9a49edd331c683f10938601a7ae3ccb1.ppt
- Количество слайдов: 54

Light Microscopy and Electronic Imaging for the Biomedical Sciences E. D. Salmon and Kerry Bloom Biology 188

History of the Microscope, Thomas E. Jones http: //www. utmem. edu/~thjones/hist_mic. htm See also Molecular Expressions, a Microscope Primer at: http: //micro. magnet. fsu. edu/primer/index. html There is one very early description of an isolated use of spectacles. Pliny the Elder wrote the following in 23 -79 A. D. : "Emeralds are usually concave so that they may concentrate the visual rays. The Emperor Nero used to watch in an Emerald the gladatorial combats. " The modern reinvention of spectacles occurred around 1280 -1285 in Florence, Italy

Janssen Microscope Was One of the First
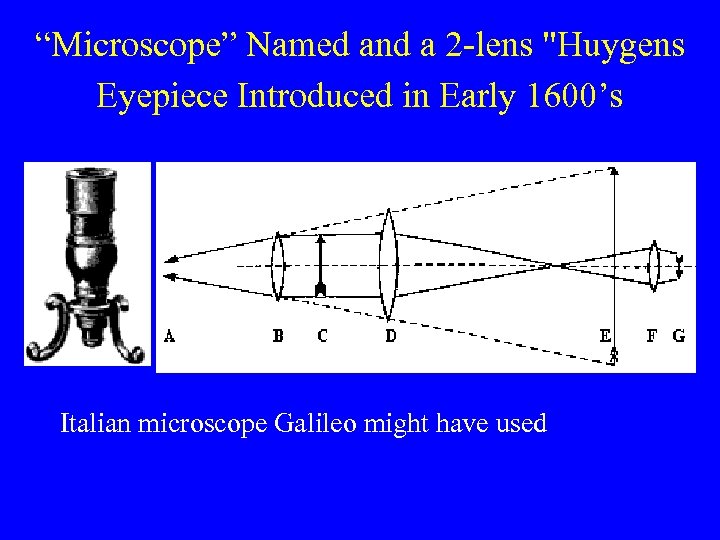
“Microscope” Named and a 2 -lens "Huygens Eyepiece Introduced in Early 1600’s Italian microscope Galileo might have used
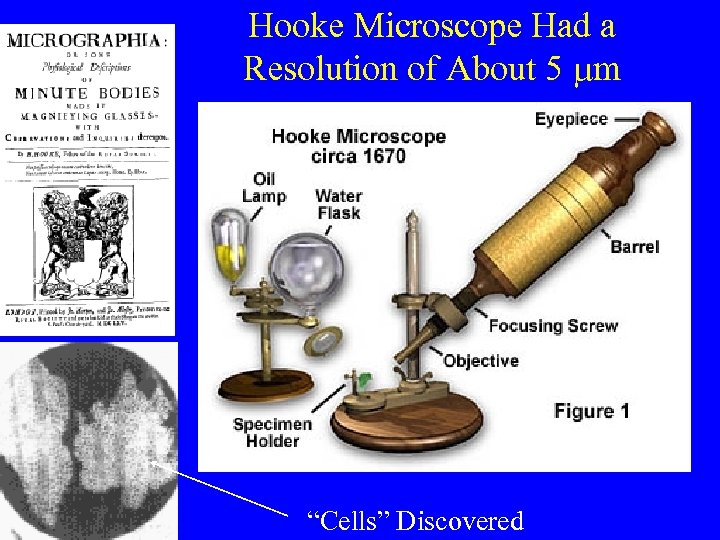
Hooke Microscope Had a Resolution of About 5 mm “Cells” Discovered

Leeuwenhoek Microscope Had Resolution of About 1 mm

Leeuwenhoek's Secret Lenses: Leeuwenhoek's method of making the tiny, high-quality and high power lenses was kept secret. A study has recently been done on the few remaining copies of Leeuwenhoek's microscopes, and it appears that some of the lenses may have been made by grinding, while the best ones were blown. Leeuwenhoek learned that when a glass bulb is blown, a small drop of thickened glass forms at the bottom of the bulb (much like a drop sits in the bottom of a blown soap bubble. ) By carefully breaking away the excess glass, this tiny drop can be used as a lens.
Chromatic and Spherical Aberration Limited Resolution While the 18 th century produced some great mechanical improvements for the microscope, making it much more sturdy and easy to use, the images obtainable remained rather blurry with colorful halos around objects. This was largely due to the problems of "Chromatic and Aspheric Aberration. " The reason the single lens "simple" microscopes remained important throughout the century was that a single lens system has much less aberration because the distortion becomes synergistic with multiple lenses. This allowed simple microscopes to attain around 2 micron resolution, while the best compound microscopes were limited to around 5 microns.
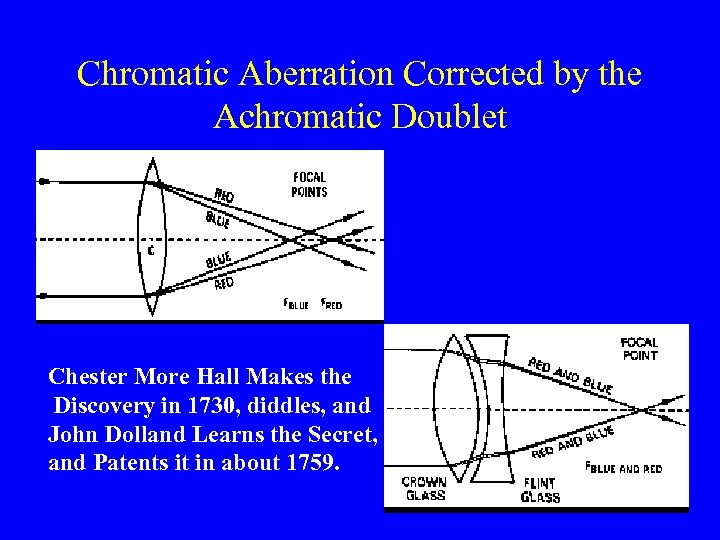
Chromatic Aberration Corrected by the Achromatic Doublet Chester More Hall Makes the Discovery in 1730, diddles, and John Dolland Learns the Secret, and Patents it in about 1759.

