c31deab0f37cd4a58e8279b9a5044d47.ppt
- Количество слайдов: 31
 Light and Temperature Astronomy: The Science of Seeing 4
Light and Temperature Astronomy: The Science of Seeing 4
 Goals • • • What is light? What are the types of light? Where does the light we see come from? Understanding the light of heat. On a sunny day: – Why does it seem hotter wearing a black T-shirt versus a white one? – Why are they different? 4
Goals • • • What is light? What are the types of light? Where does the light we see come from? Understanding the light of heat. On a sunny day: – Why does it seem hotter wearing a black T-shirt versus a white one? – Why are they different? 4
 How do you do Astronomy? • How do Chemists do Chemistry? – Make solutions, mix chemicals … • How do Biologists do Biology? – Breed fruit flies, (and whatever else biologists do). • Devise and conduct experiments in their labs. • But how do you do that for astronomy? 4
How do you do Astronomy? • How do Chemists do Chemistry? – Make solutions, mix chemicals … • How do Biologists do Biology? – Breed fruit flies, (and whatever else biologists do). • Devise and conduct experiments in their labs. • But how do you do that for astronomy? 4
 Light • Astronomy is a “passive” science. • We can’t (yet) go to the stars or other galaxies. • The Universe must come to us. • We rely on light exclusively! 4
Light • Astronomy is a “passive” science. • We can’t (yet) go to the stars or other galaxies. • The Universe must come to us. • We rely on light exclusively! 4
 What you see is all you get! • So you need to squeeze EVERY last drop of information out of the light we get. • This semester we’ll see how we can use light to: 1. Weigh a planet. 2. Take a star’s temperature. 3. Tell what’s in the center of a star a thousand lightyears away. 4. Tell what our Galaxy look like from the outside. 4
