4a47e06608a3c9f06d09fb2e7144d029.ppt
- Количество слайдов: 51
 Long Acting Opioids: Challenges in Pharmacotherapy Mary Jeanne Kreek, M. D. The Laboratory of the Biology of Addictive Diseases The Rockefeller University September 10, 2003 Anesthetic Life Support Drugs Advisory Committee Food and Drug Administration Bethesda, MD funded primarily by NIH-NIDA, NIHCRR and NYS OASAS
Long Acting Opioids: Challenges in Pharmacotherapy Mary Jeanne Kreek, M. D. The Laboratory of the Biology of Addictive Diseases The Rockefeller University September 10, 2003 Anesthetic Life Support Drugs Advisory Committee Food and Drug Administration Bethesda, MD funded primarily by NIH-NIDA, NIHCRR and NYS OASAS
 Major Issues/ Problems Related to Physician Use/Prescribing of Long-Acting Mu Opioid Receptor Agonists in Humans A. Lack of adequate or updated medical education concerning pharmacokinetics and pharmacodynamics of long-acting (intrinsic or by formulation) mu opioid receptor agonists and partial agonists. 1) Decrease in formal medical education in classical pharmacology and lack of continuing medical education in pharmacology. 2) Discovery (synthesis) and development of intrinsically longacting mu opioid agonists for treatment of opioid addiction and for treatment of chronic pain (1964 onward). 3) Increase in numbers of short-acting mu opioid agonists now formulated to be long-acting opioids for use in management of pain. Kreek, 2003
Major Issues/ Problems Related to Physician Use/Prescribing of Long-Acting Mu Opioid Receptor Agonists in Humans A. Lack of adequate or updated medical education concerning pharmacokinetics and pharmacodynamics of long-acting (intrinsic or by formulation) mu opioid receptor agonists and partial agonists. 1) Decrease in formal medical education in classical pharmacology and lack of continuing medical education in pharmacology. 2) Discovery (synthesis) and development of intrinsically longacting mu opioid agonists for treatment of opioid addiction and for treatment of chronic pain (1964 onward). 3) Increase in numbers of short-acting mu opioid agonists now formulated to be long-acting opioids for use in management of pain. Kreek, 2003
 Major Issues/ Problems Related to Physician Use/Prescribing of Long-Acting Mu Opioid Receptor Agonists in Humans (continued) B. Lack of adequate (or any) medical school education conerning any of the specific addictions and also medical approaches to assessing persons with ongoing misuse, abuse, or addiction to drugs. 1) Lack of awareness of prevalence of specific additions (10% to 20% in all US population with one or more addiction). 2) Lack of knowledge concerning, e. g. , genetic vulnerabilities; predictable chronic drug use induced changes in brain; environmental (e. g. , set and setting, peer pressure, availability) and host factors (e. g. , psychiatric comorbidity, medical comorbidity, chronic pain) contributing to the vulnerability to develop an addiction. Kreek, 2003
Major Issues/ Problems Related to Physician Use/Prescribing of Long-Acting Mu Opioid Receptor Agonists in Humans (continued) B. Lack of adequate (or any) medical school education conerning any of the specific addictions and also medical approaches to assessing persons with ongoing misuse, abuse, or addiction to drugs. 1) Lack of awareness of prevalence of specific additions (10% to 20% in all US population with one or more addiction). 2) Lack of knowledge concerning, e. g. , genetic vulnerabilities; predictable chronic drug use induced changes in brain; environmental (e. g. , set and setting, peer pressure, availability) and host factors (e. g. , psychiatric comorbidity, medical comorbidity, chronic pain) contributing to the vulnerability to develop an addiction. Kreek, 2003
 Major Issues/ Problems Related to Physician Use/Prescribing of Long-Acting Mu Opioid Receptor Agonists in Humans (continued) C. Secondary physician/healthcare worker and related enforcement issues. 1) Physicians (and related healthcare workers) with inadequate knowledge (and possibly inadequate access to information). 2) Physicians with inadequate time (due to HMO and related current healthcare system) to evaluate each patient carefully and to individualize care *majority of problems probably lay within these two realms* 3) Physicians desiring to (for profit) or willing to (for diverse reasons) become “prescription writers, ” i. e. , illicit practice of medicine. 4) Similar constraints of specific education and knowledge and time constraints often lead to inappropriate enforcement. Kreek, 2003
Major Issues/ Problems Related to Physician Use/Prescribing of Long-Acting Mu Opioid Receptor Agonists in Humans (continued) C. Secondary physician/healthcare worker and related enforcement issues. 1) Physicians (and related healthcare workers) with inadequate knowledge (and possibly inadequate access to information). 2) Physicians with inadequate time (due to HMO and related current healthcare system) to evaluate each patient carefully and to individualize care *majority of problems probably lay within these two realms* 3) Physicians desiring to (for profit) or willing to (for diverse reasons) become “prescription writers, ” i. e. , illicit practice of medicine. 4) Similar constraints of specific education and knowledge and time constraints often lead to inappropriate enforcement. Kreek, 2003
 Prevalence of Specific Drug Abuse and Vulnerability to Develop Addictions National Household Survey and Related Surveys – 1996 – 1999 Alcohol Use – ever Alcoholism ~ 177 million ~ 15 million Cocaine Use – ever Cocaine Addiction ~ 26 million ~ 1 to 2 million Heroin Use – ever Heroin Addiction ~ 2. 5 to 3 million ~ 0. 5 to 1 million Development of Addiction After Self Exposure Alcoholism Cocaine Addiction Heroin Addiction ~ 1 in 10 to 1 in 20 ~ 1 in 3 to 1 in 5 NIDA, SAMHSA Reports