Spherical Aberration Not Solved Until 1830 by Joseph Jackson Lester Tulley/Lister Corrected Lens Microscope, 1830's Adjustable Objective by Ross, circa 1840
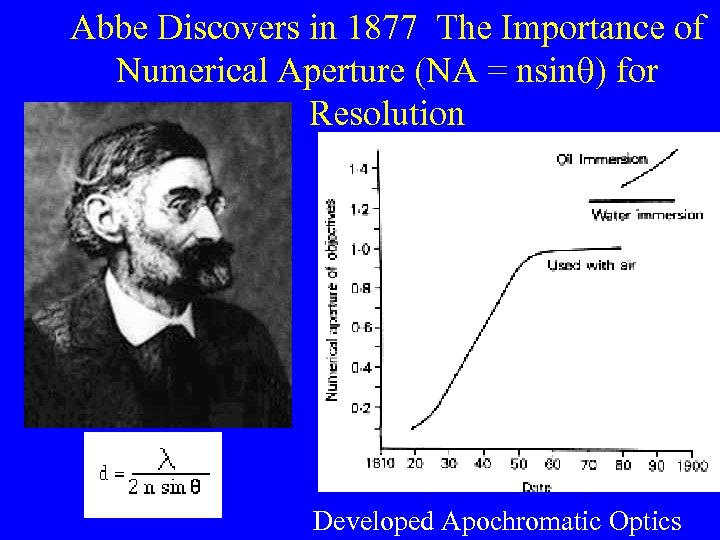
Abbe Discovers in 1877 The Importance of Numerical Aperture (NA = nsinq) for Resolution Developed Apochromatic Optics

Microscopes in the Mid-Late 1800’s Zeiss
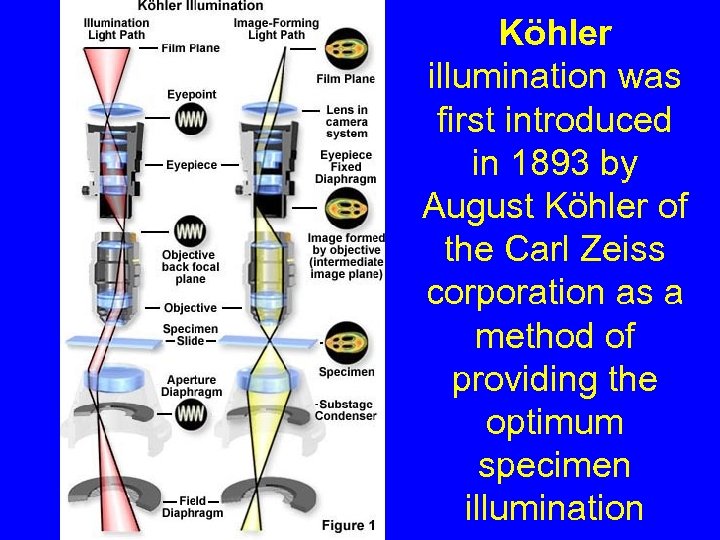
Köhler illumination was first introduced in 1893 by August Köhler of the Carl Zeiss corporation as a method of providing the optimum specimen illumination

