6ead125364fceff446a9753cb4fe9f98.ppt
- Количество слайдов: 18
 Low-Platinum Nanostructured Catalysts for Fuel Cells Karen Swider-Lyons and Peter Bouwman Naval Research Laboratory Washington, DC Wojtek Dmowski University of Tennessee Knoxville, TN
Low-Platinum Nanostructured Catalysts for Fuel Cells Karen Swider-Lyons and Peter Bouwman Naval Research Laboratory Washington, DC Wojtek Dmowski University of Tennessee Knoxville, TN
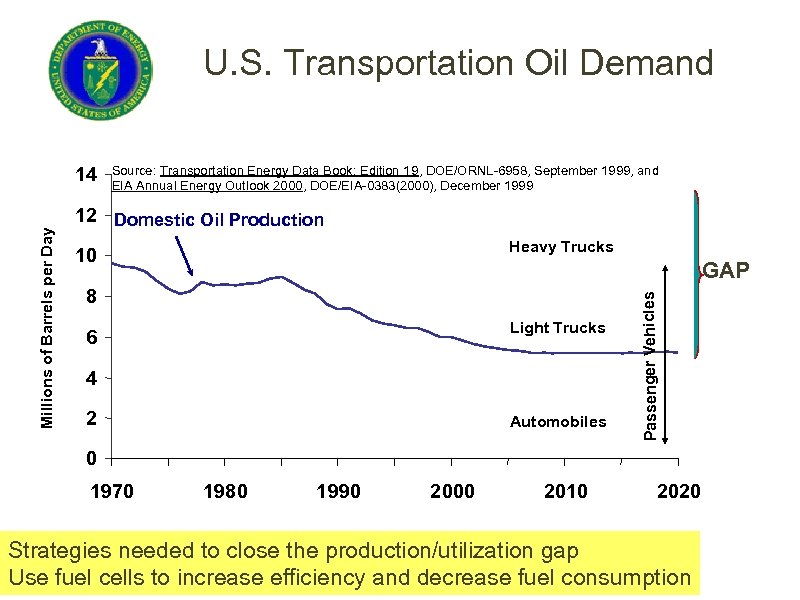 U. S. Transportation Oil Demand Source: Transportation Energy Data Book: Edition 19, DOE/ORNL-6958, September 1999, and EIA Annual Energy Outlook 2000, DOE/EIA-0383(2000), December 1999 12 Domestic Oil Production Heavy Trucks 10 GAP 8 Light Trucks 6 4 2 Automobiles Passenger Vehicles Millions of Barrels per Day 14 0 1970 1980 1990 2000 2010 2020 Strategies needed to close the production/utilization gap Use fuel cells to increase efficiency and decrease fuel consumption
U. S. Transportation Oil Demand Source: Transportation Energy Data Book: Edition 19, DOE/ORNL-6958, September 1999, and EIA Annual Energy Outlook 2000, DOE/EIA-0383(2000), December 1999 12 Domestic Oil Production Heavy Trucks 10 GAP 8 Light Trucks 6 4 2 Automobiles Passenger Vehicles Millions of Barrels per Day 14 0 1970 1980 1990 2000 2010 2020 Strategies needed to close the production/utilization gap Use fuel cells to increase efficiency and decrease fuel consumption
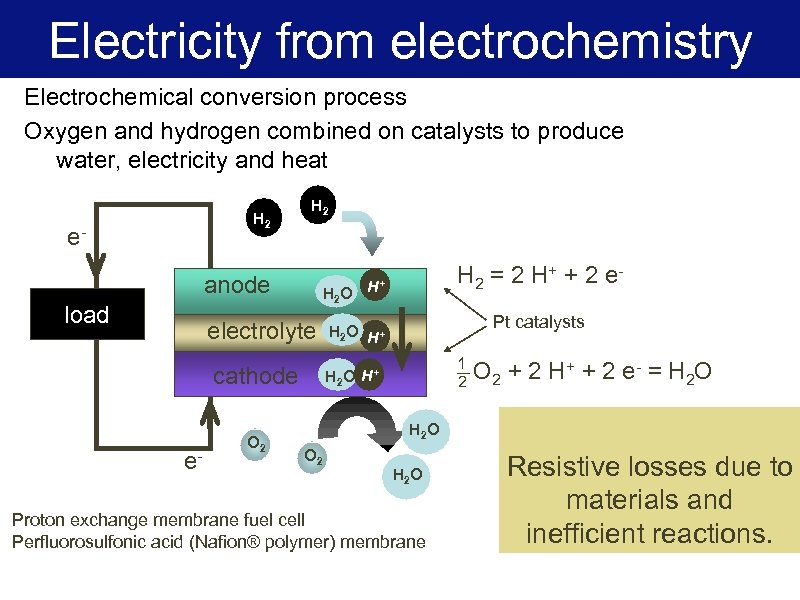 Electricity from electrochemistry Electrochemical conversion process Oxygen and hydrogen combined on catalysts to produce water, electricity and heat H 2 e- H 2 anode load H 2 O electrolyte cathode e- O 2 H 2 = 2 H + + 2 e - H+ Pt catalysts H 2 O H+ 1 2 H 2 O H+ O 2 + 2 H + + 2 e - = H 2 O H 2 O O 2 H 2 O Proton exchange membrane fuel cell Perfluorosulfonic acid (Nafion® polymer) membrane Resistive losses due to materials and inefficient reactions.
