Low-molecular biologic compounds 2.pptx
- Количество слайдов: 20
Low-molecular biologic compounds Lecture #2

Table of contents ALKANES sp 3 HYBRIDIZATION AND BONDING IN METHANE ISOMERIC ALKANES CHEMICAL PROPERTIES. COMBUSTION OF ALKANES • sp 2 HYBRIDIZATION AND BONDING IN ETHYLENE • sp HYBRIDIZATION AND BONDING IN ACETYLENE • PROBLEMS • •

ALKANES • Alkanes have the general molecular formula Cn H 2 n+2. The simplest one, methane (CH 4) , is also the most abundant. Large amounts are present in our atmosphere, in the ground, and in the oceans. • Ethane ( 10%) is the second and propane ( 5%) the third most abundant component of natural gas, which is 75% methane.
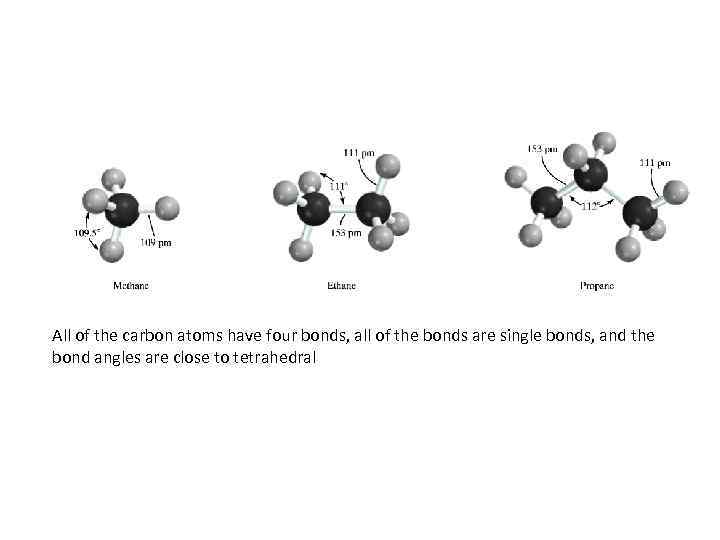
All of the carbon atoms have four bonds, all of the bonds are single bonds, and the bond angles are close to tetrahedral

sp 3 HYBRIDIZATION AND BONDING IN METHANE • methane is CH 4 and that the four bonds to carbon are directed toward the corners of a tetrahedron. Valence bond theory is based on the overlap of half-filled orbitals of the connected atoms, but with an electron configuration of 1 s 22 s 2 2 px 12 py 1 carbon has only two half-filled orbitals. • It is obtained by mixing ("hybridizing") the 2 s, 2 px, 2 py, and 2 pz orbitals, four new orbitals.
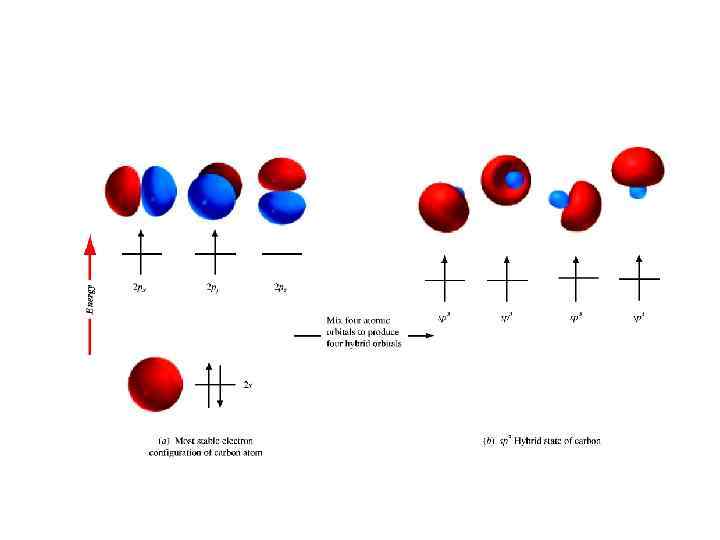