Objective Turrets Developed and Modern Condenser Design Parfocal Objectives Abbe condensers with Cond. Diaphragm and Turret
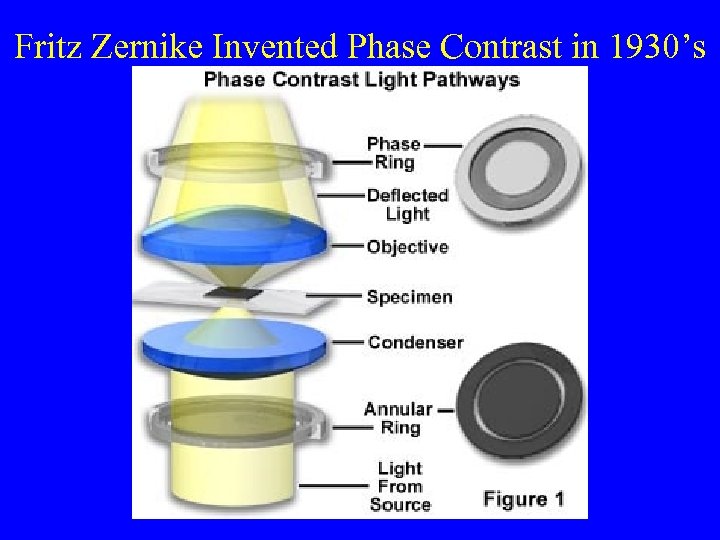
Fritz Zernike Invented Phase Contrast in 1930’s
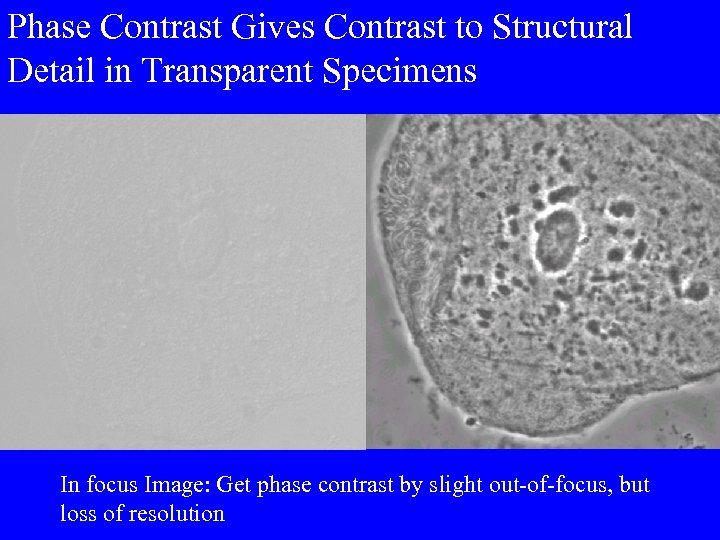
Phase Contrast Gives Contrast to Structural Detail in Transparent Specimens In focus Image: Get phase contrast by slight out-of-focus, but loss of resolution
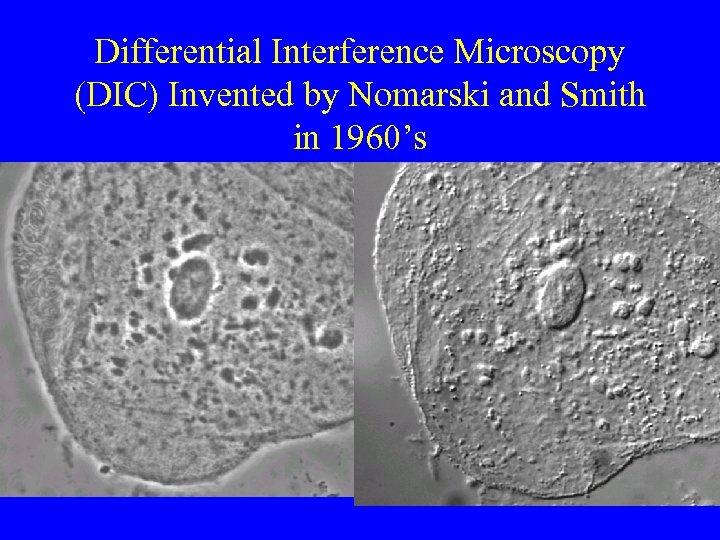
Differential Interference Microscopy (DIC) Invented by Nomarski and Smith in 1960’s
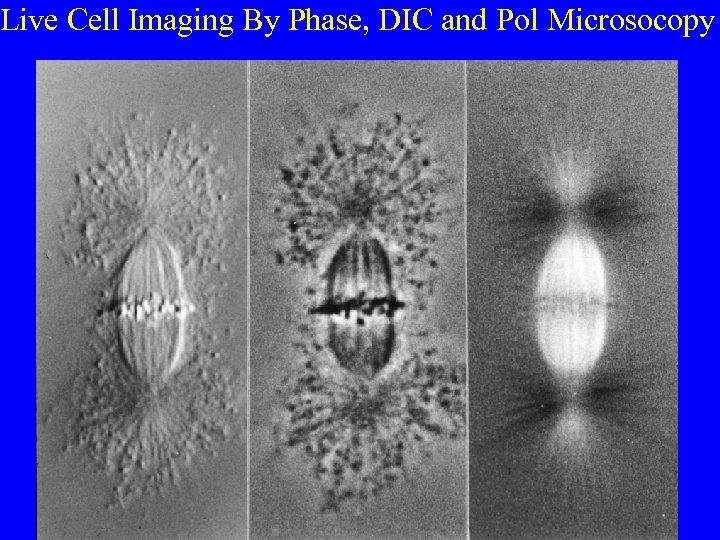
Live Cell Imaging By Phase, DIC and Pol Microsocopy
Cellular Histology Developed Over Last 150 Years
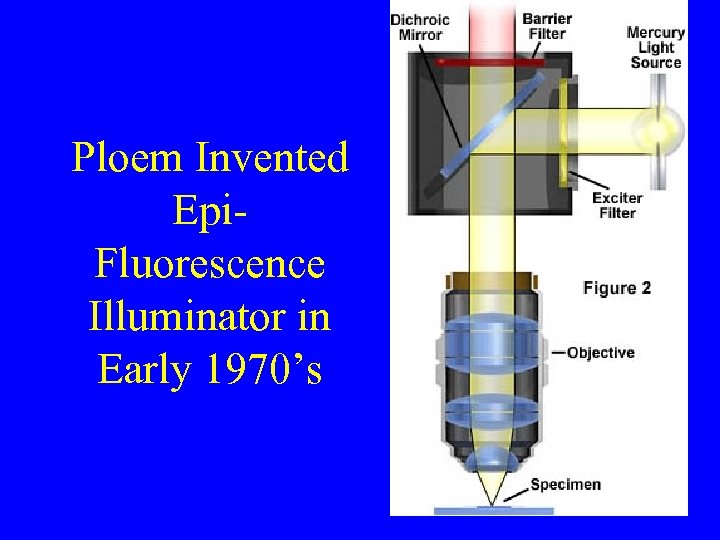
Ploem Invented Epi. Fluorescence Illuminator in Early 1970’s

Mono-Clonal and Affnitiy Purified Antibody Methods and Beginning of Molecular Probe Development Began in 1970’s Multi-Wavelength Fluorescence Microscopy: Co-Localization of Different Molecules Relative To Cellular Structures