Electricity from electrochemistry Electrochemical conversion process Oxygen and hydrogen combined on catalysts to produce water, electricity and heat H 2 e- H 2 anode load H 2 O electrolyte cathode e- O 2 H 2 = 2 H + + 2 e - H+ Pt catalysts H 2 O H+ 1 2 H 2 O H+ O 2 + 2 H + + 2 e - = H 2 O H 2 O O 2 H 2 O Proton exchange membrane fuel cell Perfluorosulfonic acid (Nafion® polymer) membrane Resistive losses due to materials and inefficient reactions.
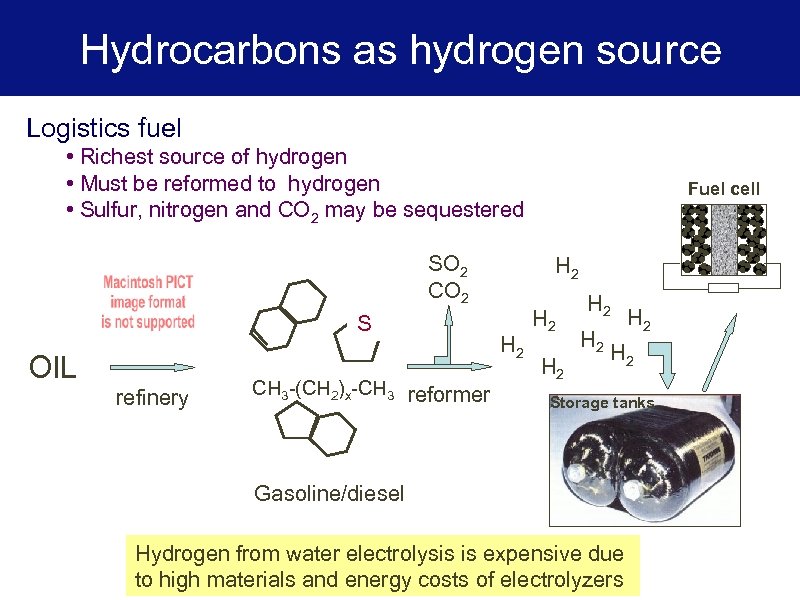 Hydrocarbons as hydrogen source Logistics fuel • Richest source of hydrogen • Must be reformed to hydrogen • Sulfur, nitrogen and CO 2 may be sequestered SO 2 CO 2 S OIL refinery CH 3 -(CH 2)x-CH 3 reformer Fuel cell H 2 H 2 Storage tanks Gasoline/diesel Hydrogen from water electrolysis is expensive due to high materials and energy costs of electrolyzers
Hydrocarbons as hydrogen source Logistics fuel • Richest source of hydrogen • Must be reformed to hydrogen • Sulfur, nitrogen and CO 2 may be sequestered SO 2 CO 2 S OIL refinery CH 3 -(CH 2)x-CH 3 reformer Fuel cell H 2 H 2 Storage tanks Gasoline/diesel Hydrogen from water electrolysis is expensive due to high materials and energy costs of electrolyzers
 Environmental Issues with Platinum Mining Pt creates a lot of waste! In fuel cell era, attention toward environmentally friendly mining and recycling From: The Lonmin Group web site
