72cf4bc6164e7d71e01f4890254519c1.ppt
- Количество слайдов: 26
Liquid Crystal Materials
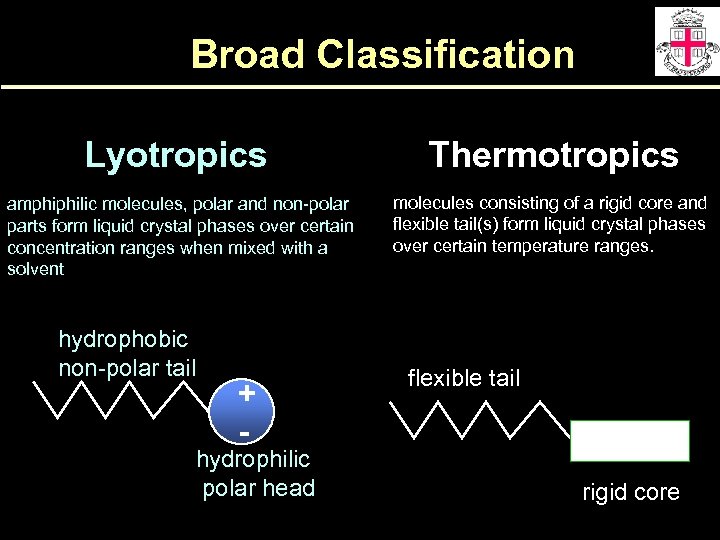
Broad Classification Lyotropics Thermotropics amphiphilic molecules, polar and non-polar parts form liquid crystal phases over certain concentration ranges when mixed with a solvent molecules consisting of a rigid core and flexible tail(s) form liquid crystal phases over certain temperature ranges. hydrophobic non-polar tail + - hydrophilic polar head flexible tail rigid core
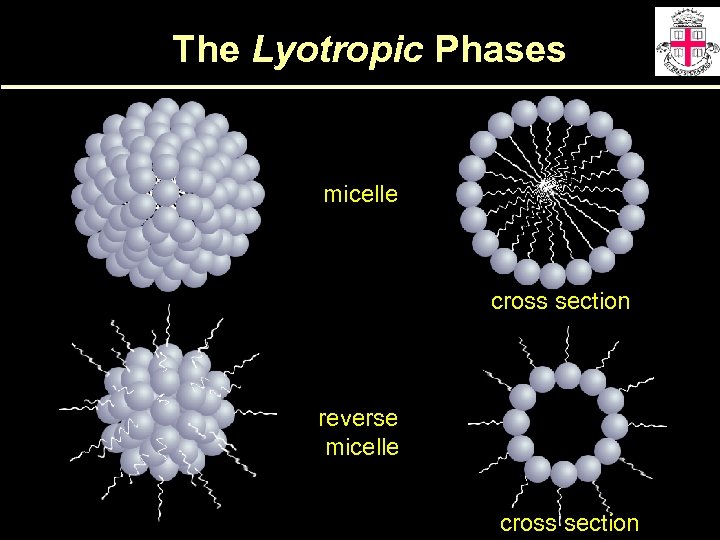
The Lyotropic Phases micelle cross section reverse micelle cross section
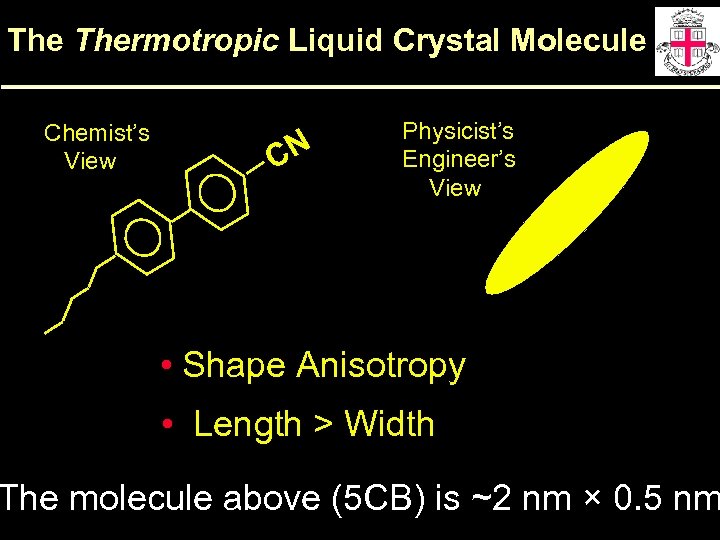
The Thermotropic Liquid Crystal Molecule Chemist’s View CN Physicist’s Engineer’s View • Shape Anisotropy • Length > Width The molecule above (5 CB) is ~2 nm × 0. 5 nm