These four new orbitals are called sp 3 hybrid orbitals because they come from one s orbital and three p orbitals. Each sp 3 hybrid orbital has 25% s character and 75% p character. Among their most important features are the following: 1. All four sp 3 orbitals are of equal energy. 2. 2. The axes of the sp 3 orbitals point toward the corners of a tetrahedron. Therefore, sp 3 hybridization of carbon is consistent with the tetrahedral structure of methane. 3. Bonds involving sp 3 hybrid orbitals of carbon are stronger than those involving unhybridized 2 s or 2 p orbitals.
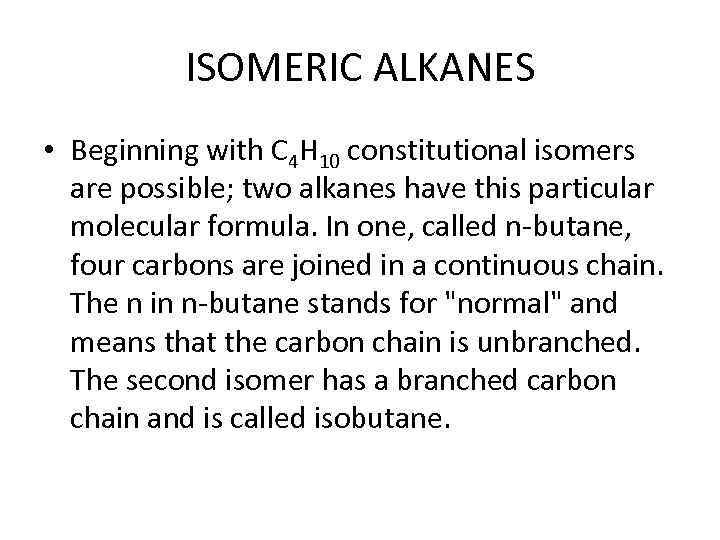
ISOMERIC ALKANES • Beginning with C 4 H 10 constitutional isomers are possible; two alkanes have this particular molecular formula. In one, called n-butane, four carbons are joined in a continuous chain. The n in n-butane stands for "normal" and means that the carbon chain is unbranched. The second isomer has a branched carbon chain and is called isobutane.
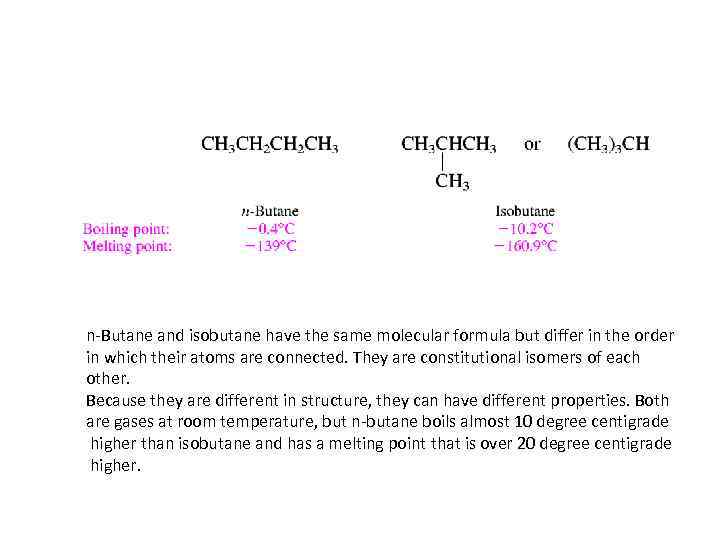
n-Butane and isobutane have the same molecular formula but differ in the order in which their atoms are connected. They are constitutional isomers of each other. Because they are different in structure, they can have different properties. Both are gases at room temperature, but n-butane boils almost 10 degree centigrade higher than isobutane and has a melting point that is over 20 degree centigrade higher.

The Number of Constitutionally Isomeric Alkanes of Particular Molecular Formulas

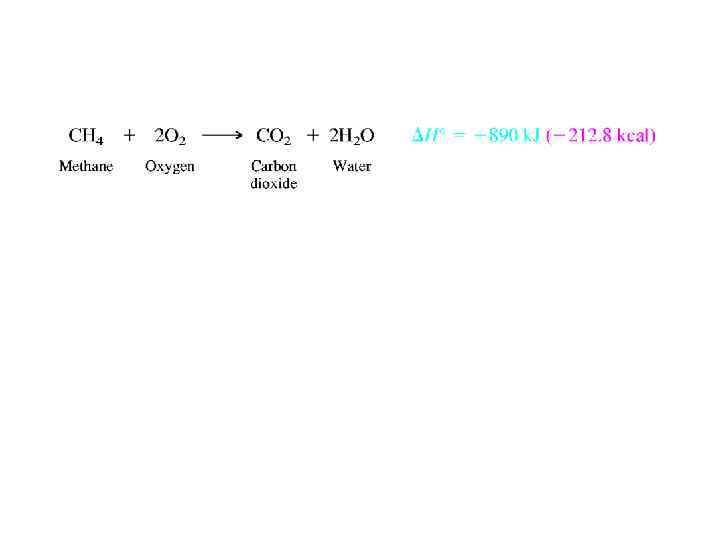
CHEMICAL PROPERTIES. COMBUSTION OF ALKANES • An older name for alkanes is paraffin hydrocarbons. Paraffin is derived from the Latin words parum affinis ("with little affinity") and testifies to the low level of reactivity of alkanes. • Burning in air (combustion) is the best known and most important example. Combustion of hydrocarbons is exothermic and gives carbon dioxide and water as the products.

