Low-molecular biologic compounds 5.pptx
- Количество слайдов: 18
Low-molecular biologic compounds Lecture #5

Table of contents • ALCOHOLS, DIOLS, AND THIOLS • Orbital hybridization model of bonding in methanol • Solubility in Water • Summary of Reactions of Alcohols • Oxidation of Alchohols • THIOLS • PROBLEMS

ALCOHOLS, DIOLS, AND THIOLS • A functional group is the atom or group in a molecule most responsible for the reaction the compound undergoes under a prescribed set of conditions. How the structure of the reactant is transformed to that of the product is what we mean by the reaction mechanism. • Organic compounds are grouped into families according to the functional groups they contain. The most important family is alcohols. Alcohols and alkyl halides are especially useful because they are versatile starting materials for preparing numerous other families. • Diols are alcohols in which two hydroxyl groups (-OH) are present; thio. Is are compounds that contain an -SH group. Phenols, compounds of the type Ar. OH, share many properties in common with alcohols but are sufficiently different from them.
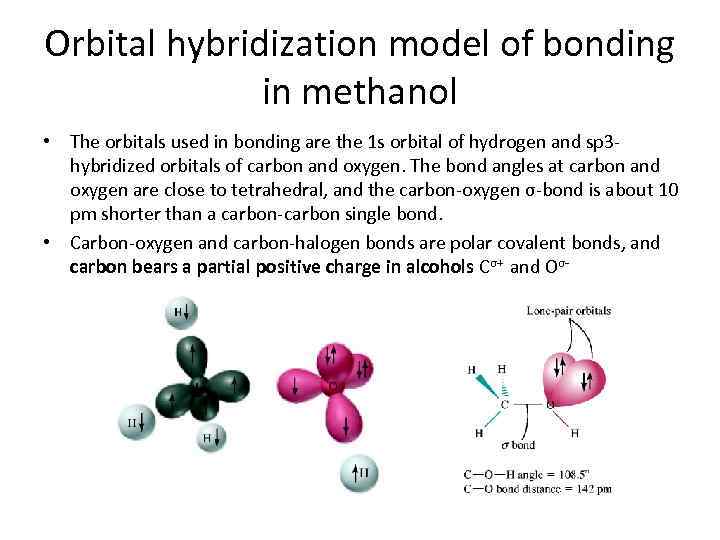
Orbital hybridization model of bonding in methanol • The orbitals used in bonding are the 1 s orbital of hydrogen and sp 3 hybridized orbitals of carbon and oxygen. The bond angles at carbon and oxygen are close to tetrahedral, and the carbon-oxygen σ-bond is about 10 pm shorter than a carbon-carbon single bond. • Carbon-oxygen and carbon-halogen bonds are polar covalent bonds, and carbon bears a partial positive charge in alcohols Cσ+ and Oσ-

Solubility in Water • Low-molecular- weight alcohols (methyl, n-propyl, and isopropyl) are soluble in water in all proportions. Their ability to participate in intermolecular hydrogen bonding not only affects the boiling points of alcohols, but also enhances their water solubility. Hydrogen-bonded networks in which alcohol and water molecules associate with one another, replace the alcohol-alcohol and water hydrogen-bonded networks present in the pure substances. Higher alcohols become more "hydrocarbonlike" and less water-soluble. 1 -Octanol, for example, dissolves to the extent of only 1 m. L in 2000 m. L of water. As the alkyl chain gets longer, the hydrophobic effect becomes more important, to the point that it, more than hydrogen bonding, governs the solubility of alcohols.
