
Lipases Alibekova Alina BT 16 -02

• Lipases (triacylglycerol acylhydrolases ) are a class of hydrolase which catalyze the hydrolysis of triglycerides to glycerol and free fatty acids over an oil–water interface. • In addition, lipases catalyze the hydrolysis and transesterification of other esters as well as the synthesis of esters and exhibit enantioselective properties. • The ability of lipases to perform very specific chemical transformation (biotransformation) has make them increasingly popular in the food, detergent, cosmetic, organic synthesis, and pharmaceutical industries

• Lipases have emerged as one of the leading biocatalysts with proven potential for contributing to the multibillion dollar underexploited lipid technology bio-industry and have been used in in situ lipid metabolism and ex situ multifaceted industrial applications The number of available lipases has increased since the 1980 s. Lipases are produced by animals, plants, and microorganisms. Microbial lipases have gained special industrial attention due to their stability, selectivity, and broad substrate specificity
Appication as industrial biocatalysis • The animal lipase most commonly used is the pancreatic lipase. • Plant lipases include papaya latex, oat seed lipase and castor seed lipase • Microbes have been found to produce high yields of lipases compare to the animal and plants. Because their bulk production is easier, commercialization of microbial lipases and their involvement in enzymatic biodiesel production are more common than animal and plant ones Lipases from microorganisms (bacterial and fungal) are the most used as biocatalysts in biotechnological applications and organic chemistry. • Lipases have been successfully used in novel biotechnological applications for the synthesis of biopolymers and the production of pharmaceuticals, flavor compounds, agrochemicals and biodiesel