What you see is all you get! • So you need to squeeze EVERY last drop of information out of the light we get. • This semester we’ll see how we can use light to: 1. Weigh a planet. 2. Take a star’s temperature. 3. Tell what’s in the center of a star a thousand lightyears away. 4. Tell what our Galaxy look like from the outside. 4
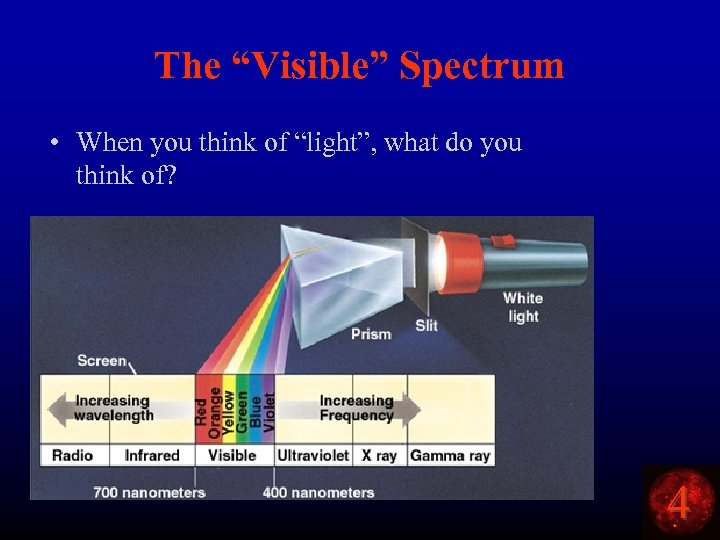 The “Visible” Spectrum • When you think of “light”, what do you think of? 4
The “Visible” Spectrum • When you think of “light”, what do you think of? 4
 What is Light? • Light is a wave of energy. • Moves through a vacuum. • Travels at the speed of light (a CONSTANT): c = 3 x 1010 cm/s • The wavelength (l) and frequency (n) are related: c = ln 4
What is Light? • Light is a wave of energy. • Moves through a vacuum. • Travels at the speed of light (a CONSTANT): c = 3 x 1010 cm/s • The wavelength (l) and frequency (n) are related: c = ln 4
 4
4
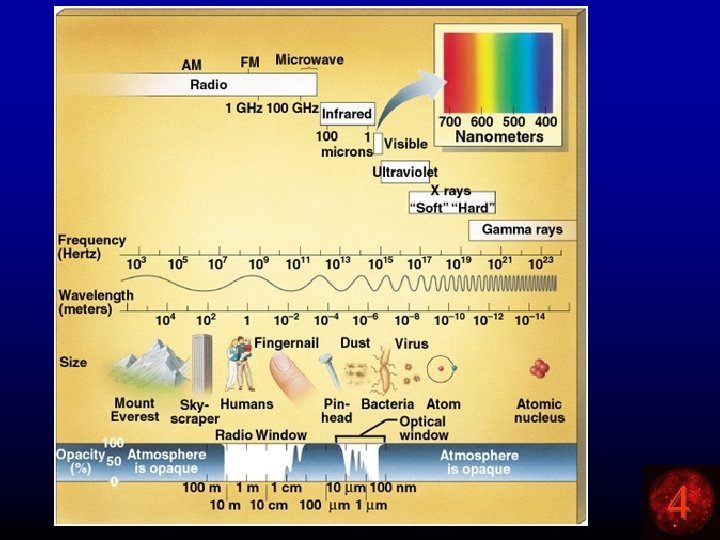
 To Sum Up… • Radio waves, microwaves, rainbows, UV waves, x -rays, etc are ALL forms of light (electromagnetic waves). • They ALL travel through space at the speed of light. c • The higher the frequency, the shorter the wavelength. c = ln • What does light look like? 4
To Sum Up… • Radio waves, microwaves, rainbows, UV waves, x -rays, etc are ALL forms of light (electromagnetic waves). • They ALL travel through space at the speed of light. c • The higher the frequency, the shorter the wavelength. c = ln • What does light look like? 4
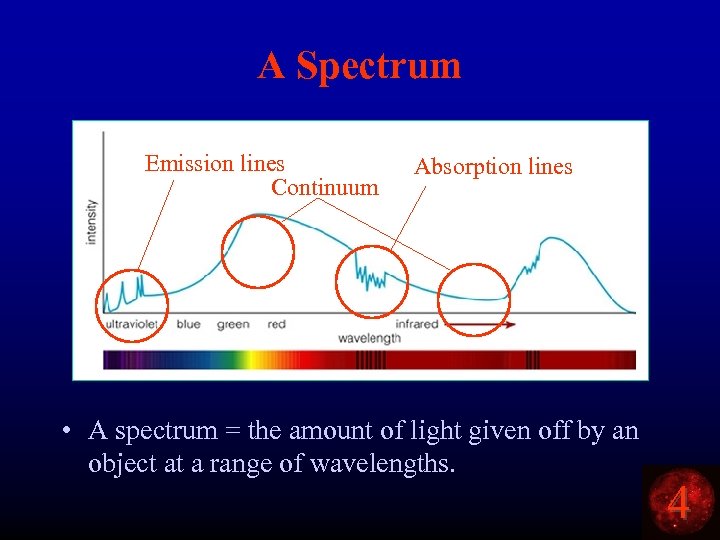 A Spectrum Emission lines Continuum Absorption lines • A spectrum = the amount of light given off by an object at a range of wavelengths. 4
A Spectrum Emission lines Continuum Absorption lines • A spectrum = the amount of light given off by an object at a range of wavelengths. 4
 Three Reasons All objects do one or more: 1. Reflect light because of color or smoothness (same as scatter) 2. Emit light because of their temperature (thermal radiation) 3. Emit or absorb light because of their composition (spectral lines) A person, house, or the Moon: reflects visible light, and because each is warm, emits infrared light. 4