Environmental Issues with Platinum Mining Pt creates a lot of waste! In fuel cell era, attention toward environmentally friendly mining and recycling From: The Lonmin Group web site
 EPA Advantages of low Pt catalysts Lowering Pt will lower the cost of fuel cells Introduce fuel cells more broadly to consumer market Lower fuel consumption Less Platinum used Less Pt mined and recycled Less chemical waste
EPA Advantages of low Pt catalysts Lowering Pt will lower the cost of fuel cells Introduce fuel cells more broadly to consumer market Lower fuel consumption Less Platinum used Less Pt mined and recycled Less chemical waste
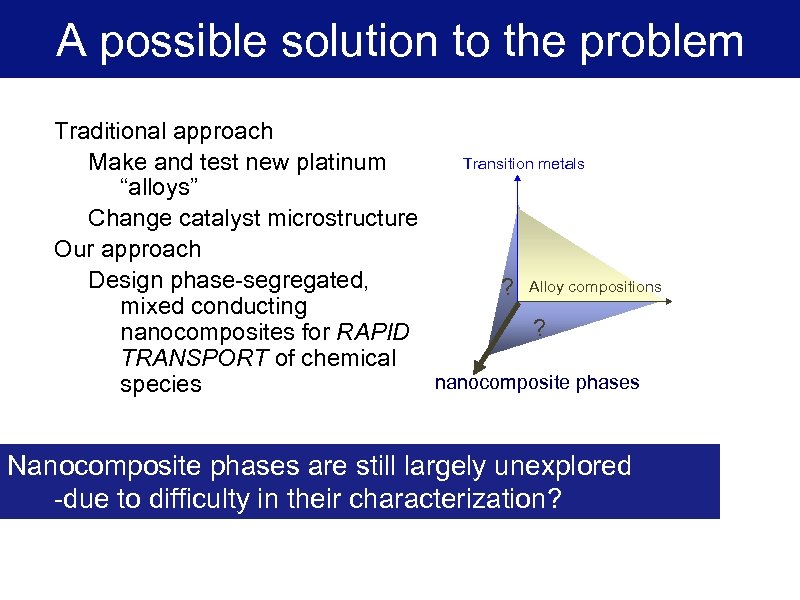 A possible solution to the problem Traditional approach Transition metals Make and test new platinum “alloys” Change catalyst microstructure Our approach Design phase-segregated, ? Alloy compositions mixed conducting ? nanocomposites for RAPID TRANSPORT of chemical nanocomposite phases species Nanocomposite phases are still largely unexplored -due to difficulty in their characterization?
A possible solution to the problem Traditional approach Transition metals Make and test new platinum “alloys” Change catalyst microstructure Our approach Design phase-segregated, ? Alloy compositions mixed conducting ? nanocomposites for RAPID TRANSPORT of chemical nanocomposite phases species Nanocomposite phases are still largely unexplored -due to difficulty in their characterization?