sp 2 HYBRIDIZATION AND BONDING IN ETHYLENE • The hybridization scheme is determined by the number of atoms to which carbon is directly attached. In sp 2 hybridization, four atoms are attached to carbon by bonds, and so four equivalent sp 2 hybrid orbitals are required. In ethylene, three atoms are attached to each carbon, so three equivalent hybrid orbitals are needed. These three orbitals are generated by mixing the carbon 2 s orbital with two of the 2 p orbitals and are called sp 2 hybrid orbitals. One of the 2 p orbitals is left unhybridized. The three sp 2 orbitals are of equal energy; each has one-third s character and two-thirds p character.
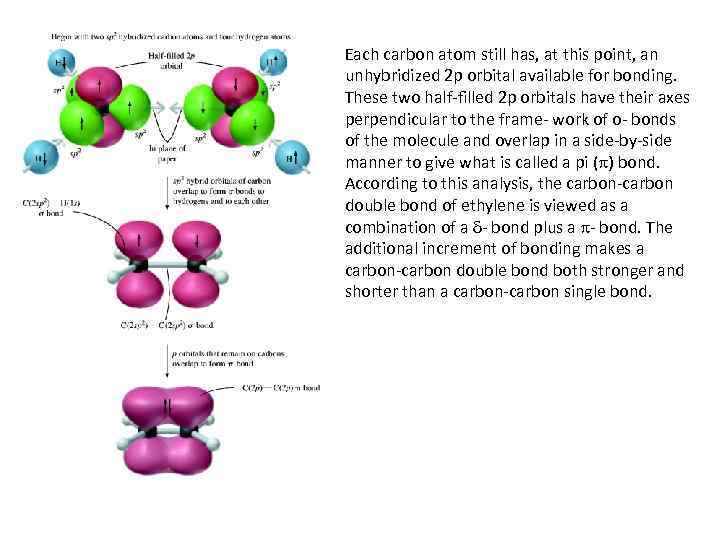
Each carbon atom still has, at this point, an unhybridized 2 p orbital available for bonding. These two half-filled 2 p orbitals have their axes perpendicular to the frame- work of o- bonds of the molecule and overlap in a side-by-side manner to give what is called a pi ( ) bond. According to this analysis, the carbon-carbon double bond of ethylene is viewed as a combination of a - bond plus a - bond. The additional increment of bonding makes a carbon-carbon double bond both stronger and shorter than a carbon-carbon single bond.

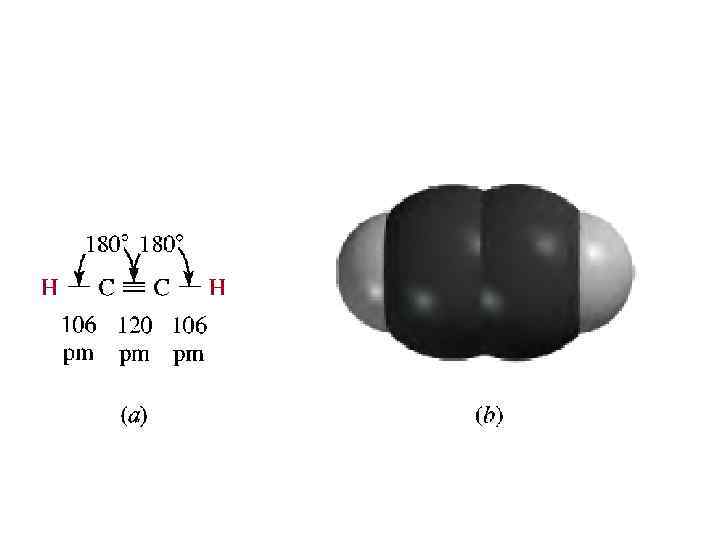
sp HYBRIDIZATION AND BONDING IN ACETYLENE • One more hybridization scheme is important in organic chemistry. It is called sp hybridization and applies when carbon is directly bonded to two atoms, as in acetylene. • Each carbon in acetylene is bonded to two other atoms, the orbital hybridization model requires each carbon to have two equivalent orbitals available for -bonds. • The carbon 2 s orbital and one of its 2 p orbitals combine to generate two sp hybrid orbitals.
SUMMARY • The classes of hydrocarbons are alkanes, alkenes, alkynes, and arenes. Alkanes are hydrocarbons in which all of the bonds are single bonds and are characterized by the molecular formula Cn. H 2 n+2. • Bonding in methane is most often described by an orbital hybridization model, which is a modified form of valence bond theory. Four equivalent sp 3 hybrid orbitals of carbon are generated by mixing the 2 s, 2 px, 2 py, and 2 pz orbitals. Overlap of each half-filled sp 3 hybrid orbital with a half-filled hydrogen 1 s orbital gives a - bond. • The alkanes have constitutionally isomeric. One has an unbranched chain and is called normale; the other has a branched chain and is called iso.