Prevalence of Specific Drug Abuse and Vulnerability to Develop Addictions National Household Survey and Related Surveys – 1996 – 1999 Alcohol Use – ever Alcoholism ~ 177 million ~ 15 million Cocaine Use – ever Cocaine Addiction ~ 26 million ~ 1 to 2 million Heroin Use – ever Heroin Addiction ~ 2. 5 to 3 million ~ 0. 5 to 1 million Development of Addiction After Self Exposure Alcoholism Cocaine Addiction Heroin Addiction ~ 1 in 10 to 1 in 20 ~ 1 in 3 to 1 in 5 NIDA, SAMHSA Reports
 Factors Contributing to Vulnerability to Develop a Specific Addiction use of the drug of abuse essential (100%) Genetic (25 -50%) Environmental (very high) • DNA • SNPs • other polymorphisms • prenatal • postnatal • contemporary • cues • comorbidity • m. RNA levels • peptides • proteomics • neurochemistry • behaviors Drug-Induced Effects (very high) Kreek et al. , 2000
Factors Contributing to Vulnerability to Develop a Specific Addiction use of the drug of abuse essential (100%) Genetic (25 -50%) Environmental (very high) • DNA • SNPs • other polymorphisms • prenatal • postnatal • contemporary • cues • comorbidity • m. RNA levels • peptides • proteomics • neurochemistry • behaviors Drug-Induced Effects (very high) Kreek et al. , 2000
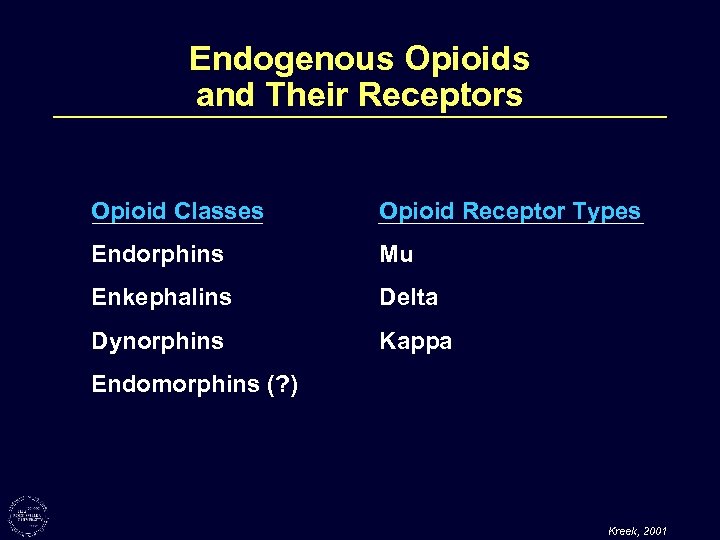 Endogenous Opioids and Their Receptors Opioid Classes Opioid Receptor Types Endorphins Mu Enkephalins Delta Dynorphins Kappa Endomorphins (? ) Kreek, 2001
Endogenous Opioids and Their Receptors Opioid Classes Opioid Receptor Types Endorphins Mu Enkephalins Delta Dynorphins Kappa Endomorphins (? ) Kreek, 2001
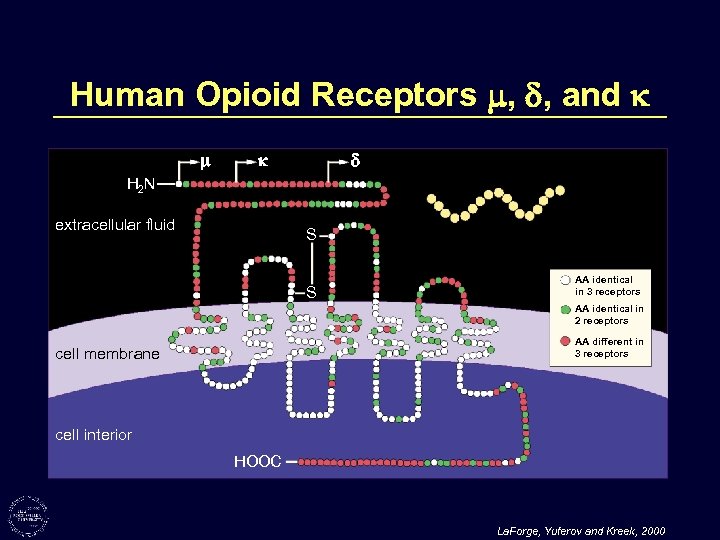 Human Opioid Receptors , , and H 2 N extracellular fluid S S AA identical in 3 receptors AA identical in 2 receptors AA different in 3 receptors cell membrane cell interior HOOC La. Forge, Yuferov and Kreek, 2000
Human Opioid Receptors , , and H 2 N extracellular fluid S S AA identical in 3 receptors AA identical in 2 receptors AA different in 3 receptors cell membrane cell interior HOOC La. Forge, Yuferov and Kreek, 2000
 Hypothesis (1963– 1964) Heroin (opiate) addiction is a disease – a “metabolic disease” – of the brain with resultant behaviors of “drug hunger” and drug self-administration, despite negative consequences to self and others. Heroin addiction is not simply a criminal behavior or due alone to antisocial personality or some other personality disorder. Dole, Nyswander and Kreek, 1966
Hypothesis (1963– 1964) Heroin (opiate) addiction is a disease – a “metabolic disease” – of the brain with resultant behaviors of “drug hunger” and drug self-administration, despite negative consequences to self and others. Heroin addiction is not simply a criminal behavior or due alone to antisocial personality or some other personality disorder. Dole, Nyswander and Kreek, 1966
 Heroin Addiction: Functional State of a Typical Addict (overdose) Functional State "High" "Straight" "Sick" AM PM AM (arrows indicate times of injection) Days Dole, Nyswander and Kreek, 1966
Heroin Addiction: Functional State of a Typical Addict (overdose) Functional State "High" "Straight" "Sick" AM PM AM (arrows indicate times of injection) Days Dole, Nyswander and Kreek, 1966
 Goals and Rationale for Specific Pharmacotherapy for an Addiction 1. Prevent withdrawal symptoms 2. Reduce drug craving 3. Normalize any physiological functions disrupted by drug use 4. Target treatment agent to specific site of action, receptor, or physiological system affected or deranged by drug of abuse Kreek, 1978; 1991; 1992; 2001
Goals and Rationale for Specific Pharmacotherapy for an Addiction 1. Prevent withdrawal symptoms 2. Reduce drug craving 3. Normalize any physiological functions disrupted by drug use 4. Target treatment agent to specific site of action, receptor, or physiological system affected or deranged by drug of abuse Kreek, 1978; 1991; 1992; 2001
 Characteristics of an Effective Pharmacotherapeutic Agent for Treatment of an Addictive Disease • Orally effective • Slow onset of action • Long duration of action • Slow offset of action Kreek, 1978; 1991; 1992; 2001
Characteristics of an Effective Pharmacotherapeutic Agent for Treatment of an Addictive Disease • Orally effective • Slow onset of action • Long duration of action • Slow offset of action Kreek, 1978; 1991; 1992; 2001
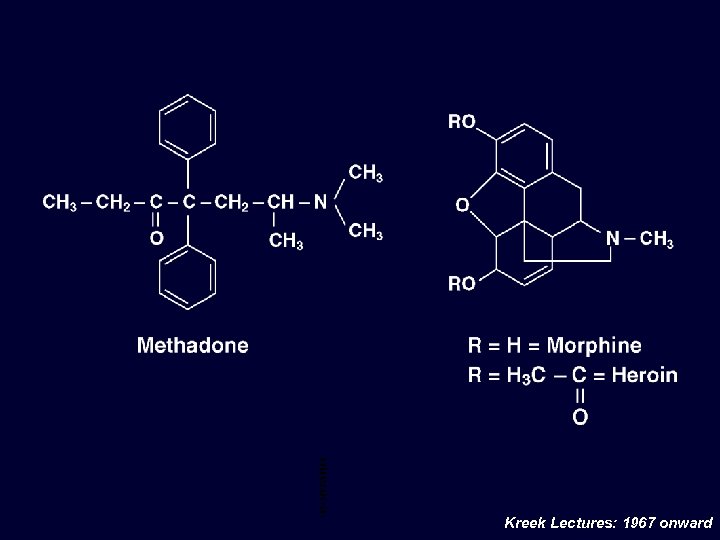 Met had one/ Mor phi ne/ Her oin Che mic al Stru ctur e Kreek Lectures: 1967 onward
Met had one/ Mor phi ne/ Her oin Che mic al Stru ctur e Kreek Lectures: 1967 onward
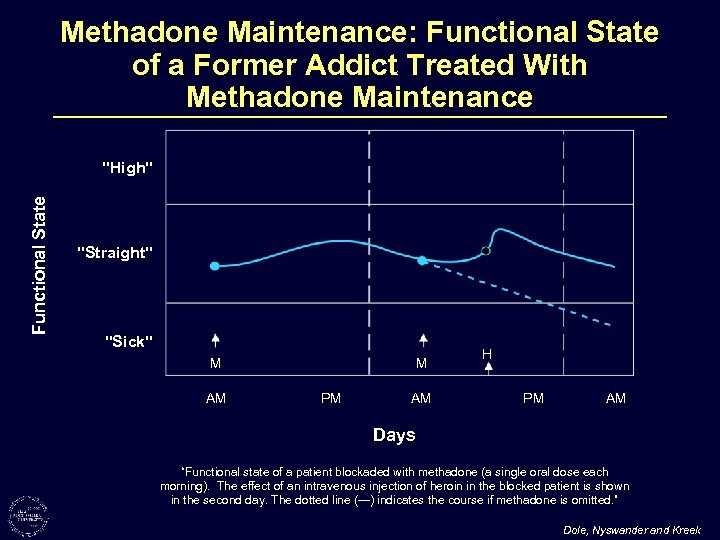 Methadone Maintenance: Functional State of a Former Addict Treated With Methadone Maintenance Functional State "High" "Straight" "Sick" M AM M PM AM H PM AM Days “Functional state of a patient blockaded with methadone (a single oral dose each morning). The effect of an intravenous injection of heroin in the blocked patient is shown in the second day. The dotted line (---) indicates the course if methadone is omitted. ” Dole, Nyswander and Kreek
Methadone Maintenance: Functional State of a Former Addict Treated With Methadone Maintenance Functional State "High" "Straight" "Sick" M AM M PM AM H PM AM Days “Functional state of a patient blockaded with methadone (a single oral dose each morning). The effect of an intravenous injection of heroin in the blocked patient is shown in the second day. The dotted line (---) indicates the course if methadone is omitted. ” Dole, Nyswander and Kreek
 Heroin versus Methadone* Heroin Methadone Route of administration intravenous oral Onset of action immediate 30 minutes Duration of action 3– 6 hrs 24– 36 hrs Euphoria first 1– 2 hrs none Withdrawal symptoms after 3– 4 hrs after 24 hrs * effects of high dosages in tolerant individuals Kreek, 1973; 1976; 1987
Heroin versus Methadone* Heroin Methadone Route of administration intravenous oral Onset of action immediate 30 minutes Duration of action 3– 6 hrs 24– 36 hrs Euphoria first 1– 2 hrs none Withdrawal symptoms after 3– 4 hrs after 24 hrs * effects of high dosages in tolerant individuals Kreek, 1973; 1976; 1987
