Low-molecular biologic compounds 8.pptx
- Количество слайдов: 34
Low-molecular biologic compounds Lecture #8

Table of contents
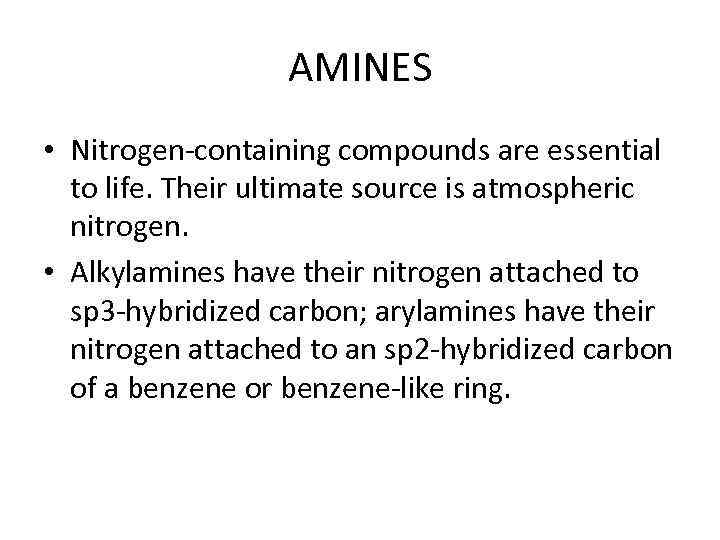
AMINES • Nitrogen-containing compounds are essential to life. Their ultimate source is atmospheric nitrogen. • Alkylamines have their nitrogen attached to sp 3 -hybridized carbon; arylamines have their nitrogen attached to an sp 2 -hybridized carbon of a benzene or benzene-like ring.
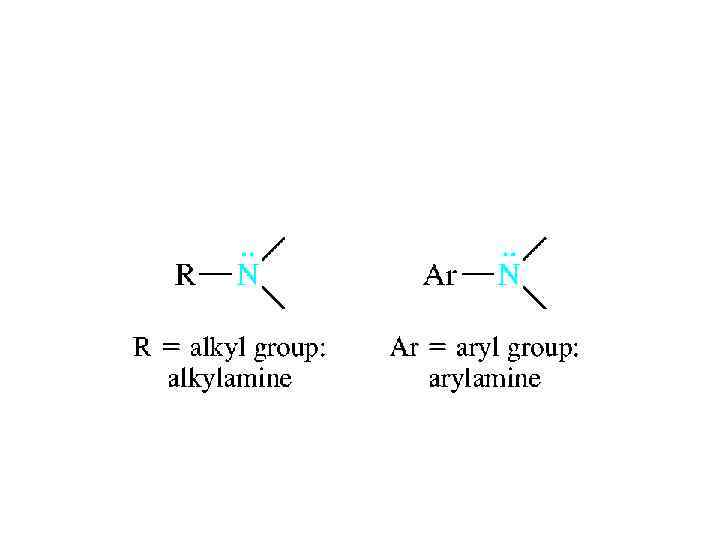
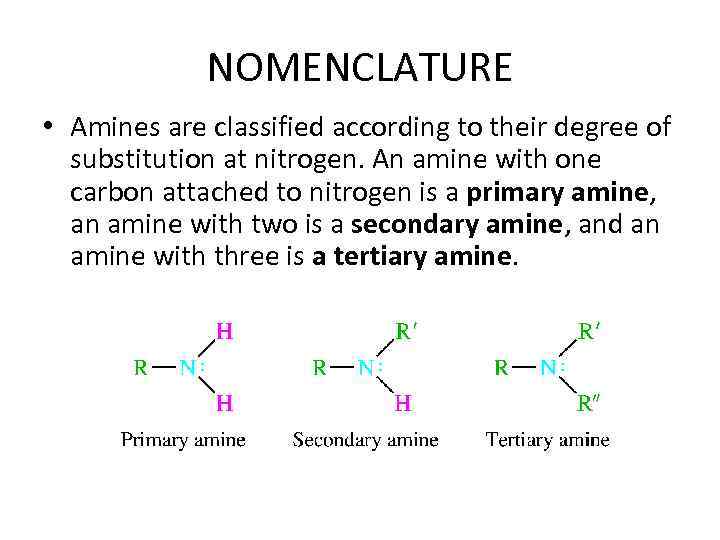
NOMENCLATURE • Amines are classified according to their degree of substitution at nitrogen. An amine with one carbon attached to nitrogen is a primary amine, an amine with two is a secondary amine, and an amine with three is a tertiary amine.