The limitations of using lipases in biodiesel production include: • (a) significant cost, • (b) the risk that glycerol inhibits the lipase by covering it, due to its accumulation in the reaction mixture; • (c) initial activity may be lost because of volume of the oil molecule
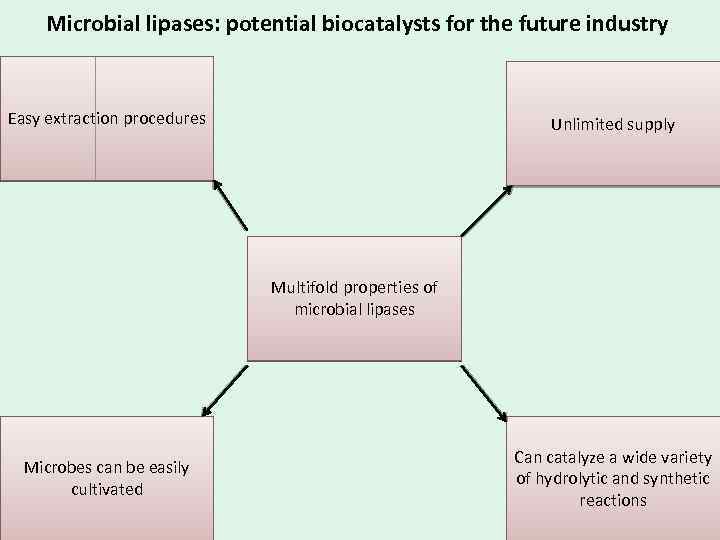
Microbial lipases: potential biocatalysts for the future industry Easy extraction procedures Unlimited supply Multifold properties of microbial lipases Microbes can be easily cultivated Can catalyze a wide variety of hydrolytic and synthetic reactions
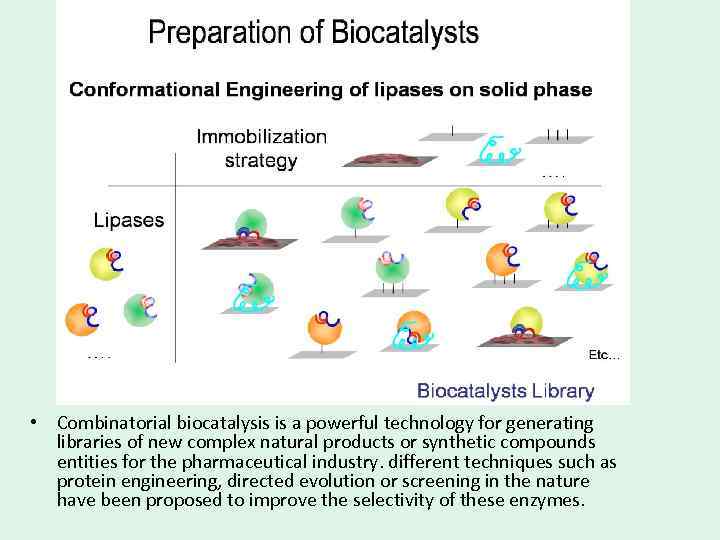
• Combinatorial biocatalysis is a powerful technology for generating libraries of new complex natural products or synthetic compounds entities for the pharmaceutical industry. different techniques such as protein engineering, directed evolution or screening in the nature have been proposed to improve the selectivity of these enzymes.
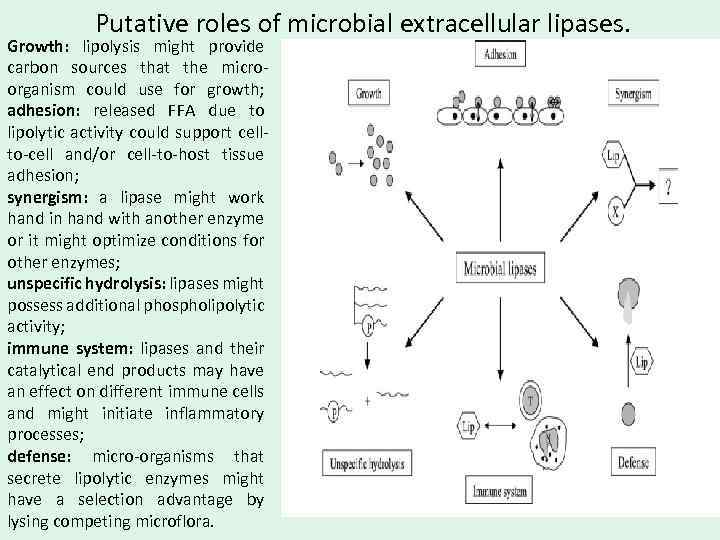
Putative roles of microbial extracellular lipases. Growth: lipolysis might provide carbon sources that the microorganism could use for growth; adhesion: released FFA due to lipolytic activity could support cellto-cell and/or cell-to-host tissue adhesion; synergism: a lipase might work hand in hand with another enzyme or it might optimize conditions for other enzymes; unspecific hydrolysis: lipases might possess additional phospholipolytic activity; immune system: lipases and their catalytical end products may have an effect on different immune cells and might initiate inflammatory processes; defense: micro-organisms that secrete lipolytic enzymes might have a selection advantage by lysing competing microflora.
