937faf59496b2824a93c2380b39816be.ppt
- Количество слайдов: 62
 Life of Synthetic CO 2, Environmental Impact, Chemical Synthesis and Industrial Applications William Schulz Bechara Charette Group - Literature Meeting May 2 nd, 2012
Life of Synthetic CO 2, Environmental Impact, Chemical Synthesis and Industrial Applications William Schulz Bechara Charette Group - Literature Meeting May 2 nd, 2012
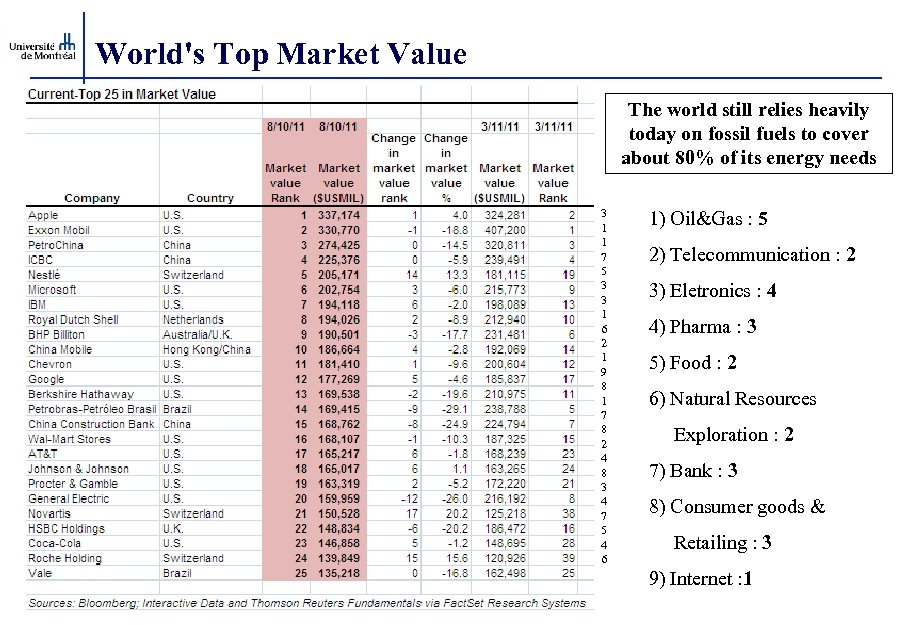 World's Top Market Value The world still relies heavily today on fossil fuels to cover about 80% of its energy needs 3 1 1 7 5 3 3 1 6 2 1 9 8 1 7 8 2 4 8 3 4 7 5 4 6 1) Oil&Gas : 5 2) Telecommunication : 2 3) Eletronics : 4 4) Pharma : 3 5) Food : 2 6) Natural Resources Exploration : 2 7) Bank : 3 8) Consumer goods & Retailing : 3 9) Internet : 1
World's Top Market Value The world still relies heavily today on fossil fuels to cover about 80% of its energy needs 3 1 1 7 5 3 3 1 6 2 1 9 8 1 7 8 2 4 8 3 4 7 5 4 6 1) Oil&Gas : 5 2) Telecommunication : 2 3) Eletronics : 4 4) Pharma : 3 5) Food : 2 6) Natural Resources Exploration : 2 7) Bank : 3 8) Consumer goods & Retailing : 3 9) Internet : 1
 CO 2 – One of the Largest Waste Product The world still relies heavily today on fossil fuels to cover about 80% of its energy needs Electricity Without Carbon, Nature News Feature, 14 August 2008, 454.
CO 2 – One of the Largest Waste Product The world still relies heavily today on fossil fuels to cover about 80% of its energy needs Electricity Without Carbon, Nature News Feature, 14 August 2008, 454.
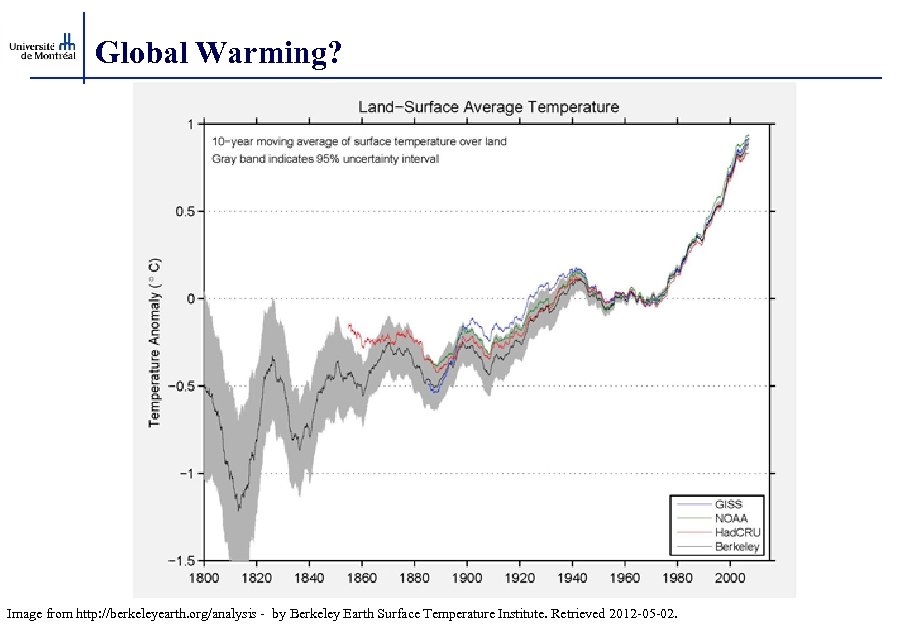 Global Warming? Image from http: //berkeleyearth. org/analysis - by Berkeley Earth Surface Temperature Institute. Retrieved 2012 -05 -02.
Global Warming? Image from http: //berkeleyearth. org/analysis - by Berkeley Earth Surface Temperature Institute. Retrieved 2012 -05 -02.
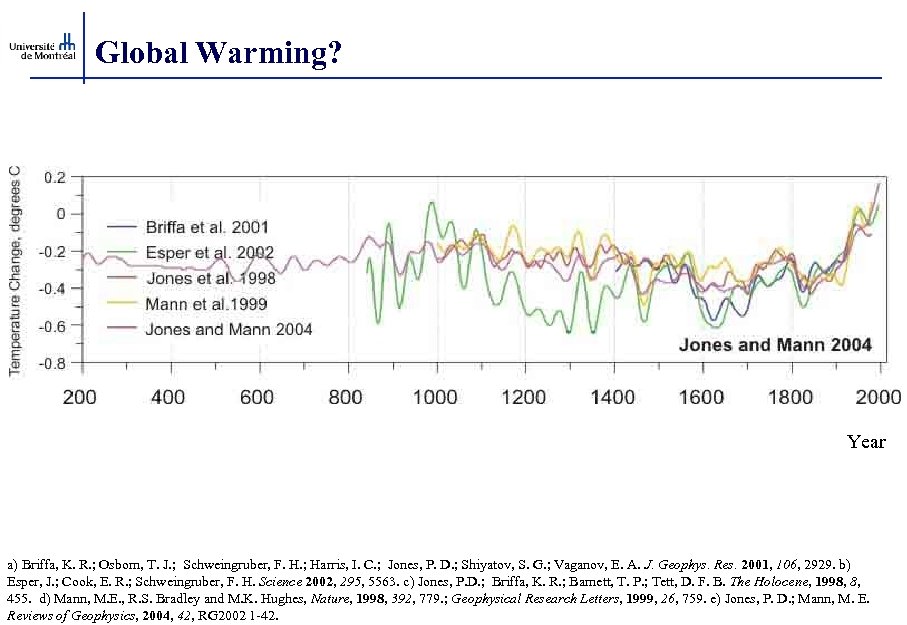 Global Warming? Year a) Briffa, K. R. ; Osborn, T. J. ; Schweingruber, F. H. ; Harris, I. C. ; Jones, P. D. ; Shiyatov, S. G. ; Vaganov, E. A. J. Geophys. Res. 2001, 106, 2929. b) Esper, J. ; Cook, E. R. ; Schweingruber, F. H. Science 2002, 295, 5563. c) Jones, P. D. ; Briffa, K. R. ; Barnett, T. P. ; Tett, D. F. B. The Holocene, 1998, 8, 455. d) Mann, M. E. , R. S. Bradley and M. K. Hughes, Nature, 1998, 392, 779. ; Geophysical Research Letters, 1999, 26, 759. e) Jones, P. D. ; Mann, M. E. Reviews of Geophysics, 2004, 42, RG 2002 1 -42.
Global Warming? Year a) Briffa, K. R. ; Osborn, T. J. ; Schweingruber, F. H. ; Harris, I. C. ; Jones, P. D. ; Shiyatov, S. G. ; Vaganov, E. A. J. Geophys. Res. 2001, 106, 2929. b) Esper, J. ; Cook, E. R. ; Schweingruber, F. H. Science 2002, 295, 5563. c) Jones, P. D. ; Briffa, K. R. ; Barnett, T. P. ; Tett, D. F. B. The Holocene, 1998, 8, 455. d) Mann, M. E. , R. S. Bradley and M. K. Hughes, Nature, 1998, 392, 779. ; Geophysical Research Letters, 1999, 26, 759. e) Jones, P. D. ; Mann, M. E. Reviews of Geophysics, 2004, 42, RG 2002 1 -42.