Video-Enhanced Contrast Methods Developed in Early 1980’s by Inoue and Allen Revealed Cellular Structures and Macromolecular Complexes Invisible by Eye or Film
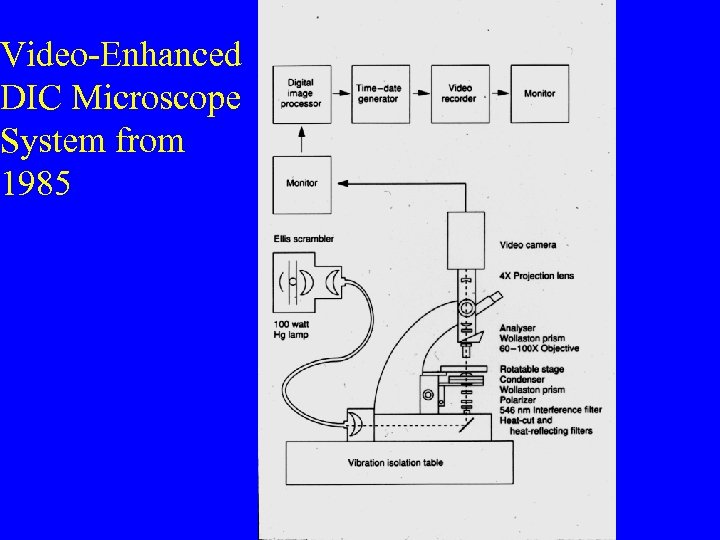
Video-Enhanced DIC Microscope System from 1985
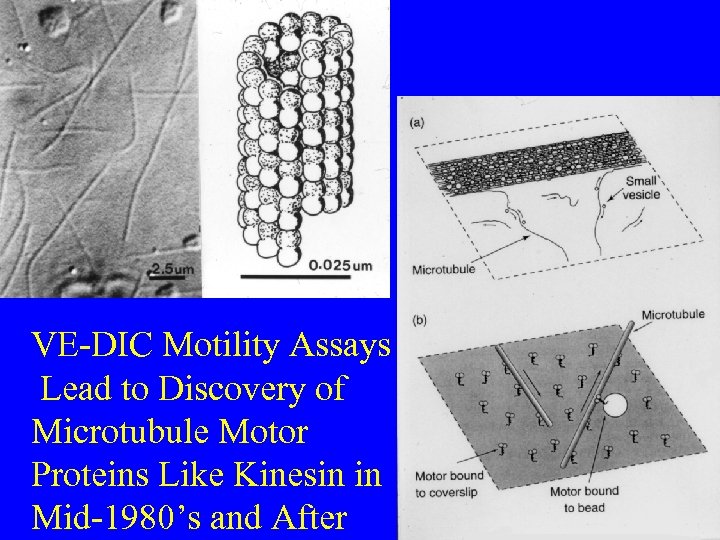
VE-DIC Motility Assays Lead to Discovery of Microtubule Motor Proteins Like Kinesin in Mid-1980’s and After
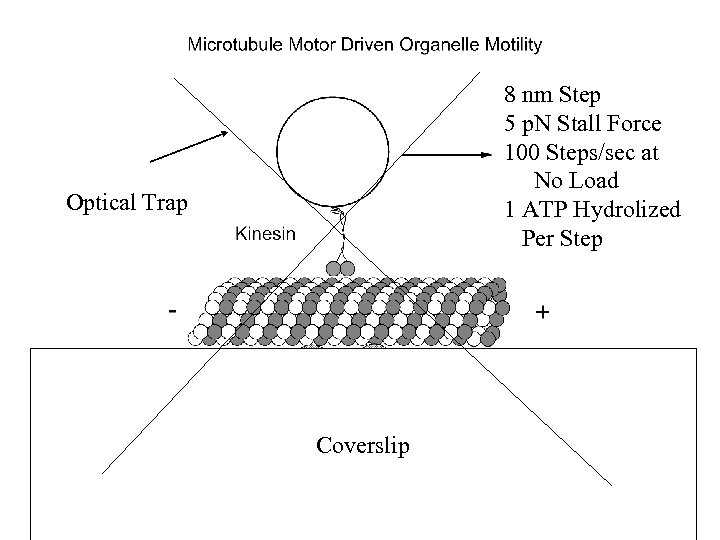
8 nm Step 5 p. N Stall Force 100 Steps/sec at No Load 1 ATP Hydrolized Per Step Optical Trap Coverslip

Simulation from Ron Milligan and Ron Vale of Kinesin Mechanochemical cycle

Fluorescence microscopy pushed forward in early 1980’s by new fluorophores (start of Molecular Probes) and intensified video cameras • • Detect fluorescence invisible to eye or film Quantitative fluorescence measurements Fluorescent protein analogs of live cells Ratio measurements for ion dynamics (e. g. Fura 2 for calcium ion…) • Molecular dynamics from Measurements of fluorescence recovery after photobleaching (FRAP)

In early 1980’s video cameras with image intensifiers:
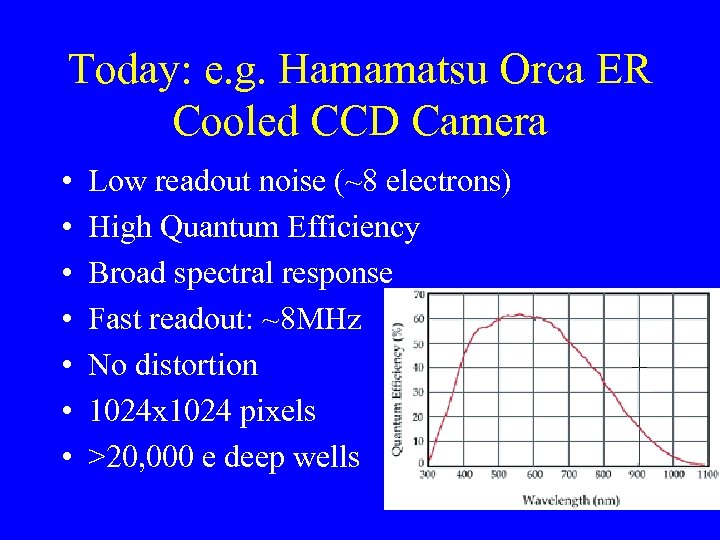
Today: e. g. Hamamatsu Orca ER Cooled CCD Camera • • Low readout noise (~8 electrons) High Quantum Efficiency Broad spectral response Fast readout: ~8 MHz No distortion 1024 x 1024 pixels >20, 000 e deep wells