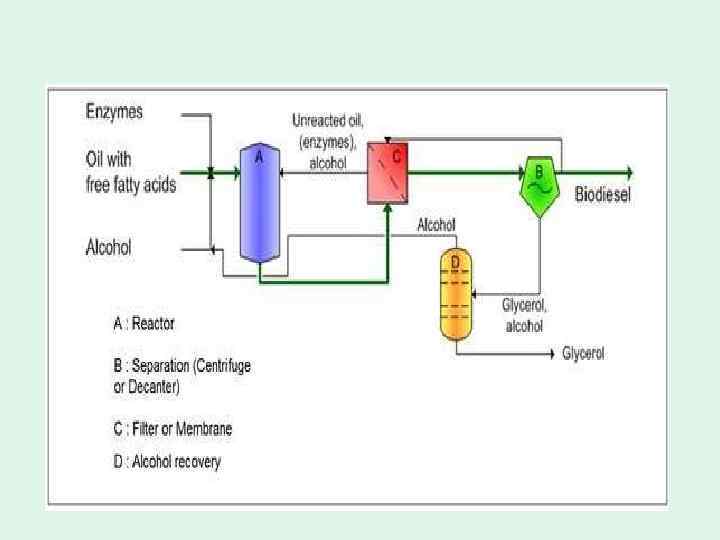
Lipases in biodiesel production: • • Biodiesel is a liquid biofuel which are esters of long chain fatty acids and short chain alcohols. Transesterification is the displacement of alcohol from an ester by another alcohol in a process similar to hydrolysis, except that an alcohol is employed instead of water. Among short chain alcohols, methanol and ethanol are usually used, especially, because of its low cost and physicochemical advantages, methanol is used frequently. This process has been widely used to reduce the viscosity of triglycerides, thereby enhancing the physical properties of renewable fuels to improve engine performance Since the cost of lipase production is the main hurdle to the commercialization of the lipase-catalyzed process, the use of intracellular lipase or cell-surface-displayed lipase as a whole-cell biocatalyst through the application of immobilization techniques has been considered as an effective way to lower the lipase production cost. However, to utilize these whole-cell biocatalysts for industrial application, a repeated methanolysis reaction cycle is required in order to produce high methyl ester content of 90 -95%. One potential solution is the use of a whole-cell biocatalyst possessing a non-specific lipase from a source such as Candida antarctica or Pseudomonas cepacia within the cell or on the cell-surface, since these lipases realize methyl ester content of more than 95%. Such a system could offer a promising prospect of realizing industrial biodiesel fuel production. However, bodiesel production by lipase is not yet commercialized.

