bb59f2c0b4da3972fed6ef1b2a977a79.ppt
- Количество слайдов: 27
 Louisville APL Diagnostics, Inc UPDATE IN THE EVALUATION OF ANTIPHOSPHOLIPID ANTIBODIES SILVIA S. PIERANGELI, Ph. D LOUISVILLE APL DIAGNOSTICS, INC DORAVILLE, GA USA
Louisville APL Diagnostics, Inc UPDATE IN THE EVALUATION OF ANTIPHOSPHOLIPID ANTIBODIES SILVIA S. PIERANGELI, Ph. D LOUISVILLE APL DIAGNOSTICS, INC DORAVILLE, GA USA
 1984 -1985 Louisville APL Diagnostics n Problems with false positive results n a. CL positive in a wide variety of infectious diseases and in non-APS related autoimmune diseases.
1984 -1985 Louisville APL Diagnostics n Problems with false positive results n a. CL positive in a wide variety of infectious diseases and in non-APS related autoimmune diseases.
 Louisville APL Diagnostics
Louisville APL Diagnostics
 ANTIPHOSPHOLIPID SYNDROME Diagnostic Testing Louisville APL Diagnostics n 1. ) Laboratory confirmation is vital for diagnosis of APS. n 2. ) Anticardiolipin antibody and lupus anticoagulant tests together diagnose vast majority of APS patients-these tests are relatively standard and well understood. n 3. ) More specific test for APS should be developed and broadly tested (include anti-cardiolipin positive/APS negative sera in testing). n 4. ) More specific tests may enable confirmation of diagnosis in equivocal and unusual clinical presentations to APS.
ANTIPHOSPHOLIPID SYNDROME Diagnostic Testing Louisville APL Diagnostics n 1. ) Laboratory confirmation is vital for diagnosis of APS. n 2. ) Anticardiolipin antibody and lupus anticoagulant tests together diagnose vast majority of APS patients-these tests are relatively standard and well understood. n 3. ) More specific test for APS should be developed and broadly tested (include anti-cardiolipin positive/APS negative sera in testing). n 4. ) More specific tests may enable confirmation of diagnosis in equivocal and unusual clinical presentations to APS.
 ANTIPHOSPHOLIPID SYNDROME Laboratory Tests Louisville APL Diagnostics n Tests should be positive in most patients with disorder (sensitivity). n Tests should be largely confined to patients with disorder (specificity) n Tests should be performed reproducibly in most laboratories.
ANTIPHOSPHOLIPID SYNDROME Laboratory Tests Louisville APL Diagnostics n Tests should be positive in most patients with disorder (sensitivity). n Tests should be largely confined to patients with disorder (specificity) n Tests should be performed reproducibly in most laboratories.
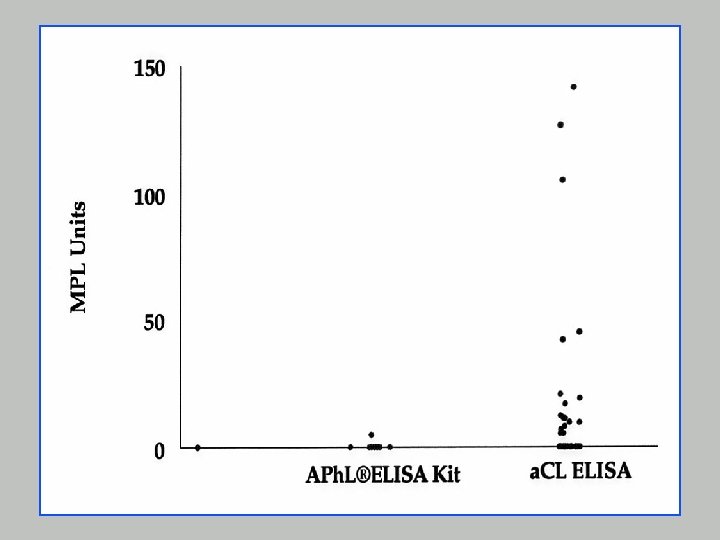
 APh. L®ELISA Kit - Principle Louisville APL Diagnostics n Based on observation that antiphospholipid antibodies crossreact with negatively charged phospholipids but syphilis and other infectious diseases sera largely limited to cardiolipin binding (no crossreactivity) n Construction of a kit with negatively charged phospholipids might eliminate non-specific binding.
APh. L®ELISA Kit - Principle Louisville APL Diagnostics n Based on observation that antiphospholipid antibodies crossreact with negatively charged phospholipids but syphilis and other infectious diseases sera largely limited to cardiolipin binding (no crossreactivity) n Construction of a kit with negatively charged phospholipids might eliminate non-specific binding.