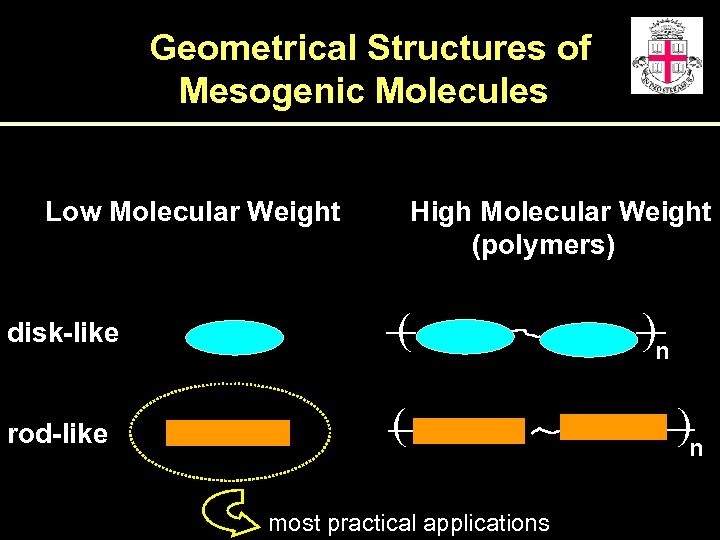
Geometrical Structures of Mesogenic Molecules Low Molecular Weight High Molecular Weight (polymers) disk-like ( rod-like ( most practical applications )n )n
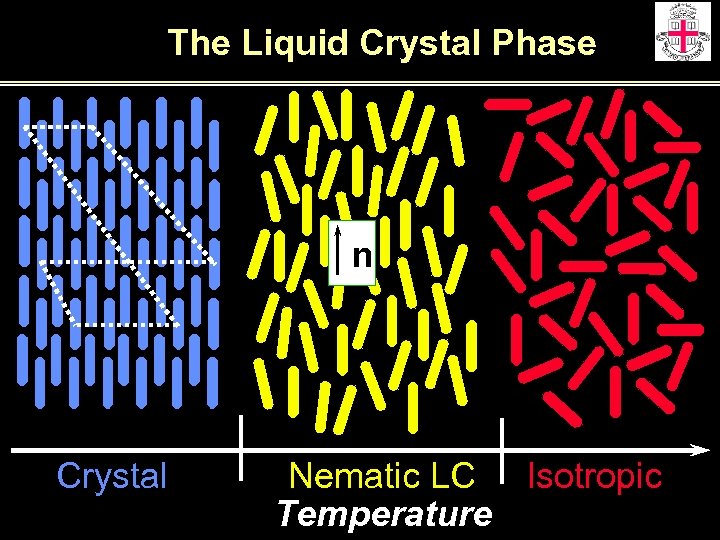
The Liquid Crystal Phase n Crystal Nematic LC Isotropic Temperature
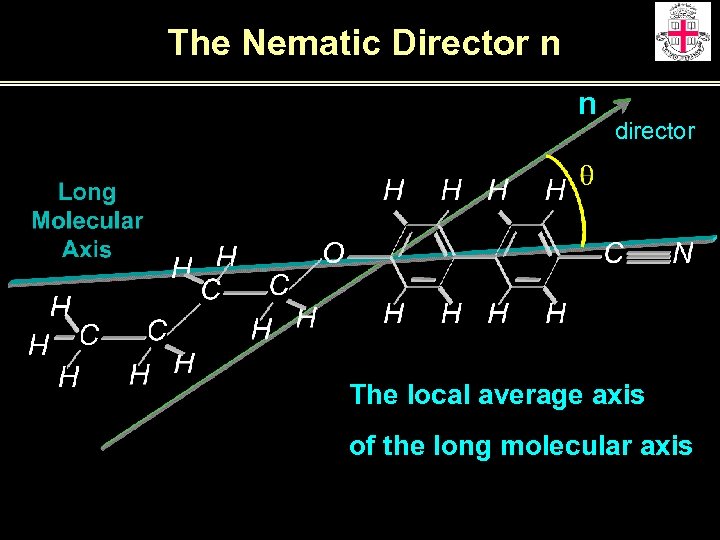
The Nematic Director n n director The local average axis of the long molecular axis
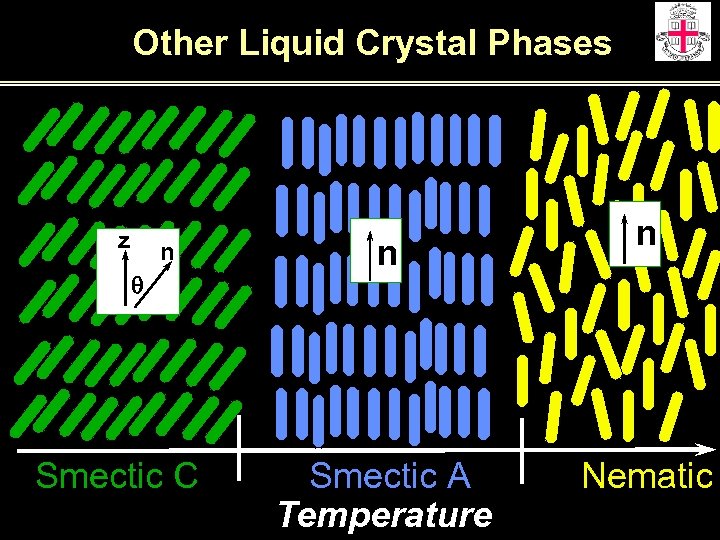
Other Liquid Crystal Phases z n q Smectic C n Smectic A Temperature n Nematic
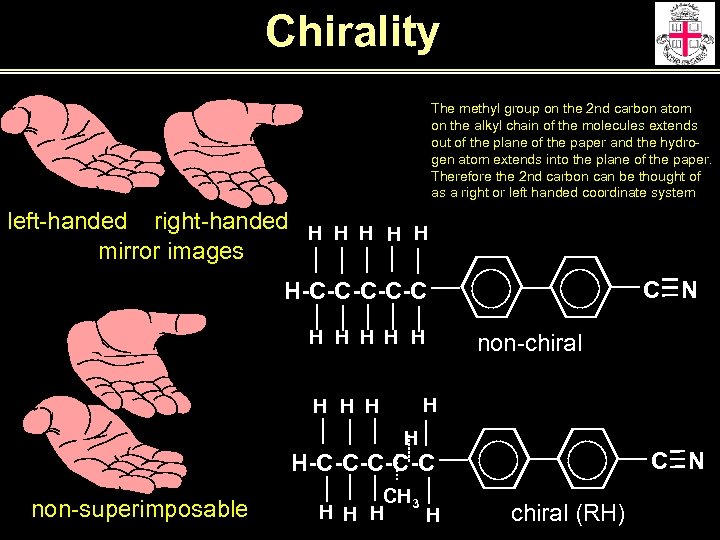