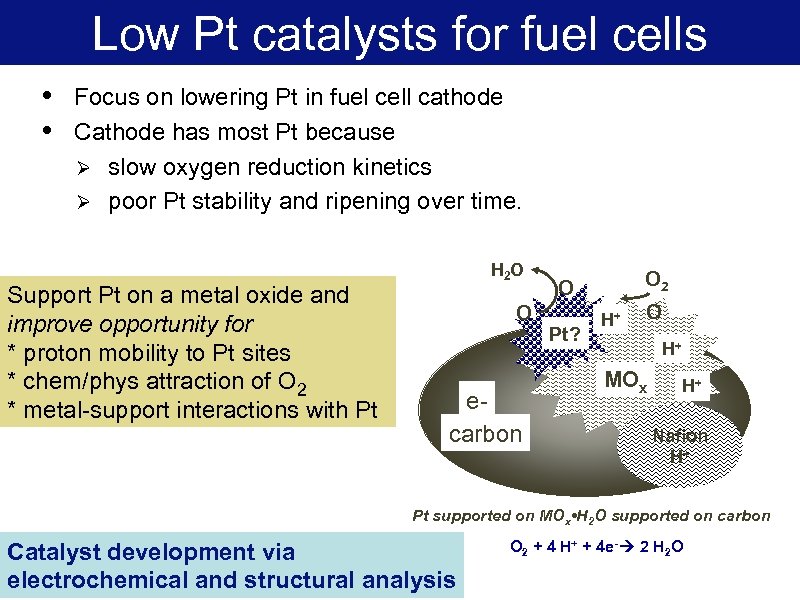 Low Pt catalysts for fuel cells Focus on lowering Pt in fuel cell cathode Cathode has most Pt because Ø slow oxygen reduction kinetics Ø poor Pt stability and ripening over time. Support Pt on a metal oxide and improve opportunity for * proton mobility to Pt sites * chem/phys attraction of O 2 * metal-support interactions with Pt H 2 O O ecarbon O 2 O Pt? H+ O • • H+ MOx H+ Nafion H+ Pt supported on MOx • H 2 O supported on carbon Catalyst development via electrochemical and structural analysis O 2 + 4 H+ + 4 e- 2 H 2 O
Low Pt catalysts for fuel cells Focus on lowering Pt in fuel cell cathode Cathode has most Pt because Ø slow oxygen reduction kinetics Ø poor Pt stability and ripening over time. Support Pt on a metal oxide and improve opportunity for * proton mobility to Pt sites * chem/phys attraction of O 2 * metal-support interactions with Pt H 2 O O ecarbon O 2 O Pt? H+ O • • H+ MOx H+ Nafion H+ Pt supported on MOx • H 2 O supported on carbon Catalyst development via electrochemical and structural analysis O 2 + 4 H+ + 4 e- 2 H 2 O
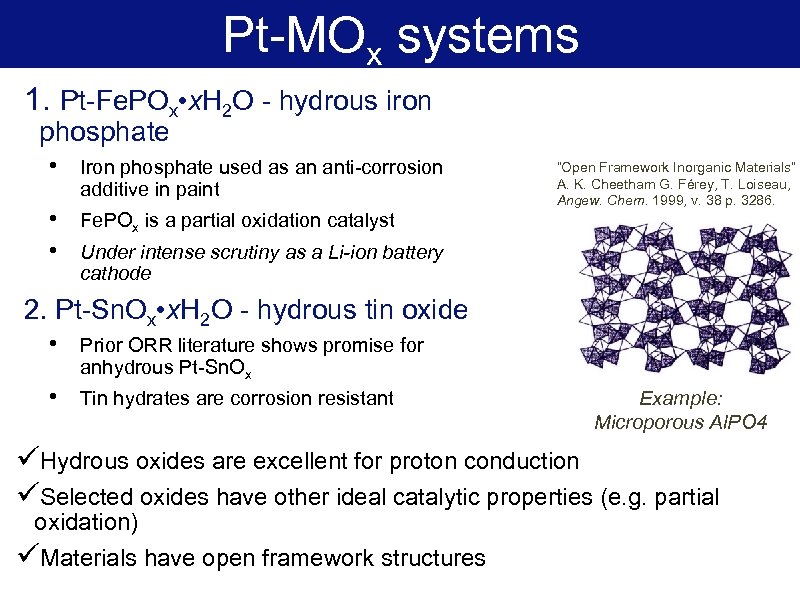 Pt-MOx systems 1. Pt-Fe. POx • x. H 2 O - hydrous iron phosphate • Iron phosphate used as an anti-corrosion additive in paint • • Fe. POx is a partial oxidation catalyst “Open Framework Inorganic Materials” A. K. Cheetham G. Férey, T. Loiseau, Angew. Chem. 1999, v. 38 p. 3286. Under intense scrutiny as a Li-ion battery cathode 2. Pt-Sn. Ox • x. H 2 O - hydrous tin oxide • Prior ORR literature shows