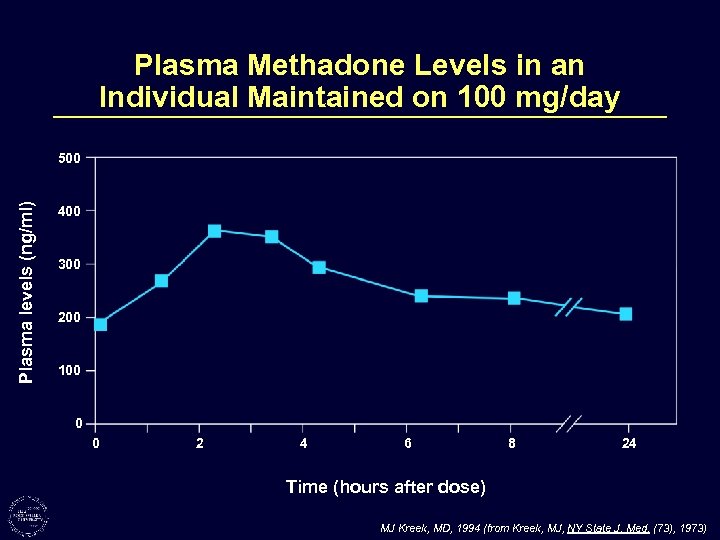 Plasma Methadone Levels in an Individual Maintained on 100 mg/day Plasma levels (ng/ml) 500 400 300 200 100 0 0 2 4 6 8 24 Time (hours after dose) MJ Kreek, MD, 1994 (from Kreek, MJ, NY State J. Med. (73), 1973)
Plasma Methadone Levels in an Individual Maintained on 100 mg/day Plasma levels (ng/ml) 500 400 300 200 100 0 0 2 4 6 8 24 Time (hours after dose) MJ Kreek, MD, 1994 (from Kreek, MJ, NY State J. Med. (73), 1973)
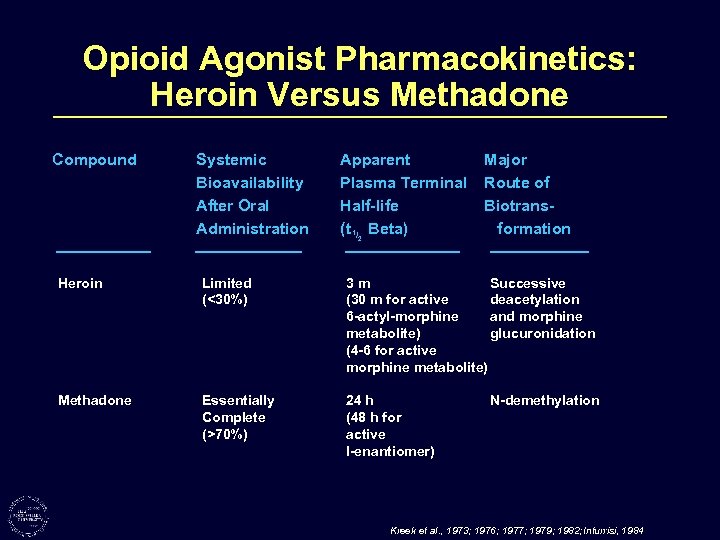 Opioid Agonist Pharmacokinetics: Heroin Versus Methadone Compound Systemic Bioavailability After Oral Administration Apparent Plasma Terminal Half-life (t 1/2 Beta) Major Route of Biotransformation Heroin Limited (<30%) 3 m Successive (30 m for active deacetylation 6 -actyl-morphine and morphine metabolite) glucuronidation (4 -6 for active morphine metabolite) Methadone Essentially Complete (>70%) 24 h (48 h for active l-enantiomer) N-demethylation Kreek et al. , 1973; 1976; 1977; 1979; 1982; Inturrisi, 1984
Opioid Agonist Pharmacokinetics: Heroin Versus Methadone Compound Systemic Bioavailability After Oral Administration Apparent Plasma Terminal Half-life (t 1/2 Beta) Major Route of Biotransformation Heroin Limited (<30%) 3 m Successive (30 m for active deacetylation 6 -actyl-morphine and morphine metabolite) glucuronidation (4 -6 for active morphine metabolite) Methadone Essentially Complete (>70%) 24 h (48 h for active l-enantiomer) N-demethylation Kreek et al. , 1973; 1976; 1977; 1979; 1982; Inturrisi, 1984
![[18 F] Cyclofoxy (a Selective Opioid Antagonist) Binding in Human Brain: Normal Volunteer PET [18 F] Cyclofoxy (a Selective Opioid Antagonist) Binding in Human Brain: Normal Volunteer PET](https://present5.com/presentation/4a47e06608a3c9f06d09fb2e7144d029/image-18.jpg) [18 F] Cyclofoxy (a Selective Opioid Antagonist) Binding in Human Brain: Normal Volunteer PET Study - NIH 116. 25 82. 50 48. 75
[18 F] Cyclofoxy (a Selective Opioid Antagonist) Binding in Human Brain: Normal Volunteer PET Study - NIH 116. 25 82. 50 48. 75
 Plasma Methadone Levels in Long-Term, Methadone-Treatment Patients Sampled Across the 90 -min PET Scan Session Plasma Methadone Levels (ng/ml) 500 400 300 200 100 n=12 0 0 10 20 30 40 50 60 70 80 90 Time (min) Kling et al. , 2000
Plasma Methadone Levels in Long-Term, Methadone-Treatment Patients Sampled Across the 90 -min PET Scan Session Plasma Methadone Levels (ng/ml) 500 400 300 200 100 n=12 0 0 10 20 30 40 50 60 70 80 90 Time (min) Kling et al. , 2000
![Specific Binding (ml plasma/ml tissue) Specific Binding of [18 F] Cyclofoxy (mean + S. Specific Binding (ml plasma/ml tissue) Specific Binding of [18 F] Cyclofoxy (mean + S.](https://present5.com/presentation/4a47e06608a3c9f06d09fb2e7144d029/image-20.jpg) Specific Binding (ml plasma/ml tissue) Specific Binding of [18 F] Cyclofoxy (mean + S. E. M. ) in 13 Brain Regions of Normal Volunteers and Long-Term, Methadone Treated Former Heroin Addicts - PET Study normal volunteers n=14 16 MTP volunteers n=14 14 12 10 * * 8 * * 6 * MT MFr 4 2 0 Thi Amy Caud Ins ACg Put Par Crb IT Hip WMt Region of Interest Kling et al. , 2000
Specific Binding (ml plasma/ml tissue) Specific Binding of [18 F] Cyclofoxy (mean + S. E. M. ) in 13 Brain Regions of Normal Volunteers and Long-Term, Methadone Treated Former Heroin Addicts - PET Study normal volunteers n=14 16 MTP volunteers n=14 14 12 10 * * 8 * * 6 * MT MFr 4 2 0 Thi Amy Caud Ins ACg Put Par Crb IT Hip WMt Region of Interest Kling et al. , 2000
 Methadone Maintenance Treatment Allows Normalization of Endogenous Opioid-Related Physiological Functions Disrupted During Chronic Heroin Use Neuroendocrine Function – Hypothalamic-Pituitary-Adrenal Axis – Stress Responsivity levels and circadian rhythm of release of POMC peptides ( Endorphin; ACTH and cortisol) – Hypothalamic-Pituitary-Gonadal Axis – Reproductive Biology levels and pulsatile release of LH and testosterone levels Immune Function – Natural Killer Cell Activity – Absolute Numbers of Cells — T cells; T cell subset levels; B cells; NK cells – Immunoglobin Levels (M and G) Kreek, 1972; 1973; 1978; 1987; 1992; 2001; Novick et al. , 1989
Methadone Maintenance Treatment Allows Normalization of Endogenous Opioid-Related Physiological Functions Disrupted During Chronic Heroin Use Neuroendocrine Function – Hypothalamic-Pituitary-Adrenal Axis – Stress Responsivity levels and circadian rhythm of release of POMC peptides ( Endorphin; ACTH and cortisol) – Hypothalamic-Pituitary-Gonadal Axis – Reproductive Biology levels and pulsatile release of LH and testosterone levels Immune Function – Natural Killer Cell Activity – Absolute Numbers of Cells — T cells; T cell subset levels; B cells; NK cells – Immunoglobin Levels (M and G) Kreek, 1972; 1973; 1978; 1987; 1992; 2001; Novick et al. , 1989
 Methadone Maintenance Treatment for Opiate (Heroin) Addiction Number of patients in treatment: 179, 000 Efficacy in “good” treatment programs using adequate doses: Voluntary retention in treatment (1 year or more) Continuing use of illicit heroin 60 – 80% 5 – 20% Actions of methadone treatment: • Prevents withdrawal symptoms and “drug hunger” • Blocks euphoric effects of short-acting narcotics • Allows normalization of disrupted physiology Mechanism of action: Long-acting narcotic provides steady levels of opioid at specific mu receptor sites (methadone found to be a full mu opioid receptor agonist which internalizes like endorphins and which also has modest NMDA receptor complex antagonism) Kreek, 1972; 1973; 2001; 2002; Inturrisi et al, in progress; Evans et al; in progress