• Natural gas is an abundant source of methane, and propane. Petroleum is a liquid mixture of many hydrocarbons, including alkanes. Alkanes also occur naturally in the waxy coating of leaves and fruits. • Alkanes and cycloalkanes are nonpolar and insoluble in water. • Alkanes and cycloalkanes burn in air to give carbon dioxide, water, and heat. This process is called combustion. • The heat evolved on burning an alkane increases with the number of carbon atoms. The relative stability of isomers may be determined by comparing their respective heats of combustion. The more stable of two isomers has the lower heat of combustion.
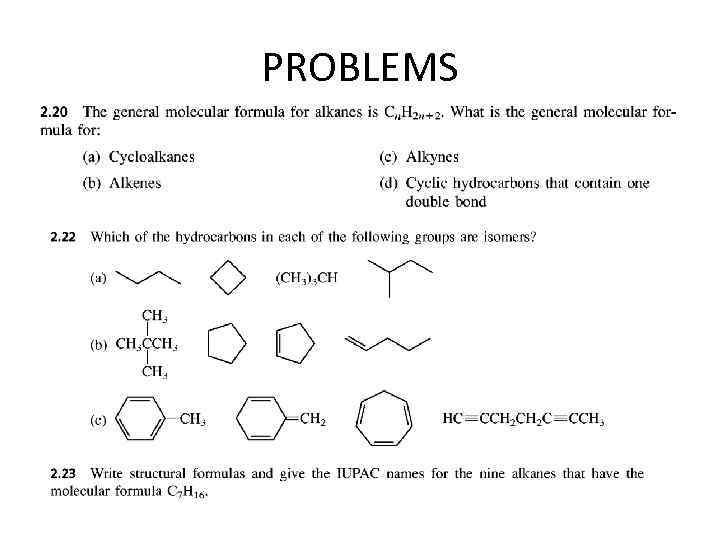
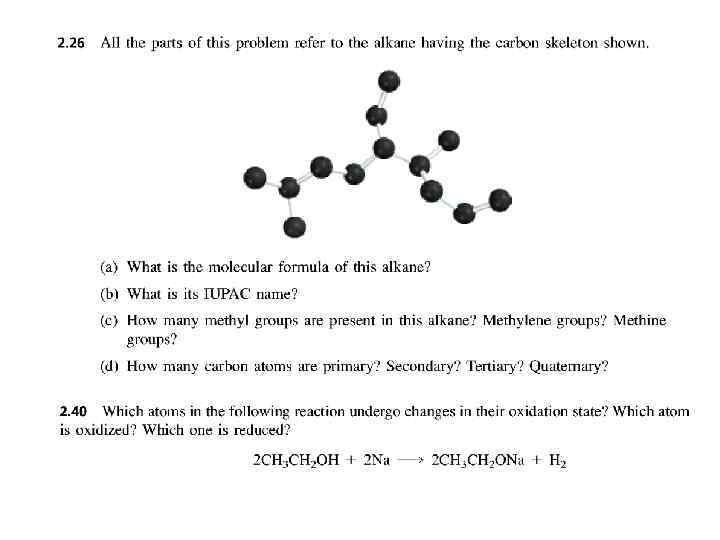
PROBLEMS
Low-molecular biologic compounds 2.pptx