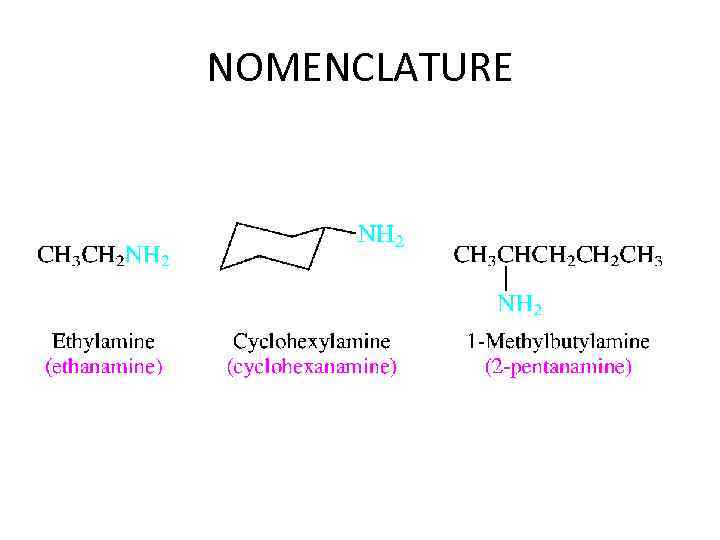
NOMENCLATURE
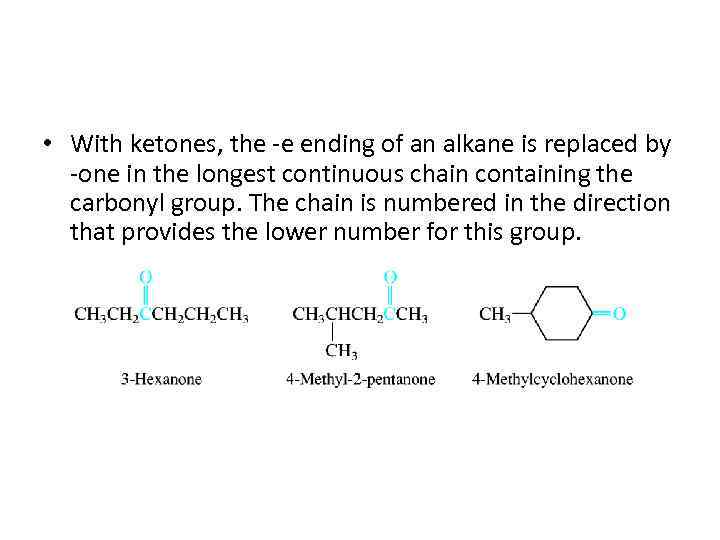
• With ketones, the -e ending of an alkane is replaced by -one in the longest continuous chain containing the carbonyl group. The chain is numbered in the direction that provides the lower number for this group.
STRUCTURE AND BONDING • Nitrogen's unshared electron pair is of major importance in understanding the structure and properties of amines. Alkylamines have a pyramidal arrangement of bonds to nitrogen, and the unshared electron pair resides in an sp 2 -hybridized orbital. • The geometry at nitrogen in arylamines is somewhat flatter than in alkylamines, and the unshared electron pair is delocalized into the π system of the ring. Delocalization binds the electron pair more strongly in arylamines than in alkylamines. Arylamines are less basic and less nucleophilic than alkylamines.

STRUCTURE AND BONDING • Amines are less polar than alcohols. Hydrogen bonding in amines is weaker than in alcohols because nitrogen is less electronegative than oxygen. • Amines have lower boiling points than alcohols, but higher boiling points than alkanes. • Primary amines have higher boiling points than isomeric secondary amines; tertiary amines, which cannot form intermolecular hydrogen bonds, have the lowest boiling points. Amines resemble alcohols in their solubility in water.