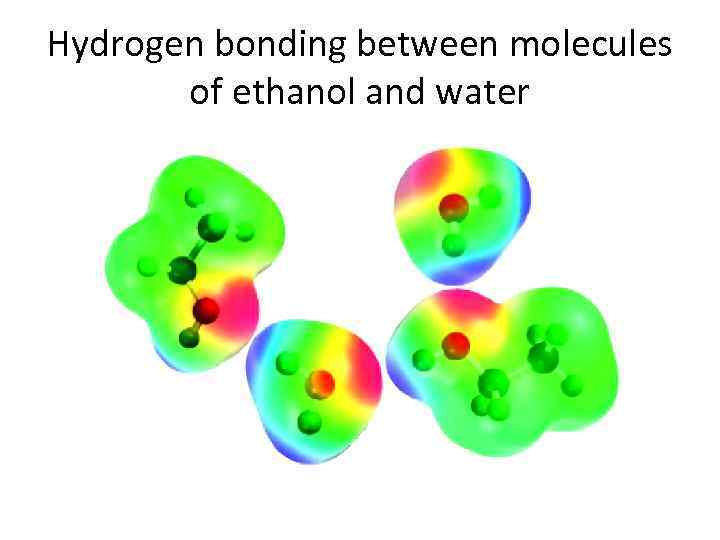
Hydrogen bonding between molecules of ethanol and water
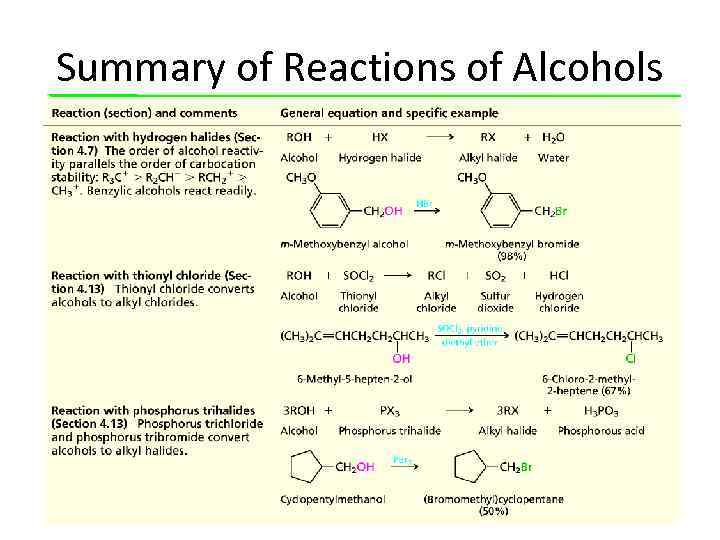
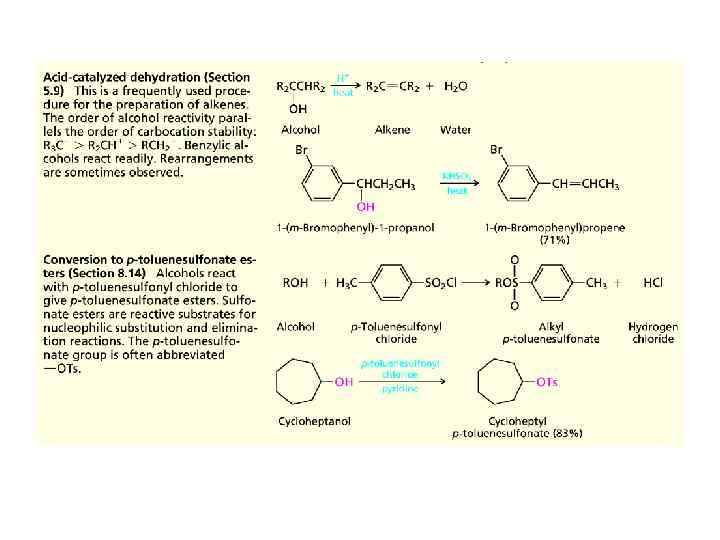
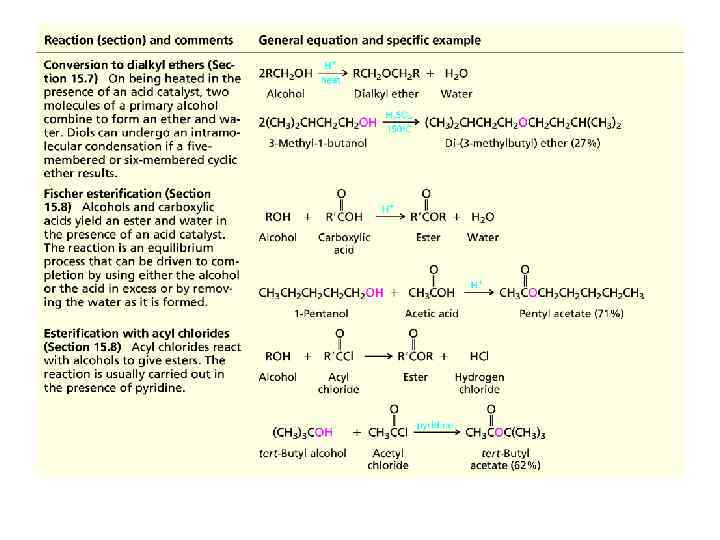
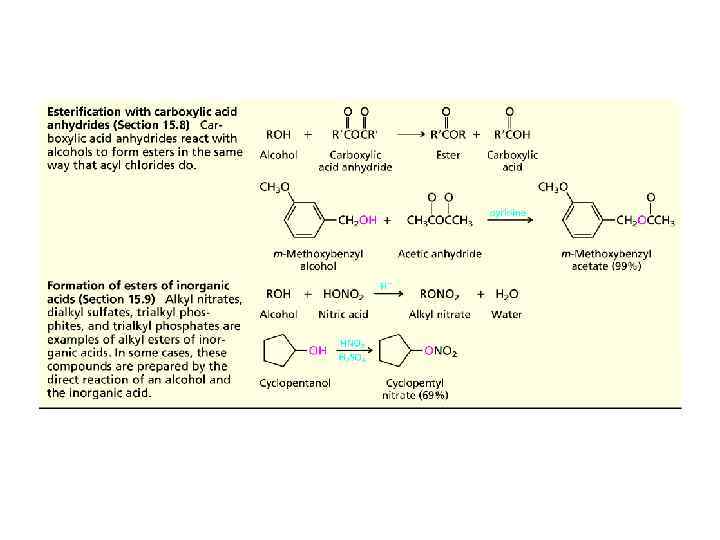
Summary of Reactions of Alcohols
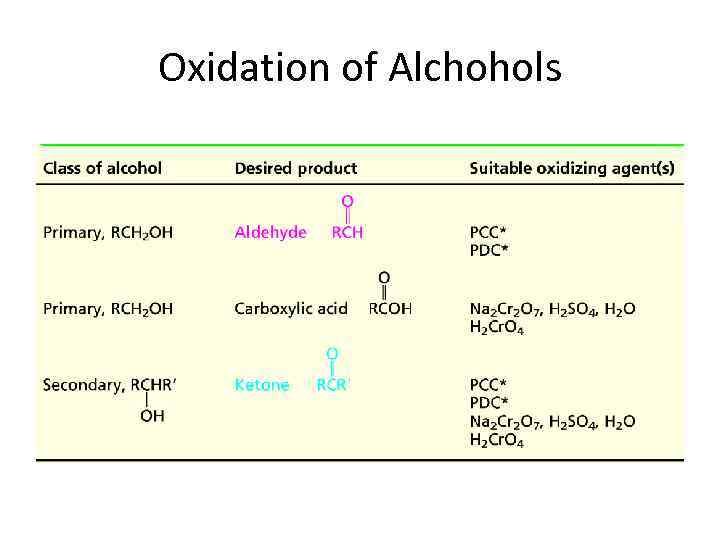
Oxidation of Alchohols
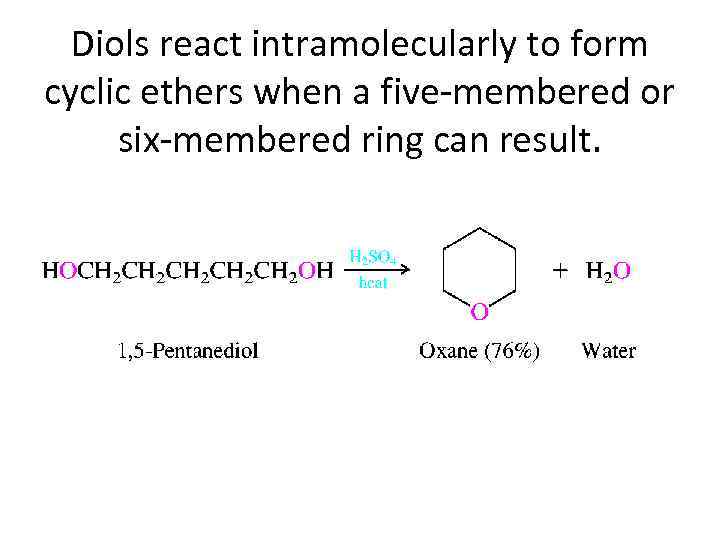
Diols react intramolecularly to form cyclic ethers when a five-membered or six-membered ring can result.

THIOLS • Sulfur lies just below oxygen in the periodic table, and many oxygen-containing organic compounds have sulfur analogs. The sulfur analogs of alcohols (ROH) are thio. Is (RSH). Thiols are given substitutive IUPAC names by appending the suffix -thiol to the name of the corresponding alkane, numbering the chain in the direction that gives the lower locant to the carbon that bears the -SH group. When the -SH group is named as a substituent, it is called a mercapto group. It is also often referred to as a sulfhydryl group, but this is a generic term, not used in systematic nomenclature.