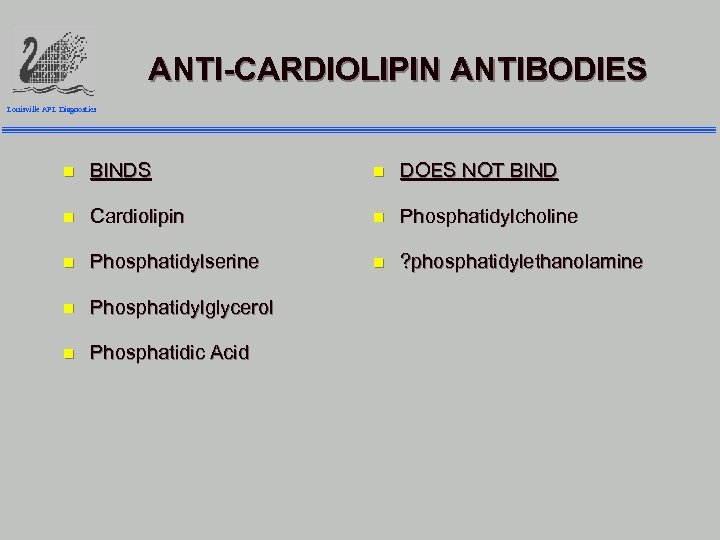 ANTI-CARDIOLIPIN ANTIBODIES Louisville APL Diagnostics n BINDS n DOES NOT BIND n Cardiolipin n Phosphatidylcholine n Phosphatidylserine n ? phosphatidylethanolamine n Phosphatidylglycerol n Phosphatidic Acid
ANTI-CARDIOLIPIN ANTIBODIES Louisville APL Diagnostics n BINDS n DOES NOT BIND n Cardiolipin n Phosphatidylcholine n Phosphatidylserine n ? phosphatidylethanolamine n Phosphatidylglycerol n Phosphatidic Acid

 Relative Sensitivity/Specificity Louisville APL Diagnostics Sensitivity of the assays
Relative Sensitivity/Specificity Louisville APL Diagnostics Sensitivity of the assays
 Prevalence of a. CL, APL and anti- 2 GPI in various infectious diseases Louisville APL Diagnostics
Prevalence of a. CL, APL and anti- 2 GPI in various infectious diseases Louisville APL Diagnostics
 Evaluation of different assays to diagnose APS: Multicenter study. Louisville APL Diagnostics n Samples tested: n APS=56 n Healthy controls=145 n Non-APS including infectious diseases (syphilis, HIV, other autoimmune diseases)= 206.
Evaluation of different assays to diagnose APS: Multicenter study. Louisville APL Diagnostics n Samples tested: n APS=56 n Healthy controls=145 n Non-APS including infectious diseases (syphilis, HIV, other autoimmune diseases)= 206.
 Evaluation of different assays to diagnose APS. Louisville APL Diagnostics n *Centers involved and assays utilized: n Univ Texas San Antonio: APh. L® ELISA test. n Morehouse School of Medicine: a. CL (in-house) and anti- 2 GPI (INOVA) n University of Milan: in-house anti- 2 GPI and anti-prothrombin. n * participating centers were blind to the identity of the samples n Univ of Utah: Coordinating center.
Evaluation of different assays to diagnose APS. Louisville APL Diagnostics n *Centers involved and assays utilized: n Univ Texas San Antonio: APh. L® ELISA test. n Morehouse School of Medicine: a. CL (in-house) and anti- 2 GPI (INOVA) n University of Milan: in-house anti- 2 GPI and anti-prothrombin. n * participating centers were blind to the identity of the samples n Univ of Utah: Coordinating center.