Chirality The methyl group on the 2 nd carbon atom on the alkyl chain of the molecules extends out of the plane of the paper and the hydrogen atom extends into the plane of the paper. Therefore the 2 nd carbon can be thought of as a right or left handed coordinate system left-handed right-handed H H H mirror images C N H-C-C-C H H H non-chiral H H H C N H-C-C-C non-superimposable CH 3 H H chiral (RH)
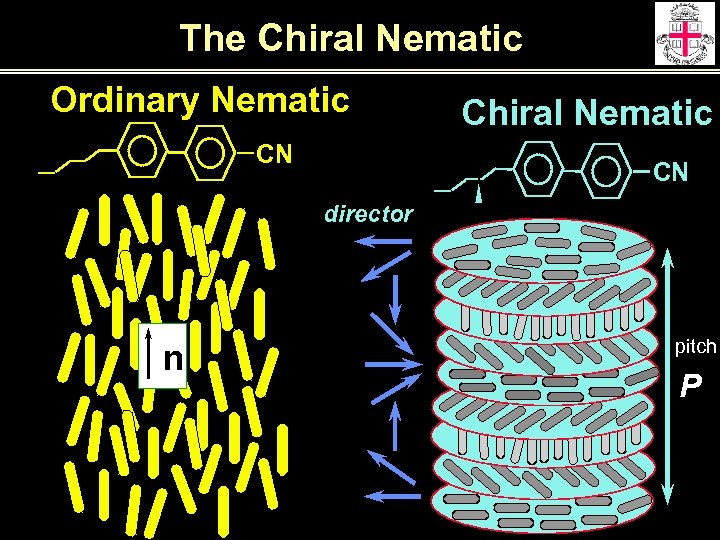
The Chiral Nematic Ordinary Nematic CN Chiral Nematic CN director n pitch P
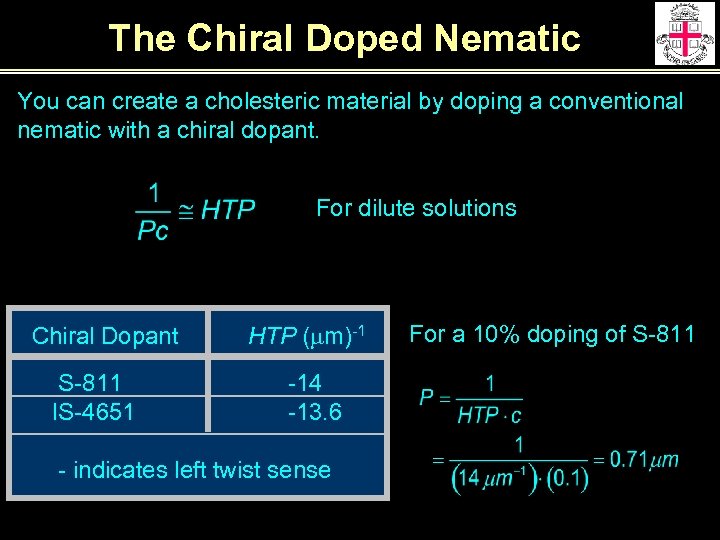
The Chiral Doped Nematic You can create a cholesteric material by doping a conventional nematic with a chiral dopant. For dilute solutions Chiral Dopant S-811 IS-4651 HTP (mm)-1 -14 -13. 6 - indicates left twist sense For a 10% doping of S-811
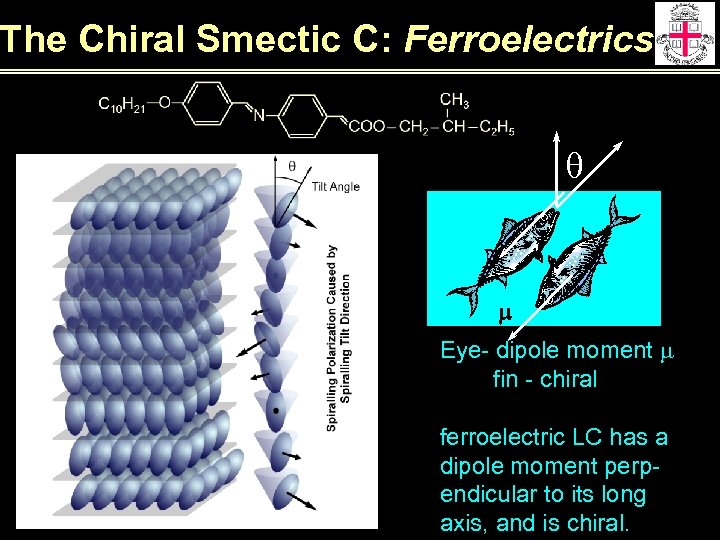