FRAP Scope with Cooled CCD Camera
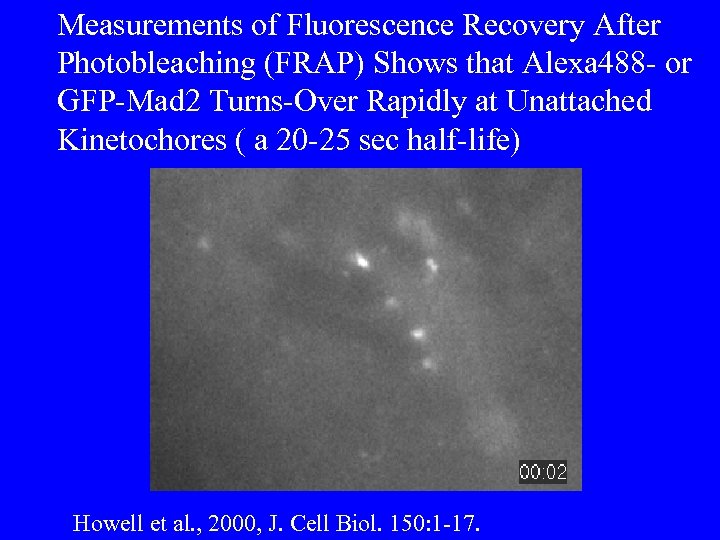
Measurements of Fluorescence Recovery After Photobleaching (FRAP) Shows that Alexa 488 - or GFP-Mad 2 Turns-Over Rapidly at Unattached Kinetochores ( a 20 -25 sec half-life) Howell et al. , 2000, J. Cell Biol. 150: 1 -17.
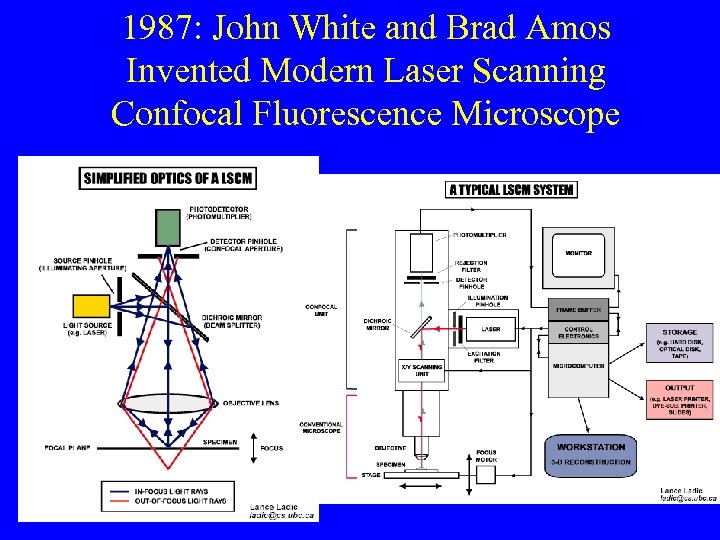
1987: John White and Brad Amos Invented Modern Laser Scanning Confocal Fluorescence Microscope
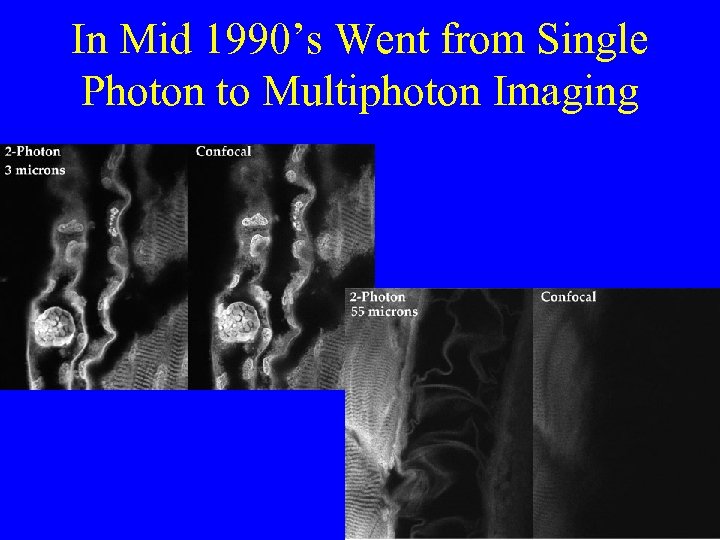
In Mid 1990’s Went from Single Photon to Multiphoton Imaging
The Modern Era of Light Microscopy • New microscope optics generate brilliant images over wide wavelengths • Computers control x-y-&z specimen position, wavelength selection, illumination and image acquisition • Electronic cameras quantitatively record light intensity of specimens invisible or undetectable by eye or film • Confocal and deconvolution methods give 3 -D views of cellular architectural dynamics • New fluorescent molecular probes and biophysical methods report on the temporal and spatial activities of the molecular machinery of living cells and single molecule imaging • Micromanipulation, ablation, force measurement