 Louisville APL Diagnostics Assay Sensitivity and Specificity of the assays APS samples Healthy controls Other diseases Non-APS combined Specificity Positive Predictive value # positives 42/56 (75%) 1/150 (0. 6%) 27/206 (13%) 28/356 (7. 8%) 91. 8% 59. 1% a GPI (INOVA) 44/56 (78. 5%) 15/143 (10. 5%) 58/197 (29. 4%) 73/340 (21. 4%) 78. 6% 37. 6% a. PT (inhouse) 13/54 (24%) 15/132 (11. 3%) 19/178 (10. 7%) 34/310 (10. 9%) 88. 4% 25. 4% 43/54 13/145 9/192 (4. 6%) 22/337 (6. 5%) 93. 2% 65. 1% (79. 6%) (8. 9%) 45/56 (80. 3%) 4/147 (2. 7%) 14/204 (6. 8%) 18/351 (5. 1%) 94. 0% 68. 1% # positives (sensitivity) a. CL (in-house) a GP(inhouse) APh. L ELISA kit
Louisville APL Diagnostics Assay Sensitivity and Specificity of the assays APS samples Healthy controls Other diseases Non-APS combined Specificity Positive Predictive value # positives 42/56 (75%) 1/150 (0. 6%) 27/206 (13%) 28/356 (7. 8%) 91. 8% 59. 1% a GPI (INOVA) 44/56 (78. 5%) 15/143 (10. 5%) 58/197 (29. 4%) 73/340 (21. 4%) 78. 6% 37. 6% a. PT (inhouse) 13/54 (24%) 15/132 (11. 3%) 19/178 (10. 7%) 34/310 (10. 9%) 88. 4% 25. 4% 43/54 13/145 9/192 (4. 6%) 22/337 (6. 5%) 93. 2% 65. 1% (79. 6%) (8. 9%) 45/56 (80. 3%) 4/147 (2. 7%) 14/204 (6. 8%) 18/351 (5. 1%) 94. 0% 68. 1% # positives (sensitivity) a. CL (in-house) a GP(inhouse) APh. L ELISA kit
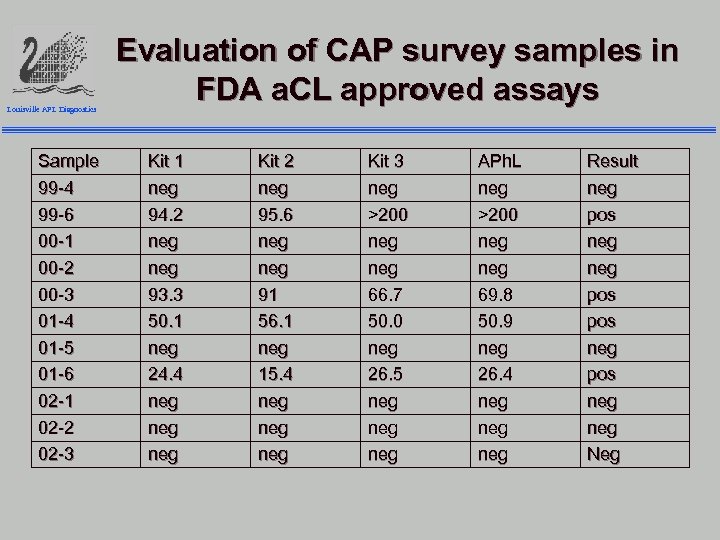 Louisville APL Diagnostics Sample 99 -4 99 -6 00 -1 00 -2 00 -3 01 -4 01 -5 01 -6 02 -1 02 -2 02 -3 Evaluation of CAP survey samples in FDA a. CL approved assays Kit 1 neg 94. 2 neg 93. 3 50. 1 neg 24. 4 neg neg Kit 2 neg 95. 6 neg 91 56. 1 neg 15. 4 neg neg Kit 3 neg >200 neg 66. 7 50. 0 neg 26. 5 neg neg APh. L neg >200 neg 69. 8 50. 9 neg 26. 4 neg neg Result neg pos neg pos neg Neg
Louisville APL Diagnostics Sample 99 -4 99 -6 00 -1 00 -2 00 -3 01 -4 01 -5 01 -6 02 -1 02 -2 02 -3 Evaluation of CAP survey samples in FDA a. CL approved assays Kit 1 neg 94. 2 neg 93. 3 50. 1 neg 24. 4 neg neg Kit 2 neg 95. 6 neg 91 56. 1 neg 15. 4 neg neg Kit 3 neg >200 neg 66. 7 50. 0 neg 26. 5 neg neg APh. L neg >200 neg 69. 8 50. 9 neg 26. 4 neg neg Result neg pos neg pos neg Neg
 CAP survey results: Ig. G APh. L ELISA Louisville APL Diagnostics
CAP survey results: Ig. G APh. L ELISA Louisville APL Diagnostics
 CAP survey: Ig. M APh. L Louisville APL Diagnostics
CAP survey: Ig. M APh. L Louisville APL Diagnostics
 APh. L®ELISA kit Louisville APL Diagnostics n Six pre-diluted calibrators (ready to use) n 3 x 30 minutes incubation steps n Peroxidase and alkaline phosphatase systems available n All other reagents in “ready-to-use” form n Determination of Ig. G and Ig. M a. PL antibodies n 12 month expiration date. n Good to be used in automated systems.