promise for anhydrous Pt-Sn. Ox • Tin hydrates are corrosion resistant Example: Microporous Al. PO 4 üHydrous oxides are excellent for proton conduction üSelected oxides have other ideal catalytic properties (e. g. partial oxidation) üMaterials have open framework structures
Pt-MOx systems 1. Pt-Fe. POx • x. H 2 O - hydrous iron phosphate • Iron phosphate used as an anti-corrosion additive in paint • • Fe. POx is a partial oxidation catalyst “Open Framework Inorganic Materials” A. K. Cheetham G. Férey, T. Loiseau, Angew. Chem. 1999, v. 38 p. 3286. Under intense scrutiny as a Li-ion battery cathode 2. Pt-Sn. Ox • x. H 2 O - hydrous tin oxide • Prior ORR literature shows promise for anhydrous Pt-Sn. Ox • Tin hydrates are corrosion resistant Example: Microporous Al. PO 4 üHydrous oxides are excellent for proton conduction üSelected oxides have other ideal catalytic properties (e. g. partial oxidation) üMaterials have open framework structures
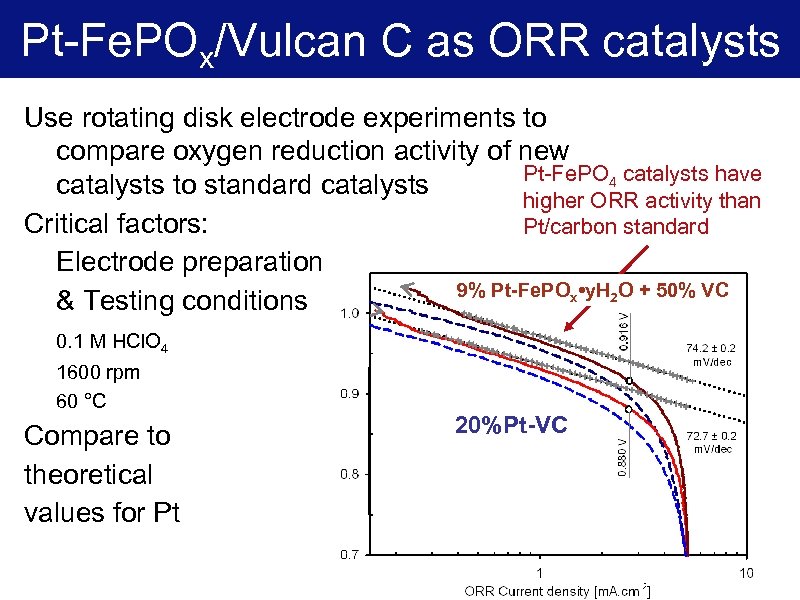 Pt-Fe. POx/Vulcan C as ORR catalysts Use rotating disk electrode experiments to compare oxygen reduction activity of new Pt-Fe. PO 4 catalysts have catalysts to standard catalysts higher ORR activity than Critical factors: Pt/carbon standard Electrode preparation 9% Pt-Fe. POx • y. H 2 O + 50% VC & Testing conditions 0. 1 M HCl. O 4 1600 rpm 60 °C Compare to theoretical values for Pt 20%Pt-VC