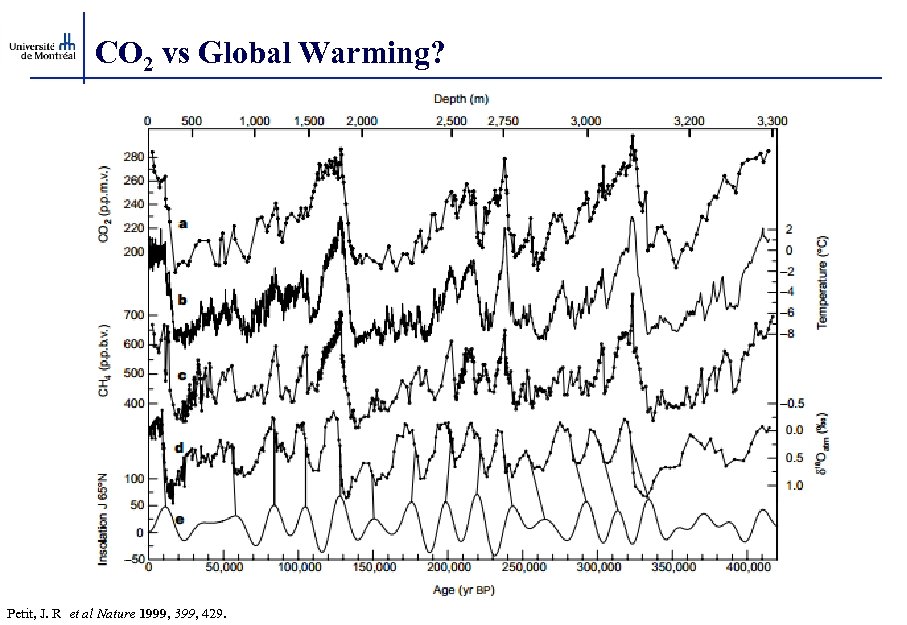 CO 2 vs Global Warming? Petit, J. R et al Nature 1999, 399, 429.
CO 2 vs Global Warming? Petit, J. R et al Nature 1999, 399, 429.
![CO 2 and Global Warming? [. . . ] records suggests a close link CO 2 and Global Warming? [. . . ] records suggests a close link](https://present5.com/presentation/937faf59496b2824a93c2380b39816be/image-7.jpg) CO 2 and Global Warming? [. . . ] records suggests a close link between CO 2 and climate [. . . ] The role and relative importance of CO 2 in producing these climate changes remains unclear [. . . ] a) Petit, J. R et al. Nature 1999, 399, 429. b) Barnola, J. -M. ; Raynaud, d. ; Korotkevich, Y. S. ; Lorius C. Nature, 1987, 329, 408. c) Lorius, C. ; Jouzel, J. ; Raynaud, D. ; Hansen, J. ; Le Treut, H. Nature, 1990, 347, 139. d) Martıinez-Garcia, A. et al. Nature 2011, 476, 312. e) Tripati, A. K. et all. Science 2009, 326, 1394. f) Shakun, J. D. et al. Nature 2012, 484, 49.
CO 2 and Global Warming? [. . . ] records suggests a close link between CO 2 and climate [. . . ] The role and relative importance of CO 2 in producing these climate changes remains unclear [. . . ] a) Petit, J. R et al. Nature 1999, 399, 429. b) Barnola, J. -M. ; Raynaud, d. ; Korotkevich, Y. S. ; Lorius C. Nature, 1987, 329, 408. c) Lorius, C. ; Jouzel, J. ; Raynaud, D. ; Hansen, J. ; Le Treut, H. Nature, 1990, 347, 139. d) Martıinez-Garcia, A. et al. Nature 2011, 476, 312. e) Tripati, A. K. et all. Science 2009, 326, 1394. f) Shakun, J. D. et al. Nature 2012, 484, 49.
 CO 2 Emissions Going Up Aresta, M. Carbon Dioxide as Chemical Feedstock 2010 Wiley, Weinheim.
CO 2 Emissions Going Up Aresta, M. Carbon Dioxide as Chemical Feedstock 2010 Wiley, Weinheim.
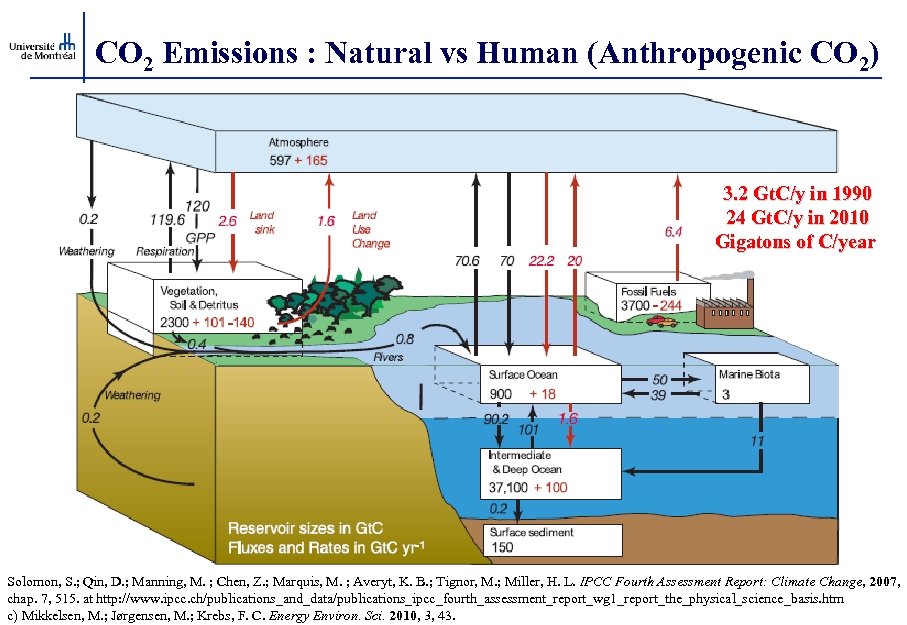 CO 2 Emissions : Natural vs Human (Anthropogenic CO 2) 3. 2 Gt. C/y in 1990 24 Gt. C/y in 2010 Gigatons of C/year Solomon, S. ; Qin, D. ; Manning, M. ; Chen, Z. ; Marquis, M. ; Averyt, K. B. ; Tignor, M. ; Miller, H. L. IPCC Fourth Assessment Report: Climate Change, 2007, chap. 7, 515. at http: //www. ipcc. ch/publications_and_data/publications_ipcc_fourth_assessment_report_wg 1_report_the_physical_science_basis. htm c) Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43.
CO 2 Emissions : Natural vs Human (Anthropogenic CO 2) 3. 2 Gt. C/y in 1990 24 Gt. C/y in 2010 Gigatons of C/year Solomon, S. ; Qin, D. ; Manning, M. ; Chen, Z. ; Marquis, M. ; Averyt, K. B. ; Tignor, M. ; Miller, H. L. IPCC Fourth Assessment Report: Climate Change, 2007, chap. 7, 515. at http: //www. ipcc. ch/publications_and_data/publications_ipcc_fourth_assessment_report_wg 1_report_the_physical_science_basis. htm c) Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43.
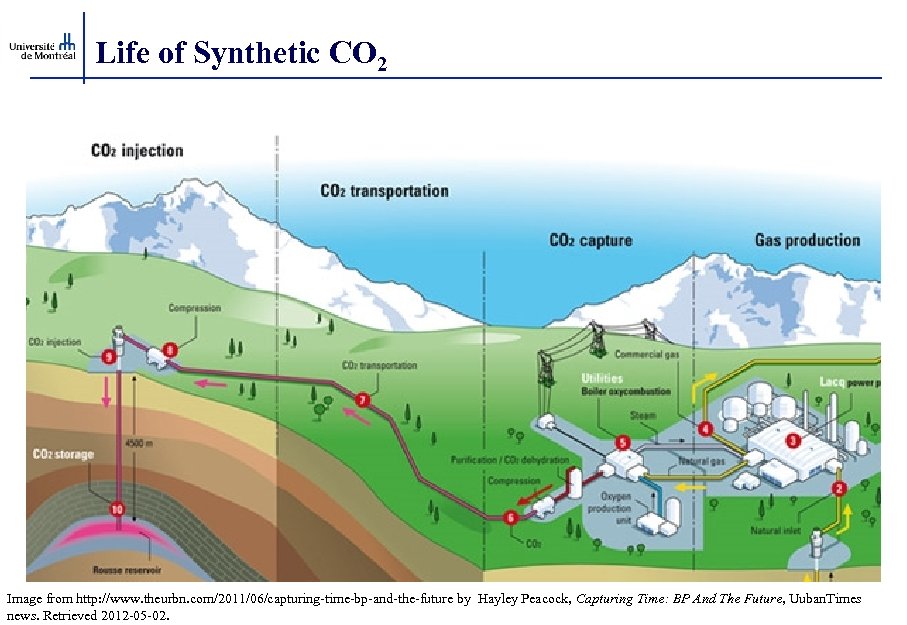 Life of Synthetic CO 2 Image from http: //www. theurbn. com/2011/06/capturing-time-bp-and-the-future by Hayley Peacock, Capturing Time: BP And The Future, Uuban. Times news. Retrieved 2012 -05 -02.
Life of Synthetic CO 2 Image from http: //www. theurbn. com/2011/06/capturing-time-bp-and-the-future by Hayley Peacock, Capturing Time: BP And The Future, Uuban. Times news. Retrieved 2012 -05 -02.