BASICITY OF AMINES • The more basic the amine, the weaker its conjugate acid. • The more basic the amine, the larger the p. K a of its conjugate acid.
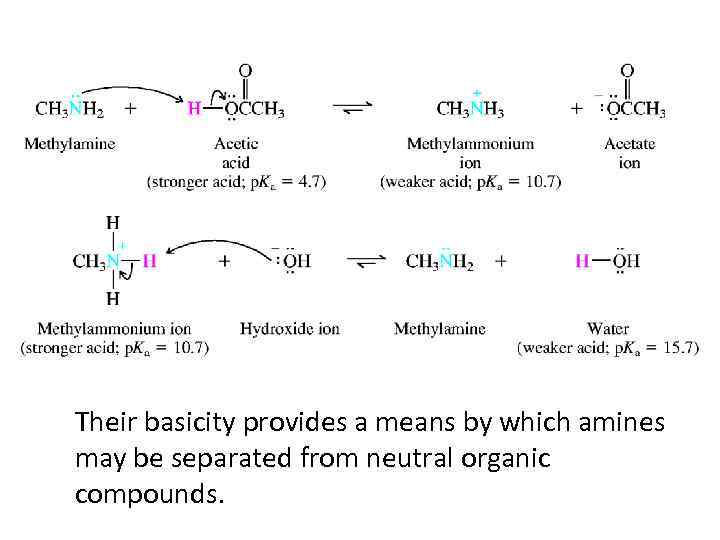
Their basicity provides a means by which amines may be separated from neutral organic compounds.

1. Alkylamines are slightly stronger bases than ammonia. 2. Alkylamines differ very little among themselves in basicity. Their basicities cover a range of less than 10 in equilibrium constant (1 p. K unit). 3. Arylamines are about 1 million times (6 p. K units) weaker bases than ammonia and alkylamines.
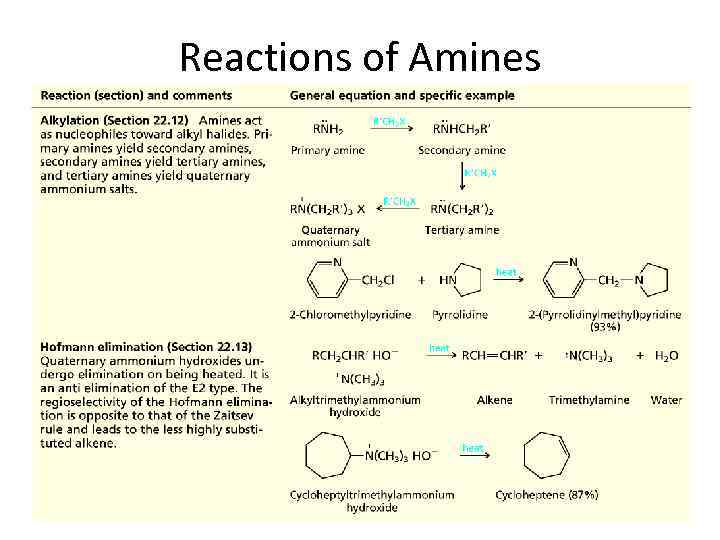