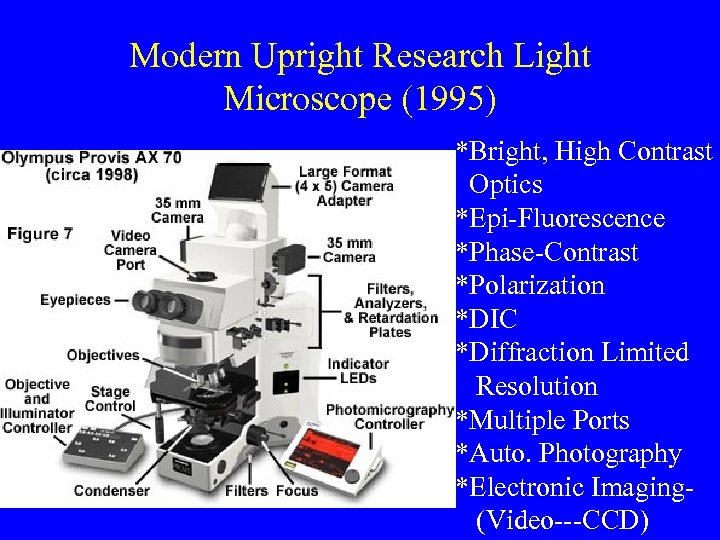
Modern Upright Research Light Microscope (1995) *Bright, High Contrast Optics *Epi-Fluorescence *Phase-Contrast *Polarization *DIC *Diffraction Limited Resolution *Multiple Ports *Auto. Photography *Electronic Imaging(Video---CCD)
The Modern Era of Light Microscopy • New microscope optics generate brilliant images over wide wavelengths • Computers control x-y-&z specimen position, wavelength selection, illumination and image acquisition • Electronic cameras quantitatively record light intensity of specimens invisible or undetectable by eye or film • Confocal and deconvolution methods give 3 -D views of cellular architectural dynamics • New fluorescent molecular probes and biophysical methods report on the temporal and spatial activities of the molecular machinery of living cells and single molecule imaging • Micromanipulation, ablation, force measurement
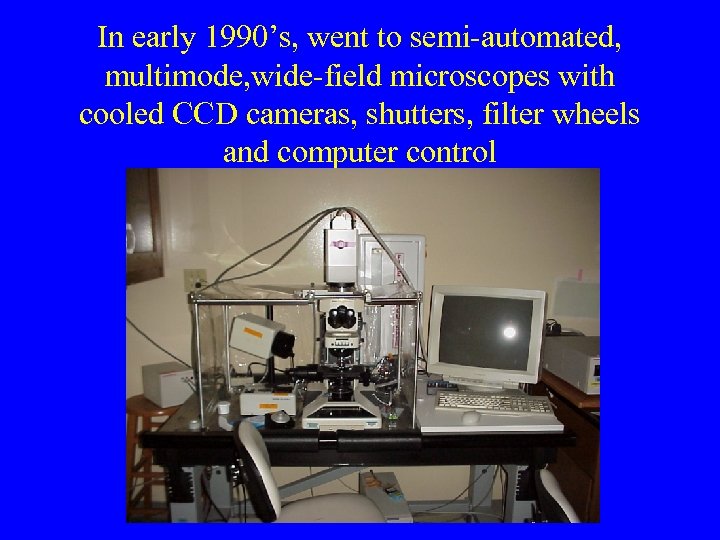