Bioreactors • Chen and Wu achieved 70 % conversion in continuous packed-bed bioreactor in the absence of organic solvent, but with periodical regeneration of the immobilized lipase with t-butanol washing. • Nie used lipase immobilized on cheap cotton fibers in a series of three packed-bed bioreactors with stepwise addition of methanol to produce biodiesel from SO and WO and achieved 93% and 92% conversions. • The use of membrane bioreactors for the enzymatic processing of fats and oils is increasingly becoming more attractive to substitute conventional stirred tanks or packed-bed bioreactors

• Lipase production has been mainly performed in tray bioreactors (TB) and packed-bed bioreactor (PBB). • Tray bioreactors consist of a chamber where controlled air (flow, temperature and relative humidity) is circulated around a number of trays. Intermittent mixing of the solid substrate by hand could be carried out but in general this will occur only once per day. The main drawback of TB is their low volumetric efficiency compared to column bioreactor • PBB typically involves a static bed on top of a perforated plate through which conditioned air is blown Over the last 25 years, PBB has received much experimental and modeling attention. The main attractive characteristic is that it has no mechanical (i. e. moving) parts, thus reducing the cost of construction, operation and maintenance. • In PBB processes, axial dynamical temperature profiles could be observed. evaporation phenomenon can remove up to 65% of the heat generated by the cellular metabolism, it lowers the moisture of the solid substrate and thus limit the cellular growth In PBB, moisture regulation is almost impossible since the bed is unmixed.
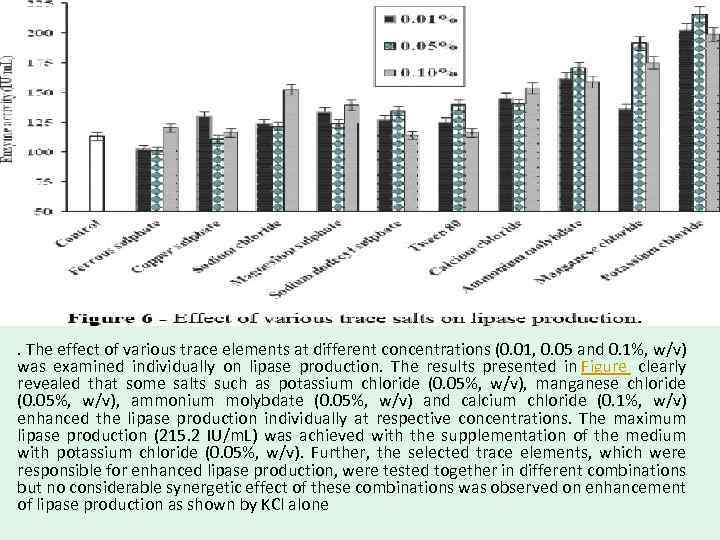
. The effect of various trace elements at different concentrations (0. 01, 0. 05 and 0. 1%, w/v) was examined individually on lipase production. The results presented in Figure clearly revealed that some salts such as potassium chloride (0. 05%, w/v), manganese chloride (0. 05%, w/v), ammonium molybdate (0. 05%, w/v) and calcium chloride (0. 1%, w/v) enhanced the lipase production individually at respective concentrations. The maximum lipase production (215. 2 IU/m. L) was achieved with the supplementation of the medium with potassium chloride (0. 05%, w/v). Further, the selected trace elements, which were responsible for enhanced lipase production, were tested together in different combinations but no considerable synergetic effect of these combinations was observed on enhancement of lipase production as shown by KCl alone
Genetic engineering • Current attention in lipase production is focused on genetic engineering as there is a hope that the cost can be reduced by gene technology such as gene amplification. The first and essential step of genetic manipulation is cloning of gene involved in the enzyme’s biosynthesis. Recently a gene of bacterial lipases and mammalian phospholipase A 2 has been cloned. A number of genes of lipase will be cloned rapidly in the coming years. The use of recombinant DNA technology to produce large quantities of recombinant lipases will help lower the enzyme cost.
• For example, the immobilized lipase producing Rhizopus orizae whole cells developed by Matsumoto have already been overexpressed in Sacharomyces cerevisiae. Isoform/isoenzyme of C. rugosa lipase named Lip 2 has been engineered and produced, which may be useful in biodiesel production
Yang produced a methanol resistant and thermostable recombinant lipase in B. sepacia strain. The optimum temperature of the purified lipase was 70 C and was highly tolerant methanol Recently, Gao cloned lipase gene from a lipase-producing Proteus sp. Strain bacterium in a heterologous host, E. Coli. The recombinant E. Coli was applied in biodiesel production in the form of whole-cell biocatalyst
Harnessing biodiesel-producing microbes: from genetic engineering of lipase to metabolic engineering of fatty acid biosynthetic pathway • Genetically modified microbial cells expressing lipases for in vitro biodiesel production The most frequently used E. coli host was explored as microbial whole cells overexpressing intracellular heterologous lipases for biodiesel production via in vitro route. • Subsequently, an E. coli whole-cell biocatalyst expressing intracellular Serratia marcescens lipase was constructed to catalyze biodiesel production from grease via esterification and transesterification . More interestingly, considering that Candida antarctica lipase B and Thermomyces lanuginosus lipase demonstrated different preferences for esterification and transesterification reactions, recombinant E. coli whole-cell biocatalysts co-expressing these two lipases were developed as a tandem catalysis system for producing biodiesel from high-FFAcontaining grease . These latter two promising E. coli whole cell systems without permeabilization afforded satisfactory biodiesel yield (higher than 90%) and recyclability (more than 70%)
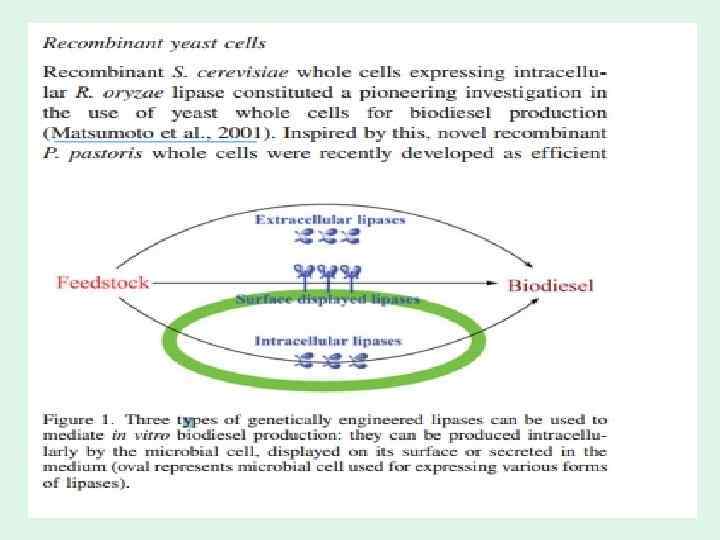