APh. L®ELISA kit Louisville APL Diagnostics n Six pre-diluted calibrators (ready to use) n 3 x 30 minutes incubation steps n Peroxidase and alkaline phosphatase systems available n All other reagents in “ready-to-use” form n Determination of Ig. G and Ig. M a. PL antibodies n 12 month expiration date. n Good to be used in automated systems.
 APh. L®ELISA kit Louisville APL Diagnostics n Antigen composed fo mixture of phospholipids ß 2 GP 1 n Sensitivity of APS (greater than 90%) n More specific than anticardiolipin test and at least as specific (or more) compared to anti-ß 2 GP 1 n Incorporation of an in-house positive control n Can be utilized for first line testing, and certainly important in confirmation of APS
APh. L®ELISA kit Louisville APL Diagnostics n Antigen composed fo mixture of phospholipids ß 2 GP 1 n Sensitivity of APS (greater than 90%) n More specific than anticardiolipin test and at least as specific (or more) compared to anti-ß 2 GP 1 n Incorporation of an in-house positive control n Can be utilized for first line testing, and certainly important in confirmation of APS
 Conclusion Louisville APL Diagnostics n Since sensitivity of APh. L®ELISA kit is comparable to anticardiolipin test, it can be used for first line testing in place of the anticardiolipin test. The APh. L®ELISA kit will enable greater specificity - APS diagnosis
Conclusion Louisville APL Diagnostics n Since sensitivity of APh. L®ELISA kit is comparable to anticardiolipin test, it can be used for first line testing in place of the anticardiolipin test. The APh. L®ELISA kit will enable greater specificity - APS diagnosis
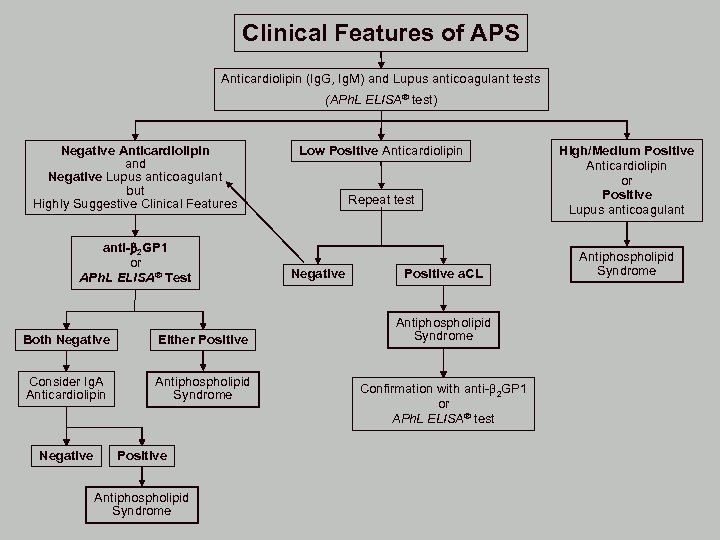 Clinical Features of APS Anticardiolipin (Ig. G, Ig. M) and Lupus anticoagulant tests (APh. L ELISA® test) Negative Anticardiolipin and Negative Lupus anticoagulant but Highly Suggestive Clinical Features anti- 2 GP 1 or APh. L ELISA® Test Both Negative Either Positive Consider Ig. A Anticardiolipin Antiphospholipid Syndrome Negative Positive Antiphospholipid Syndrome Low Positive Anticardiolipin Repeat test Negative Positive a. CL Antiphospholipid Syndrome Confirmation with anti- 2 GP 1 or APh. L ELISA® test High/Medium Positive Anticardiolipin or Positive Lupus anticoagulant Antiphospholipid Syndrome
Clinical Features of APS Anticardiolipin (Ig. G, Ig. M) and Lupus anticoagulant tests (APh. L ELISA® test) Negative Anticardiolipin and Negative Lupus anticoagulant but Highly Suggestive Clinical Features anti- 2 GP 1 or APh. L ELISA® Test Both Negative Either Positive Consider Ig. A Anticardiolipin Antiphospholipid Syndrome Negative Positive Antiphospholipid Syndrome Low Positive Anticardiolipin Repeat test Negative Positive a. CL Antiphospholipid Syndrome Confirmation with anti- 2 GP 1 or APh. L ELISA® test High/Medium Positive Anticardiolipin or Positive Lupus anticoagulant Antiphospholipid Syndrome