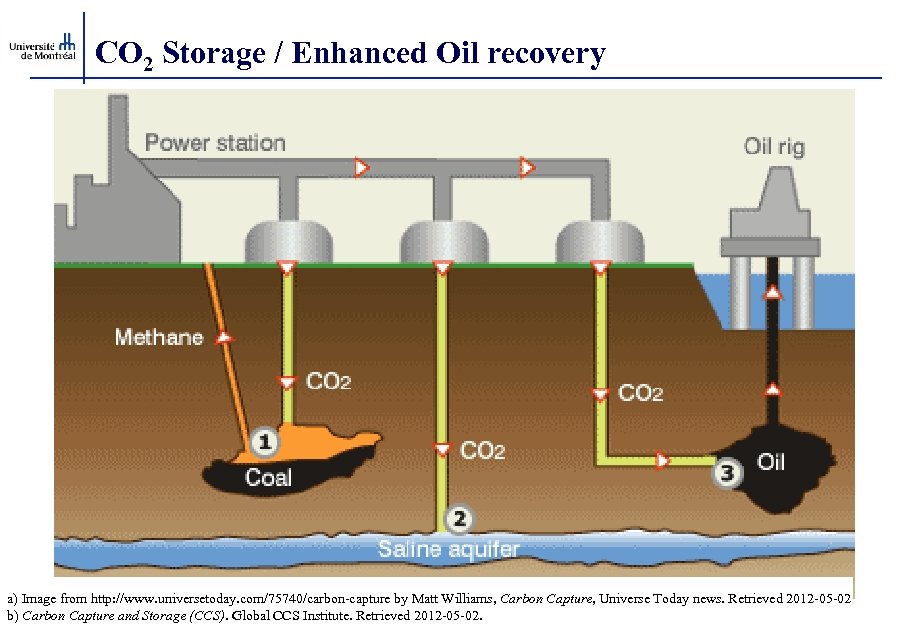 CO 2 Storage / Enhanced Oil recovery a) Image from http: //www. universetoday. com/75740/carbon-capture by Matt Williams, Carbon Capture, Universe Today news. Retrieved 2012 -05 -02 b) Carbon Capture and Storage (CCS). Global CCS Institute. Retrieved 2012 -05 -02.
CO 2 Storage / Enhanced Oil recovery a) Image from http: //www. universetoday. com/75740/carbon-capture by Matt Williams, Carbon Capture, Universe Today news. Retrieved 2012 -05 -02 b) Carbon Capture and Storage (CCS). Global CCS Institute. Retrieved 2012 -05 -02.
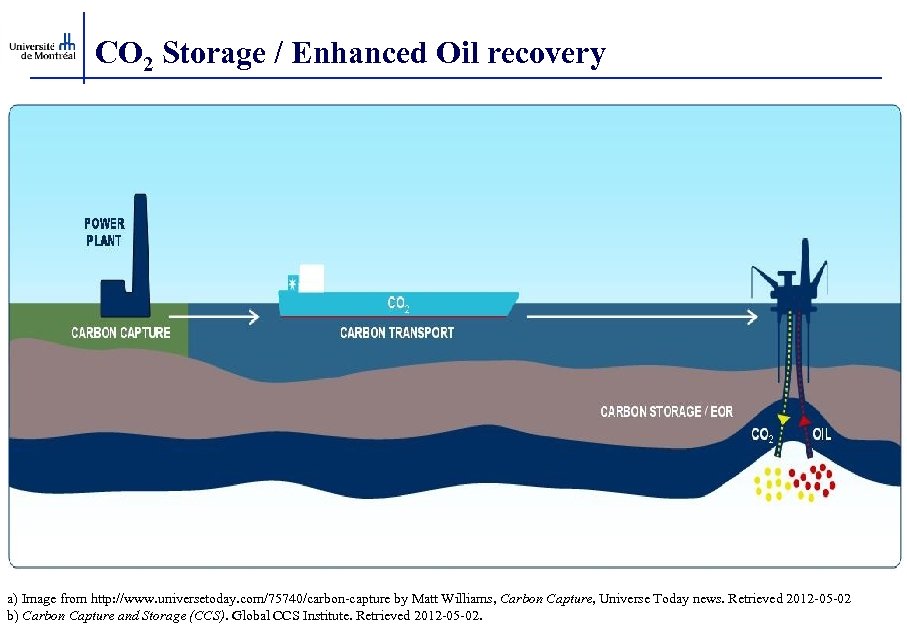 CO 2 Storage / Enhanced Oil recovery a) Image from http: //www. universetoday. com/75740/carbon-capture by Matt Williams, Carbon Capture, Universe Today news. Retrieved 2012 -05 -02 b) Carbon Capture and Storage (CCS). Global CCS Institute. Retrieved 2012 -05 -02.
CO 2 Storage / Enhanced Oil recovery a) Image from http: //www. universetoday. com/75740/carbon-capture by Matt Williams, Carbon Capture, Universe Today news. Retrieved 2012 -05 -02 b) Carbon Capture and Storage (CCS). Global CCS Institute. Retrieved 2012 -05 -02.
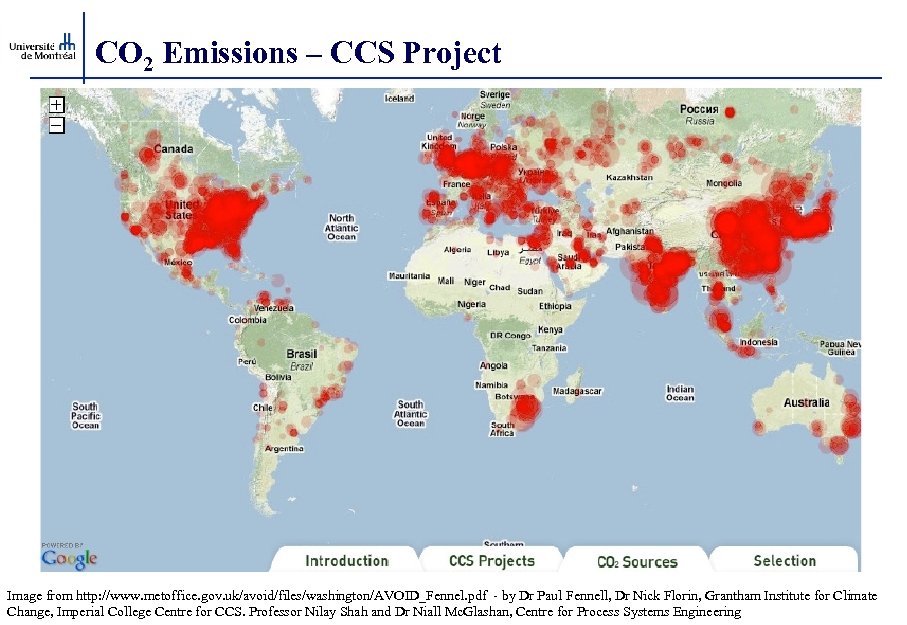 CO 2 Emissions – CCS Project Image from http: //www. metoffice. gov. uk/avoid/files/washington/AVOID_Fennel. pdf - by Dr Paul Fennell, Dr Nick Florin, Grantham Institute for Climate Change, Imperial College Centre for CCS. Professor Nilay Shah and Dr Niall Mc. Glashan, Centre for Process Systems Engineering
CO 2 Emissions – CCS Project Image from http: //www. metoffice. gov. uk/avoid/files/washington/AVOID_Fennel. pdf - by Dr Paul Fennell, Dr Nick Florin, Grantham Institute for Climate Change, Imperial College Centre for CCS. Professor Nilay Shah and Dr Niall Mc. Glashan, Centre for Process Systems Engineering
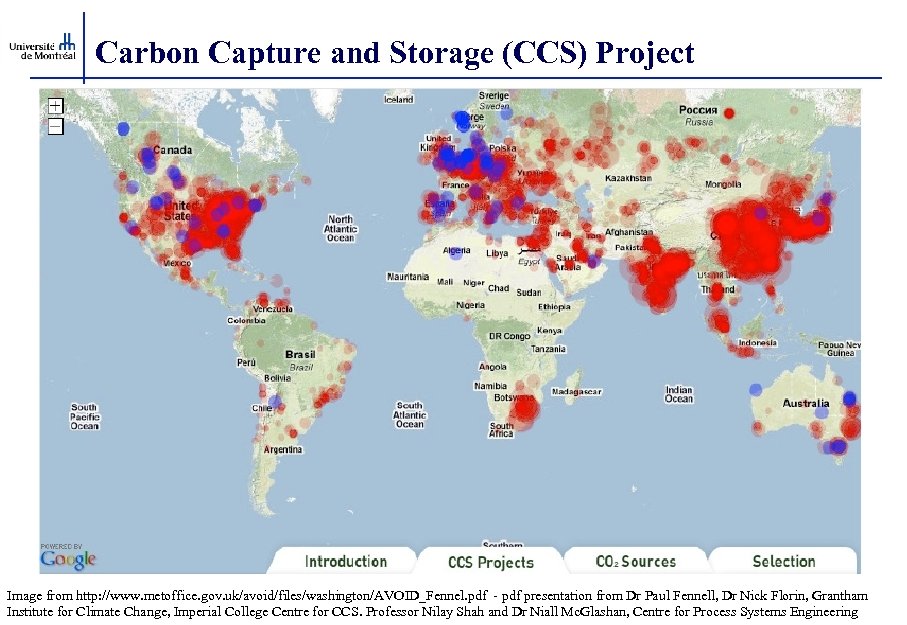 Carbon Capture and Storage (CCS) Project Image from http: //www. metoffice. gov. uk/avoid/files/washington/AVOID_Fennel. pdf - pdf presentation from Dr Paul Fennell, Dr Nick Florin, Grantham Institute for Climate Change, Imperial College Centre for CCS. Professor Nilay Shah and Dr Niall Mc. Glashan, Centre for Process Systems Engineering
Carbon Capture and Storage (CCS) Project Image from http: //www. metoffice. gov. uk/avoid/files/washington/AVOID_Fennel. pdf - pdf presentation from Dr Paul Fennell, Dr Nick Florin, Grantham Institute for Climate Change, Imperial College Centre for CCS. Professor Nilay Shah and Dr Niall Mc. Glashan, Centre for Process Systems Engineering
 CCS Project - Operational Image from http: //www. metoffice. gov. uk/avoid/files/washington/AVOID_Fennel. pdf - pdf presentation from Dr Paul Fennell, Dr Nick Florin, Grantham Institute for Climate Change, Imperial College Centre for CCS. Professor Nilay Shah and Dr Niall Mc. Glashan, Centre for Process Systems Engineering