Methadone Maintenance Treatment for Opiate (Heroin) Addiction Number of patients in treatment: 179, 000 Efficacy in “good” treatment programs using adequate doses: Voluntary retention in treatment (1 year or more) Continuing use of illicit heroin 60 – 80% 5 – 20% Actions of methadone treatment: • Prevents withdrawal symptoms and “drug hunger” • Blocks euphoric effects of short-acting narcotics • Allows normalization of disrupted physiology Mechanism of action: Long-acting narcotic provides steady levels of opioid at specific mu receptor sites (methadone found to be a full mu opioid receptor agonist which internalizes like endorphins and which also has modest NMDA receptor complex antagonism) Kreek, 1972; 1973; 2001; 2002; Inturrisi et al, in progress; Evans et al; in progress
 Reinforcing or “Reward” Effects of Drugs of Abuse Initial exposure to a drug of abuse may produce effects which are interpreted by the individual as “desirable” or “pleasurable”, i. e. , “rewarding”. These effects may lead to “craving” or “hunger” for the drug, with resultant spontaneous activity or work for drug acquisition and self-administration. Primary sites of actions of drugs of abuse with respect to their reward or reinforcing effects have been identified as specific brain regions, rich in dopamine nerve terminals or cell bodies, the mesolimbic and mesocortical dopamine systems especially the nucleus accumbens, as well as the amygdala and the anterior cingulate. Kreek, 1987; 2001
Reinforcing or “Reward” Effects of Drugs of Abuse Initial exposure to a drug of abuse may produce effects which are interpreted by the individual as “desirable” or “pleasurable”, i. e. , “rewarding”. These effects may lead to “craving” or “hunger” for the drug, with resultant spontaneous activity or work for drug acquisition and self-administration. Primary sites of actions of drugs of abuse with respect to their reward or reinforcing effects have been identified as specific brain regions, rich in dopamine nerve terminals or cell bodies, the mesolimbic and mesocortical dopamine systems especially the nucleus accumbens, as well as the amygdala and the anterior cingulate. Kreek, 1987; 2001
 Relationship Between Blood (and Brain) Levels of Drugs of Abuse and Their Effects on Events Related to Addictions Rates of rise of blood (and presumable brain) levels of drugs of abuse are related positively to their reinforcing effects Rates of fall of blood (and presumably brain) levels of drugs of abuse are related positively to the onset of withdrawal symptoms and/or acute “craving” Kreek, 1978; 1991, 1992; 2001
Relationship Between Blood (and Brain) Levels of Drugs of Abuse and Their Effects on Events Related to Addictions Rates of rise of blood (and presumable brain) levels of drugs of abuse are related positively to their reinforcing effects Rates of fall of blood (and presumably brain) levels of drugs of abuse are related positively to the onset of withdrawal symptoms and/or acute “craving” Kreek, 1978; 1991, 1992; 2001
 “On-Off” versus “Steady-State” Disruption versus Normalization • levels of gene expression • receptor mediated events • physiology • behaviors Kreek, 1987; 2001
“On-Off” versus “Steady-State” Disruption versus Normalization • levels of gene expression • receptor mediated events • physiology • behaviors Kreek, 1987; 2001
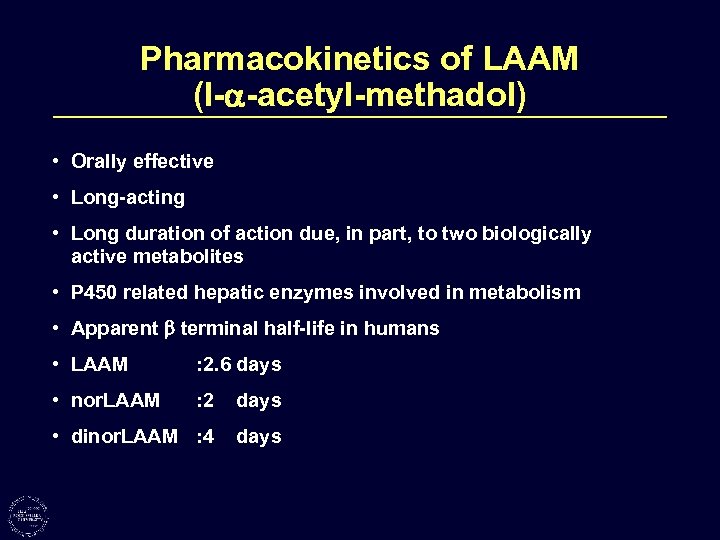 Pharmacokinetics of LAAM (l- -acetyl-methadol) • Orally effective • Long-acting • Long duration of action due, in part, to two biologically active metabolites • P 450 related hepatic enzymes involved in metabolism • Apparent terminal half-life in humans • LAAM : 2. 6 days • nor. LAAM : 2 days • dinor. LAAM : 4 days
Pharmacokinetics of LAAM (l- -acetyl-methadol) • Orally effective • Long-acting • Long duration of action due, in part, to two biologically active metabolites • P 450 related hepatic enzymes involved in metabolism • Apparent terminal half-life in humans • LAAM : 2. 6 days • nor. LAAM : 2 days • dinor. LAAM : 4 days
 Buprenorphine Hydrochloride* HO O N HCl CH 3 O HO CH 3 C(CH 3)3 *Buprenex
Buprenorphine Hydrochloride* HO O N HCl CH 3 O HO CH 3 C(CH 3)3 *Buprenex
 Buprenorphine— Pharmacokinetics and Dynamics in Humans • Mu opioid receptor partial agonist. • No oral preparation— (ineffective: “first pass” rapid biotransformation by GI mucosa and by liver). • Sublingual preparation– (without, and with, naloxone to reduce intravenous abuse liability). – Approved by FDA– maximum 16 mg/day for treatment of opiate addiction (only); no approval for management of pain yet. – Maximal dose effective in humans 24 to 32 mg. – Sublingual liquid or tablet; tablet ~55% plasma levels of liquid. • Plasma β terminal half-life: “ 3 to 5 hours. ” – Around 120 minutes to peak after sublingual dose. • Pharmacodynamic sustained mu opioid effect, 24 to 48 hours due to very long mu opioid receptor occupancy. • Mu opioid agonist effects NOT reversible with opioid antagonist naloxone (because of very high affinity to and quasi-irreversible binding at mu opioid receptor. Kreek, 2003
Buprenorphine— Pharmacokinetics and Dynamics in Humans • Mu opioid receptor partial agonist. • No oral preparation— (ineffective: “first pass” rapid biotransformation by GI mucosa and by liver). • Sublingual preparation– (without, and with, naloxone to reduce intravenous abuse liability). – Approved by FDA– maximum 16 mg/day for treatment of opiate addiction (only); no approval for management of pain yet. – Maximal dose effective in humans 24 to 32 mg. – Sublingual liquid or tablet; tablet ~55% plasma levels of liquid. • Plasma β terminal half-life: “ 3 to 5 hours. ” – Around 120 minutes to peak after sublingual dose. • Pharmacodynamic sustained mu opioid effect, 24 to 48 hours due to very long mu opioid receptor occupancy. • Mu opioid agonist effects NOT reversible with opioid antagonist naloxone (because of very high affinity to and quasi-irreversible binding at mu opioid receptor. Kreek, 2003
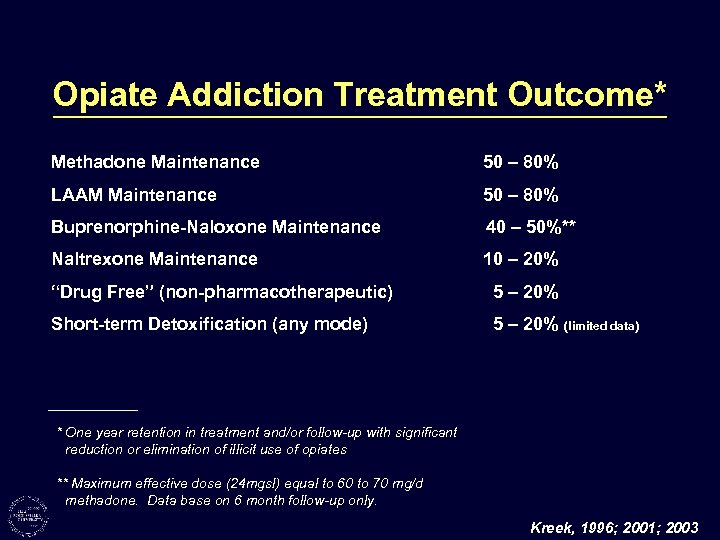 Opiate Addiction Treatment Outcome* Methadone Maintenance 50 – 80% LAAM Maintenance 50 – 80% Buprenorphine-Naloxone Maintenance 40 – 50%** Naltrexone Maintenance 10 – 20% “Drug Free” (non-pharmacotherapeutic) 5 – 20% Short-term Detoxification (any mode) 5 – 20% (limited data) * One year retention in treatment and/or follow-up with significant reduction or elimination of illicit use of opiates ** Maximum effective dose (24 mgsl) equal to 60 to 70 mg/d methadone. Data base on 6 month follow-up only. Kreek, 1996; 2001; 2003