The Chiral Smectic C: Ferroelectrics q m Eye- dipole moment m fin - chiral ferroelectric LC has a dipole moment perpendicular to its long axis, and is chiral.
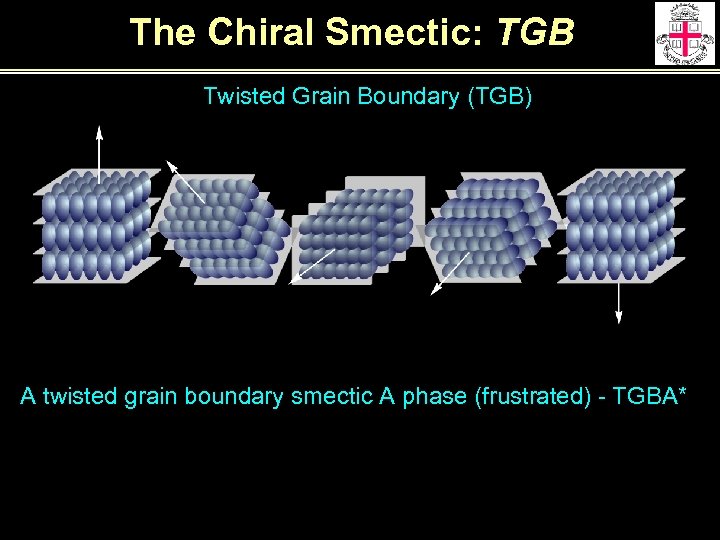
The Chiral Smectic: TGB Twisted Grain Boundary (TGB) A twisted grain boundary smectic A phase (frustrated) - TGBA*
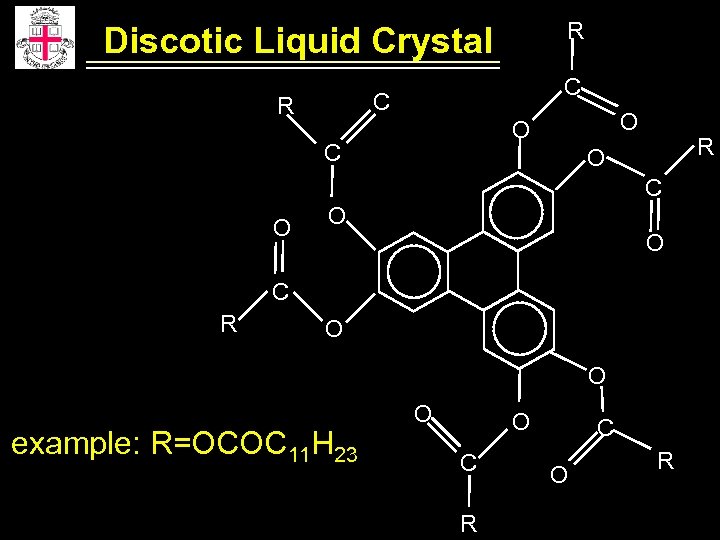
R Discotic Liquid Crystal C C R O O C R O C O O O C R O O example: R=OCOC 11 H 23 O O C R C O R
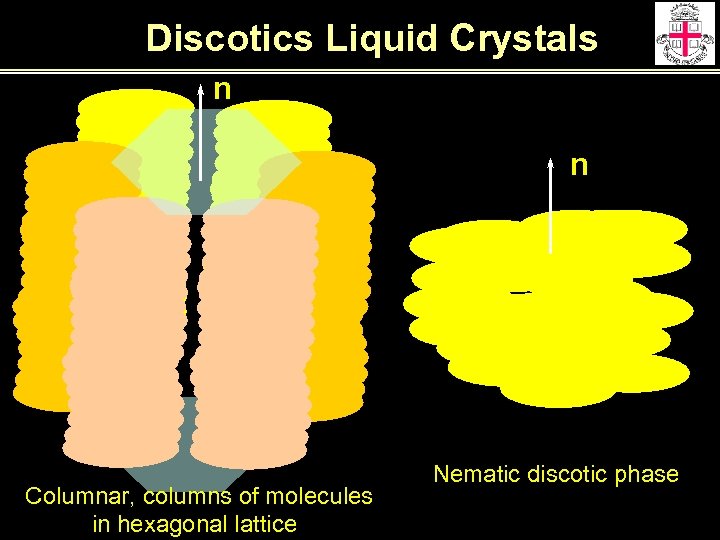
Discotics Liquid Crystals n n Columnar, columns of molecules in hexagonal lattice Nematic discotic phase
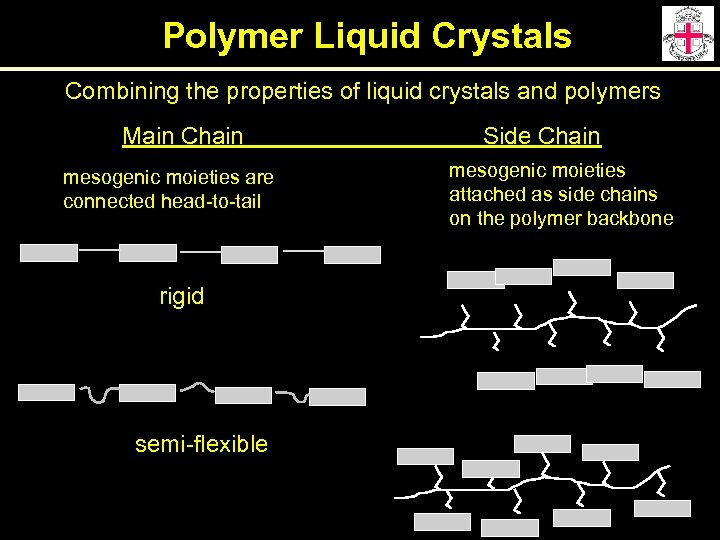
Polymer Liquid Crystals Combining the properties of liquid crystals and polymers Main Chain mesogenic moieties are connected head-to-tail rigid semi-flexible Side Chain mesogenic moieties attached as side chains on the polymer backbone