• Microbial cells with secretion of extracellular lipases P. pastoris yeast has already been developed as an efficient expression/secretion host for heterologous proteins. • Extracellular lipases secreted by recombinant P. pastoris have been used to catalyze biodiesel production • Recombinant R. oryzae lipases secreted by P. pastoris, used directly from crude fermentation supernatant, could mediate biodiesel production with a greater than 90% yield • Rhizomucor miehei lipase with 1, 3 -specific selectivity and Penicillium cyclopium lipase with non-specific mono- and diacylglycerol selectivity were individually overexpressed and secreted by P. pastoris, and the combination of both lipasecontaining supernatants from respective fermentation broths, exhibiting different specificities, converted complex oil feedstock to biodiesel with a greater than 95% yield • More interestingly, inspired by the consolidated bioprocessing of lignocellulose to generate bioethanol, we recently developed an integrated process by coupling lipase production and in situ biodiesel synthesis in a recombinant P. pastoris yeast. This novel and efficient dual biocatalytic system, based on recombinant T. lanuginosus lipase, took advantage of both cell-free enzymes (extracellular lipases) and whole-cell catalysts (intracellular lipases).
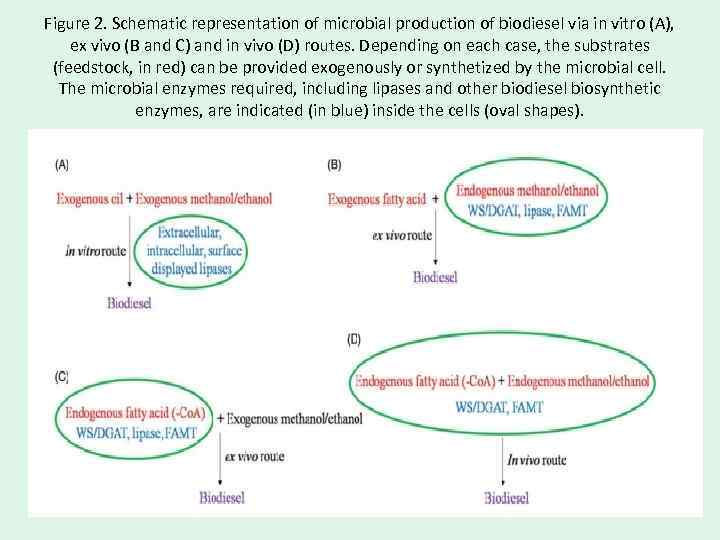
Figure 2. Schematic representation of microbial production of biodiesel via in vitro (A), ex vivo (B and C) and in vivo (D) routes. Depending on each case, the substrates (feedstock, in red) can be provided exogenously or synthetized by the microbial cell. The microbial enzymes required, including lipases and other biodiesel biosynthetic enzymes, are indicated (in blue) inside the cells (oval shapes).
Conclusion and perspectives • • Microbial whole cells with expression of various forms of lipases (intracellular, extracellular or surfacedisplayed) are now widely used for producing biodiesel. Microbial synthesis based on protein engineering, metabolic engineering, synthetic biology and systems biology approaches has the potential to push the frontiers of biodiesel production. We identify several hot spots or trends in the genetic or metabolic engineering of microorganisms for producing biodiesel: (1) genetically tractable oleaginous microorganisms are more easy-to-use for production of FFA- or TAGderived biodiesel via genetically engineered lipase whole-cell-mediated in vitro route, or could be developed as microbial cell factory for in vivo biodiesel synthesis via metabolically engineered fatty acid biosynthetic pathway; (2) consolidated bioprocessing of lignocellulosic biomass for biodiesel production, via combination of introducing cellulose- or hemicelluloses-utilizing genes with biodiesel producing pathway into a genetically tractable microorganism host, would be more cost efficient; (3) protein engineering of biodiesel producing enzymes to alter selectivity or activity in the context of metabolic engineering would be a powerful tool for developing microorganisms into efficient cell factories for biodiesel production; (4) regulation of the entire biodiesel biosynthesis network through developing systematic metabolic engineering and synthetic biology approaches, such as regulator bio-sensing, could constitute a tool for balancing cell growth and biodiesel production, thus representing a new frontier with interesting potentialities. (5) We expect that the combination of lipase engineering with fatty-acid-derived pathway metabolic engineering will allow designing highly efficient microbial cell factories for the production of biodiesel in a near future.