Three Reasons All objects do one or more: 1. Reflect light because of color or smoothness (same as scatter) 2. Emit light because of their temperature (thermal radiation) 3. Emit or absorb light because of their composition (spectral lines) A person, house, or the Moon: reflects visible light, and because each is warm, emits infrared light. 4
 Reflection, absorption, and scatter • • • Why do you see me? Why do I see you? Why is your shirt blue? Why is this paper white? Why is the table top black? 4
Reflection, absorption, and scatter • • • Why do you see me? Why do I see you? Why is your shirt blue? Why is this paper white? Why is the table top black? 4
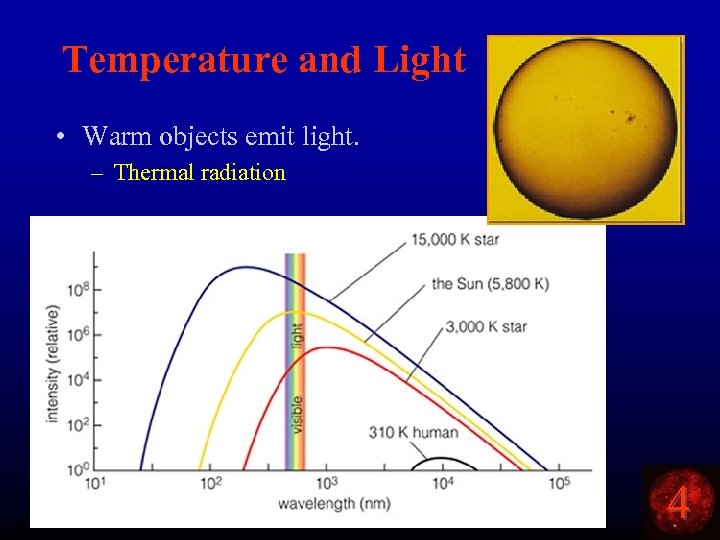 Temperature and Light • Warm objects emit light. – Thermal radiation 4
Temperature and Light • Warm objects emit light. – Thermal radiation 4
 Kelvin Temperature • • • Kelvin: an absolute scale. Kelvin is Celsius + 273 degrees. Water freezes: 0 C 273 K Water Boils: 100 C 373 K Room Temp: 80 F 27 C 300 K Surface Sun: 6000 K 4
Kelvin Temperature • • • Kelvin: an absolute scale. Kelvin is Celsius + 273 degrees. Water freezes: 0 C 273 K Water Boils: 100 C 373 K Room Temp: 80 F 27 C 300 K Surface Sun: 6000 K 4
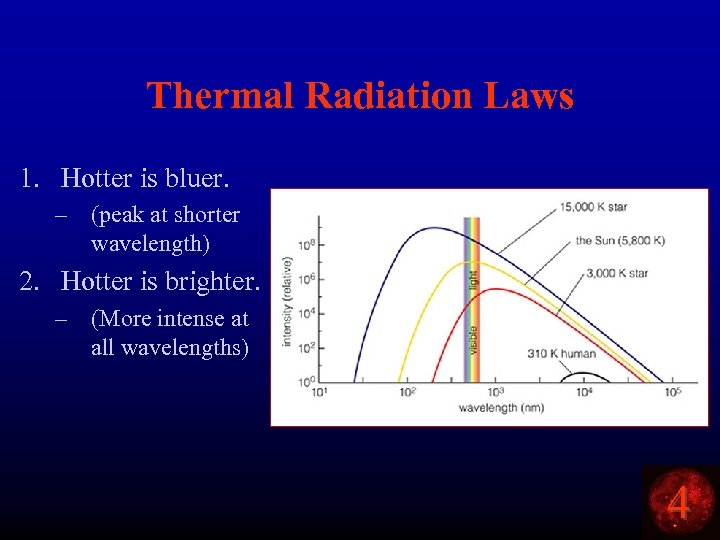 Thermal Radiation Laws 1. Hotter is bluer. – (peak at shorter wavelength) 2. Hotter is brighter. – (More intense at all wavelengths) 4
Thermal Radiation Laws 1. Hotter is bluer. – (peak at shorter wavelength) 2. Hotter is brighter. – (More intense at all wavelengths) 4
 Atoms in Motion • Everything is composed of atoms which are constantly in motion. 4
Atoms in Motion • Everything is composed of atoms which are constantly in motion. 4
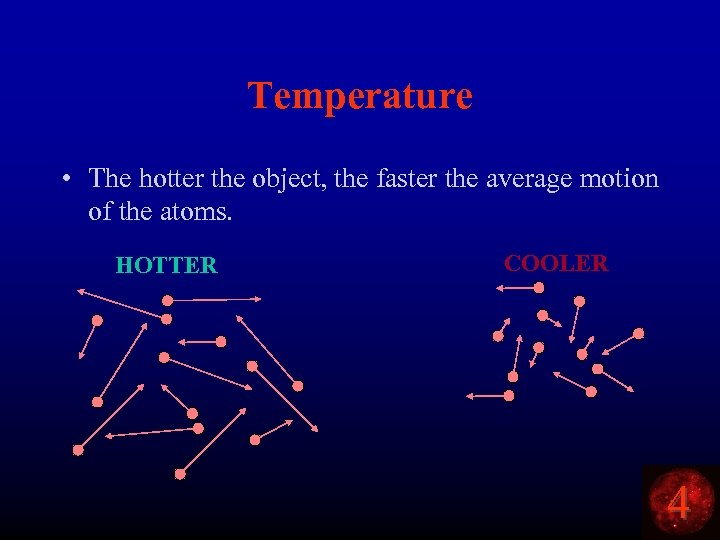 Temperature • The hotter the object, the faster the average motion of the atoms. HOTTER COOLER 4
Temperature • The hotter the object, the faster the average motion of the atoms. HOTTER COOLER 4