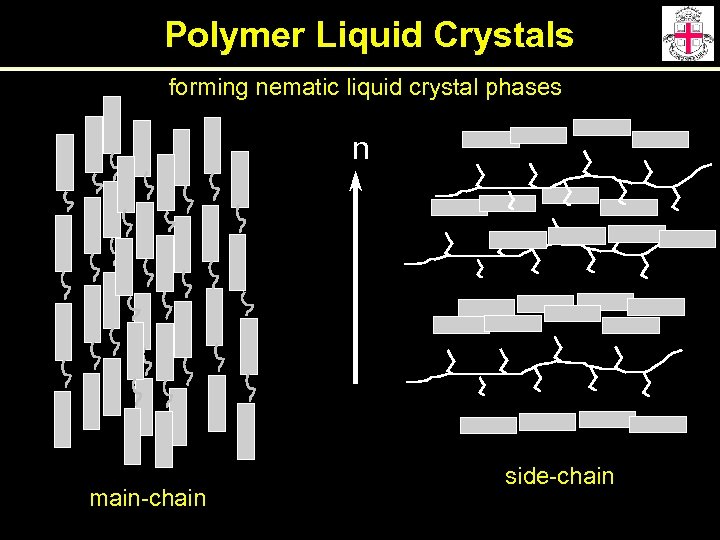
Polymer Liquid Crystals forming nematic liquid crystal phases n main-chain side-chain
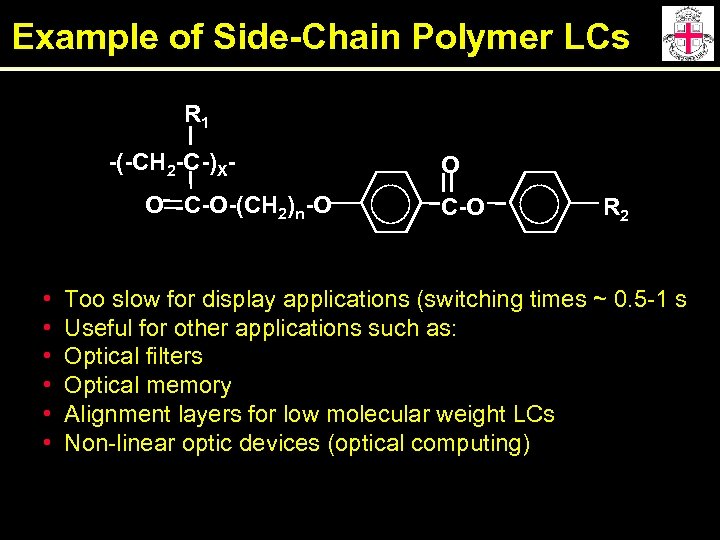
Example of Side-Chain Polymer LCs R 1 -(-CH 2 -C-)XO C-O-(CH 2)n-O • • • O C-O R 2 Too slow for display applications (switching times ~ 0. 5 -1 s Useful for other applications such as: Optical filters Optical memory Alignment layers for low molecular weight LCs Non-linear optic devices (optical computing)
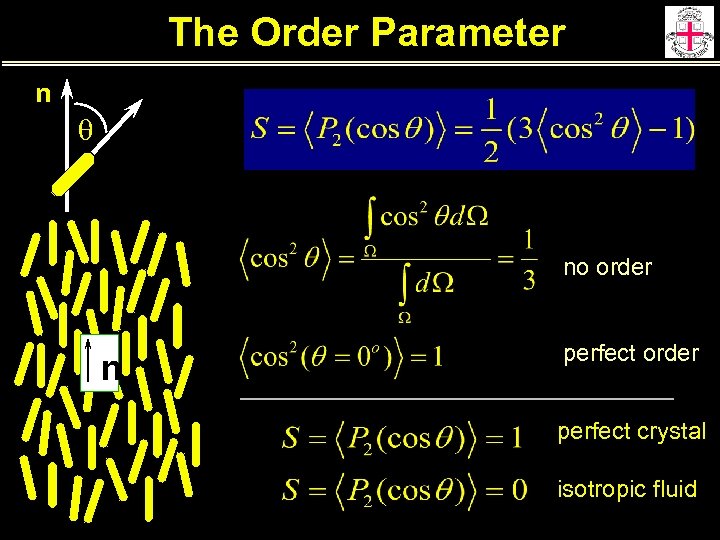
The Order Parameter n q no order n perfect order perfect crystal isotropic fluid
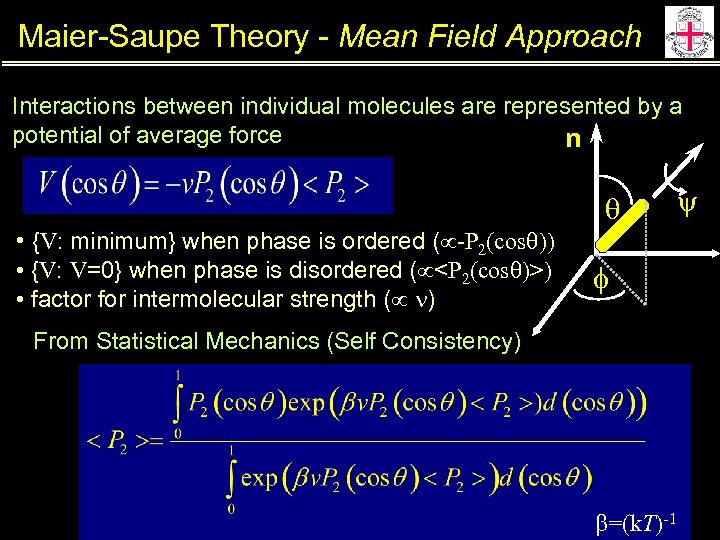