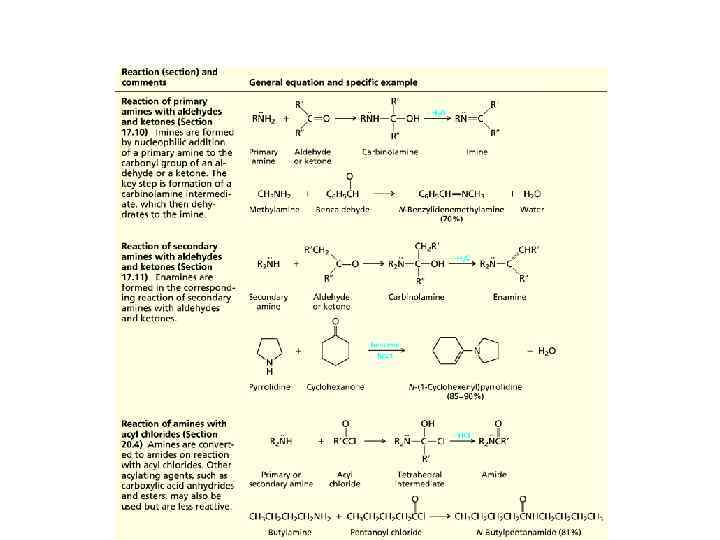
Reactions of Amines
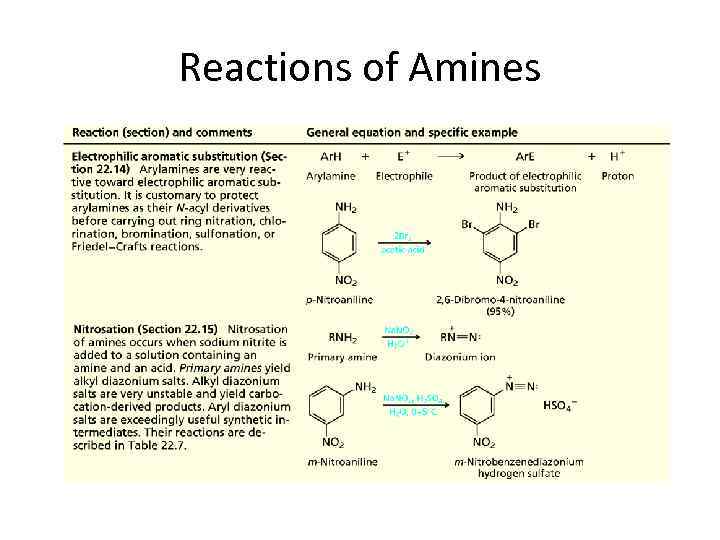
Reactions of Amines
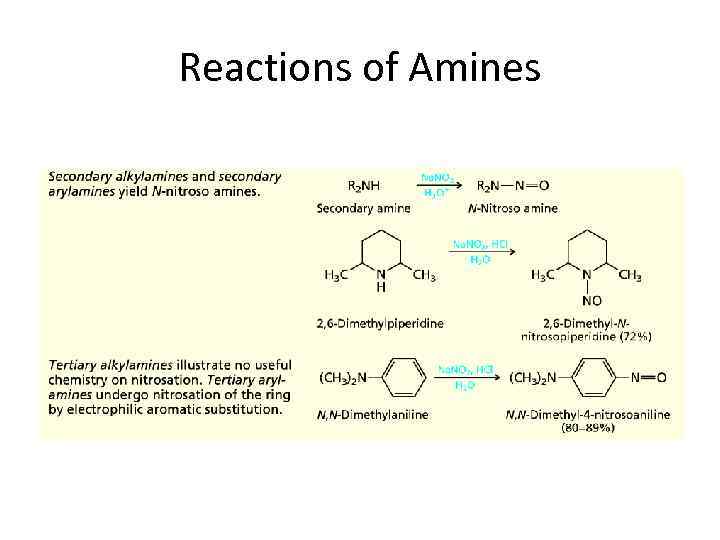
Reactions of Amines

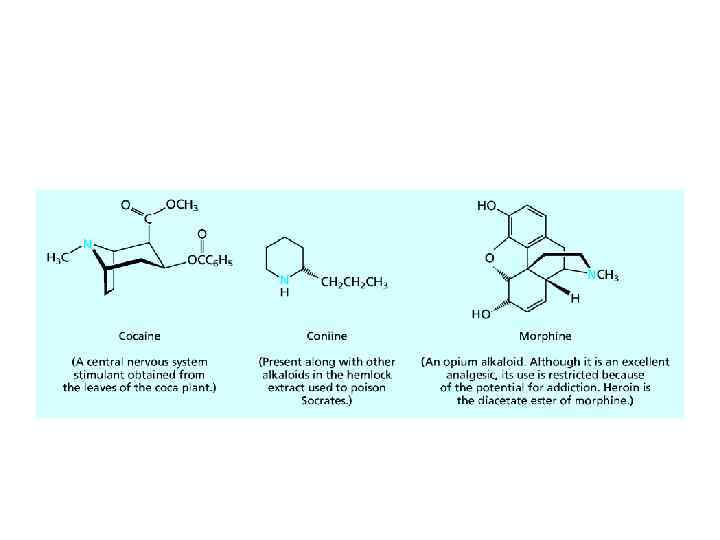
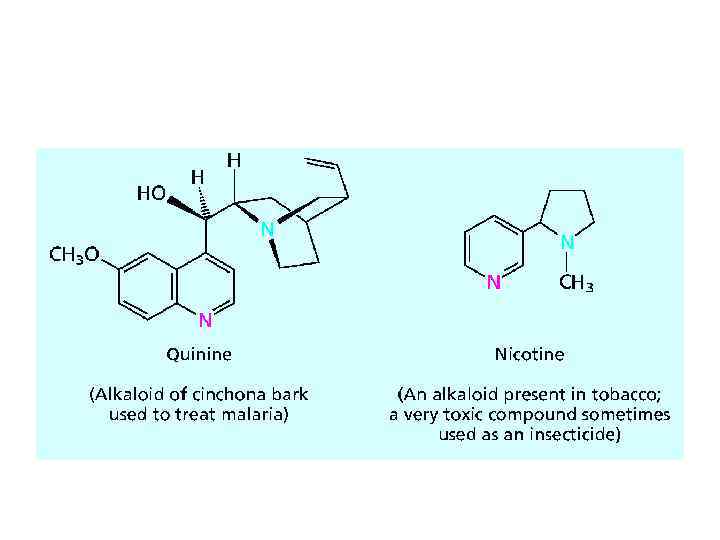
AMINES AS NATURAL PRODUCTS • The amines obtained from plants to be called alkaloids. The number of known alkaloids exceeds 5000. They are of special interest because most are characterized by a high level of biological activity. Some examples include cocaine, coniine, and morphine.
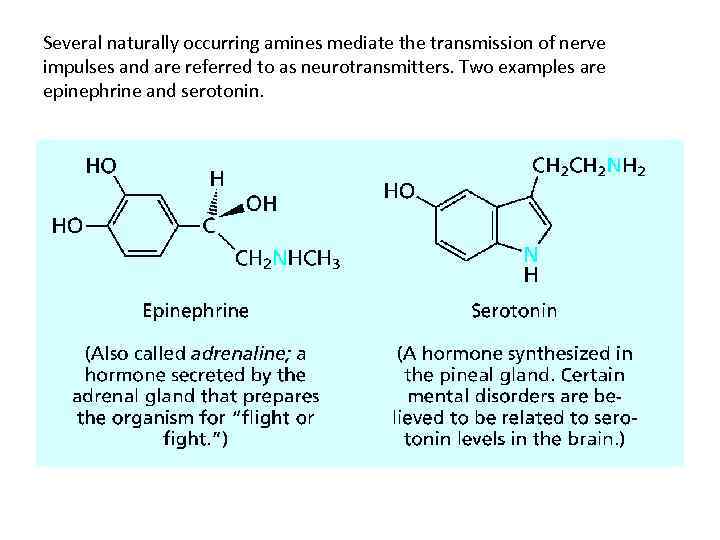
Several naturally occurring amines mediate the transmission of nerve impulses and are referred to as neurotransmitters. Two examples are epinephrine and serotonin.

PROBLEMS
PHENOLS • Phenols are compounds that have a hydroxyl group bonded directly to a benzene or benzenoid ring. The parent compound of this group, C 6 H 6 OH, called simply phenol, is an important industrial chemical. • Many of the properties of phenols are analogous to those of alcohols, but this similarity is something of an oversimplification. • Like arylamines, phenols are difunctional compounds; the hydroxyl group and the aromatic ring interact strongly, affecting each other's reactivity.