CCS Project - Operational Image from http: //www. metoffice. gov. uk/avoid/files/washington/AVOID_Fennel. pdf - pdf presentation from Dr Paul Fennell, Dr Nick Florin, Grantham Institute for Climate Change, Imperial College Centre for CCS. Professor Nilay Shah and Dr Niall Mc. Glashan, Centre for Process Systems Engineering
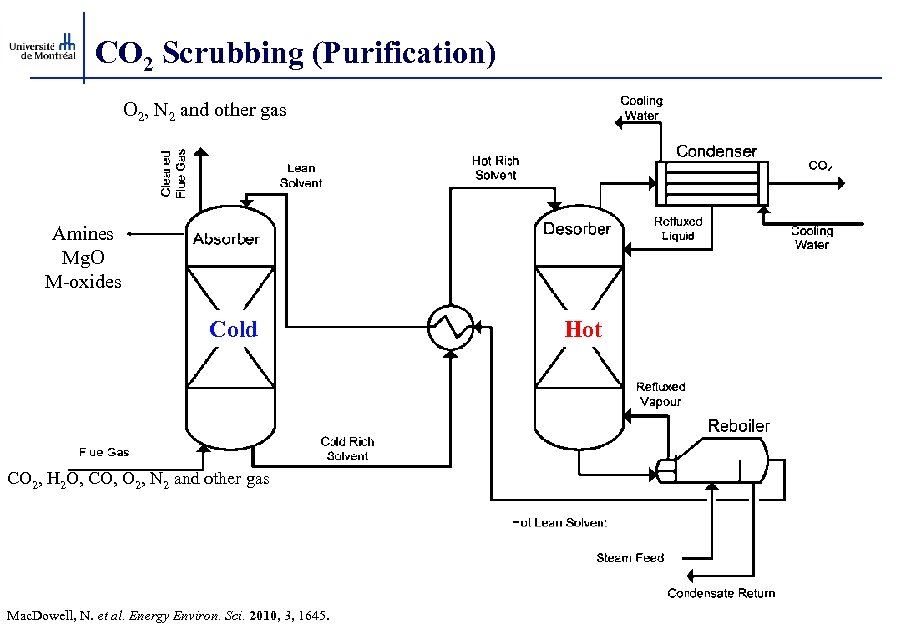 CO 2 Scrubbing (Purification) O 2, N 2 and other gas Amines Mg. O M-oxides Cold CO 2, H 2 O, CO, O 2, N 2 and other gas Mac. Dowell, N. et al. Energy Environ. Sci. 2010, 3, 1645. Hot
CO 2 Scrubbing (Purification) O 2, N 2 and other gas Amines Mg. O M-oxides Cold CO 2, H 2 O, CO, O 2, N 2 and other gas Mac. Dowell, N. et al. Energy Environ. Sci. 2010, 3, 1645. Hot
 Recycling CO 2 Only 1% of the total CO 2 on Earth is currently being used for chemical synthesis : - Chemical inertness, - CO 2 capture and storage is expensive. Recycling CO 2 for the production of chemicals not only lower the impact on global climate changes but also provides a grand challenge in exploring new concepts and opportunities for catalytic and industrial development. a) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703. b) Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43. c) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365. c) Gibson, D. H. Chem. Rev. 1996, 2063.
Recycling CO 2 Only 1% of the total CO 2 on Earth is currently being used for chemical synthesis : - Chemical inertness, - CO 2 capture and storage is expensive. Recycling CO 2 for the production of chemicals not only lower the impact on global climate changes but also provides a grand challenge in exploring new concepts and opportunities for catalytic and industrial development. a) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703. b) Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43. c) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365. c) Gibson, D. H. Chem. Rev. 1996, 2063.
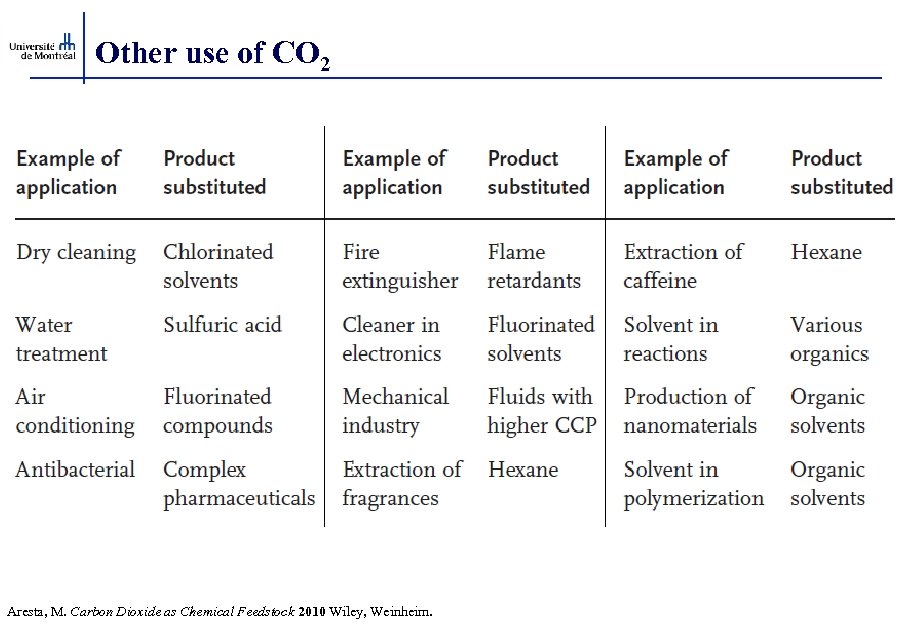 Other use of CO 2 Aresta, M. Carbon Dioxide as Chemical Feedstock 2010 Wiley, Weinheim.
Other use of CO 2 Aresta, M. Carbon Dioxide as Chemical Feedstock 2010 Wiley, Weinheim.
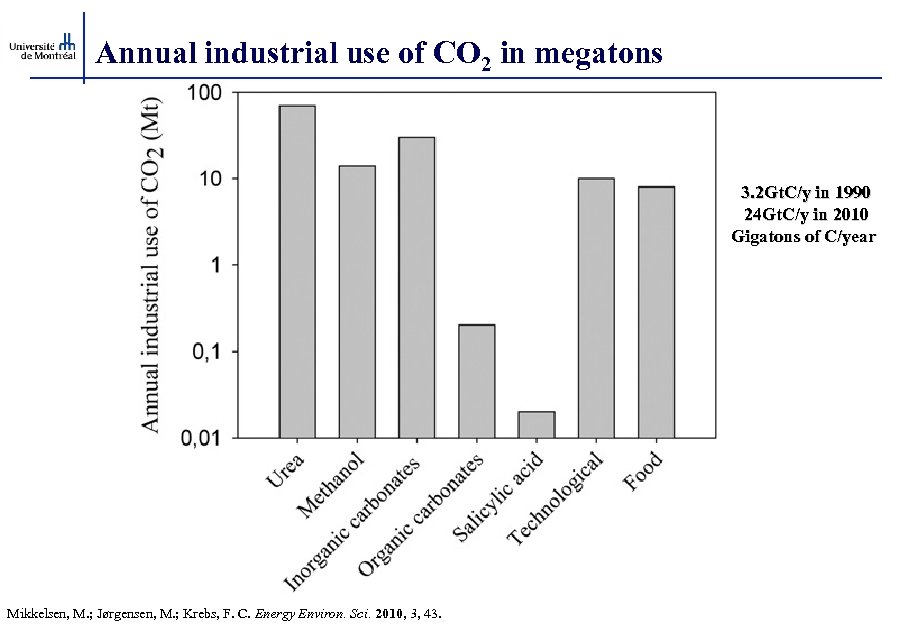 Annual industrial use of CO 2 in megatons 3. 2 Gt. C/y in 1990 24 Gt. C/y in 2010 Gigatons of C/year Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43.
Annual industrial use of CO 2 in megatons 3. 2 Gt. C/y in 1990 24 Gt. C/y in 2010 Gigatons of C/year Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43.
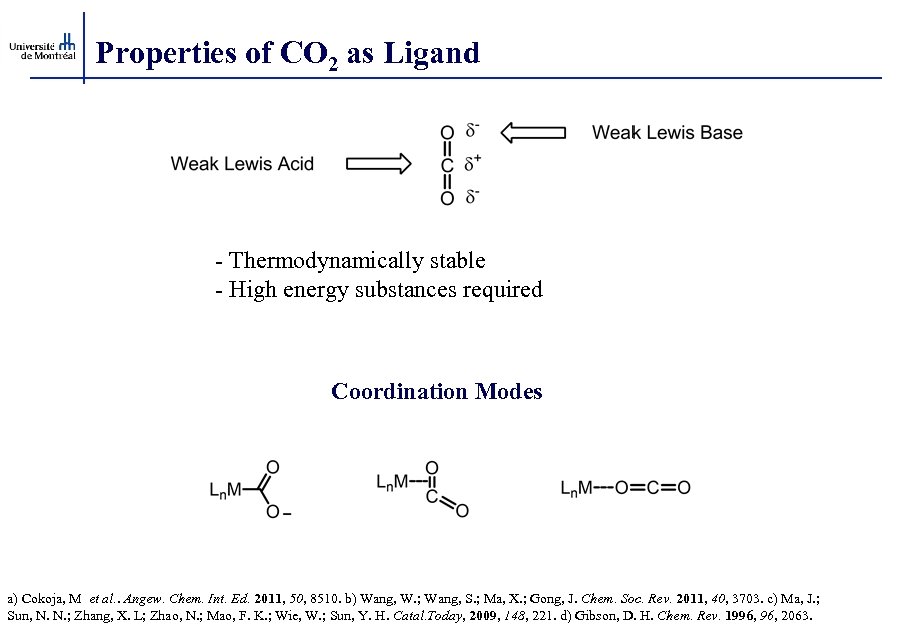 Properties of CO 2 as Ligand - Thermodynamically stable - High energy substances required Coordination Modes a) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. b) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703. c) Ma, J. ; Sun, N. N. ; Zhang, X. L; Zhao, N. ; Mao, F. K. ; Wie, W. ; Sun, Y. H. Catal. Today, 2009, 148, 221. d) Gibson, D. H. Chem. Rev. 1996, 2063.