Opiate Addiction Treatment Outcome* Methadone Maintenance 50 – 80% LAAM Maintenance 50 – 80% Buprenorphine-Naloxone Maintenance 40 – 50%** Naltrexone Maintenance 10 – 20% “Drug Free” (non-pharmacotherapeutic) 5 – 20% Short-term Detoxification (any mode) 5 – 20% (limited data) * One year retention in treatment and/or follow-up with significant reduction or elimination of illicit use of opiates ** Maximum effective dose (24 mgsl) equal to 60 to 70 mg/d methadone. Data base on 6 month follow-up only. Kreek, 1996; 2001; 2003
 Continuing Urgent Medical Needs 1) To provide the most effective treatment for a major addictive disease, long term heroin (or other short-acting opiate) addiction, the use of long-acting mu opioid agonists or partial agonists is usually essential. 2) To provide the most effective treatment for chronic pain, neoplastic or other, the use of long-acting mu opioid agonists or partial agonists are often essential. Kreek, 2003
Continuing Urgent Medical Needs 1) To provide the most effective treatment for a major addictive disease, long term heroin (or other short-acting opiate) addiction, the use of long-acting mu opioid agonists or partial agonists is usually essential. 2) To provide the most effective treatment for chronic pain, neoplastic or other, the use of long-acting mu opioid agonists or partial agonists are often essential. Kreek, 2003
 Specific Critical Questions 1) Is this mu opioid agonist medication and/or this specific formulation short- or long-acting? 2) Is the patient opioid naïve, modestly exposed, or long-term exposed and, thus, tolerant? 3) Does this patient already have a problem with some type of drug abuse or addiction or other indicators suggesting risk of increased vulnerability to develop an addiction? Kreek, 2003
Specific Critical Questions 1) Is this mu opioid agonist medication and/or this specific formulation short- or long-acting? 2) Is the patient opioid naïve, modestly exposed, or long-term exposed and, thus, tolerant? 3) Does this patient already have a problem with some type of drug abuse or addiction or other indicators suggesting risk of increased vulnerability to develop an addiction? Kreek, 2003
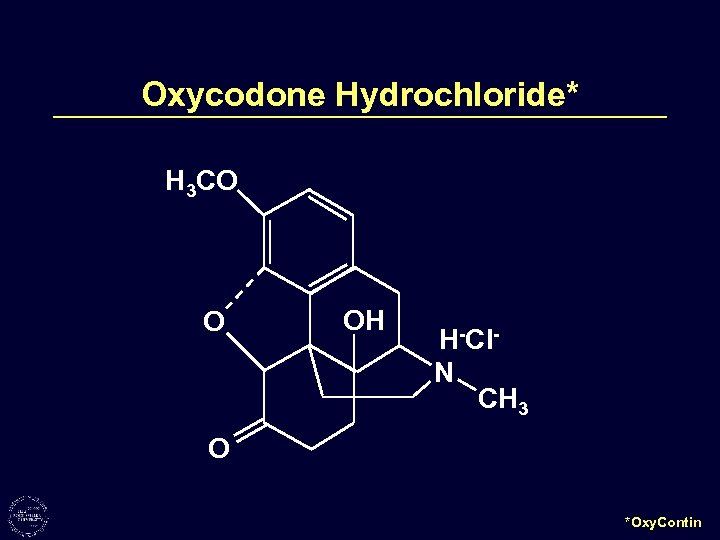 Oxycodone Hydrochloride* H 3 CO O OH H-Cl. N CH 3 O *Oxy. Contin
Oxycodone Hydrochloride* H 3 CO O OH H-Cl. N CH 3 O *Oxy. Contin
 Hydromorphone Hydrochloride OH HCl O H CH 2 O H N CH 2 CH 3
Hydromorphone Hydrochloride OH HCl O H CH 2 O H N CH 2 CH 3
 Fentanyl CH 3 CH 2 CON N CH 2
Fentanyl CH 3 CH 2 CON N CH 2
 Generic Name (Trade Name of Immediate Release Preparation) (Dosing Interval) (Dosage) Trade Name(s) of Extended Release Preparation (Dosing Interval) (Dosages) (higher doses for tolerant individuals only) 1) Oxycodone (q 4 h) (5 mg; 10 mg) e. g. , Oxy. Contin® (q 12 h) (10, 20, 40, 80 mg)* 2. 7 h 12 h duration (t½ absorption—bimodal, e. g. , 0. 6 and 6. 9 h) 2) Hydromorphone (e. g. , Dilaudid ®) (q 4 h) (2 mg; 4 mg) e. g. , Hydromorphin Contin ® (q 12 h) (8, 16, 32, 64 mg)* 2. 6 h 12 h duration 3) Morphine (q 4 h) (multiple) e. g. , MSContin ® e. g. , Kadian Reg ® (q 12 h) (15, 30, 60, 100, 200 mg)* 2 to 4 h 12 h duration 4) Fentanyl e. g. , Duragesic ® (q 72 h) (25, 50, 75, 100μg/h)* (2. 5, 5. 0, 7. 5, 10 mg) 3 -12 h (? ) 72 h duration Plasma t½ (h) Dynamics/Duration of Extended Release Formulation *“Taken broken, chewed, crushed xxx (compound formulations 1, 2, or 3) could lead to rapid release… toxic dose. ” Kreek, 2003
Generic Name (Trade Name of Immediate Release Preparation) (Dosing Interval) (Dosage) Trade Name(s) of Extended Release Preparation (Dosing Interval) (Dosages) (higher doses for tolerant individuals only) 1) Oxycodone (q 4 h) (5 mg; 10 mg) e. g. , Oxy. Contin® (q 12 h) (10, 20, 40, 80 mg)* 2. 7 h 12 h duration (t½ absorption—bimodal, e. g. , 0. 6 and 6. 9 h) 2) Hydromorphone (e. g. , Dilaudid ®) (q 4 h) (2 mg; 4 mg) e. g. , Hydromorphin Contin ® (q 12 h) (8, 16, 32, 64 mg)* 2. 6 h 12 h duration 3) Morphine (q 4 h) (multiple) e. g. , MSContin ® e. g. , Kadian Reg ® (q 12 h) (15, 30, 60, 100, 200 mg)* 2 to 4 h 12 h duration 4) Fentanyl e. g. , Duragesic ® (q 72 h) (25, 50, 75, 100μg/h)* (2. 5, 5. 0, 7. 5, 10 mg) 3 -12 h (? ) 72 h duration Plasma t½ (h) Dynamics/Duration of Extended Release Formulation *“Taken broken, chewed, crushed xxx (compound formulations 1, 2, or 3) could lead to rapid release… toxic dose. ” Kreek, 2003
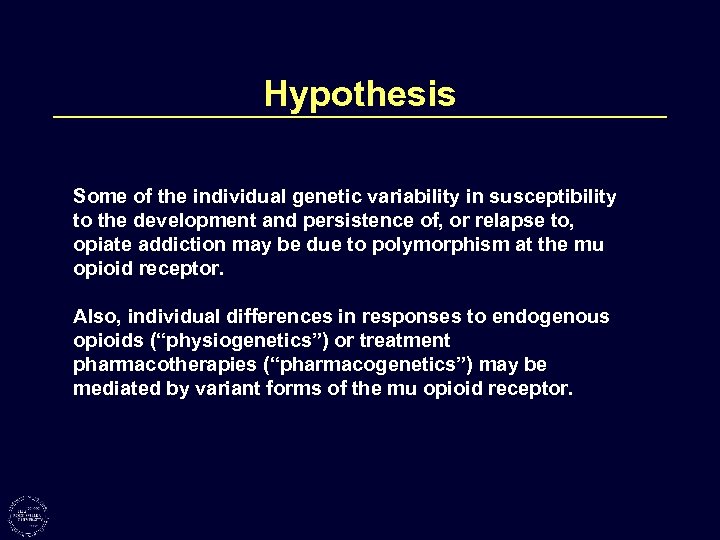 Hypothesis Some of the individual genetic variability in susceptibility to the development and persistence of, or relapse to, opiate addiction may be due to polymorphism at the mu opioid receptor. Also, individual differences in responses to endogenous opioids (“physiogenetics”) or treatment pharmacotherapies (“pharmacogenetics”) may be mediated by variant forms of the mu opioid receptor.
Hypothesis Some of the individual genetic variability in susceptibility to the development and persistence of, or relapse to, opiate addiction may be due to polymorphism at the mu opioid receptor. Also, individual differences in responses to endogenous opioids (“physiogenetics”) or treatment pharmacotherapies (“pharmacogenetics”) may be mediated by variant forms of the mu opioid receptor.
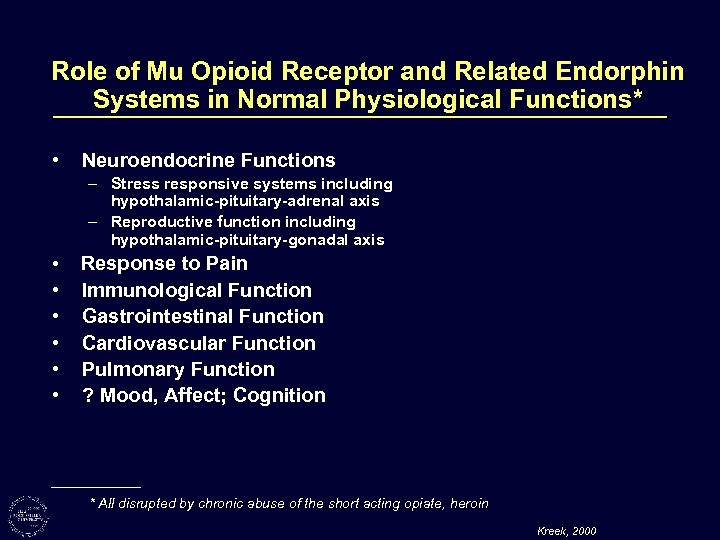 Role of Mu Opioid Receptor and Related Endorphin Systems in Normal Physiological Functions* • Neuroendocrine Functions – Stress responsive systems including hypothalamic-pituitary-adrenal axis – Reproductive function including hypothalamic-pituitary-gonadal axis • • • Response to Pain Immunological Function Gastrointestinal Function Cardiovascular Function Pulmonary Function ? Mood, Affect; Cognition * All disrupted by chronic abuse of the short acting opiate, heroin Kreek, 2000