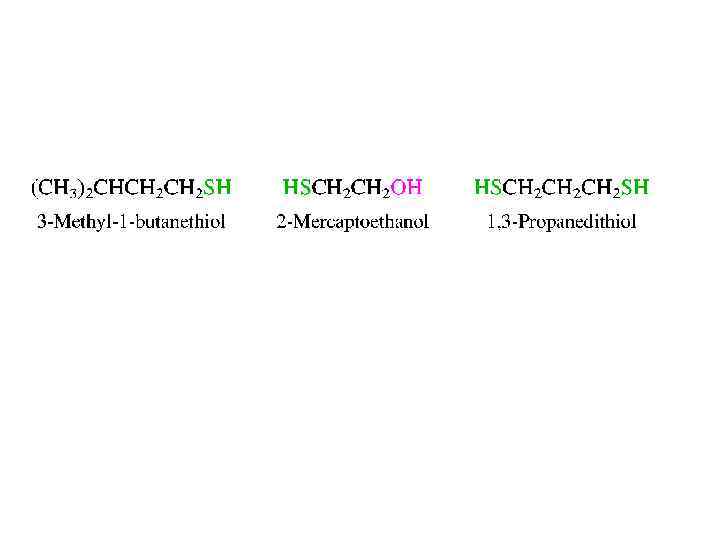

• The S-H bond is less polar than the O-H bond. • In spite of S-H bonds being less polar than O-H bonds, thiols are stronger acids than alcohols. This is largely because S-H bonds are weaker than O-H bonds.
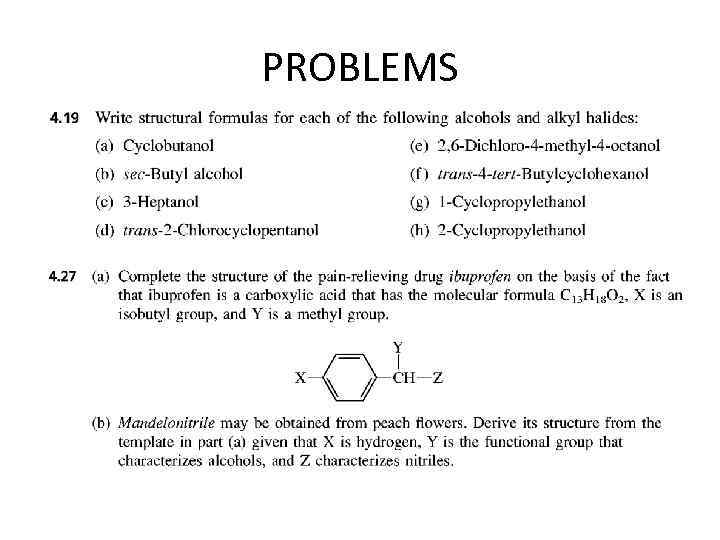
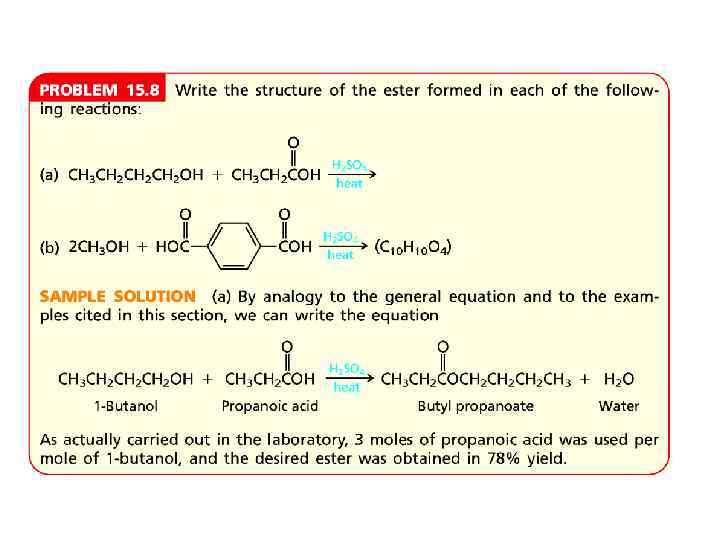
PROBLEMS
Low-molecular biologic compounds 5.pptx