Maier-Saupe Theory - Mean Field Approach Interactions between individual molecules are represented by a potential of average force n q • {V: minimum} when phase is ordered ( -P 2(cosq)) • {V: V=0} when phase is disordered ( <P 2(cosq)>) • factor for intermolecular strength ( n) y f From Statistical Mechanics (Self Consistency) b=(k. T)-1
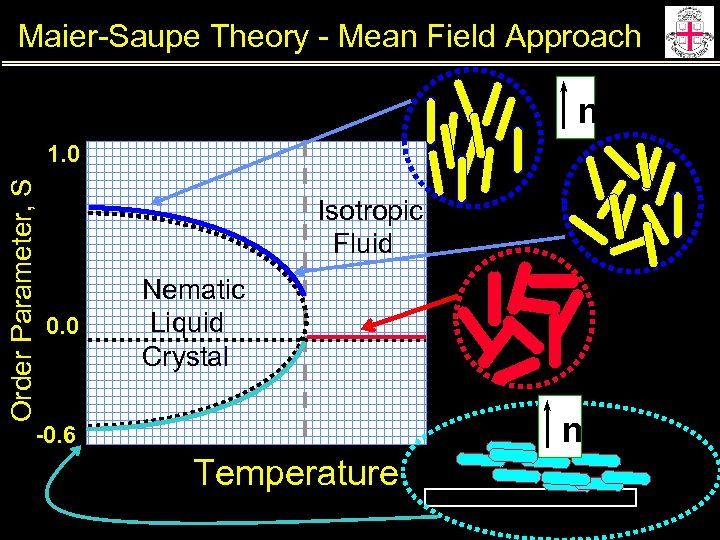
Maier-Saupe Theory - Mean Field Approach n Order Parameter, S 1. 0 Isotropic Fluid 0. 0 Nematic Liquid Crystal n -0. 6 Temperature
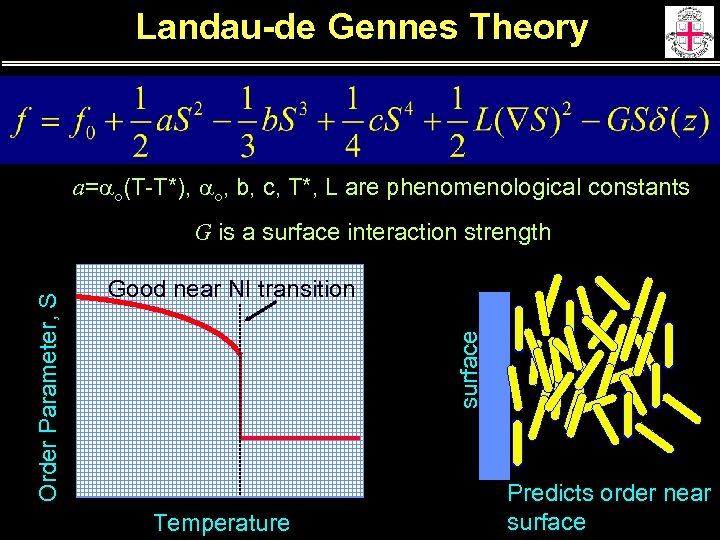
Landau-de Gennes Theory a=ao(T-T*), ao, b, c, T*, L are phenomenological constants Good near NI transition surface Order Parameter, S G is a surface interaction strength Temperature Predicts order near surface
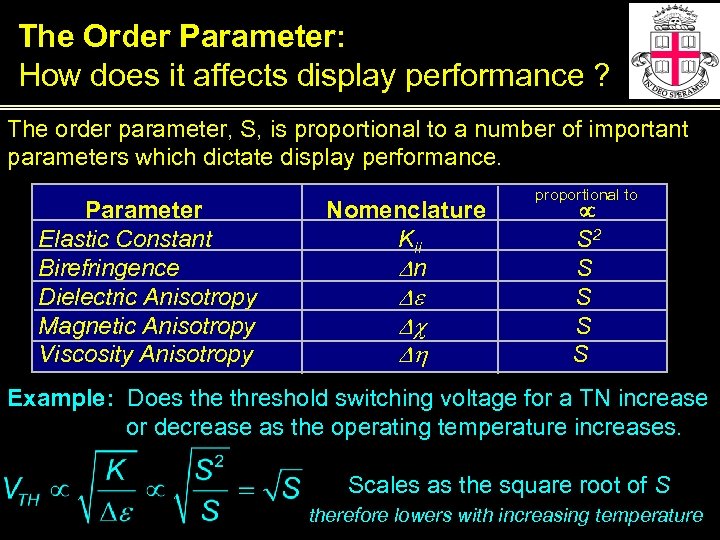