In early 1990’s, went to semi-automated, multimode, wide-field microscopes with cooled CCD cameras, shutters, filter wheels and computer control
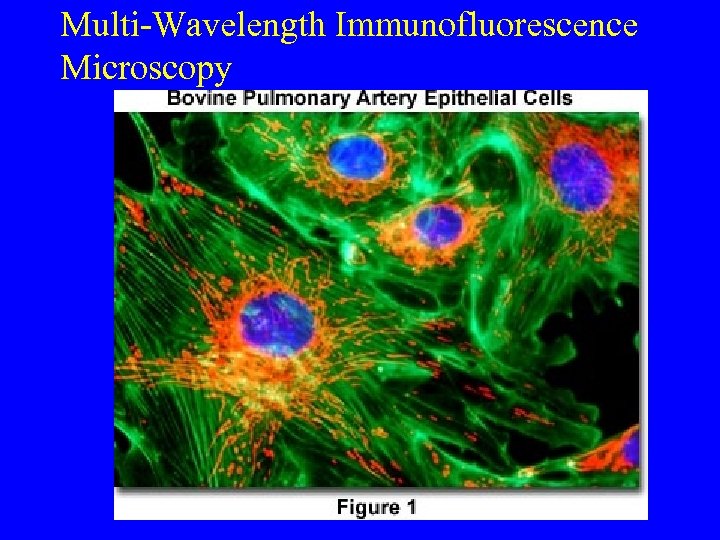
Multi-Wavelength Immunofluorescence Microscopy
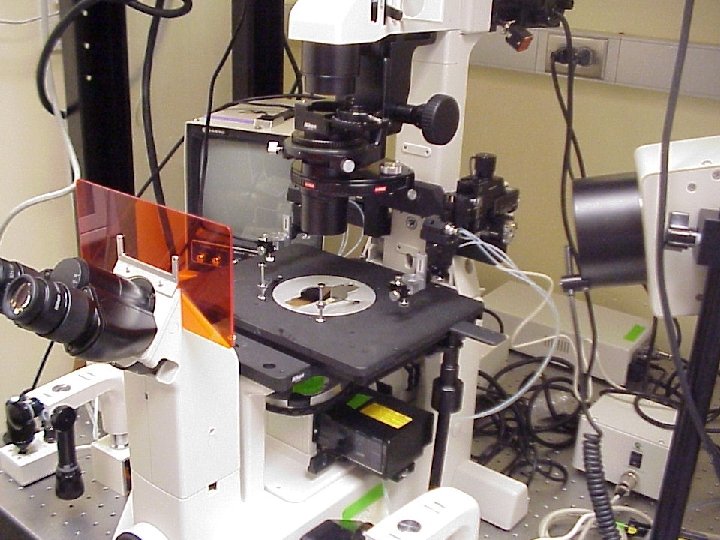
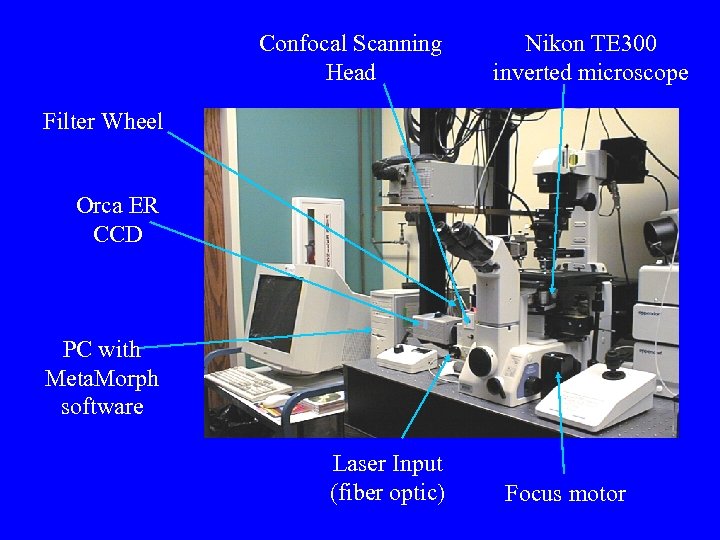
Confocal Scanning Head Nikon TE 300 inverted microscope Filter Wheel Orca ER CCD PC with Meta. Morph software Laser Input (fiber optic) Focus motor
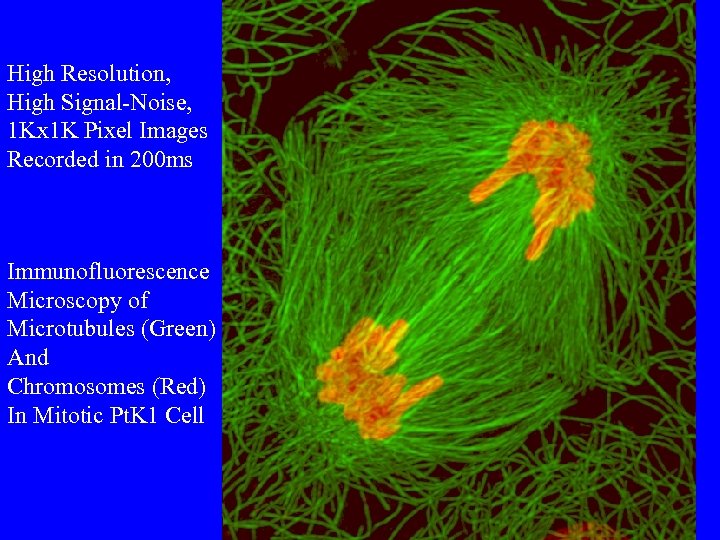
High Resolution, High Signal-Noise, 1 Kx 1 K Pixel Images Recorded in 200 ms Immunofluorescence Microscopy of Microtubules (Green) And Chromosomes (Red) In Mitotic Pt. K 1 Cell
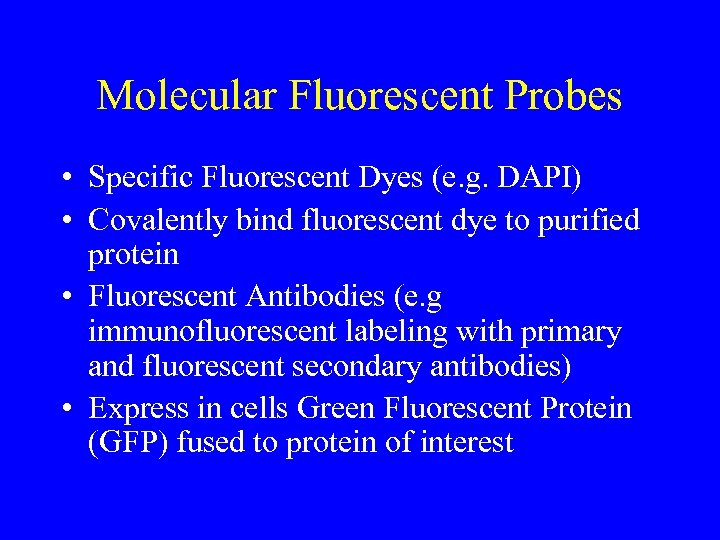
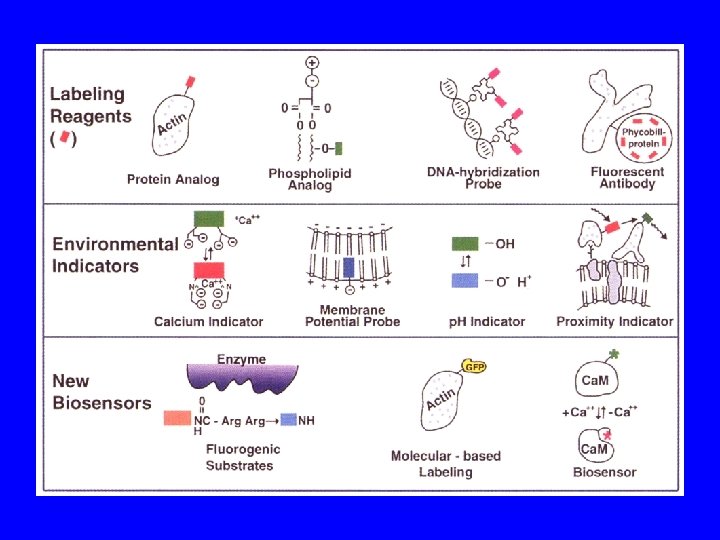
Molecular Fluorescent Probes • Specific Fluorescent Dyes (e. g. DAPI) • Covalently bind fluorescent dye to purified protein • Fluorescent Antibodies (e. g immunofluorescent labeling with primary and fluorescent secondary antibodies) • Express in cells Green Fluorescent Protein (GFP) fused to protein of interest