Pt-Fe. POx/Vulcan C as ORR catalysts Use rotating disk electrode experiments to compare oxygen reduction activity of new Pt-Fe. PO 4 catalysts have catalysts to standard catalysts higher ORR activity than Critical factors: Pt/carbon standard Electrode preparation 9% Pt-Fe. POx • y. H 2 O + 50% VC & Testing conditions 0. 1 M HCl. O 4 1600 rpm 60 °C Compare to theoretical values for Pt 20%Pt-VC
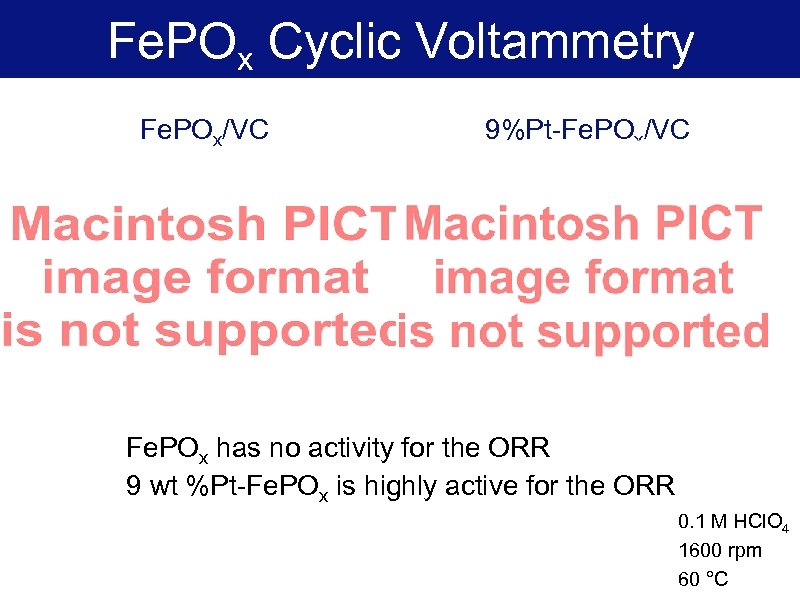 Fe. POx Cyclic Voltammetry Fe. POx/VC 9%Pt-Fe. POx/VC Fe. POx has no activity for the ORR 9 wt %Pt-Fe. POx is highly active for the ORR 0. 1 M HCl. O 4 1600 rpm 60 °C
Fe. POx Cyclic Voltammetry Fe. POx/VC 9%Pt-Fe. POx/VC Fe. POx has no activity for the ORR 9 wt %Pt-Fe. POx is highly active for the ORR 0. 1 M HCl. O 4 1600 rpm 60 °C
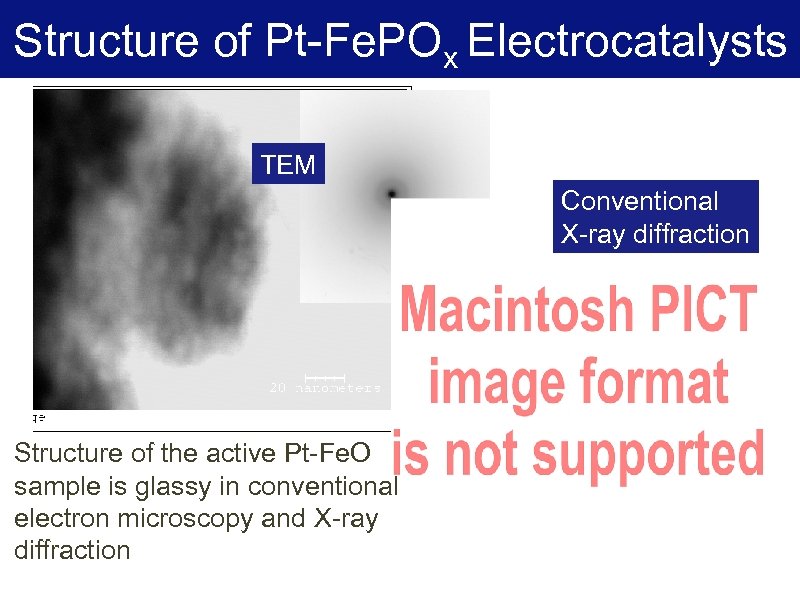 Structure of Pt-Fe. POx Electrocatalysts TEM Conventional X-ray diffraction Structure of the active Pt-Fe. O sample is glassy in conventional electron microscopy and X-ray diffraction
Structure of Pt-Fe. POx Electrocatalysts TEM Conventional X-ray diffraction Structure of the active Pt-Fe. O sample is glassy in conventional electron microscopy and X-ray diffraction
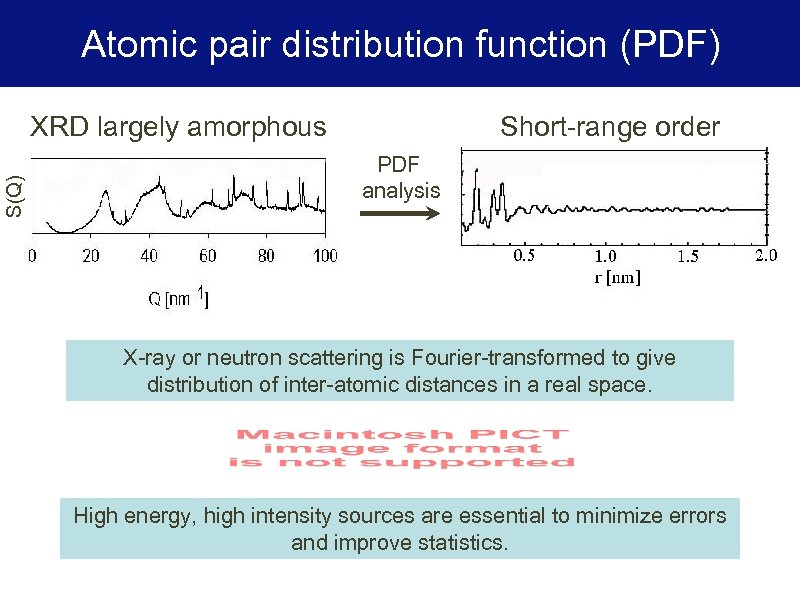 Atomic pair distribution function (PDF) S(Q) XRD largely amorphous Short-range order PDF analysis X-ray or neutron scattering is Fourier-transformed to give distribution of inter-atomic distances in a real space. High energy, high intensity sources are essential to minimize errors and improve statistics.
