 80 mg N=90 160 mg N=63 240 mg N=21 Total N=253 AE by preferred term occur in at least 15% of patients overall Diarrhea 5 (24) 24 (41) 30 (33) 43 (68) 16 (76) 118 (47) Rash (grouped term) 5 (24) 13 (22) 29 (32) 40 (63) 15 (71) 102 (40) Nausea 3 (14) 10 (17) 16 (18) 19 (30) 7 (33) 55 (22) Decreased appetite 7 (33) 11 (19) 14 (16) 16 (25) 6 (29) 54 (21) Dry skin 2 (10) 9 (16) 10 (11) 25 (40) 5 (24) 51 (20) Pruritus 2 (10) 11 (19) 15 (17) 12 (19) 7 (33) 47 (19) Fatigue 4 (19) 15 (26) 9 (10) 11 (17) 5 (24) 44 (17) Paronychia 2 (10) 5 (9) 11 (12) 18 (29) 6 (29) 42 (17) Constipation 1 (5) 13 (22) 15 (17) 10 (16) 1 (5) 40 (16) Cough 3 (14) 9 (16) 12 (13) 13 (21) 0 37 (15) Hyperglycemia 0 1 (2) 3 (3) 2 (3) 0 6 (2) QT prolongation 0 2 (3) 4 (4) 4 (6) 1 (5) 11 (4) Pneumonitis-like event*# 0 0 2 (2) 4 (6) 0 0 Select AEs of interest *All pneumonitis-like events are undergoing full investigation and subject to change. Data cut-off 1 Aug 2014. #As of 12 September 2014, out of more than 620 patients across all studies dosed with AZD 9291, pneumonitis grouped term events have been reported in 2. 09% of patients (13 events). Of these events, seven were Grade 1– 2, three were Grade 3, and one Grade 5 (0. 16%) and two have no CTCAE grade reported yet. Ten events were reported in second line+ patients (five cases 160 mg, five cases 80 mg) and three events in first-line patients (all at 80 mg). Pneumonitis-like events are under investigation and subject to change Presented at: ESMO 2014; Poster Discussion, Developmental therapeutics; Sunday, September 28, 2014.
Osimertinib (AZD 9291): All-causality Adverse Events, All Grades Patients with an AE, n (%) 20 mg N=21 40 mg N=58 80 mg N=90 160 mg N=63 240 mg N=21 Total N=253 AE by preferred term occur in at least 15% of patients overall Diarrhea 5 (24) 24 (41) 30 (33) 43 (68) 16 (76) 118 (47) Rash (grouped term) 5 (24) 13 (22) 29 (32) 40 (63) 15 (71) 102 (40) Nausea 3 (14) 10 (17) 16 (18) 19 (30) 7 (33) 55 (22) Decreased appetite 7 (33) 11 (19) 14 (16) 16 (25) 6 (29) 54 (21) Dry skin 2 (10) 9 (16) 10 (11) 25 (40) 5 (24) 51 (20) Pruritus 2 (10) 11 (19) 15 (17) 12 (19) 7 (33) 47 (19) Fatigue 4 (19) 15 (26) 9 (10) 11 (17) 5 (24) 44 (17) Paronychia 2 (10) 5 (9) 11 (12) 18 (29) 6 (29) 42 (17) Constipation 1 (5) 13 (22) 15 (17) 10 (16) 1 (5) 40 (16) Cough 3 (14) 9 (16) 12 (13) 13 (21) 0 37 (15) Hyperglycemia 0 1 (2) 3 (3) 2 (3) 0 6 (2) QT prolongation 0 2 (3) 4 (4) 4 (6) 1 (5) 11 (4) Pneumonitis-like event*# 0 0 2 (2) 4 (6) 0 0 Select AEs of interest *All pneumonitis-like events are undergoing full investigation and subject to change. Data cut-off 1 Aug 2014. #As of 12 September 2014, out of more than 620 patients across all studies dosed with AZD 9291, pneumonitis grouped term events have been reported in 2. 09% of patients (13 events). Of these events, seven were Grade 1– 2, three were Grade 3, and one Grade 5 (0. 16%) and two have no CTCAE grade reported yet. Ten events were reported in second line+ patients (five cases 160 mg, five cases 80 mg) and three events in first-line patients (all at 80 mg). Pneumonitis-like events are under investigation and subject to change Presented at: ESMO 2014; Poster Discussion, Developmental therapeutics; Sunday, September 28, 2014.