Selection of Strains Producing Lipase with Transesterification Activity and Its Characterization • Among the microorganisms selected for lipase production, the most productive strains were Burkholderia cepacia (isolated), wild yeast, Aspergillus fumigatus NRRL 164 (using medium containing soy and rice brans), Aspergillus fumigatus NRRL 166 (in medium containing wheat bran) and Aspergillus (not identified). The other strains did not show satisfactory lipase activity.
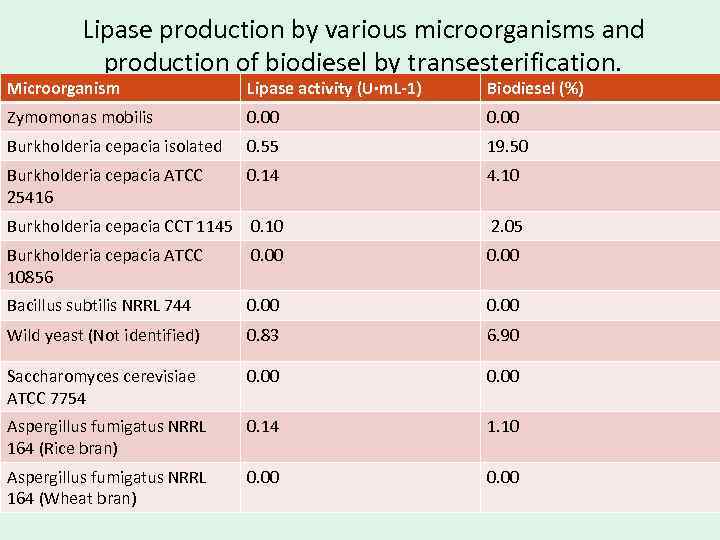
Lipase production by various microorganisms and production of biodiesel by transesterification. Microorganism Lipase activity (U·m. L-1) Biodiesel (%) Zymomonas mobilis 0. 00 Burkholderia cepacia isolated 0. 55 19. 50 Burkholderia cepacia ATCC 25416 0. 14 4. 10 Burkholderia cepacia CCT 1145 0. 10 2. 05 Burkholderia cepacia ATCC 10856 0. 00 Bacillus subtilis NRRL 744 0. 00 Wild yeast (Not identified) 0. 83 6. 90 Saccharomyces cerevisiae ATCC 7754 0. 00 Aspergillus fumigatus NRRL 164 (Rice bran) 0. 14 1. 10 Aspergillus fumigatus NRRL 164 (Wheat bran) 0. 00
• Thus, it can be noted that the experiments conducted with different compositions of culture medium were determinant for the production of lipase from Aspergillus fumigatus. The addition of wheat bran in the fermentation medium with the strain NRRL 166 as well as the addition of soybean bran for the strain NRRL 164 significantly increased the enzyme production.