Properties of CO 2 as Ligand - Thermodynamically stable - High energy substances required Coordination Modes a) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. b) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703. c) Ma, J. ; Sun, N. N. ; Zhang, X. L; Zhao, N. ; Mao, F. K. ; Wie, W. ; Sun, Y. H. Catal. Today, 2009, 148, 221. d) Gibson, D. H. Chem. Rev. 1996, 2063.
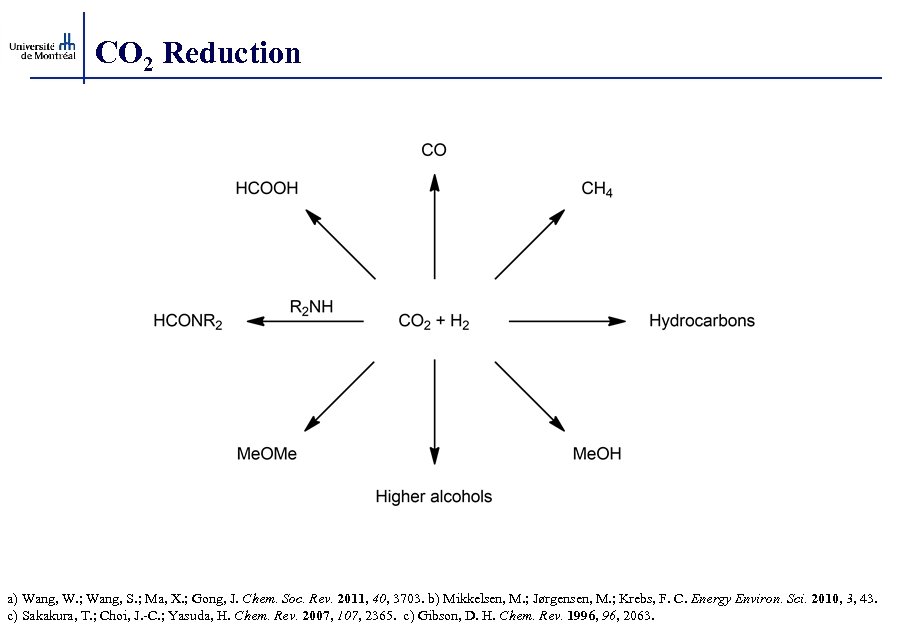 CO 2 Reduction a) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703. b) Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43. c) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365. c) Gibson, D. H. Chem. Rev. 1996, 2063.
CO 2 Reduction a) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703. b) Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43. c) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365. c) Gibson, D. H. Chem. Rev. 1996, 2063.
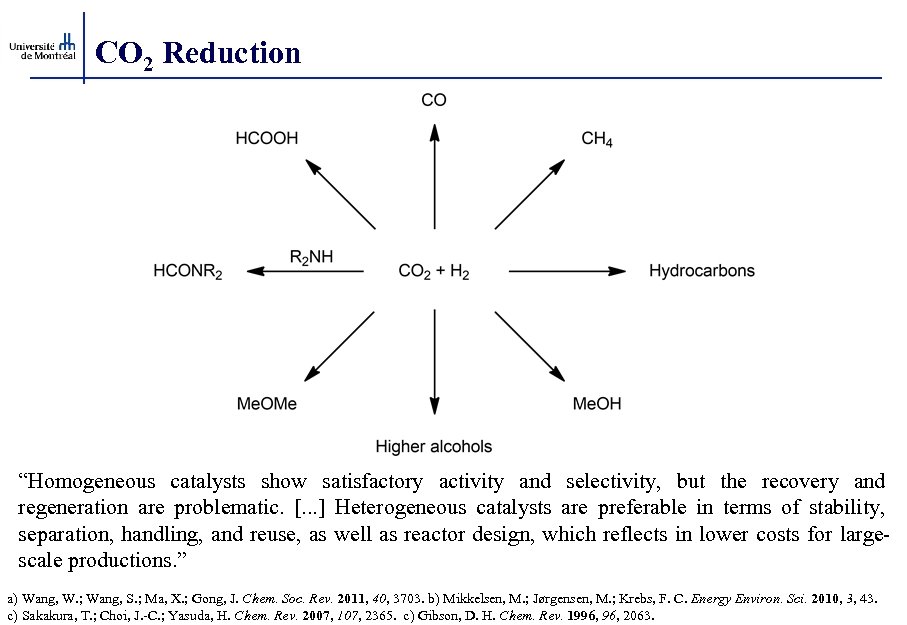 CO 2 Reduction “Homogeneous catalysts show satisfactory activity and selectivity, but the recovery and regeneration are problematic. [. . . ] Heterogeneous catalysts are preferable in terms of stability, separation, handling, and reuse, as well as reactor design, which reflects in lower costs for largescale productions. ” a) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703. b) Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43. c) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365. c) Gibson, D. H. Chem. Rev. 1996, 2063.
CO 2 Reduction “Homogeneous catalysts show satisfactory activity and selectivity, but the recovery and regeneration are problematic. [. . . ] Heterogeneous catalysts are preferable in terms of stability, separation, handling, and reuse, as well as reactor design, which reflects in lower costs for largescale productions. ” a) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703. b) Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43. c) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365. c) Gibson, D. H. Chem. Rev. 1996, 2063.
 Reduction Potential of CO 2 at p. H=7 CO 2 + 1 e- CO 2 + 2 H+ 2 e. CO 2 + 4 H+ 4 e. CO 2 + 6 H+ 6 e. CO 2 + 8 H+ 8 e- → → → CO 2 • HCO 2 H CO + H 2 O H 2 CO + H 2 O CH 3 OH + H 2 O CH 4 + 2 H 2 O E 0 = -1. 90 V E 0 = -0. 61 V E 0 = -0. 53 V E 0 = -0. 48 V E 0 = -0. 38 V E 0 = -0. 24 V a) Benson, E. E. ; Kubiak, C. P. ; Sathrum, A. J. ; Smieja. , J. M. Chem. Soc. Rev. 2009, 38, 89. b) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703. c) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
Reduction Potential of CO 2 at p. H=7 CO 2 + 1 e- CO 2 + 2 H+ 2 e. CO 2 + 4 H+ 4 e. CO 2 + 6 H+ 6 e. CO 2 + 8 H+ 8 e- → → → CO 2 • HCO 2 H CO + H 2 O H 2 CO + H 2 O CH 3 OH + H 2 O CH 4 + 2 H 2 O E 0 = -1. 90 V E 0 = -0. 61 V E 0 = -0. 53 V E 0 = -0. 48 V E 0 = -0. 38 V E 0 = -0. 24 V a) Benson, E. E. ; Kubiak, C. P. ; Sathrum, A. J. ; Smieja. , J. M. Chem. Soc. Rev. 2009, 38, 89. b) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703. c) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
 Reduction of CO 2 to CO Reverse water gas shift (RWGS) is the most promising process : - Metal : Cu, Cu/Si. O 2, Cu–Ni/Al 2 O 3, Cu/Zn. O, Cu–Zn/Al 2 O 3, Pd/Al 2 O 3, Pt/Al 2 O 3, Pt/Ce. O 2, Ni/Ce. O 2, Rh/Si. O 2 (from Rh 2(OAc)4) - Temperature : >600 °C - Cu-based systems remain mostly used. - Often reduction to CH 4 occurs since CO is a better ligand than CO 2 a) Xiaoding, X. ; Moulijn, J. A. Energy Fuels, 1996, 10, 305. b) Kusama, H. ; Bando, K. K. ; Okabe, K. ; Arakawa, H. Appl. Catal. , A 2001, 205, 285. c) Bando, K. K. ; Soga, K. ; Kunimori, K. ; Arakawa, H. Appl. Catal. , A 1998, 175, 67. d) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703.
Reduction of CO 2 to CO Reverse water gas shift (RWGS) is the most promising process : - Metal : Cu, Cu/Si. O 2, Cu–Ni/Al 2 O 3, Cu/Zn. O, Cu–Zn/Al 2 O 3, Pd/Al 2 O 3, Pt/Al 2 O 3, Pt/Ce. O 2, Ni/Ce. O 2, Rh/Si. O 2 (from Rh 2(OAc)4) - Temperature : >600 °C - Cu-based systems remain mostly used. - Often reduction to CH 4 occurs since CO is a better ligand than CO 2 a) Xiaoding, X. ; Moulijn, J. A. Energy Fuels, 1996, 10, 305. b) Kusama, H. ; Bando, K. K. ; Okabe, K. ; Arakawa, H. Appl. Catal. , A 2001, 205, 285. c) Bando, K. K. ; Soga, K. ; Kunimori, K. ; Arakawa, H. Appl. Catal. , A 1998, 175, 67. d) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703.