Role of Mu Opioid Receptor and Related Endorphin Systems in Normal Physiological Functions* • Neuroendocrine Functions – Stress responsive systems including hypothalamic-pituitary-adrenal axis – Reproductive function including hypothalamic-pituitary-gonadal axis • • • Response to Pain Immunological Function Gastrointestinal Function Cardiovascular Function Pulmonary Function ? Mood, Affect; Cognition * All disrupted by chronic abuse of the short acting opiate, heroin Kreek, 2000
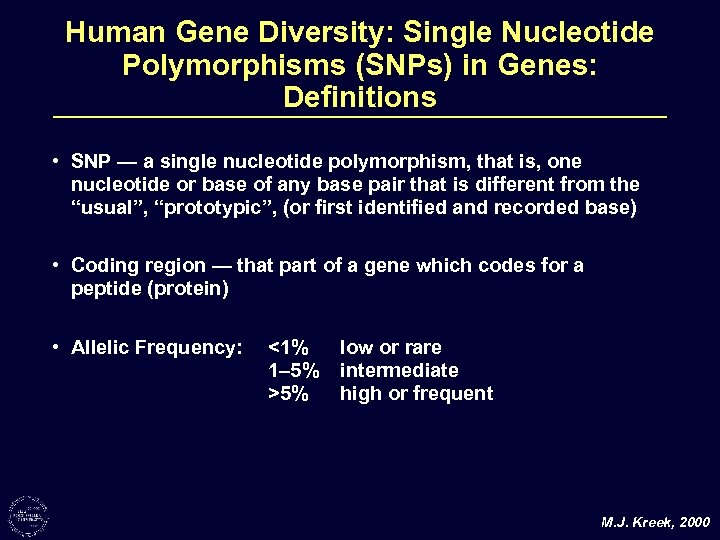 Human Gene Diversity: Single Nucleotide Polymorphisms (SNPs) in Genes: Definitions • SNP — a single nucleotide polymorphism, that is, one nucleotide or base of any base pair that is different from the “usual”, “prototypic”, (or first identified and recorded base) • Coding region — that part of a gene which codes for a peptide (protein) • Allelic Frequency: <1% 1– 5% >5% low or rare intermediate high or frequent M. J. Kreek, 2000
Human Gene Diversity: Single Nucleotide Polymorphisms (SNPs) in Genes: Definitions • SNP — a single nucleotide polymorphism, that is, one nucleotide or base of any base pair that is different from the “usual”, “prototypic”, (or first identified and recorded base) • Coding region — that part of a gene which codes for a peptide (protein) • Allelic Frequency: <1% 1– 5% >5% low or rare intermediate high or frequent M. J. Kreek, 2000
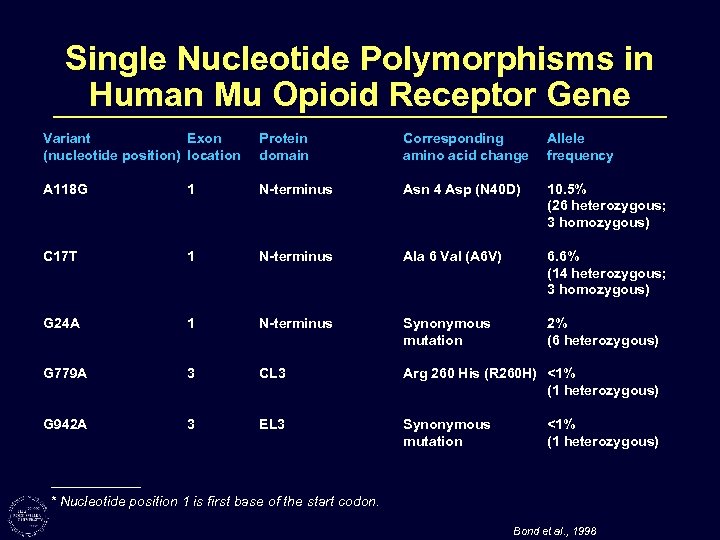 Single Nucleotide Polymorphisms in Human Mu Opioid Receptor Gene Variant Exon (nucleotide position) location Protein domain Corresponding amino acid change Allele frequency A 118 G 1 N-terminus Asn 4 Asp (N 40 D) 10. 5% (26 heterozygous; 3 homozygous) C 17 T 1 N-terminus Ala 6 Val (A 6 V) 6. 6% (14 heterozygous; 3 homozygous) G 24 A 1 N-terminus Synonymous mutation 2% (6 heterozygous) G 779 A 3 CL 3 Arg 260 His (R 260 H) <1% (1 heterozygous) G 942 A 3 EL 3 Synonymous mutation <1% (1 heterozygous) * Nucleotide position 1 is first base of the start codon. Bond et al. , 1998
Single Nucleotide Polymorphisms in Human Mu Opioid Receptor Gene Variant Exon (nucleotide position) location Protein domain Corresponding amino acid change Allele frequency A 118 G 1 N-terminus Asn 4 Asp (N 40 D) 10. 5% (26 heterozygous; 3 homozygous) C 17 T 1 N-terminus Ala 6 Val (A 6 V) 6. 6% (14 heterozygous; 3 homozygous) G 24 A 1 N-terminus Synonymous mutation 2% (6 heterozygous) G 779 A 3 CL 3 Arg 260 His (R 260 H) <1% (1 heterozygous) G 942 A 3 EL 3 Synonymous mutation <1% (1 heterozygous) * Nucleotide position 1 is first base of the start codon. Bond et al. , 1998
 Allelic Frequency Associations Opioid Dependence – C 17 T and A 118 G C T A G Total Dependent 207 (0. 916) 19 (0. 084) 206 (0. 912) 20 (0. 088) 226 Non-dependent 77 (0. 987) 1 (0. 013) 66 (0. 846) 12 (0. 154) 78 Yate's corrected c 2 (1) = 3. 70 (p = 0. 054)* 1. 98 (p = 0. 159) † * This finding is similar to that obtained by Berrettini et al. (1997) c 22 = 4. 1 [p=0. 05] † However, within the Hispanic study subjects groups (total n=67), the A 118 G variant allele was present in a significantly higher proportion of non-opioid dependent subjects compared to opioid dependent subjects. (Yates corrected Chi-square c 22(1) = 8. 22 [p=0. 0041]) (1) Bond et al. , 1998
Allelic Frequency Associations Opioid Dependence – C 17 T and A 118 G C T A G Total Dependent 207 (0. 916) 19 (0. 084) 206 (0. 912) 20 (0. 088) 226 Non-dependent 77 (0. 987) 1 (0. 013) 66 (0. 846) 12 (0. 154) 78 Yate's corrected c 2 (1) = 3. 70 (p = 0. 054)* 1. 98 (p = 0. 159) † * This finding is similar to that obtained by Berrettini et al. (1997) c 22 = 4. 1 [p=0. 05] † However, within the Hispanic study subjects groups (total n=67), the A 118 G variant allele was present in a significantly higher proportion of non-opioid dependent subjects compared to opioid dependent subjects. (Yates corrected Chi-square c 22(1) = 8. 22 [p=0. 0041]) (1) Bond et al. , 1998
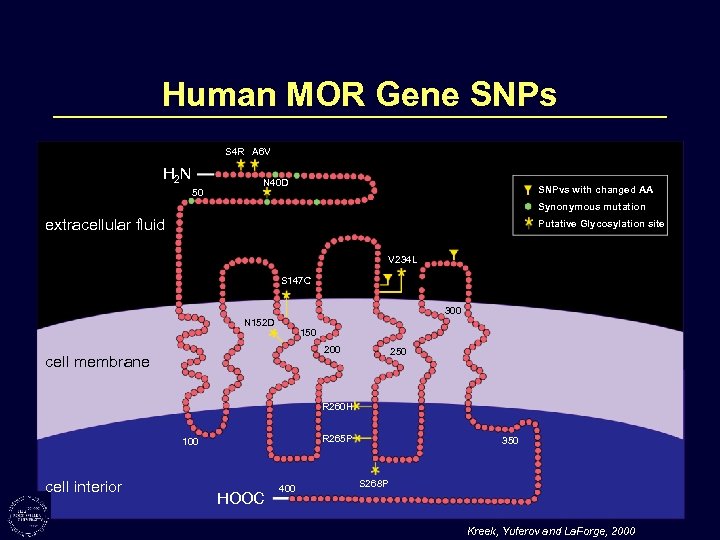 Human MOR Gene SNPs S 4 R A 6 V H 2 N 50 N 40 D SNPvs with changed AA Synonymous mutation extracellular fluid Putative Glycosylation site V 234 L S 147 C 300 N 152 D 150 200 cell membrane 250 R 260 H R 265 P 100 cell interior HOOC 400 350 S 268 P Kreek, Yuferov and La. Forge, 2000
Human MOR Gene SNPs S 4 R A 6 V H 2 N 50 N 40 D SNPvs with changed AA Synonymous mutation extracellular fluid Putative Glycosylation site V 234 L S 147 C 300 N 152 D 150 200 cell membrane 250 R 260 H R 265 P 100 cell interior HOOC 400 350 S 268 P Kreek, Yuferov and La. Forge, 2000
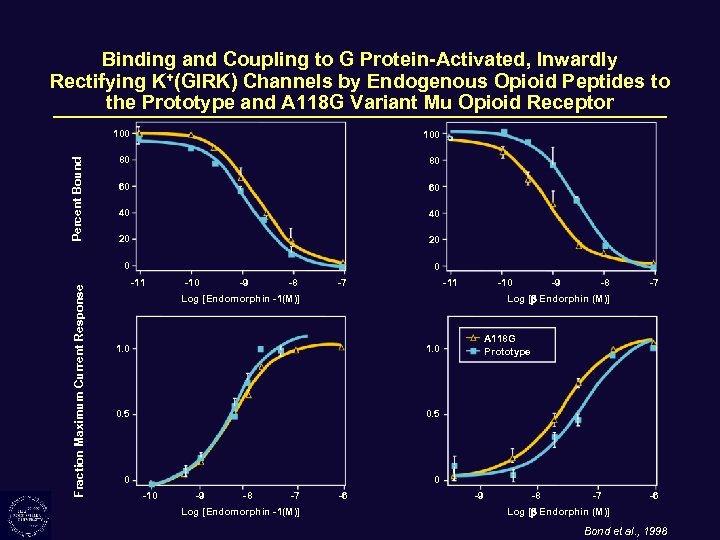 Binding and Coupling to G Protein-Activated, Inwardly Rectifying K+(GIRK) Channels by Endogenous Opioid Peptides to the Prototype and A 118 G Variant Mu Opioid Receptor Fraction Maximum Current Response 100 80 80 60 60 40 40 20 20 0 Percent Bound 100 0 -11 -10 -9 -8 -7 -11 -10 Log [Endomorphin -1(M)] -9 -8 -7 Log [ Endorphin (M)] 1. 0 0. 5 0 A 118 G Prototype 1. 0 0 -10 -9 -8 -7 Log [Endomorphin -1(M)] -6 -9 -8 -7 -6 Log [ Endorphin (M)] Bond et al. , 1998
Binding and Coupling to G Protein-Activated, Inwardly Rectifying K+(GIRK) Channels by Endogenous Opioid Peptides to the Prototype and A 118 G Variant Mu Opioid Receptor Fraction Maximum Current Response 100 80 80 60 60 40 40 20 20 0 Percent Bound 100 0 -11 -10 -9 -8 -7 -11 -10 Log [Endomorphin -1(M)] -9 -8 -7 Log [ Endorphin (M)] 1. 0 0. 5 0 A 118 G Prototype 1. 0 0 -10 -9 -8 -7 Log [Endomorphin -1(M)] -6 -9 -8 -7 -6 Log [ Endorphin (M)] Bond et al. , 1998
 Serum Cortisol (ug/dl) “Physiogenetics”— Cortisol Levels After Incremental Naloxone Administration: Mu Opioid Receptor A 118 G Heterozygote Individuals 24 A/A (n=29) A/G (n=7) 22 20 N N 18 N 16 14 PI 12 N 10 8 50 0 50 100 Time (min) 150 200 Kreek, 1999; Wand et al, 2002
Serum Cortisol (ug/dl) “Physiogenetics”— Cortisol Levels After Incremental Naloxone Administration: Mu Opioid Receptor A 118 G Heterozygote Individuals 24 A/A (n=29) A/G (n=7) 22 20 N N 18 N 16 14 PI 12 N 10 8 50 0 50 100 Time (min) 150 200 Kreek, 1999; Wand et al, 2002