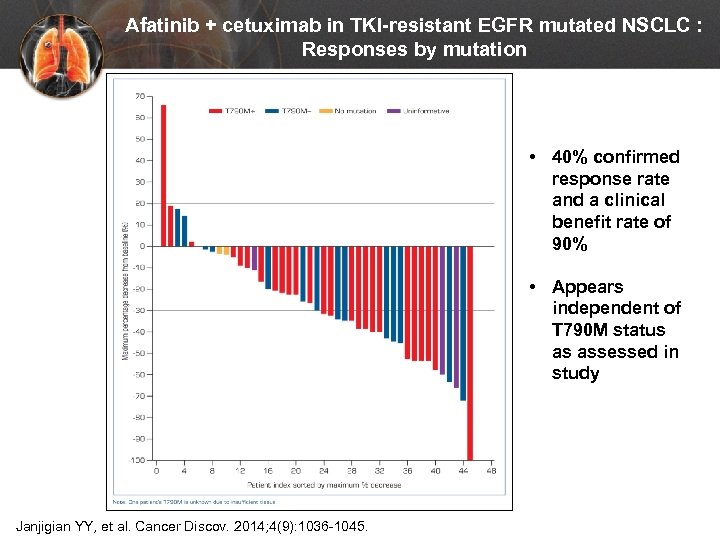 Afatinib + cetuximab in TKI-resistant EGFR mutated NSCLC : Responses by mutation • 40% confirmed response rate and a clinical benefit rate of 90% N = 45 Janjigian YY, et al. Cancer Discov. 2014; 4(9): 1036 -1045. • Appears independent of T 790 M status as assessed in study
Afatinib + cetuximab in TKI-resistant EGFR mutated NSCLC : Responses by mutation • 40% confirmed response rate and a clinical benefit rate of 90% N = 45 Janjigian YY, et al. Cancer Discov. 2014; 4(9): 1036 -1045. • Appears independent of T 790 M status as assessed in study
 Management of EGFR-mutation positive patients: overall conclusions • Test all non-squamous and selected squamous • Standard of care = first line EGFR TKI (erlotinib, afatinib, or gefitinib) • At progression: – Think local therapy (radiation) if limited progression – Tolerate “slow PD” – Re-biopsy growing areas at progression for T 790 M (can consider “liquid biopsy” too) • Don’t continue TKI with chemotherapy • Responses to anti-PD 1 “average”
Management of EGFR-mutation positive patients: overall conclusions • Test all non-squamous and selected squamous • Standard of care = first line EGFR TKI (erlotinib, afatinib, or gefitinib) • At progression: – Think local therapy (radiation) if limited progression – Tolerate “slow PD” – Re-biopsy growing areas at progression for T 790 M (can consider “liquid biopsy” too) • Don’t continue TKI with chemotherapy • Responses to anti-PD 1 “average”
 What if… Jack had stage IV NSCLC with non-squamous cell histology and an ALK rearrangement – what are treatment options?
What if… Jack had stage IV NSCLC with non-squamous cell histology and an ALK rearrangement – what are treatment options?
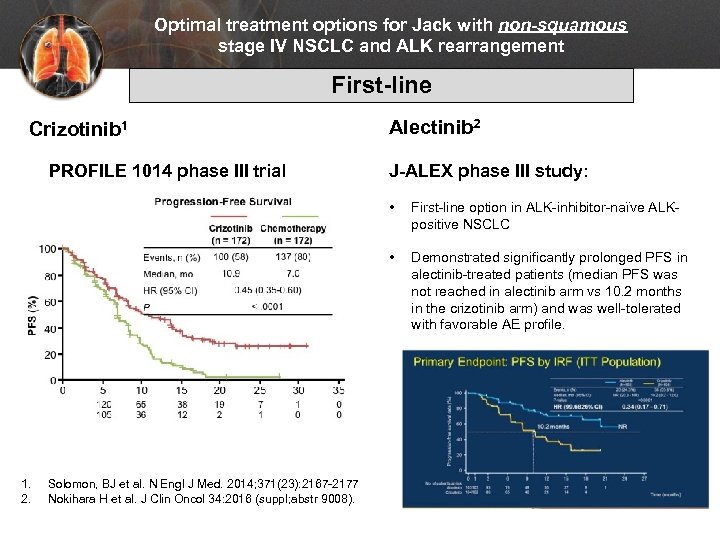 Optimal treatment options for Jack with non-squamous stage IV NSCLC and ALK rearrangement First-line Crizotinib 1 PROFILE 1014 phase III trial Alectinib 2 J-ALEX phase III study: • • 1. 2. Solomon, BJ et al. N Engl J Med. 2014; 371(23): 2167 -2177 Nokihara H et al. J Clin Oncol 34: 2016 (suppl; abstr 9008). First-line option in ALK-inhibitor-naïve ALKpositive NSCLC Demonstrated significantly prolonged PFS in alectinib-treated patients (median PFS was not reached in alectinib arm vs 10. 2 months in the crizotinib arm) and was well-tolerated with favorable AE profile.