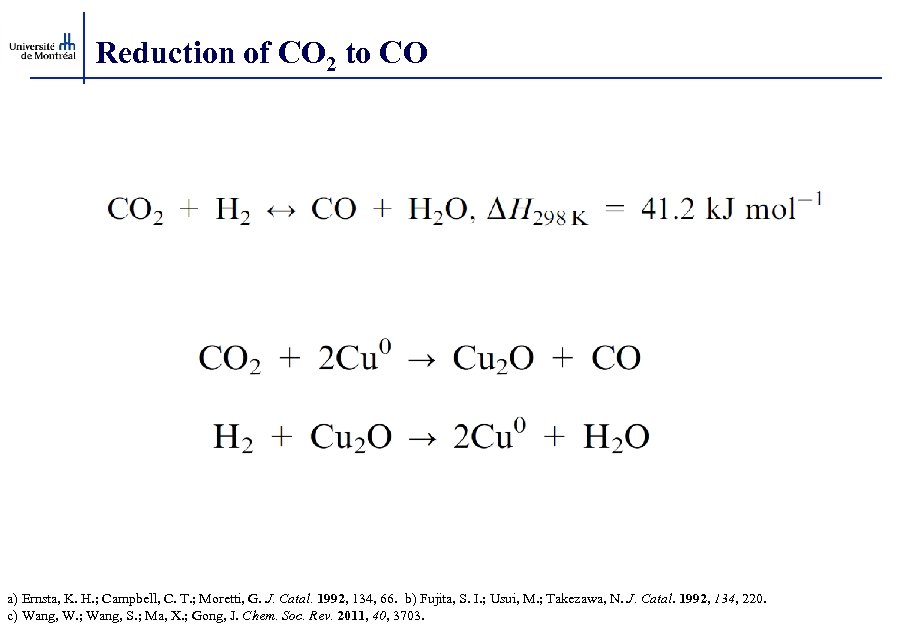 Reduction of CO 2 to CO a) Ernsta, K. H. ; Campbell, C. T. ; Moretti, G. J. Catal. 1992, 134, 66. b) Fujita, S. I. ; Usui, M. ; Takezawa, N. J. Catal. 1992, 134, 220. c) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703.
Reduction of CO 2 to CO a) Ernsta, K. H. ; Campbell, C. T. ; Moretti, G. J. Catal. 1992, 134, 66. b) Fujita, S. I. ; Usui, M. ; Takezawa, N. J. Catal. 1992, 134, 220. c) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703.
 Reduction of CO 2 to CO Mechanism with Pt/Ce. O 2 a) Goguet, A. ; Meunier, F. C. ; Tibiletti, D. ; Breen, J. P. ; Burch, R. J. Phys. Chem. B 2004, 108, 20240. c) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703.
Reduction of CO 2 to CO Mechanism with Pt/Ce. O 2 a) Goguet, A. ; Meunier, F. C. ; Tibiletti, D. ; Breen, J. P. ; Burch, R. J. Phys. Chem. B 2004, 108, 20240. c) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703.
 Photochemical Reduction of CO 2 to CO Takeda, H. ; Ishitani, O. Coordination Chemistry Reviews 2010, 254, 346
Photochemical Reduction of CO 2 to CO Takeda, H. ; Ishitani, O. Coordination Chemistry Reviews 2010, 254, 346
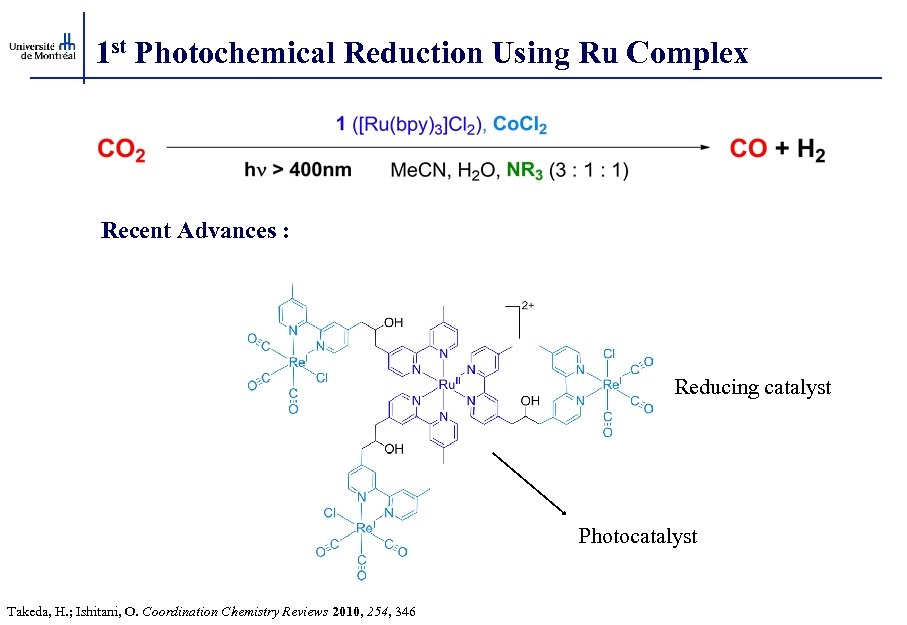 1 st Photochemical Reduction Using Ru Complex Recent Advances : Reducing catalyst Photocatalyst Takeda, H. ; Ishitani, O. Coordination Chemistry Reviews 2010, 254, 346
1 st Photochemical Reduction Using Ru Complex Recent Advances : Reducing catalyst Photocatalyst Takeda, H. ; Ishitani, O. Coordination Chemistry Reviews 2010, 254, 346
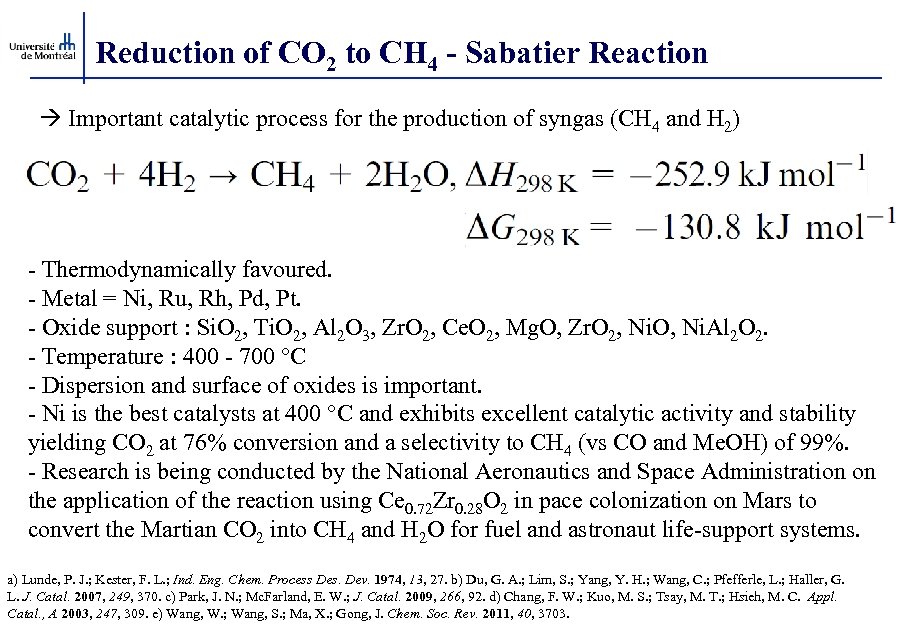 Reduction of CO 2 to CH 4 - Sabatier Reaction Important catalytic process for the production of syngas (CH 4 and H 2) - Thermodynamically favoured. - Metal = Ni, Ru, Rh, Pd, Pt. - Oxide support : Si. O 2, Ti. O 2, Al 2 O 3, Zr. O 2, Ce. O 2, Mg. O, Zr. O 2, Ni. O, Ni. Al 2 O 2. - Temperature : 400 - 700 °C - Dispersion and surface of oxides is important. - Ni is the best catalysts at 400 °C and exhibits excellent catalytic activity and stability yielding CO 2 at 76% conversion and a selectivity to CH 4 (vs CO and Me. OH) of 99%. - Research is being conducted by the National Aeronautics and Space Administration on the application of the reaction using Ce 0. 72 Zr 0. 28 O 2 in pace colonization on Mars to convert the Martian CO 2 into CH 4 and H 2 O for fuel and astronaut life-support systems. a) Lunde, P. J. ; Kester, F. L. ; Ind. Eng. Chem. Process Des. Dev. 1974, 13, 27. b) Du, G. A. ; Lim, S. ; Yang, Y. H. ; Wang, C. ; Pfefferle, L. ; Haller, G. L. J. Catal. 2007, 249, 370. c) Park, J. N. ; Mc. Farland, E. W. ; J. Catal. 2009, 266, 92. d) Chang, F. W. ; Kuo, M. S. ; Tsay, M. T. ; Hsieh, M. C. Appl. Catal. , A 2003, 247, 309. e) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703.
Reduction of CO 2 to CH 4 - Sabatier Reaction Important catalytic process for the production of syngas (CH 4 and H 2) - Thermodynamically favoured. - Metal = Ni, Ru, Rh, Pd, Pt. - Oxide support : Si. O 2, Ti. O 2, Al 2 O 3, Zr. O 2, Ce. O 2, Mg. O, Zr. O 2, Ni. O, Ni. Al 2 O 2. - Temperature : 400 - 700 °C - Dispersion and surface of oxides is important. - Ni is the best catalysts at 400 °C and exhibits excellent catalytic activity and stability yielding CO 2 at 76% conversion and a selectivity to CH 4 (vs CO and Me. OH) of 99%. - Research is being conducted by the National Aeronautics and Space Administration on the application of the reaction using Ce 0. 72 Zr 0. 28 O 2 in pace colonization on Mars to convert the Martian CO 2 into CH 4 and H 2 O for fuel and astronaut life-support systems. a) Lunde, P. J. ; Kester, F. L. ; Ind. Eng. Chem. Process Des. Dev. 1974, 13, 27. b) Du, G. A. ; Lim, S. ; Yang, Y. H. ; Wang, C. ; Pfefferle, L. ; Haller, G. L. J. Catal. 2007, 249, 370. c) Park, J. N. ; Mc. Farland, E. W. ; J. Catal. 2009, 266, 92. d) Chang, F. W. ; Kuo, M. S. ; Tsay, M. T. ; Hsieh, M. C. Appl. Catal. , A 2003, 247, 309. e) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703.
 Potential Bifunctional Model for Pd/Mg. O Catalysis a) Park, J. N. ; Mc. Farland, E. W. J. Catal. 2009, 266, 92. b) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703.
Potential Bifunctional Model for Pd/Mg. O Catalysis a) Park, J. N. ; Mc. Farland, E. W. J. Catal. 2009, 266, 92. b) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703.