Aequorea victoria
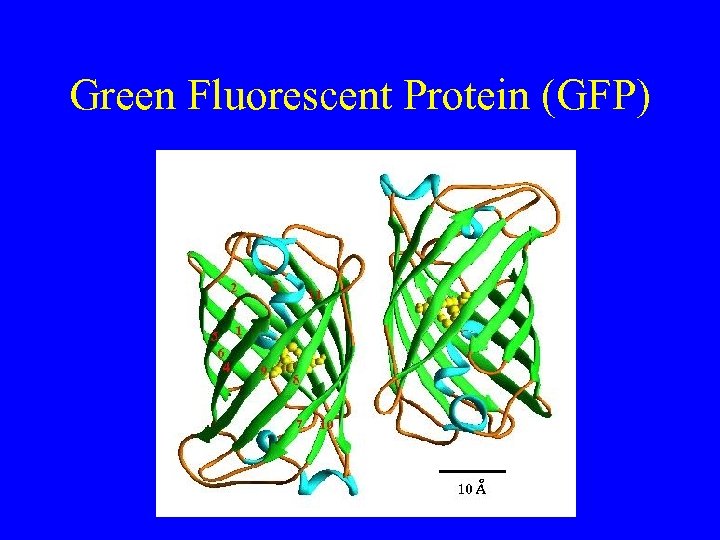
Green Fluorescent Protein (GFP)
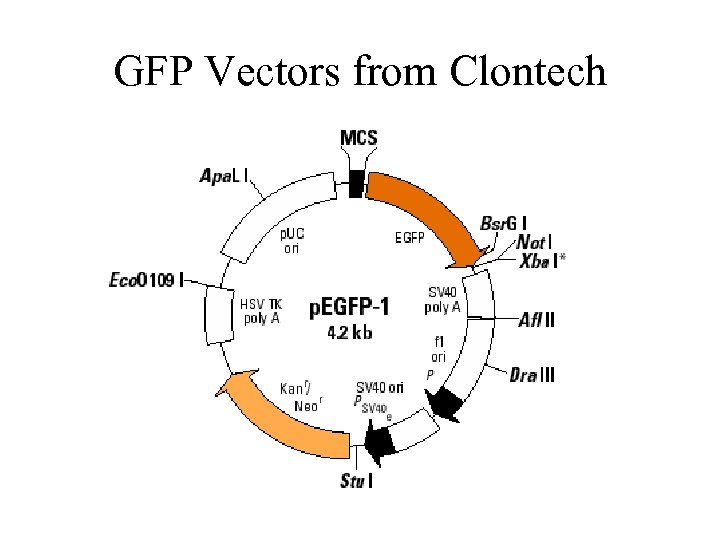
GFP Vectors from Clontech
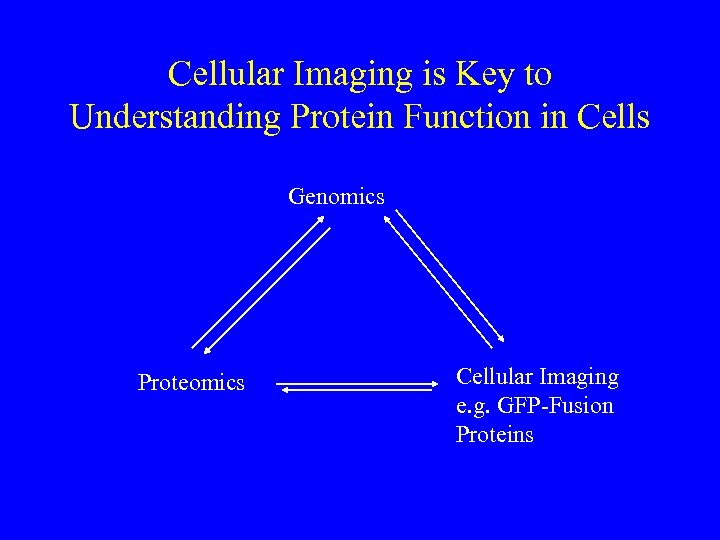
Cellular Imaging is Key to Understanding Protein Function in Cells Genomics Proteomics Cellular Imaging e. g. GFP-Fusion Proteins
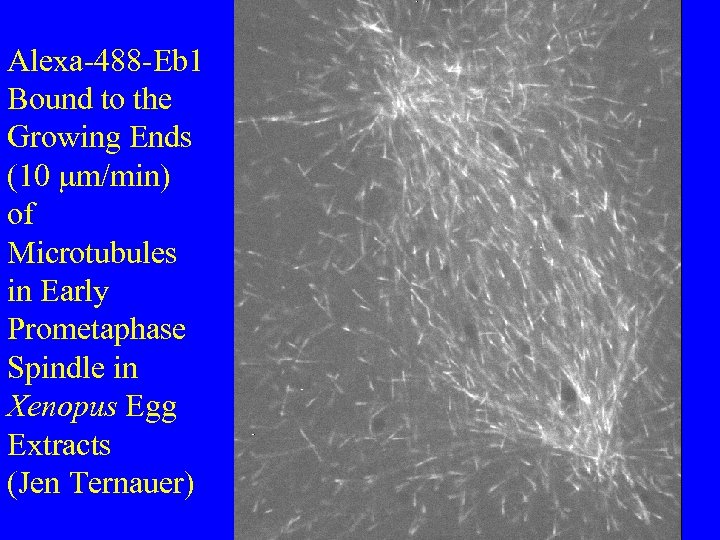
Alexa-488 -Eb 1 Bound to the Growing Ends (10 mm/min) of Microtubules in Early Prometaphase Spindle in Xenopus Egg Extracts (Jen Ternauer)
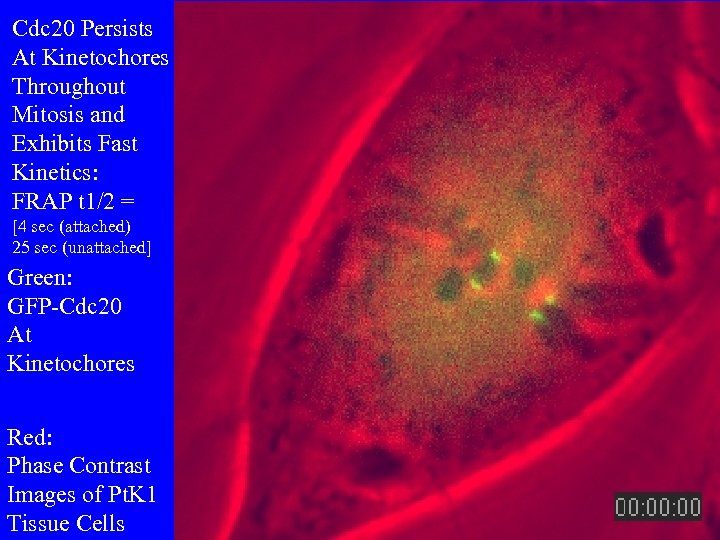
Cdc 20 Persists At Kinetochores Throughout Mitosis and Exhibits Fast Kinetics: FRAP t 1/2 = [4 sec (attached) 25 sec (unattached] Green: GFP-Cdc 20 At Kinetochores Red: Phase Contrast Images of Pt. K 1 Tissue Cells
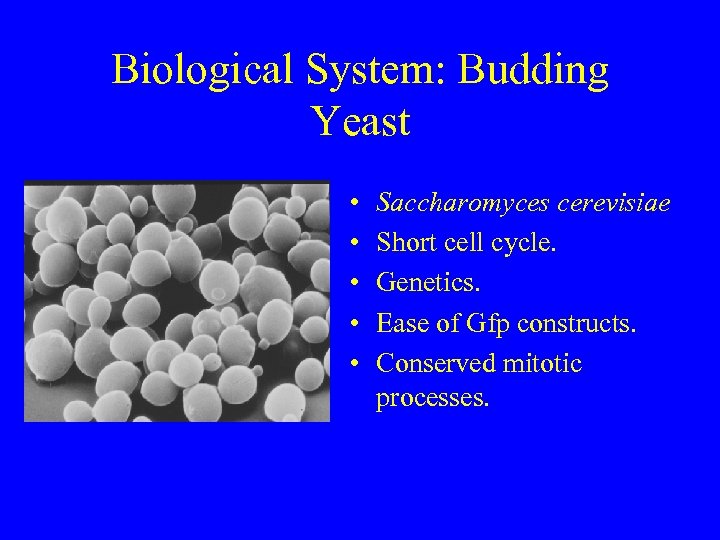
Biological System: Budding Yeast • • • Saccharomyces cerevisiae Short cell cycle. Genetics. Ease of Gfp constructs. Conserved mitotic processes.

Budding Yeast Anaphase and Cytokinesis: GFP-Tubulin and CFP-Myo 1(Myosin) Paul Maddox

GFP-Microtubule Dynamics in A First Division C. elegans Embryo Karen Oogema And Paul Maddox
9a49edd331c683f10938601a7ae3ccb1.ppt