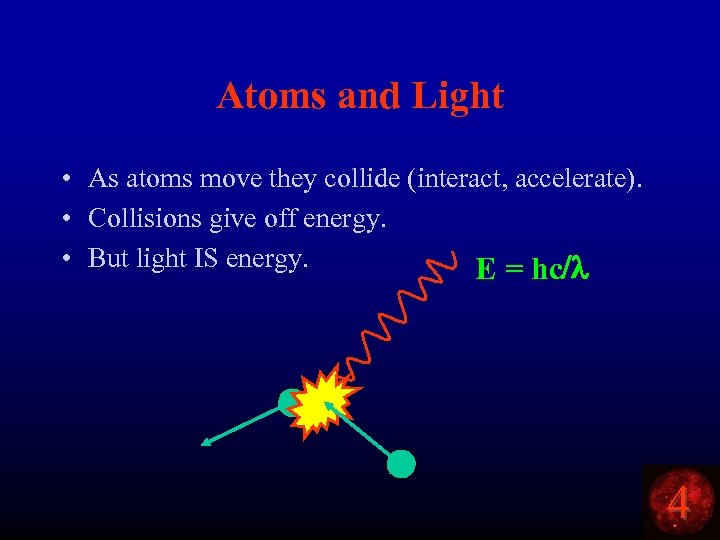 Atoms and Light • As atoms move they collide (interact, accelerate). • Collisions give off energy. • But light IS energy. E = hc/l 4
Atoms and Light • As atoms move they collide (interact, accelerate). • Collisions give off energy. • But light IS energy. E = hc/l 4
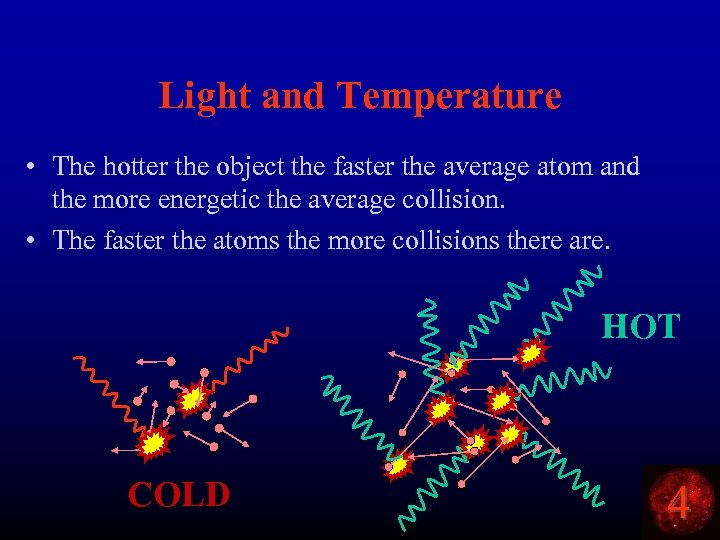 Light and Temperature • The hotter the object the faster the average atom and the more energetic the average collision. • The faster the atoms the more collisions there are. HOT COLD 4
Light and Temperature • The hotter the object the faster the average atom and the more energetic the average collision. • The faster the atoms the more collisions there are. HOT COLD 4
 Energy and Intensity • The more energetic the average collision the bluer the average light that is given off. – Since E = hc/l • The more collisions that occur the more light that is given off per surface area. 1. Hotter is bluer. (peak at shorter wavelength) 2. Hotter is brighter. (more intense at all wavelengths) 4
Energy and Intensity • The more energetic the average collision the bluer the average light that is given off. – Since E = hc/l • The more collisions that occur the more light that is given off per surface area. 1. Hotter is bluer. (peak at shorter wavelength) 2. Hotter is brighter. (more intense at all wavelengths) 4
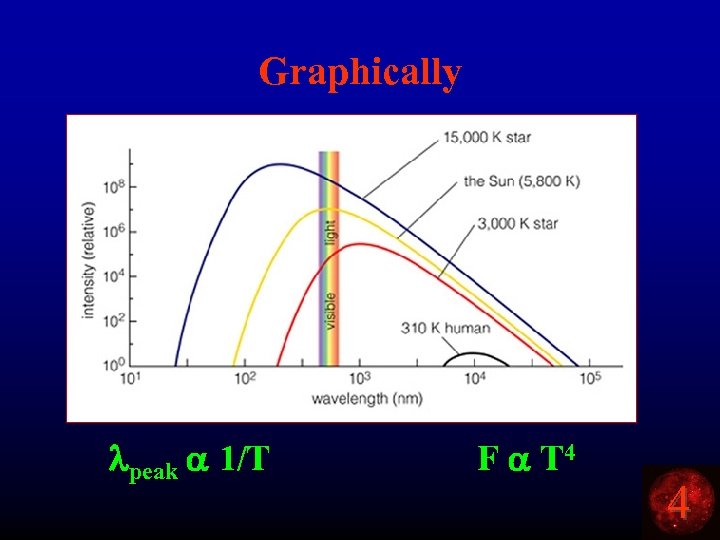 Graphically lpeak a 1/T F a T 4 4
Graphically lpeak a 1/T F a T 4 4
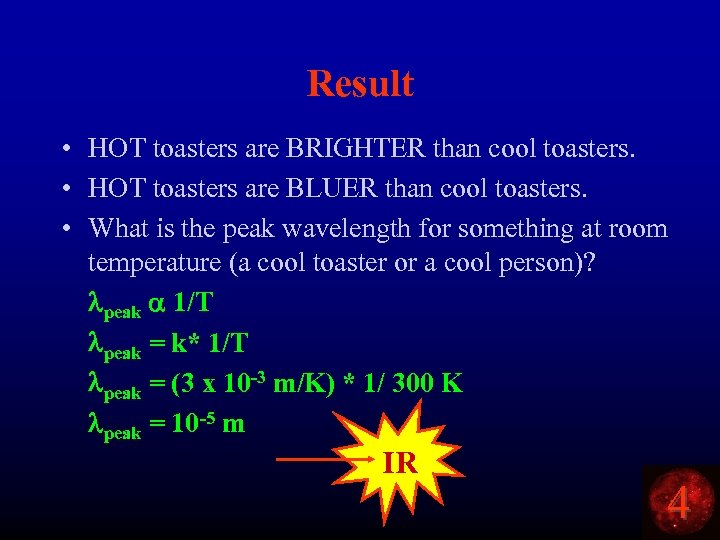 Result • HOT toasters are BRIGHTER than cool toasters. • HOT toasters are BLUER than cool toasters. • What is the peak wavelength for something at room temperature (a cool toaster or a cool person)? lpeak a 1/T lpeak = k* 1/T lpeak = (3 x 10 -3 m/K) * 1/ 300 K lpeak = 10 -5 m IR 4
Result • HOT toasters are BRIGHTER than cool toasters. • HOT toasters are BLUER than cool toasters. • What is the peak wavelength for something at room temperature (a cool toaster or a cool person)? lpeak a 1/T lpeak = k* 1/T lpeak = (3 x 10 -3 m/K) * 1/ 300 K lpeak = 10 -5 m IR 4