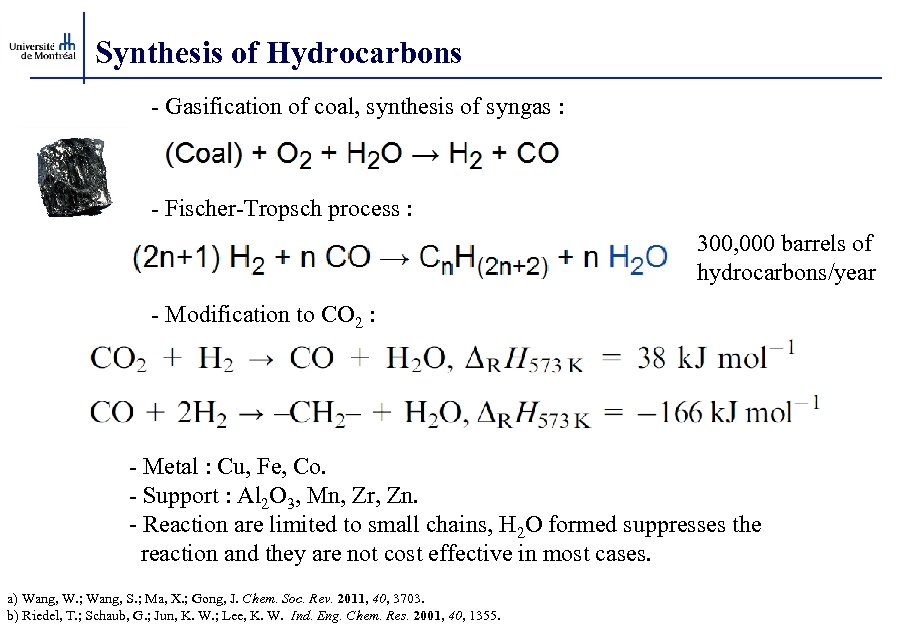 Synthesis of Hydrocarbons - Gasification of coal, synthesis of syngas : - Fischer-Tropsch process : 300, 000 barrels of hydrocarbons/year - Modification to CO 2 : - Metal : Cu, Fe, Co. - Support : Al 2 O 3, Mn, Zr, Zn. - Reaction are limited to small chains, H 2 O formed suppresses the reaction and they are not cost effective in most cases. a) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703. b) Riedel, T. ; Schaub, G. ; Jun, K. W. ; Lee, K. W. Ind. Eng. Chem. Res. 2001, 40, 1355.
Synthesis of Hydrocarbons - Gasification of coal, synthesis of syngas : - Fischer-Tropsch process : 300, 000 barrels of hydrocarbons/year - Modification to CO 2 : - Metal : Cu, Fe, Co. - Support : Al 2 O 3, Mn, Zr, Zn. - Reaction are limited to small chains, H 2 O formed suppresses the reaction and they are not cost effective in most cases. a) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703. b) Riedel, T. ; Schaub, G. ; Jun, K. W. ; Lee, K. W. Ind. Eng. Chem. Res. 2001, 40, 1355.
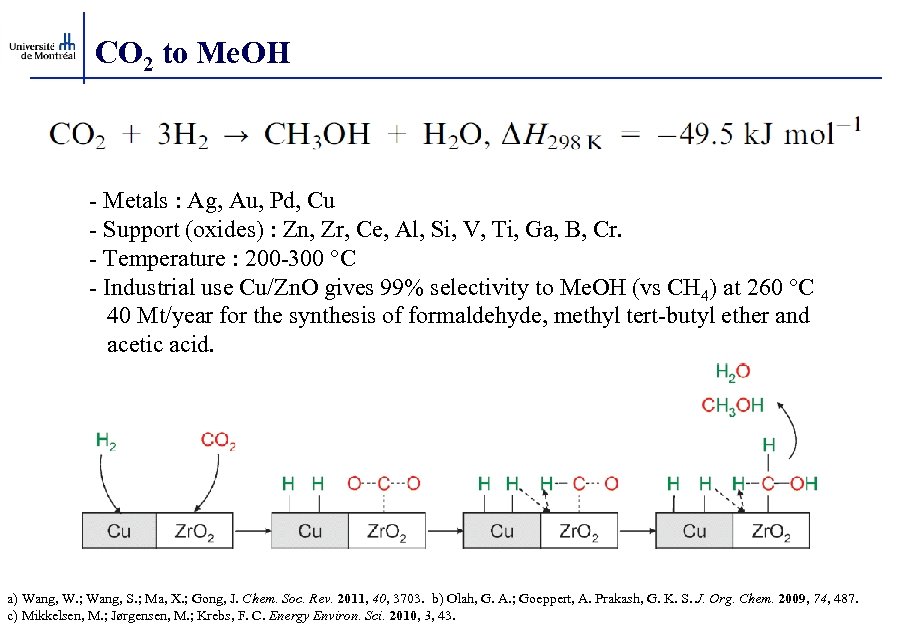 CO 2 to Me. OH - Metals : Ag, Au, Pd, Cu - Support (oxides) : Zn, Zr, Ce, Al, Si, V, Ti, Ga, B, Cr. - Temperature : 200 -300 °C - Industrial use Cu/Zn. O gives 99% selectivity to Me. OH (vs CH 4) at 260 °C 40 Mt/year for the synthesis of formaldehyde, methyl tert-butyl ether and acetic acid. a) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703. b) Olah, G. A. ; Goeppert, A. Prakash, G. K. S. J. Org. Chem. 2009, 74, 487. c) Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43.
CO 2 to Me. OH - Metals : Ag, Au, Pd, Cu - Support (oxides) : Zn, Zr, Ce, Al, Si, V, Ti, Ga, B, Cr. - Temperature : 200 -300 °C - Industrial use Cu/Zn. O gives 99% selectivity to Me. OH (vs CH 4) at 260 °C 40 Mt/year for the synthesis of formaldehyde, methyl tert-butyl ether and acetic acid. a) Wang, W. ; Wang, S. ; Ma, X. ; Gong, J. Chem. Soc. Rev. 2011, 40, 3703. b) Olah, G. A. ; Goeppert, A. Prakash, G. K. S. J. Org. Chem. 2009, 74, 487. c) Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43.
 Potential CO 2 to Me. OH in Industry 82% of conversion a) Olah, G. A. ; Goeppert, A. Prakash, G. K. S. J. Org. Chem. 2009, 74, 487. b) Shulenberger, A. M. ; Jonsson, F. R. ; Ingolfsson, O. ; Tran, K. -C. Process for Producing Liquid Fuel from Carbon Dioxide and Water. US Patent Appl. 2007/0244208 A 1, 2007. c) Tremblay, J. -F. Chem. Eng. News 2008, 86, 13. d) Image from http: /newenergyandfuel/com/2008/08/29/a-new-leading-process-for-co 2 -to-methanol – A New Leading Process For CO 2 to Methanol, Mitsui Chemicals Inc. , New energy and fuel news.
Potential CO 2 to Me. OH in Industry 82% of conversion a) Olah, G. A. ; Goeppert, A. Prakash, G. K. S. J. Org. Chem. 2009, 74, 487. b) Shulenberger, A. M. ; Jonsson, F. R. ; Ingolfsson, O. ; Tran, K. -C. Process for Producing Liquid Fuel from Carbon Dioxide and Water. US Patent Appl. 2007/0244208 A 1, 2007. c) Tremblay, J. -F. Chem. Eng. News 2008, 86, 13. d) Image from http: /newenergyandfuel/com/2008/08/29/a-new-leading-process-for-co 2 -to-methanol – A New Leading Process For CO 2 to Methanol, Mitsui Chemicals Inc. , New energy and fuel news.
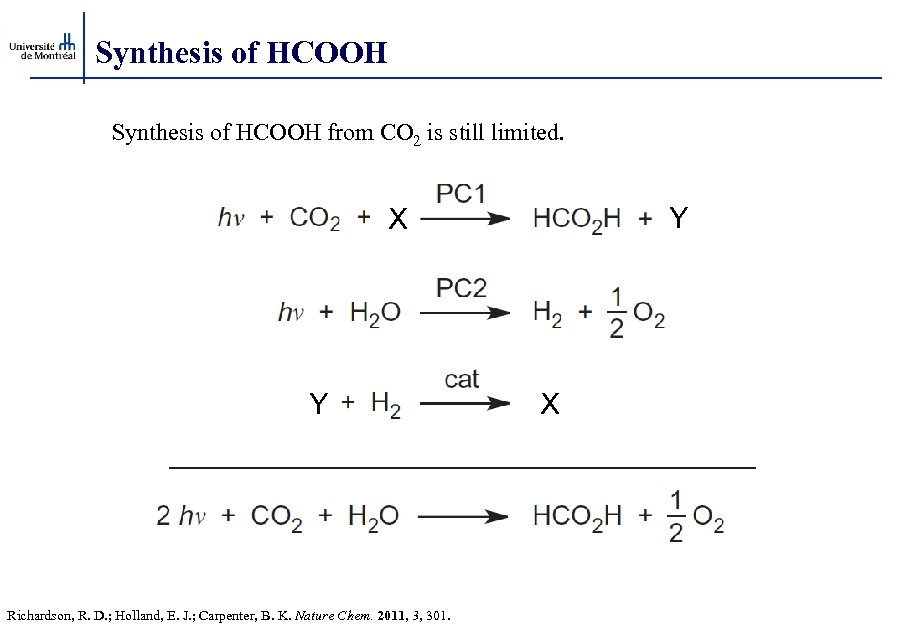 Synthesis of HCOOH from CO 2 is still limited. Y X Y Richardson, R. D. ; Holland, E. J. ; Carpenter, B. K. Nature Chem. 2011, 3, 301. X
Synthesis of HCOOH from CO 2 is still limited. Y X Y Richardson, R. D. ; Holland, E. J. ; Carpenter, B. K. Nature Chem. 2011, 3, 301. X
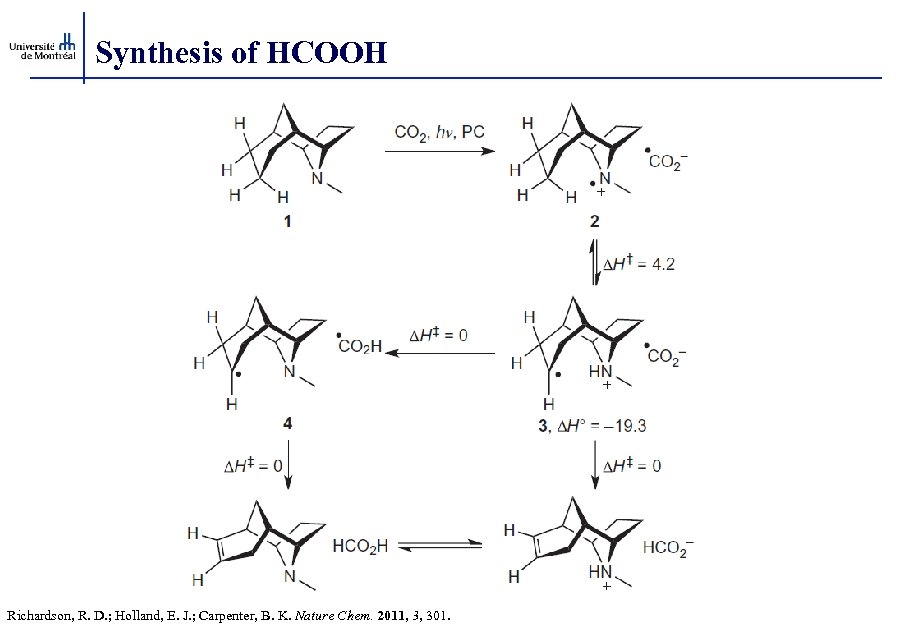 Synthesis of HCOOH Richardson, R. D. ; Holland, E. J. ; Carpenter, B. K. Nature Chem. 2011, 3, 301.