Optimal treatment options for Jack with non-squamous stage IV NSCLC and ALK rearrangement First-line Crizotinib 1 PROFILE 1014 phase III trial Alectinib 2 J-ALEX phase III study: • • 1. 2. Solomon, BJ et al. N Engl J Med. 2014; 371(23): 2167 -2177 Nokihara H et al. J Clin Oncol 34: 2016 (suppl; abstr 9008). First-line option in ALK-inhibitor-naïve ALKpositive NSCLC Demonstrated significantly prolonged PFS in alectinib-treated patients (median PFS was not reached in alectinib arm vs 10. 2 months in the crizotinib arm) and was well-tolerated with favorable AE profile.
 Optimal treatment options for Jack with non-squamous stage IV NSCLC and ALK rearrangement Second-line • Ceritinib, a 2 nd generation ALK inhibitor approved in Apr 2014 for patients who have progressed on or are intolerant of crizotinib • Alectinib, a highly potent-selective ALK inhibitor approved in Dec 2015 for patients who have progressed on or are intolerant of crizotinib – Significant reduction in metastatic brain tumors observed Solomon, BJ et al. N Engl J Med. 2014; 371(23): 2167 -2177
Optimal treatment options for Jack with non-squamous stage IV NSCLC and ALK rearrangement Second-line • Ceritinib, a 2 nd generation ALK inhibitor approved in Apr 2014 for patients who have progressed on or are intolerant of crizotinib • Alectinib, a highly potent-selective ALK inhibitor approved in Dec 2015 for patients who have progressed on or are intolerant of crizotinib – Significant reduction in metastatic brain tumors observed Solomon, BJ et al. N Engl J Med. 2014; 371(23): 2167 -2177
 What if… Jack had stage IV NSCLC with non-squamous cell histology and another “actionable” genomic alteration? What are considerations for management?
What if… Jack had stage IV NSCLC with non-squamous cell histology and another “actionable” genomic alteration? What are considerations for management?
 Selected “Other” Mutations in NSCLC Oncogene KRAS mutations Prevalence % Available drugs with potential benefit** 25 -30 Selumetinib, trametinib, cobimetinib KRAS G 12 C inhibitors, MEK and PI 3 K inhibitors, JAK/TBK 1/KK inhibitors Therapeutic pathways being targeted ROS 1 rearrangements 1 -3 Crizotinib**, ceritinib ROS inhibitors, Hsp 90 inhibitors HER 2 mutations 1 -3 afatinib, trastuzumab, T-DM 1 HER 2 inhibitors, Hsp 90 inhibitors BRAF mutations 1 -3 Vemurafenib, dabrafenib, selumetinib, trametinib BRAF inhibitors, MEK inhibitors RET rearrangements 1 Cabozantinib, vandetanib, RET inhibitors, Hsp 90 inhibitors MET amplification 1 Crizotinib, cabozantinib MET inhibitors MET exon 14 mutation 3 Crizotinib, cabozantinib MET inhibitors NTRK 1 rearrangements <1 Crizotinib TRK inhibitors * Evidence level: Bold –Strong clinical evidence, Plain – weak clinical evidence ** FDA approved
Selected “Other” Mutations in NSCLC Oncogene KRAS mutations Prevalence % Available drugs with potential benefit** 25 -30 Selumetinib, trametinib, cobimetinib KRAS G 12 C inhibitors, MEK and PI 3 K inhibitors, JAK/TBK 1/KK inhibitors Therapeutic pathways being targeted ROS 1 rearrangements 1 -3 Crizotinib**, ceritinib ROS inhibitors, Hsp 90 inhibitors HER 2 mutations 1 -3 afatinib, trastuzumab, T-DM 1 HER 2 inhibitors, Hsp 90 inhibitors BRAF mutations 1 -3 Vemurafenib, dabrafenib, selumetinib, trametinib BRAF inhibitors, MEK inhibitors RET rearrangements 1 Cabozantinib, vandetanib, RET inhibitors, Hsp 90 inhibitors MET amplification 1 Crizotinib, cabozantinib MET inhibitors MET exon 14 mutation 3 Crizotinib, cabozantinib MET inhibitors NTRK 1 rearrangements <1 Crizotinib TRK inhibitors * Evidence level: Bold –Strong clinical evidence, Plain – weak clinical evidence ** FDA approved
 Summary – Chemotherapy • Cytotoxic chemotherapy improves survival and palliates symptoms in first line, maintenance, and subsequent treatment settings • Histology important at diagnosis to select agents: Pemetrexed effective in non-squamous, necitumumab effective in squamous • Targeting angiogenesis improves survival in 1 st and 2 nd line treatment