Atomic pair distribution function (PDF) S(Q) XRD largely amorphous Short-range order PDF analysis X-ray or neutron scattering is Fourier-transformed to give distribution of inter-atomic distances in a real space. High energy, high intensity sources are essential to minimize errors and improve statistics.
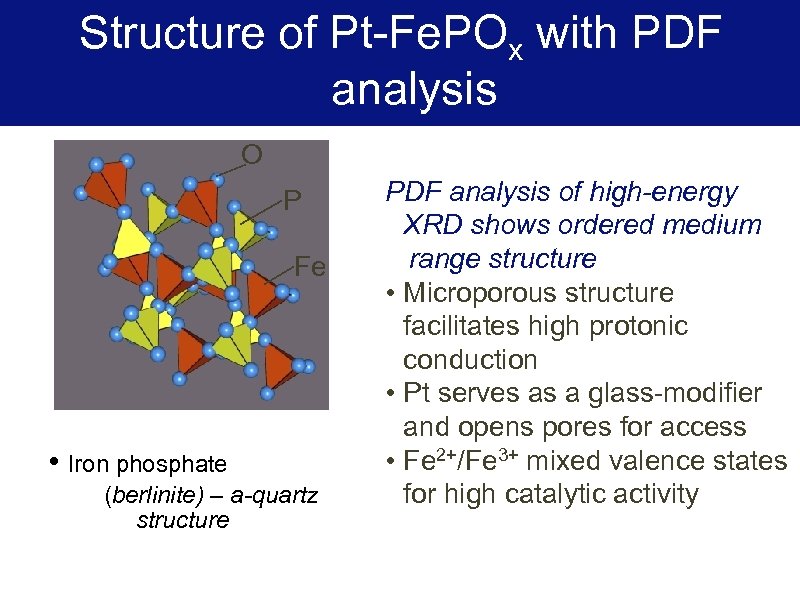 Structure of Pt-Fe. POx with PDF s analysis O P Fe • Iron phosphate (berlinite) – a-quartz structure PDF analysis of high-energy XRD shows ordered medium range structure • Microporous structure facilitates high protonic conduction • Pt serves as a glass-modifier and opens pores for access • Fe 2+/Fe 3+ mixed valence states for high catalytic activity
Structure of Pt-Fe. POx with PDF s analysis O P Fe • Iron phosphate (berlinite) – a-quartz structure PDF analysis of high-energy XRD shows ordered medium range structure • Microporous structure facilitates high protonic conduction • Pt serves as a glass-modifier and opens pores for access • Fe 2+/Fe 3+ mixed valence states for high catalytic activity
 Next steps… Solution-filtered Pt-Fe. POx VC impregnated with Pt. Fe. POx Reduce particle size of oxides to improve electrical properties of Fe. POx
Next steps… Solution-filtered Pt-Fe. POx VC impregnated with Pt. Fe. POx Reduce particle size of oxides to improve electrical properties of Fe. POx
 Effect of particle size Smaller (nano)particle size leads to: • Higher electronic conductivity (tunneling from carbon) • Higher surface area (more sites for catalysis) Pt-Fe. POx mixed with Vulcan carbon vs. Pt-Fe. POx impregnated on Vulcan carbon
Effect of particle size Smaller (nano)particle size leads to: • Higher electronic conductivity (tunneling from carbon) • Higher surface area (more sites for catalysis) Pt-Fe. POx mixed with Vulcan carbon vs. Pt-Fe. POx impregnated on Vulcan carbon
 Summary and Outlook • Fuel cells are efficient fuel conversion systems that may lead to significant fuel savings - less pollution • Lower greenhouse gases at a central fuel reforming site and hydrogen stored in fuel tanks • Nanomaterials may be useful routes to lowering Pt content of fuel cells and lowering their cost • New analytical techniques may be needed for the accurate study of new nanomaterials
Summary and Outlook • Fuel cells are efficient fuel conversion systems that may lead to significant fuel savings - less pollution • Lower greenhouse gases at a central fuel reforming site and hydrogen stored in fuel tanks • Nanomaterials may be useful routes to lowering Pt content of fuel cells and lowering their cost • New analytical techniques may be needed for the accurate study of new nanomaterials
 Acknowledgements Department of Energy, EERE Office of Naval Research The synchrotron experiments were carried out at the NSLS - Brookhaven National Lab
Acknowledgements Department of Energy, EERE Office of Naval Research The synchrotron experiments were carried out at the NSLS - Brookhaven National Lab