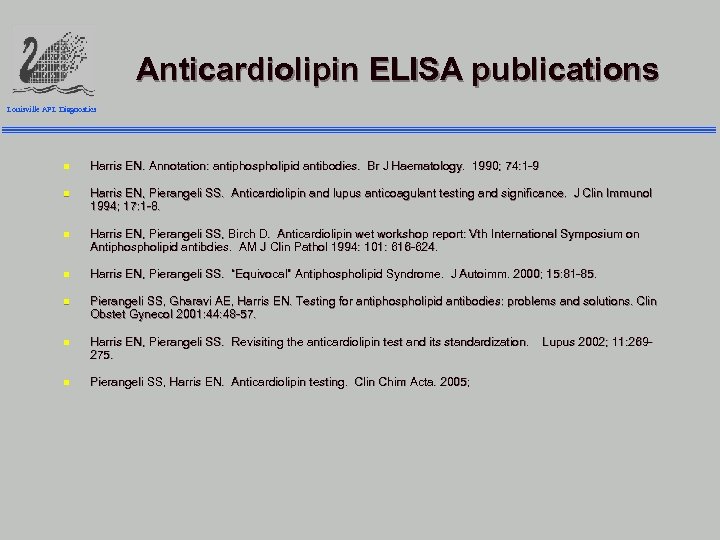 Anticardiolipin ELISA publications Louisville APL Diagnostics n Harris EN. Annotation: antiphospholipid antibodies. Br J Haematology. 1990; 74: 1 -9 Haematology. n Harris EN, Pierangeli SS. Anticardiolipin and lupus anticoagulant testing and significance. J Clin Immunol 1994; 17: 1 -8. n Harris EN, Pierangeli SS, Birch D. Anticardiolipin wet workshop report: Vth International Symposium on Antiphospholipid antibdies. AM J Clin Pathol 1994: 101: 616 -624. antibdies. n Harris EN, Pierangeli SS. “Equivocal” Antiphospholipid Syndrome. J Autoimm. 2000; 15: 81 -85. Autoimm. n Pierangeli SS, Gharavi AE, Harris EN. Testing for antiphospholipid antibodies: problems and solutions. Clin Obstet Gynecol 2001: 44: 48 -57. n Harris EN, Pierangeli SS. Revisiting the anticardiolipin test and its standardization. 275. n Pierangeli SS, Harris EN. Anticardiolipin testing. Clin Chim Acta. 2005; Acta. Lupus 2002; 11: 269 -
Anticardiolipin ELISA publications Louisville APL Diagnostics n Harris EN. Annotation: antiphospholipid antibodies. Br J Haematology. 1990; 74: 1 -9 Haematology. n Harris EN, Pierangeli SS. Anticardiolipin and lupus anticoagulant testing and significance. J Clin Immunol 1994; 17: 1 -8. n Harris EN, Pierangeli SS, Birch D. Anticardiolipin wet workshop report: Vth International Symposium on Antiphospholipid antibdies. AM J Clin Pathol 1994: 101: 616 -624. antibdies. n Harris EN, Pierangeli SS. “Equivocal” Antiphospholipid Syndrome. J Autoimm. 2000; 15: 81 -85. Autoimm. n Pierangeli SS, Gharavi AE, Harris EN. Testing for antiphospholipid antibodies: problems and solutions. Clin Obstet Gynecol 2001: 44: 48 -57. n Harris EN, Pierangeli SS. Revisiting the anticardiolipin test and its standardization. 275. n Pierangeli SS, Harris EN. Anticardiolipin testing. Clin Chim Acta. 2005; Acta. Lupus 2002; 11: 269 -
 ELISAs for Ig. G and Ig. M a. PL Louisville APL Diagnostics
ELISAs for Ig. G and Ig. M a. PL Louisville APL Diagnostics
 ELISAs for Ig. G or Ig. M a. PL Louisville APL Diagnostics
ELISAs for Ig. G or Ig. M a. PL Louisville APL Diagnostics
 ELISAs for Ig. A a. PL Louisville APL Diagnostics
ELISAs for Ig. A a. PL Louisville APL Diagnostics
 Louisville APL Diagnostics, Inc web site: www. louisvilleapl. com email: support@louisvilleapl. com
Louisville APL Diagnostics, Inc web site: www. louisvilleapl. com email: support@louisvilleapl. com
 Silvia S. Pierangeli, Ph. D. Louisville APL Diagnostics
Silvia S. Pierangeli, Ph. D. Louisville APL Diagnostics