The Order Parameter: How does it affects display performance ? The order parameter, S, is proportional to a number of important parameters which dictate display performance. Parameter Elastic Constant Birefringence Dielectric Anisotropy Magnetic Anisotropy Viscosity Anisotropy Nomenclature Kii Dn De Dc Dh proportional to S 2 S S Example: Does the threshold switching voltage for a TN increase or decrease as the operating temperature increases. Scales as the square root of S therefore lowers with increasing temperature
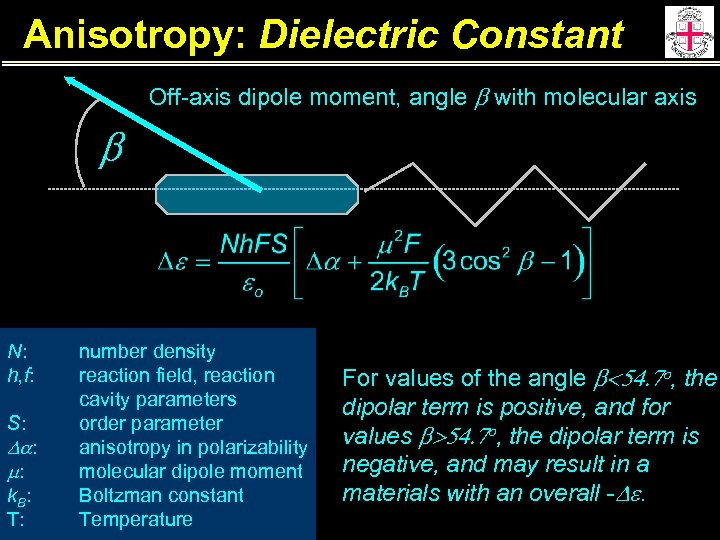
Anisotropy: Dielectric Constant Off-axis dipole moment, angle b with molecular axis b N: h, f: S: Da: m: k. B: T: number density reaction field, reaction cavity parameters order parameter