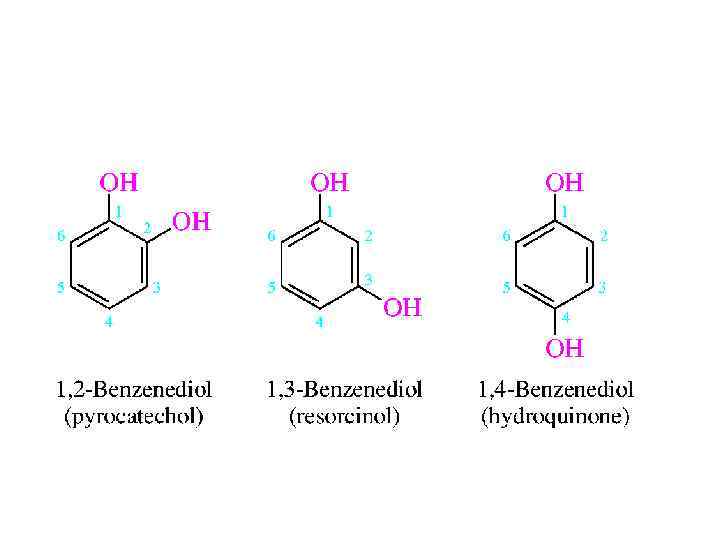
NOMENCLATURE • An old name for benzene was phene, and its hydroxyl derivative came to be called phenol. Likewise, o-, m-, and p-cresol are acceptable names for the various ring-substituted hydroxyl derivatives of toluene. More highly substituted compounds are named as derivatives of phenol. Numbering of the ring begins at the hydroxylsubstituted carbon and proceeds in the direction that gives the lower number to the next substituted carbon. Substituents are cited in alphabetical order.
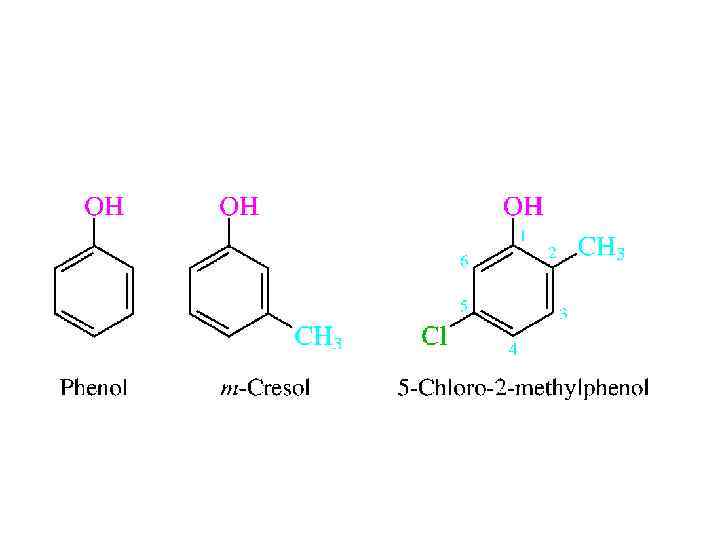
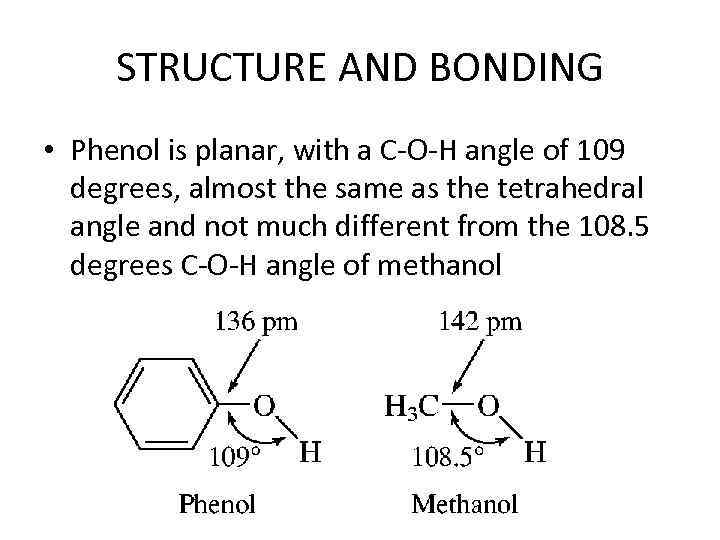
STRUCTURE AND BONDING • Phenol is planar, with a C-O-H angle of 109 degrees, almost the same as the tetrahedral angle and not much different from the 108. 5 degrees C-O-H angle of methanol

ACIDITY OF PHENOLS • The most characteristic property of phenols is their acidity. • Phenols are more acidic than alcohols but less acidic than carboxylic acids. • Recall that carboxylic acids have p. Ka's of approximately 5, whereas the p. Ka's of alcohols are in the 16 -20 range. The p. Ka for most phenols is about 10.