 Thermal versus Reflection • Thermal radiation is light given off because of an object’s temperature. • Don’t confuse with reflected light: – Buses are yellow not because they are hot enough to emit visible radiation but rather they reflect the yellow light given off by the Sun. • What kinds of thermal radiation do we see in our everyday life? 4
Thermal versus Reflection • Thermal radiation is light given off because of an object’s temperature. • Don’t confuse with reflected light: – Buses are yellow not because they are hot enough to emit visible radiation but rather they reflect the yellow light given off by the Sun. • What kinds of thermal radiation do we see in our everyday life? 4
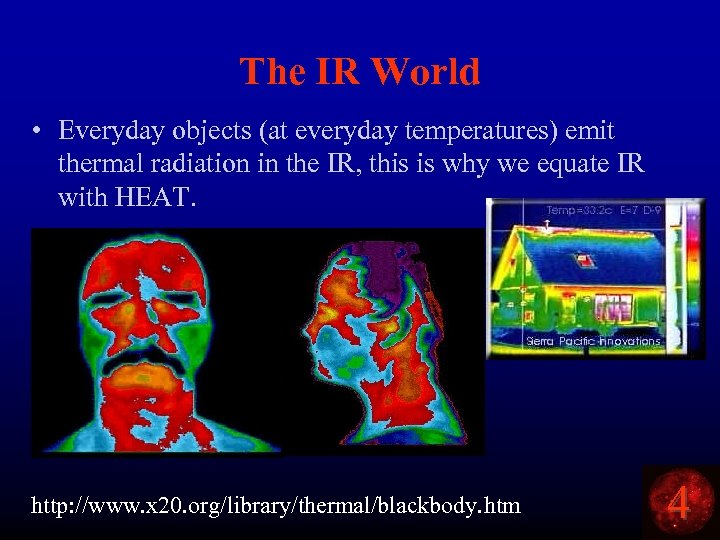 The IR World • Everyday objects (at everyday temperatures) emit thermal radiation in the IR, this is why we equate IR with HEAT. http: //www. x 20. org/library/thermal/blackbody. htm 4
The IR World • Everyday objects (at everyday temperatures) emit thermal radiation in the IR, this is why we equate IR with HEAT. http: //www. x 20. org/library/thermal/blackbody. htm 4
 The IR Universe Orion - visible Orion – by IRAS • Everyday things that are hot radiate in the IR: • Dust – There are interstellar clouds of dust. 4
The IR Universe Orion - visible Orion – by IRAS • Everyday things that are hot radiate in the IR: • Dust – There are interstellar clouds of dust. 4
 The IR Universe Io from IRTF. Orion – by IRAS • Molten Rock – There are lava flows on a moon of Jupiter. 4
The IR Universe Io from IRTF. Orion – by IRAS • Molten Rock – There are lava flows on a moon of Jupiter. 4
 The IR Universe The Moon in eclipse. R. Gendler Orion – by IRAS • In eclipse, there is no reflected light. • Only thermal radiation. • Differences in composition lead to differences in temperature. 4
