Low-molecular biologic compounds 1.pptx
- Количество слайдов: 19
Low-molecular biologic compounds Lecture #1

Table of contents INTRODUCTION STRUCTURE DETERMINES PROPERTIES ACIDS AND BASES: THE ARRHENIUS VIEW ACIDS AND BASES: THE BRONSTED-LOWRY VIEW • PROBLEMS • •

Introduction • Low-molecular biologic compounds are carbon compounds. As one of the tools that fostered an increased understanding of our world.
Structure determines properties • The properties of a substance depend on the atoms it contains and the way the atoms are connected. Someone who is trained in chemistry can look at a structural formula of a substance and tell you a lot about its properties.

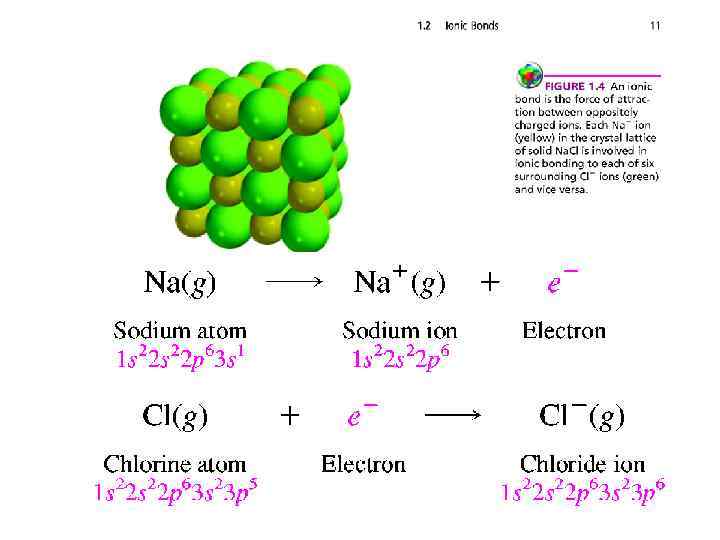
IONIC BONDS • Atoms combine with one another to give compounds having properties different from the atoms they contain. The attractive force between atoms in a compound is a chemical bond. One type of chemical bond, called an ionic bond, is the force of attraction between oppositely charged species (ions). Ions that are positively charged are referred to as cations; those that are negatively charged are anions.

COVALENT BONDS • The covalent, or shared electron pair, model of chemical bonding was first suggested by G. N. Lewis of the University of California in 1916. Lewis proposed that a sharing of two electrons by two hydrogen atoms permits each one to have a stable closed-shell electron configuration analogous to helium.

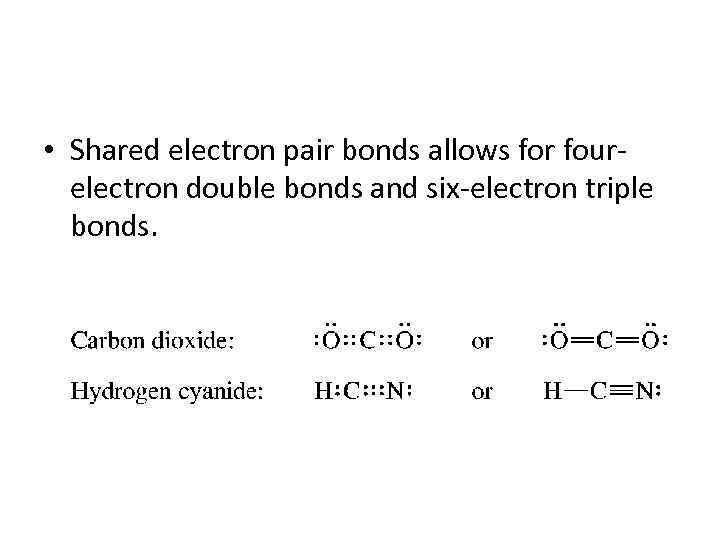
• Shared electron pair bonds allows for fourelectron double bonds and six-electron triple bonds.