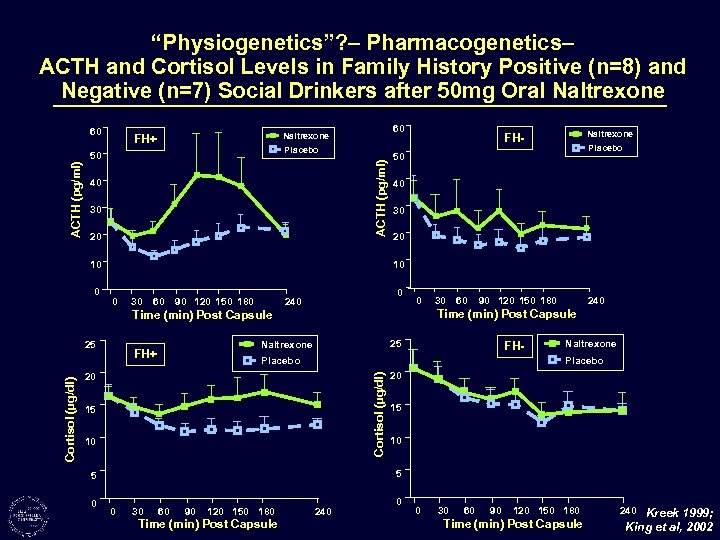 “Physiogenetics”? – Pharmacogenetics– ACTH and Cortisol Levels in Family History Positive (n=8) and Negative (n=7) Social Drinkers after 50 mg Oral Naltrexone 60 Placebo ACTH (pg/ml) 50 ACTH (pg/ml) 60 Naltrexone FH+ 40 30 20 10 0 25 30 60 90 120 150 180 0 240 0 60 90 120 150 180 25 Naltrexone FH+ 30 240 Time (min) Post Capsule FH- Placebo Naltrexone Placebo 20 Cortisol (µg/dl) Placebo 50 Time (min) Post Capsule 15 10 20 15 10 5 5 0 Naltrexone FH- 0 30 60 90 120 150 180 Time (min) Post Capsule 240 0 0 30 60 90 120 150 180 Time (min) Post Capsule 240 Kreek 1999; King et al, 2002
“Physiogenetics”? – Pharmacogenetics– ACTH and Cortisol Levels in Family History Positive (n=8) and Negative (n=7) Social Drinkers after 50 mg Oral Naltrexone 60 Placebo ACTH (pg/ml) 50 ACTH (pg/ml) 60 Naltrexone FH+ 40 30 20 10 0 25 30 60 90 120 150 180 0 240 0 60 90 120 150 180 25 Naltrexone FH+ 30 240 Time (min) Post Capsule FH- Placebo Naltrexone Placebo 20 Cortisol (µg/dl) Placebo 50 Time (min) Post Capsule 15 10 20 15 10 5 5 0 Naltrexone FH- 0 30 60 90 120 150 180 Time (min) Post Capsule 240 0 0 30 60 90 120 150 180 Time (min) Post Capsule 240 Kreek 1999; King et al, 2002
 Pharmacogenetics: Mu Opioid Receptor A 118 G Polymorphism— Naltrexone Treatment Response Study 1: UPENN (Monterosso et al. , 2001)* 183 alcohol dependent outpatient subjects Randomized to 3 groups: a) 9 months of naltrexone (100 mg/day) b) 12 weeks of naltrexone (100 mg/day), followed by 6 months of placebo c) 9 months of placebo Study 2: UPENN (unpublished)* 240 alcohol dependent outpatient subjects Randomized to 6 groups: a) either naltrexone (100 mg/day) for 24 weeks or b) placebo for 24 weeks c) all subjects in (a) or (b) randomized to 3 different psychosocial interventions Study 3: University of Connecticut Health Center (Kranzler et al. , 2000)* 183 alcohol dependent outpatient subjects After Initial treatment of 1 week single-blind placebo, randomized to 3 groups: a) naltrexone (50 mg/day) b) placebo c) nefazine (up to 600 mg/day) Oslin, Berrettini, Volpicelli, Kranzler, O’Brien, et al. , 2003 *All subjects consented for genetics studies either at the time of naltrexone study or at a later date
Pharmacogenetics: Mu Opioid Receptor A 118 G Polymorphism— Naltrexone Treatment Response Study 1: UPENN (Monterosso et al. , 2001)* 183 alcohol dependent outpatient subjects Randomized to 3 groups: a) 9 months of naltrexone (100 mg/day) b) 12 weeks of naltrexone (100 mg/day), followed by 6 months of placebo c) 9 months of placebo Study 2: UPENN (unpublished)* 240 alcohol dependent outpatient subjects Randomized to 6 groups: a) either naltrexone (100 mg/day) for 24 weeks or b) placebo for 24 weeks c) all subjects in (a) or (b) randomized to 3 different psychosocial interventions Study 3: University of Connecticut Health Center (Kranzler et al. , 2000)* 183 alcohol dependent outpatient subjects After Initial treatment of 1 week single-blind placebo, randomized to 3 groups: a) naltrexone (50 mg/day) b) placebo c) nefazine (up to 600 mg/day) Oslin, Berrettini, Volpicelli, Kranzler, O’Brien, et al. , 2003 *All subjects consented for genetics studies either at the time of naltrexone study or at a later date
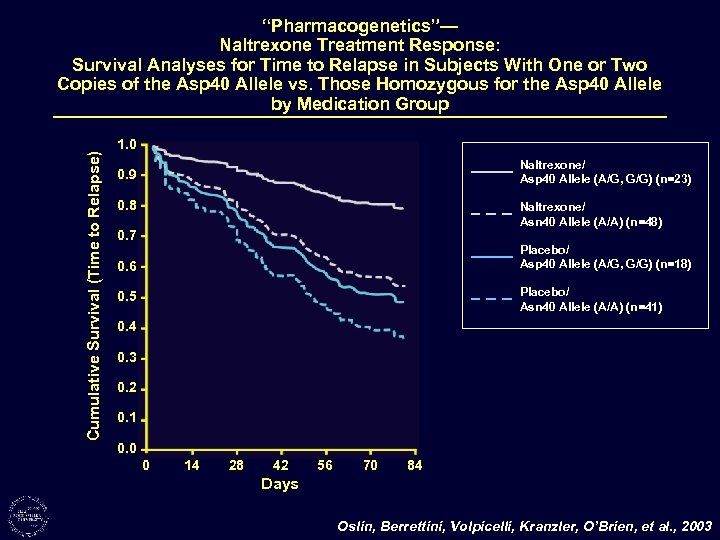 Cumulative Survival (Time to Relapse) “Pharmacogenetics”— Naltrexone Treatment Response: Survival Analyses for Time to Relapse in Subjects With One or Two Copies of the Asp 40 Allele vs. Those Homozygous for the Asp 40 Allele by Medication Group 1. 0 Naltrexone/ Asp 40 Allele (A/G, G/G) (n=23) 0. 9 0. 8 Naltrexone/ Asn 40 Allele (A/A) (n=48) 0. 7 Placebo/ Asp 40 Allele (A/G, G/G) (n=18) 0. 6 Placebo/ Asn 40 Allele (A/A) (n=41) 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0 14 28 42 56 70 84 Days Oslin, Berrettini, Volpicelli, Kranzler, O’Brien, et al. , 2003
Cumulative Survival (Time to Relapse) “Pharmacogenetics”— Naltrexone Treatment Response: Survival Analyses for Time to Relapse in Subjects With One or Two Copies of the Asp 40 Allele vs. Those Homozygous for the Asp 40 Allele by Medication Group 1. 0 Naltrexone/ Asp 40 Allele (A/G, G/G) (n=23) 0. 9 0. 8 Naltrexone/ Asn 40 Allele (A/A) (n=48) 0. 7 Placebo/ Asp 40 Allele (A/G, G/G) (n=18) 0. 6 Placebo/ Asn 40 Allele (A/A) (n=41) 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0 14 28 42 56 70 84 Days Oslin, Berrettini, Volpicelli, Kranzler, O’Brien, et al. , 2003
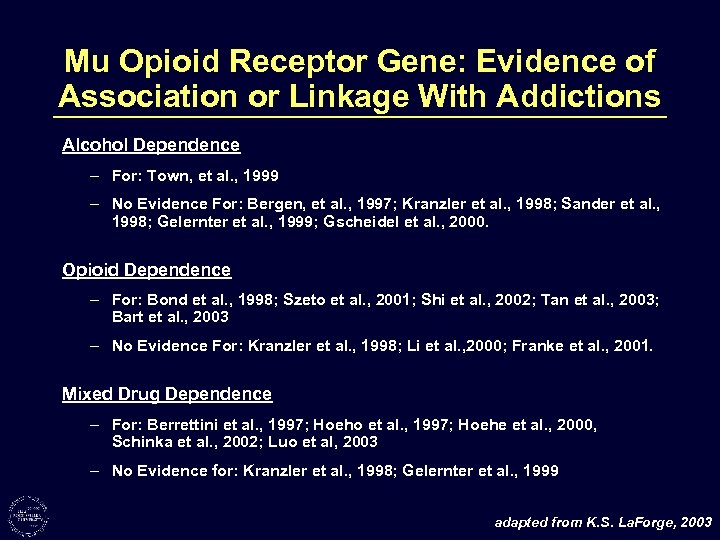 Mu Opioid Receptor Gene: Evidence of Association or Linkage With Addictions Alcohol Dependence – For: Town, et al. , 1999 – No Evidence For: Bergen, et al. , 1997; Kranzler et al. , 1998; Sander et al. , 1998; Gelernter et al. , 1999; Gscheidel et al. , 2000. Opioid Dependence – For: Bond et al. , 1998; Szeto et al. , 2001; Shi et al. , 2002; Tan et al. , 2003; Bart et al. , 2003 – No Evidence For: Kranzler et al. , 1998; Li et al. , 2000; Franke et al. , 2001. Mixed Drug Dependence – For: Berrettini et al. , 1997; Hoeho et al. , 1997; Hoehe et al. , 2000, Schinka et al. , 2002; Luo et al, 2003 – No Evidence for: Kranzler et al. , 1998; Gelernter et al. , 1999 adapted from K. S. La. Forge, 2003
Mu Opioid Receptor Gene: Evidence of Association or Linkage With Addictions Alcohol Dependence – For: Town, et al. , 1999 – No Evidence For: Bergen, et al. , 1997; Kranzler et al. , 1998; Sander et al. , 1998; Gelernter et al. , 1999; Gscheidel et al. , 2000. Opioid Dependence – For: Bond et al. , 1998; Szeto et al. , 2001; Shi et al. , 2002; Tan et al. , 2003; Bart et al. , 2003 – No Evidence For: Kranzler et al. , 1998; Li et al. , 2000; Franke et al. , 2001. Mixed Drug Dependence – For: Berrettini et al. , 1997; Hoeho et al. , 1997; Hoehe et al. , 2000, Schinka et al. , 2002; Luo et al, 2003 – No Evidence for: Kranzler et al. , 1998; Gelernter et al. , 1999 adapted from K. S. La. Forge, 2003