• The strain of Burkholderia cepacia was the microorganism among those tested with the highest potential for lipase production for transesterification reaction. The characterization has shown this lipase to be a small molecule capable of a more ready catalysis of the hydrolysis at 37 °C and p. H 8. 0. However, in the transesterification reactions, the p. H exerts a positive significant effect in low acidity conditions in the presence of approximately 42% water in the system.

• Both lipases have been purified to homogeneity and were tested for their potential to catalyze biotechnologically important reactions. S. marcescens lipase stereo selectively hydrolyzed racemic isopropylideneglycerol acetate which is a basic building block in a variety of organic synthesis reactions. • P. aeruginosa lipase was successfully used for kinetic resolution of chiral alcohols and amines giving enantiomeric excess values of 2 95% at reaction rates of 4050%. Our results demonstrate that both lipases can be produced at levels of 100 mg/l for S. marcescens and 150 mg/l for P. arruginosa. The recombinant lipase proteins are promising candidates for biotechnological applications.


Choice of host organism (extremophiles) 15% • Lipases from extremophiles can provide special features that make them more suitable for specific applications where a lipolytic biocatalyst is required. In some cases this would be an advantage, e. g. enhanced stability that permits the recycling of the biocatalyst with no loss of activity, but in other cases it would be a requisite, e. g. high levels of catalytic activity at low temperatures for processing thermolabile compounds. • Here we present different extreme environments from which lipases/esterases have been isolated through metagenomics in recent years, and the new knowledge that these discoveries have brought to the field of lipolytic enzymes: bifunctional enzymes, several completely new families, protein engineering of metagenome-derived lipases and some very extreme features.

• Extremophiles have the potential to produce uniquely valuable biocatalysts that function under conditions in which usually the enzymes of their nonextremophilic counterparts could not. Among novel enzymes isolated from extremophilic microorganisms, hydrolases, and particularly lipases and esterases are experiencing a growing demand. Lipases (EC 3. 1. 1. 3) and esterases (EC 3. 1. 1. 1) catalyze the cleavage of ester bounds in aqueous media and the reverse reaction in organic solvents.

Thermophilic and psychrophilic lipases of microbial origin: • The optima activity of lipases obtained from conventional sources range from 30 and 60°C. However, currently, lipases were obtained from extremophiles, i. e. , organisms adapted to life in high temperature, with maximum activity over 70°C (Bacillus thermocatenulatus) or with high activity at low temperature as is the case for enzymes produced by Antarctic bacteria, such as Pseudomonas and Moraxella sp. Such extreme and unusual features open the possibility to apply these enzymes without further modification using molecular engineering approaches to adapt them for use in reactions carried out at high temperatures or, conversely low temperature processes such as that of detergents (low temperature washes) or in food processing. • Generally, lipases are further divided into three based on their degree of temperature stability; namely psychrophilic, mesophilic and thermophilic. Thermostable enzymes can be obtained from mesophilic and thermophilic organisms; even psycrophiles have some thermostable enzymes . Currently, lipases from thermophilic and psychrophilic organisms have been proved to be more useful for biotechnological applications. •