POLAR COVALENT BONDS AND ELECTRONEGATIVITY • Electrons in covalent bonds are not necessarily shared equally by the two atoms that they connect. If one atom has a greater tendency to attract electrons toward itself than the other, we say the electron distribution is polarized, and the bond is referred to as a polar covalent bond. Hydrogen fluoride, for example, has a polar covalent bond. Because fluorine attracts electrons more strongly than hydrogen, the electrons in the H-F bond are pulled toward fluorine, giving it a partial negative charge, and away from hydrogen giving it a partial positive charge.
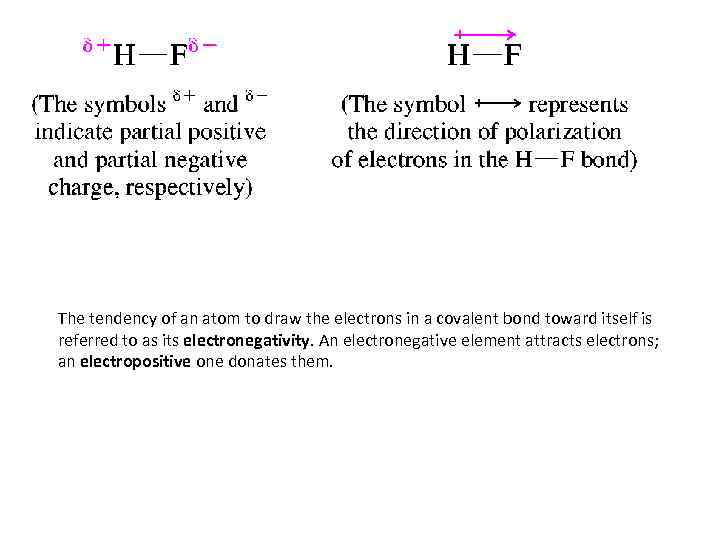
The tendency of an atom to draw the electrons in a covalent bond toward itself is referred to as its electronegativity. An electronegative element attracts electrons; an electropositive one donates them.
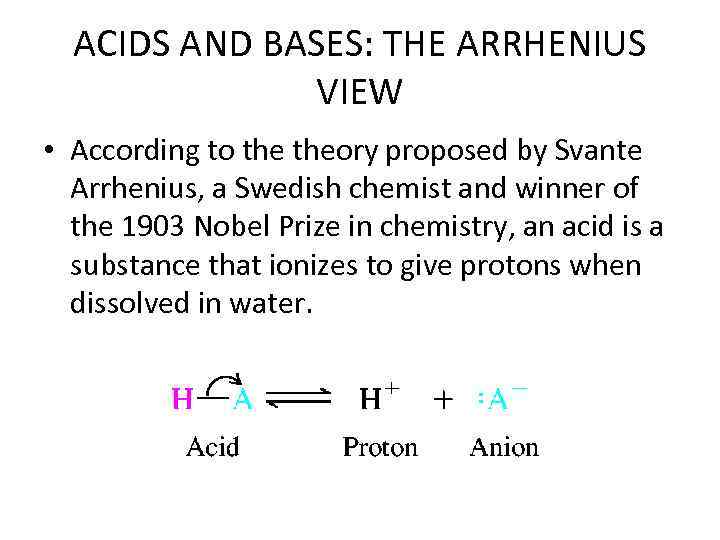
ACIDS AND BASES: THE ARRHENIUS VIEW • According to theory proposed by Svante Arrhenius, a Swedish chemist and winner of the 1903 Nobel Prize in chemistry, an acid is a substance that ionizes to give protons when dissolved in water.
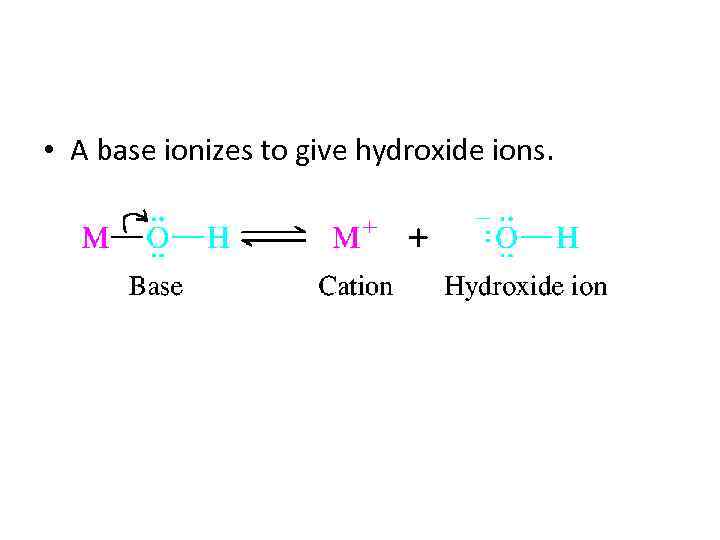
• A base ionizes to give hydroxide ions.
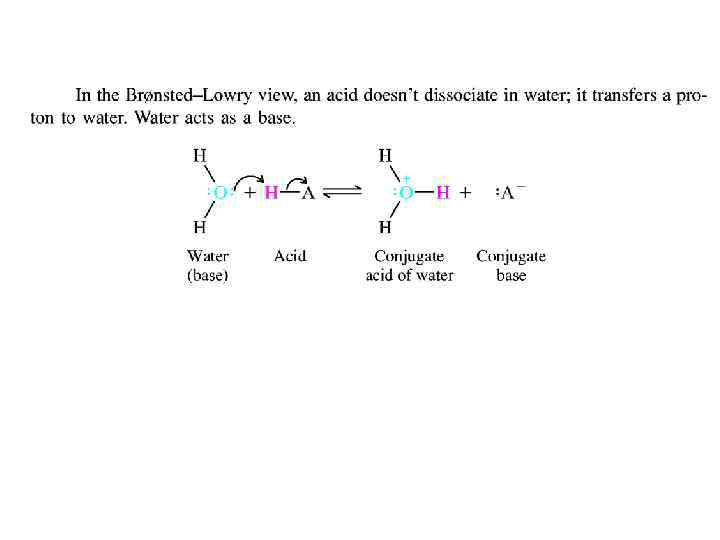