anisotropy in polarizability molecular dipole moment Boltzman constant Temperature For values of the angle b<54. 7 o, the dipolar term is positive, and for values b>54. 7 o, the dipolar term is negative, and may result in a materials with an overall -De.
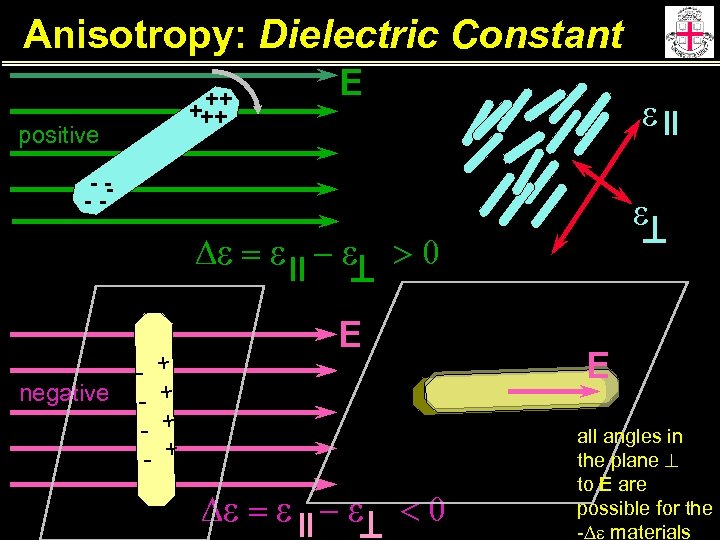
Anisotropy: Dielectric Constant ++ +++ positive E e -- -- De = e - e negative - + - + >0 E De = e - e e E <0 all angles in the plane to E are possible for the -De materials
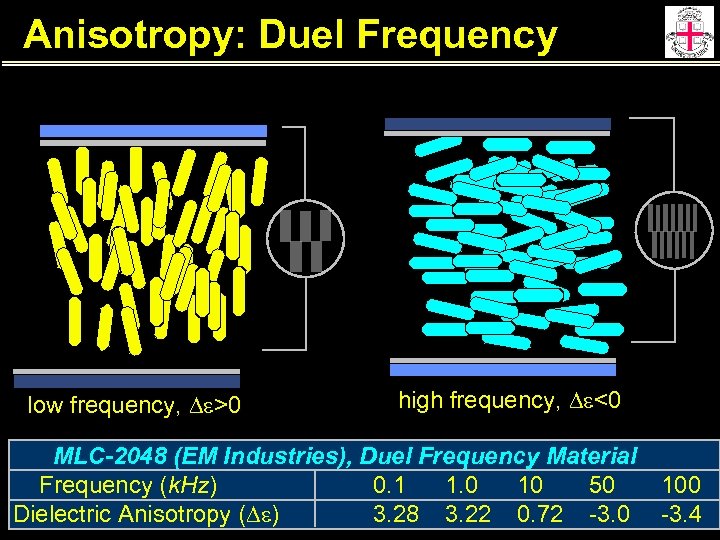
Anisotropy: Duel Frequency low frequency, De>0 high frequency, De<0 MLC-2048 (EM Industries), Duel Frequency Material Frequency (k. Hz) 0. 1 1. 0 10 50 Dielectric Anisotropy (De) 3. 28 3. 22 0. 72 -3. 0 100 -3. 4
72cf4bc6164e7d71e01f4890254519c1.ppt