The IR Universe The Moon in eclipse. R. Gendler Orion – by IRAS • In eclipse, there is no reflected light. • Only thermal radiation. • Differences in composition lead to differences in temperature. 4
 The Greenhouse Effect • Why is my car hot on a summer day? • At T = 6000 K, the Sun radiates mostly visible light. Windshield is transparent to visible light. • Car seat absorbs this visible light and warms up to 400 K. • At T = 400 K, my seat radiates mostly at longer wavelengths in the IR. Windshield is opaque in the IR. • Result: Energy is TRAPPED inside the car! 4
The Greenhouse Effect • Why is my car hot on a summer day? • At T = 6000 K, the Sun radiates mostly visible light. Windshield is transparent to visible light. • Car seat absorbs this visible light and warms up to 400 K. • At T = 400 K, my seat radiates mostly at longer wavelengths in the IR. Windshield is opaque in the IR. • Result: Energy is TRAPPED inside the car! 4
 Venus and Earth • Certain gases act the same way as your windshield: Carbon Dioxide (CO 2). • Venus – Runaway greenhouse effect. • Earth – Could that happen here? 4
Venus and Earth • Certain gases act the same way as your windshield: Carbon Dioxide (CO 2). • Venus – Runaway greenhouse effect. • Earth – Could that happen here? 4
 Color Why’s • • • Why is that shirt blue? Why is the Sun yellow? Why is this paper white? Why is the light filament orange? Why is Mars red? On a sunny day: – Why does it seem hotter wearing a black T-shirt versus a white one? 4
Color Why’s • • • Why is that shirt blue? Why is the Sun yellow? Why is this paper white? Why is the light filament orange? Why is Mars red? On a sunny day: – Why does it seem hotter wearing a black T-shirt versus a white one? 4
 Homework #4 • For Tuesday 1/27: • Read B 6. 4 – 6. 5 • Do B 6: Problems 4, 8, 12, 13, 14, 17, 18 – (PHYS 170: replace problem 13, 17 with 22, 23) 4
Homework #4 • For Tuesday 1/27: • Read B 6. 4 – 6. 5 • Do B 6: Problems 4, 8, 12, 13, 14, 17, 18 – (PHYS 170: replace problem 13, 17 with 22, 23) 4