ACIDS AND BASES: THE BRONSTEDLOWRY VIEW • A more general theory of acids and bases was devised independently by Johannes Br 0 n- sted (Denmark) and Thomas M. Lowry (England) in 1923. In the Br 0 nsted-Lowry approach, an acid is a proton donor, and a base is a proton acceptor. The reaction that occurs between an acid and a base is proton transfen
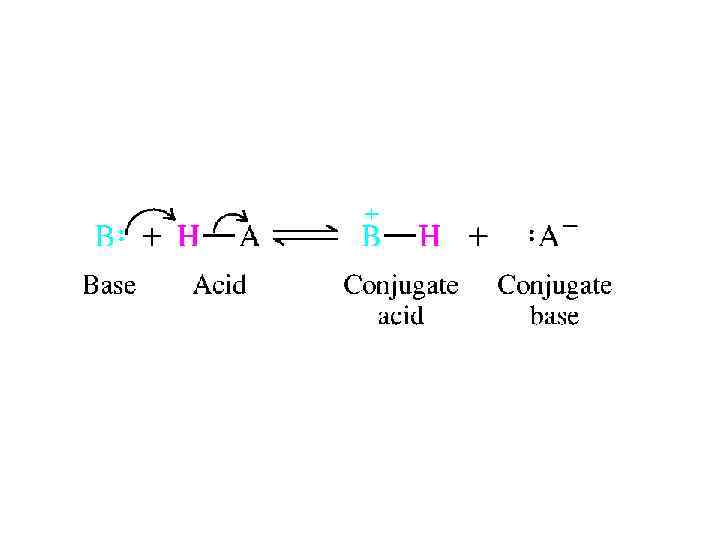
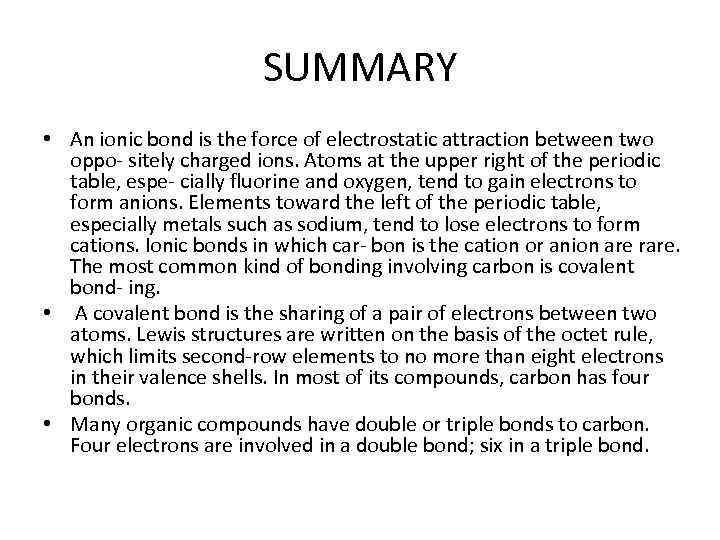
SUMMARY • An ionic bond is the force of electrostatic attraction between two oppo- sitely charged ions. Atoms at the upper right of the periodic table, espe- cially fluorine and oxygen, tend to gain electrons to form anions. Elements toward the left of the periodic table, especially metals such as sodium, tend to lose electrons to form cations. Ionic bonds in which car- bon is the cation or anion are rare. The most common kind of bonding involving carbon is covalent bond- ing. • A covalent bond is the sharing of a pair of electrons between two atoms. Lewis structures are written on the basis of the octet rule, which limits second-row elements to no more than eight electrons in their valence shells. In most of its compounds, carbon has four bonds. • Many organic compounds have double or triple bonds to carbon. Four electrons are involved in a double bond; six in a triple bond.
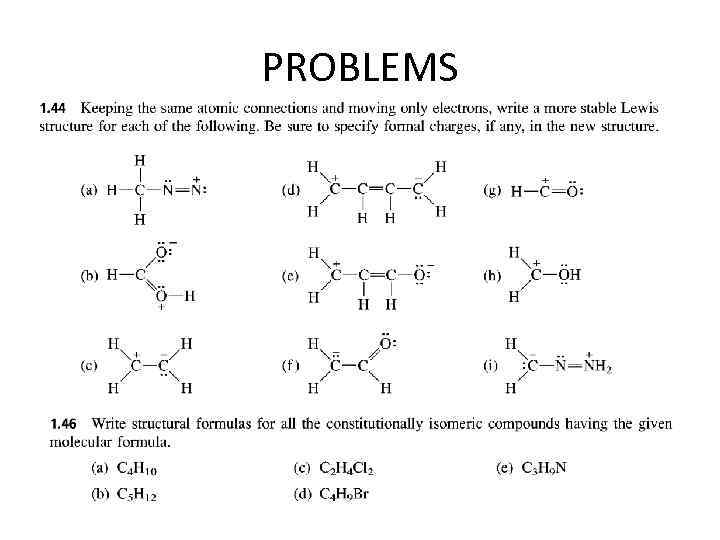
PROBLEMS

Low-molecular biologic compounds 1.pptx