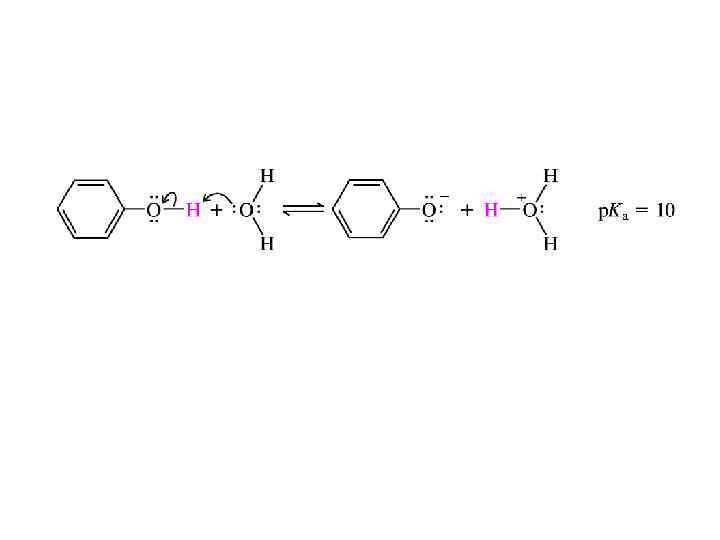

• The -OH group of phenols makes it possible for them to participate in hydrogen bonding. This contributes to the higher boiling points and greater water-solubility of phenolic compounds compared with arenes and aryl halides. • With p. Ka's of approximately 10, phenols are stronger acids than alcohols, but weaker than carboxylic acids. They are converted quantitatively to phenoxide anions on treatment with aqueous sodium hydroxide. • Electron-releasing substituents attached to the ring have a negligible effect on the acidity of