Thermophilic lipases: • The demand of thermostable lipases for different applications has been growing rapidly. Most of the studies were carried out to produce lipases from mesophilic microorganisms. Many lipases from mesophiles are stable at elevated temperatures. Proteins from thermophilic organisms have also been proved to be more useful for biotechnological applications than similar proteins from mesophiles due to their stability at high temperature. Enzymes with high thermostability are important to have higher reaction rate at higher operation temperature. This is because higher temperature can increase solubility of substrates and also help to lower substrate viscosity and thereby avoid environmental contamination • Thermostable lipases from such microbial sources are highly advantageous for biotechnological applications, since they can be produced at low cost and exhibit improved stability at high extreme temperature Currently, there has been a great demand for thermophilic and thermostable enzymes in various industrial fields. Thus, thermostable lipases from various sources have been purified and characterized using appropriate procedures
Lipases operating chemical reaction at elevated temperatures have the following advantages. • (1) A higher diffusion rates. • (2) Increased solubility of lipids and other hydrophobic substrates in water. • (3) Decreased substrate viscosities. • (4) Increased reactant solubility. • (5) Higher temperature faster reaction rates. • (6) Reduced risk of microbial contamination Thermophile microorganisms are a valuable source of thermostable lipase with desired properties usually associated with stability in solvents and detergents for potential biotechnological and industrial applications These enzymes have been applied to synthesis biopolymers, pharmaceutical chemicals, agrochemicals, cosmetics, flavours and biodiesel
Currently, thermostable lipases have been isolated from many sources, including Pseudomonas fluorescens Bacillus sp. B coagulans and B. cereus
• B. stearothermophilus Aeromonas sobria Geotrichum sp. • • The enzyme from P. aeruginosa was significantly stabilized by Ca 2+ and was inactivated by EDTA. This inactivation could be overcome by adding Ca. Cl 2, suggesting the existence of a calciumbinding site in P. aeruginosa lipase.

• One of the more notable thermostable enzymes was isolated by from a Bacillus strain. This enzyme had maximum activity at 60°C and retained 100% of the original activity after being held at 75°C for 30 min. The half-life of the enzyme was 8 h at 75°C • Thermal stability of a lipase is clearly related with its structure. Thermostability is also influenced by environmental factors such as p. H and the presence of metal ions. enhanced by immobilization For instance, C. antarctica lipase B could be thermally stabilized by immobilization

Psychrophilic lipases: • Cold adapted lipases are largely distributed in microorganisms existing at low temperatures nearly 5°C. Cold adapted bacterial strains were isolated mostly from Antarctic and Polar regions which represent a permanently cold (0± 2°C). A marine bacterium Aeromonas hydrophila growing at a temperature range between 4 and 37°C was found to produce cold active lipolytic enzyme. • Few bacterial genera have been isolated and characterized from deep-sea sediments where temperature is below 3°C. They include Aeromonas sp. Pseudoalteromonas sp. and Psychrobacter sp. and Photobacterium lipolyticum • The soil and ice in Alpine region also harbor psychrophilic microorganisms which produces cold active lipases.

• The cold enzymes along with the producing microorganisms cover a broad spectrum of biotechnological applications. Their current application include additives in deter-gents (cold washing), additives in food industries (fermentation, cheese manufacture, bakery, meat tenderizing), environmental bioremediations (digesters, composting, oil degradation or xenobiotic biology applications and molecular biology applications), biotransformation and heterologous gene expression in psychrophilic hosts to prevent formation of inclusion bodies A number of relatively straightforward reasons for applications of cold active enzymes in biotechnology have been mentioned by various authors • In the food industry, reaction needs to be carried out at low temperature in order to avoid changes in food ingredients caused by undesirable side-reaction that

• Lipases have become an integral part of the modern food industry. The use of enzymes to improve the traditional chemical processes of food manufacture has been developed in the past few years. The use of cold active lipase in the formulation of detergents would be of great advantage for cold washing that would reduce the energy consumption and wear and tear of textile fibers . The industrial dehairing of hides and skin at low temperature using psychrophilic lipase together with protease or keratinase would not only save energy but also reduce the impacts of toxic chemicals used in dehairing. This is because they have no negative impact on sewage treatment processes and do not present a risk to aquatic life. The other common commercial application of lipase as detergent includes in dish washing, clearing of drains clogged by lipids in food processing or domestic/industrial effluent treatment plants (Bailey and Ollis, 1986). As determined by Buchon et al. (2000), cold adapted lipases have great potential in the field of wastewater treatment, bioremediation in fat contaminated cold environment and active compounds synthesis in cold condition.