Summary – Chemotherapy • Cytotoxic chemotherapy improves survival and palliates symptoms in first line, maintenance, and subsequent treatment settings • Histology important at diagnosis to select agents: Pemetrexed effective in non-squamous, necitumumab effective in squamous • Targeting angiogenesis improves survival in 1 st and 2 nd line treatment
 Summary – Targeted therapy • Molecular genotyping is now the standard in advanced NSCLC (adenocarcinoma) – Multidisciplinary approach assuring adequate tissue for molecular testing • Histology and selected molecular biomarkers (EGFR, ALK, ROS 1, others) drive therapeutic choices – EGFR and ALK - molecularly targeted therapies offer longer time to progression in the first line setting than conventional chemotherapy, but chemo can be effective too – Other targetable drivers may also respond to off label use of available targeted therapies, which is reasonable following chemotherapy failure – Re-biopsy should be considered routinely after progression on targeted agents, and drugs to overcome initial resistance are available
Summary – Targeted therapy • Molecular genotyping is now the standard in advanced NSCLC (adenocarcinoma) – Multidisciplinary approach assuring adequate tissue for molecular testing • Histology and selected molecular biomarkers (EGFR, ALK, ROS 1, others) drive therapeutic choices – EGFR and ALK - molecularly targeted therapies offer longer time to progression in the first line setting than conventional chemotherapy, but chemo can be effective too – Other targetable drivers may also respond to off label use of available targeted therapies, which is reasonable following chemotherapy failure – Re-biopsy should be considered routinely after progression on targeted agents, and drugs to overcome initial resistance are available
 Summary – Immunotherapy • Immunotherapy is now a reality in the treatment of advanced NSCLC • Clearly effective in the 2 nd+ line setting and will likely be part of 1 st line therapy in the near future for selected patients • PD-L 1 IHC testing is available and can be helpful clinically to estimate probability of response and to select therapeutic • Toxicity patterns are unique; grade > 3 toxicities occur in <5% of patients • Education at all levels (MD, PA/NP, nurses and patients & caregivers) about the nature of immune-related adverse events is necessary 89
Summary – Immunotherapy • Immunotherapy is now a reality in the treatment of advanced NSCLC • Clearly effective in the 2 nd+ line setting and will likely be part of 1 st line therapy in the near future for selected patients • PD-L 1 IHC testing is available and can be helpful clinically to estimate probability of response and to select therapeutic • Toxicity patterns are unique; grade > 3 toxicities occur in <5% of patients • Education at all levels (MD, PA/NP, nurses and patients & caregivers) about the nature of immune-related adverse events is necessary 89
 DON’T FORGET Please be sure to return the completed POST-TEST/EVALUATION/REQUEST FOR CREDIT FORM (found in the handout package) to any staff member managing the meeting. Even if you don’t need credit, this data is very valuable to us. All information must be clearly filled out (BOTH SIDES!) in order for your certificate to be issued. Thank you! 90
DON’T FORGET Please be sure to return the completed POST-TEST/EVALUATION/REQUEST FOR CREDIT FORM (found in the handout package) to any staff member managing the meeting. Even if you don’t need credit, this data is very valuable to us. All information must be clearly filled out (BOTH SIDES!) in order for your certificate to be issued. Thank you! 90
 Questions? 91
Questions? 91