phenols. Strongly electron-withdrawing groups increase the acidity. The compound 4 -nitro-3(trifluoromethyl)phenol, for example, is 10, 000 times more acidic than phenol.
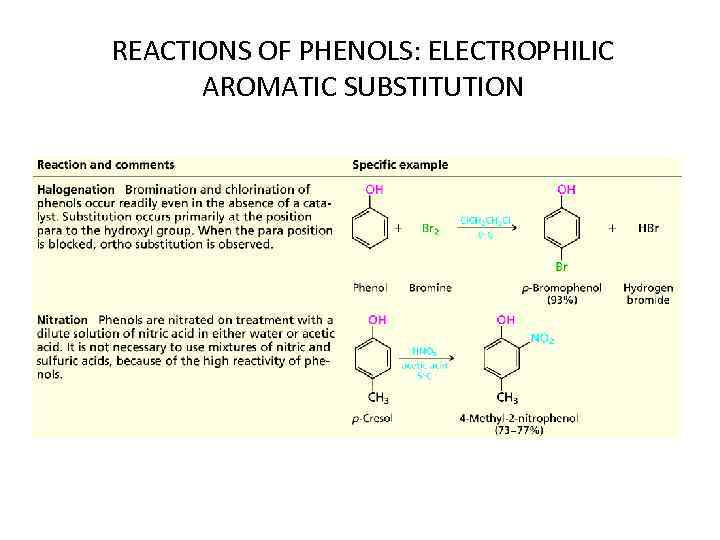
REACTIONS OF PHENOLS: ELECTROPHILIC AROMATIC SUBSTITUTION
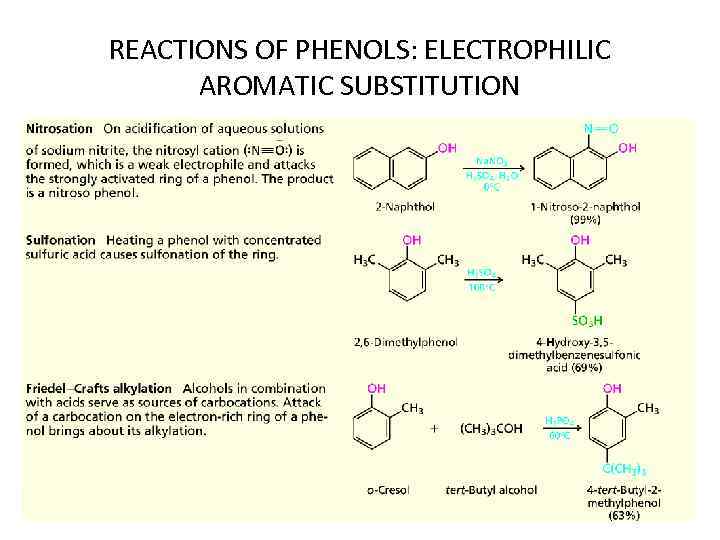
REACTIONS OF PHENOLS: ELECTROPHILIC AROMATIC SUBSTITUTION
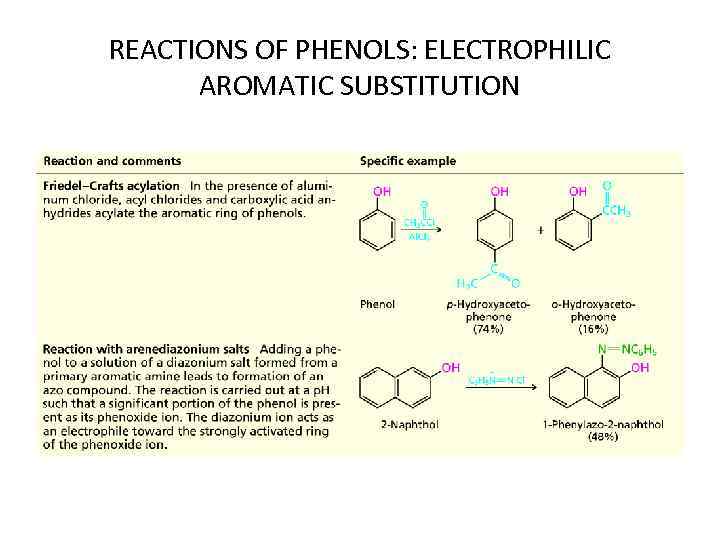
REACTIONS OF PHENOLS: ELECTROPHILIC AROMATIC SUBSTITUTION
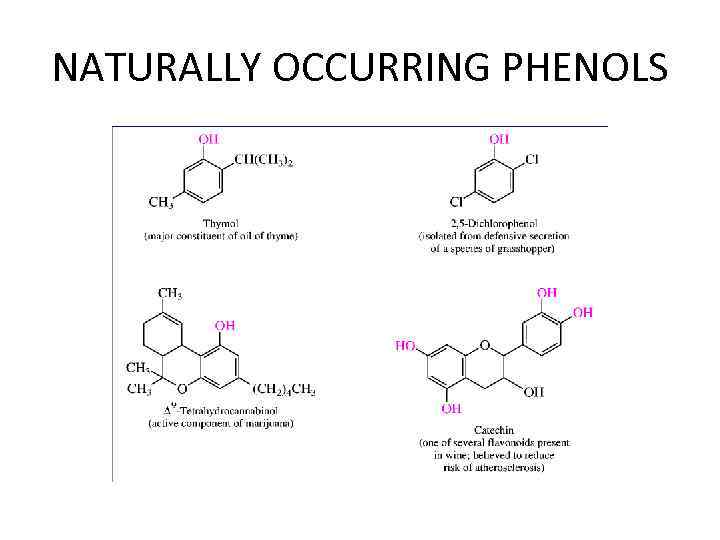
NATURALLY OCCURRING PHENOLS
PROBLEMS
Low-molecular biologic compounds 8.pptx