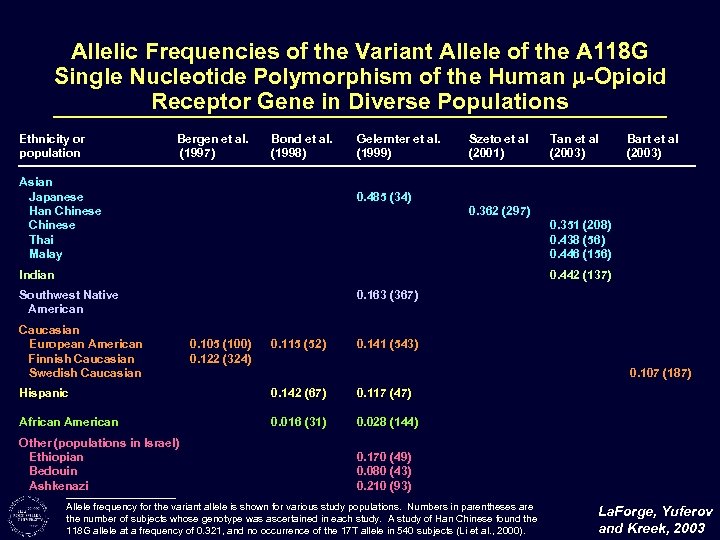 Allelic Frequencies of the Variant Allele of the A 118 G Single Nucleotide Polymorphism of the Human -Opioid Receptor Gene in Diverse Populations Ethnicity or population Bergen et al. (1997) Bond et al. (1998) Asian Japanese Han Chinese Thai Malay Gelernter et al. (1999) Szeto et al (2001) Tan et al (2003) Bart et al (2003) 0. 485 (34) 0. 362 (297) 0. 351 (208) 0. 438 (56) 0. 446 (156) Indian 0. 442 (137) Southwest Native American Caucasian European American Finnish Caucasian Swedish Caucasian 0. 163 (367) 0. 105 (100) 0. 122 (324) 0. 115 (52) 0. 141 (543) 0. 107 (187) Hispanic 0. 142 (67) 0. 117 (47) African American 0. 016 (31) 0. 028 (144) Other (populations in Israel) Ethiopian Bedouin Ashkenazi 0. 170 (49) 0. 080 (43) 0. 210 (93) Allele frequency for the variant allele is shown for various study populations. Numbers in parentheses are the number of subjects whose genotype was ascertained in each study. A study of Han Chinese found the 118 G allele at a frequency of 0. 321, and no occurrence of the 17 T allele in 540 subjects (Li et al. , 2000). La. Forge, Yuferov and Kreek, 2003
Allelic Frequencies of the Variant Allele of the A 118 G Single Nucleotide Polymorphism of the Human -Opioid Receptor Gene in Diverse Populations Ethnicity or population Bergen et al. (1997) Bond et al. (1998) Asian Japanese Han Chinese Thai Malay Gelernter et al. (1999) Szeto et al (2001) Tan et al (2003) Bart et al (2003) 0. 485 (34) 0. 362 (297) 0. 351 (208) 0. 438 (56) 0. 446 (156) Indian 0. 442 (137) Southwest Native American Caucasian European American Finnish Caucasian Swedish Caucasian 0. 163 (367) 0. 105 (100) 0. 122 (324) 0. 115 (52) 0. 141 (543) 0. 107 (187) Hispanic 0. 142 (67) 0. 117 (47) African American 0. 016 (31) 0. 028 (144) Other (populations in Israel) Ethiopian Bedouin Ashkenazi 0. 170 (49) 0. 080 (43) 0. 210 (93) Allele frequency for the variant allele is shown for various study populations. Numbers in parentheses are the number of subjects whose genotype was ascertained in each study. A study of Han Chinese found the 118 G allele at a frequency of 0. 321, and no occurrence of the 17 T allele in 540 subjects (Li et al. , 2000). La. Forge, Yuferov and Kreek, 2003
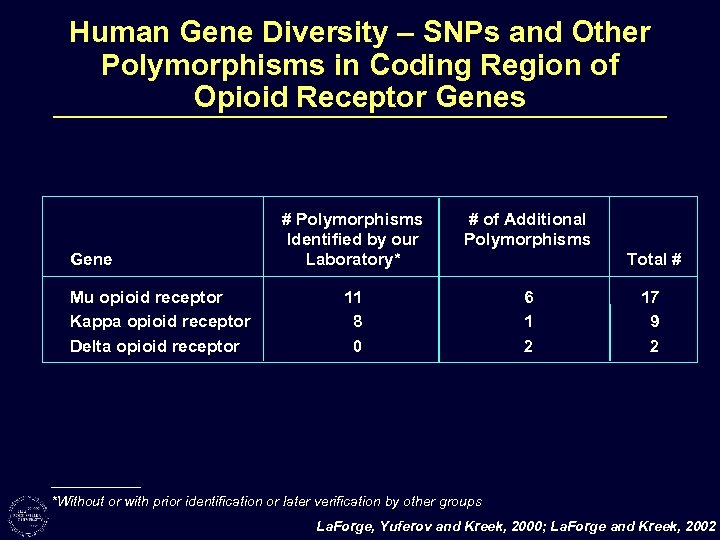 Human Gene Diversity – SNPs and Other Polymorphisms in Coding Region of Opioid Receptor Genes Gene Mu opioid receptor Kappa opioid receptor Delta opioid receptor # Polymorphisms Identified by our Laboratory* # of Additional Polymorphisms 11 8 0 6 1 2 Total # 17 9 2 *Without or with prior identification or later verification by other groups La. Forge, Yuferov and Kreek, 2000; La. Forge and Kreek, 2002
Human Gene Diversity – SNPs and Other Polymorphisms in Coding Region of Opioid Receptor Genes Gene Mu opioid receptor Kappa opioid receptor Delta opioid receptor # Polymorphisms Identified by our Laboratory* # of Additional Polymorphisms 11 8 0 6 1 2 Total # 17 9 2 *Without or with prior identification or later verification by other groups La. Forge, Yuferov and Kreek, 2000; La. Forge and Kreek, 2002
 The Laboratory of the Biology of Addictive Diseases Mary Jeanne Kreek, M. D. – Professor and Head 2003 Laboratory Scientists Ann Ho K. Steven La. Forge Eduardo Butelman Vadim Yuferov David Nielsen Yan Zhou Alexis Bailey Thomas Kroslak Dmitri Proudnikov Brian Reed Stefan Schlussman Adena Svingos Yong Zhang Laboratory Adjunct Scientists Charles Inturrisi Roberto Picetti Virginia Pickel Ellen Unterwald Vanya Quinones-Jenab Clinical Scientists Gavin Bart Lisa Borg Scott Kellogg Charles Lilly Clinical Adjunct Scientists Paola Piccolo Joan Culpepper-Morgan Lawrence Brown James Kocsis Mark Green Lynette Benjamin Elizabeth Khuri Aaron Wells Research Nurses Kathy Bell Elizabeth Ducat Dorothy Melia Administrative Staff Kitt Lavoie Janesse Rojas Susan Russo Assistants for Research Jonathan Ball Lauren Bence Jason Choi Wan-Xin Feng David Fussell Rob Gianotti Todd Harris Lauren Hofmann Elizabeth Oosterhuis Laboratory Worker Laura Nunez
The Laboratory of the Biology of Addictive Diseases Mary Jeanne Kreek, M. D. – Professor and Head 2003 Laboratory Scientists Ann Ho K. Steven La. Forge Eduardo Butelman Vadim Yuferov David Nielsen Yan Zhou Alexis Bailey Thomas Kroslak Dmitri Proudnikov Brian Reed Stefan Schlussman Adena Svingos Yong Zhang Laboratory Adjunct Scientists Charles Inturrisi Roberto Picetti Virginia Pickel Ellen Unterwald Vanya Quinones-Jenab Clinical Scientists Gavin Bart Lisa Borg Scott Kellogg Charles Lilly Clinical Adjunct Scientists Paola Piccolo Joan Culpepper-Morgan Lawrence Brown James Kocsis Mark Green Lynette Benjamin Elizabeth Khuri Aaron Wells Research Nurses Kathy Bell Elizabeth Ducat Dorothy Melia Administrative Staff Kitt Lavoie Janesse Rojas Susan Russo Assistants for Research Jonathan Ball Lauren Bence Jason Choi Wan-Xin Feng David Fussell Rob Gianotti Todd Harris Lauren Hofmann Elizabeth Oosterhuis Laboratory Worker Laura Nunez
 NIH-NIDA K 05 -DA 00049 “Addictive Drugs: Pharmacology and Physiology” NIH-NIDA P 60 -DA 05130 “Treatment of Addictions: Biological Correlates” NIH-NIDA R 01 -DA 09444 “Mu Opioid Receptor Gene in Heroin Addicts” NIH-NIDA R 01 -DA 11113 “Effects of Systematically Administered Dynorphins” NIH-NIDA R 01 -DA 12848 “Addictions: Genotypes, Polymorphisms and Function” NIH-NIDA U 10 -DA 13046 “NIDA Clinical Trials Network – NYC” New York State OASAS NIH-NCRR M 01 -RR 00102 GCRC – The Rockefeller University Hospital
NIH-NIDA K 05 -DA 00049 “Addictive Drugs: Pharmacology and Physiology” NIH-NIDA P 60 -DA 05130 “Treatment of Addictions: Biological Correlates” NIH-NIDA R 01 -DA 09444 “Mu Opioid Receptor Gene in Heroin Addicts” NIH-NIDA R 01 -DA 11113 “Effects of Systematically Administered Dynorphins” NIH-NIDA R 01 -DA 12848 “Addictions: Genotypes, Polymorphisms and Function” NIH-NIDA U 10 -DA 13046 “NIDA Clinical Trials Network – NYC” New York State OASAS NIH-NCRR M 01 -RR 00102 GCRC – The Rockefeller University Hospital