Synthesis of HCOOH Richardson, R. D. ; Holland, E. J. ; Carpenter, B. K. Nature Chem. 2011, 3, 301.
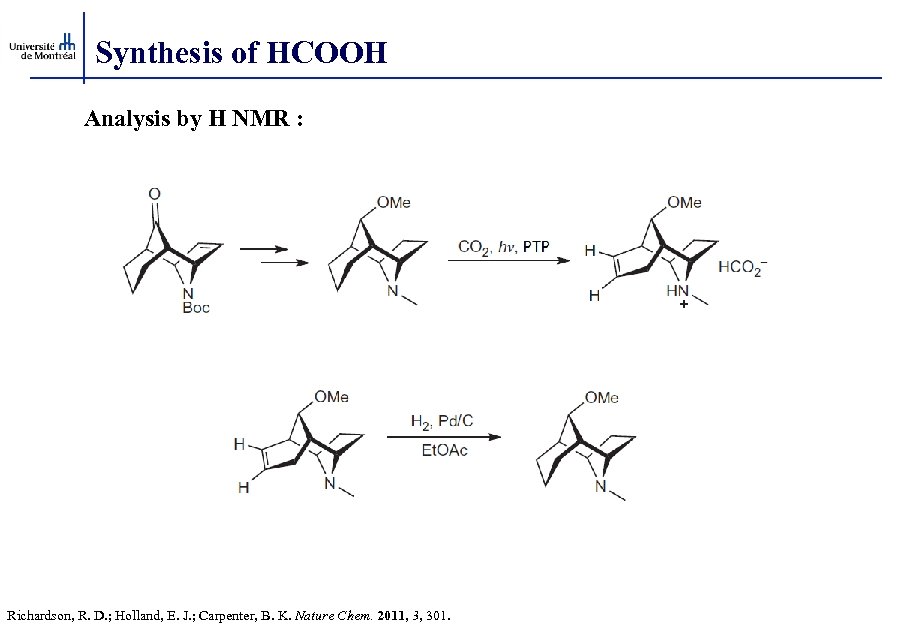 Synthesis of HCOOH Analysis by H NMR : Richardson, R. D. ; Holland, E. J. ; Carpenter, B. K. Nature Chem. 2011, 3, 301.
Synthesis of HCOOH Analysis by H NMR : Richardson, R. D. ; Holland, E. J. ; Carpenter, B. K. Nature Chem. 2011, 3, 301.
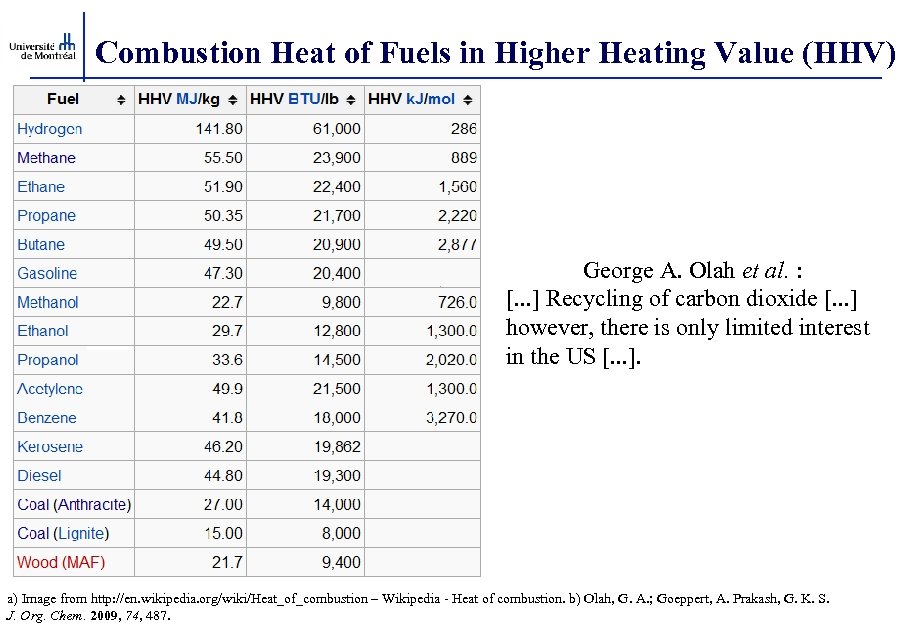 Combustion Heat of Fuels in Higher Heating Value (HHV) George A. Olah et al. : [. . . ] Recycling of carbon dioxide [. . . ] however, there is only limited interest in the US [. . . ]. a) Image from http: //en. wikipedia. org/wiki/Heat_of_combustion – Wikipedia - Heat of combustion. b) Olah, G. A. ; Goeppert, A. Prakash, G. K. S. J. Org. Chem. 2009, 74, 487.
Combustion Heat of Fuels in Higher Heating Value (HHV) George A. Olah et al. : [. . . ] Recycling of carbon dioxide [. . . ] however, there is only limited interest in the US [. . . ]. a) Image from http: //en. wikipedia. org/wiki/Heat_of_combustion – Wikipedia - Heat of combustion. b) Olah, G. A. ; Goeppert, A. Prakash, G. K. S. J. Org. Chem. 2009, 74, 487.
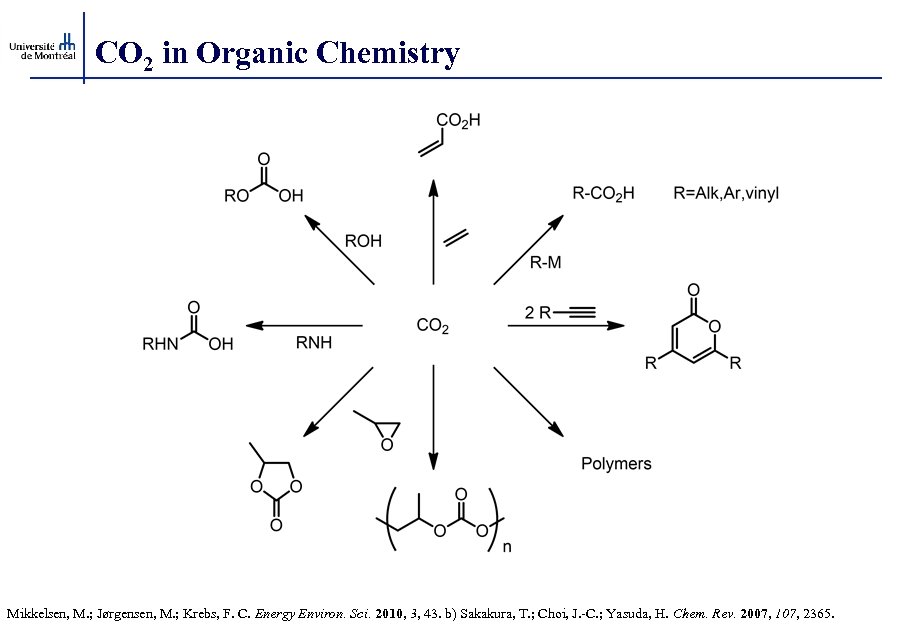 CO 2 in Organic Chemistry Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
CO 2 in Organic Chemistry Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
 Industrial Synthesis of Salicylic Acid a) Xiaoding, X. ; Moulijn, J. A. Energy Fuels, 1996, 10, 305. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
Industrial Synthesis of Salicylic Acid a) Xiaoding, X. ; Moulijn, J. A. Energy Fuels, 1996, 10, 305. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
 Urea Synthesis and Derivatives Mesoporous silica a) Xiaoding, X. ; Moulijn, J. A. Energy Fuels, 1996, 10, 305. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
Urea Synthesis and Derivatives Mesoporous silica a) Xiaoding, X. ; Moulijn, J. A. Energy Fuels, 1996, 10, 305. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
 Reaction of CO 2 with Organometallic Reagents a) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
Reaction of CO 2 with Organometallic Reagents a) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
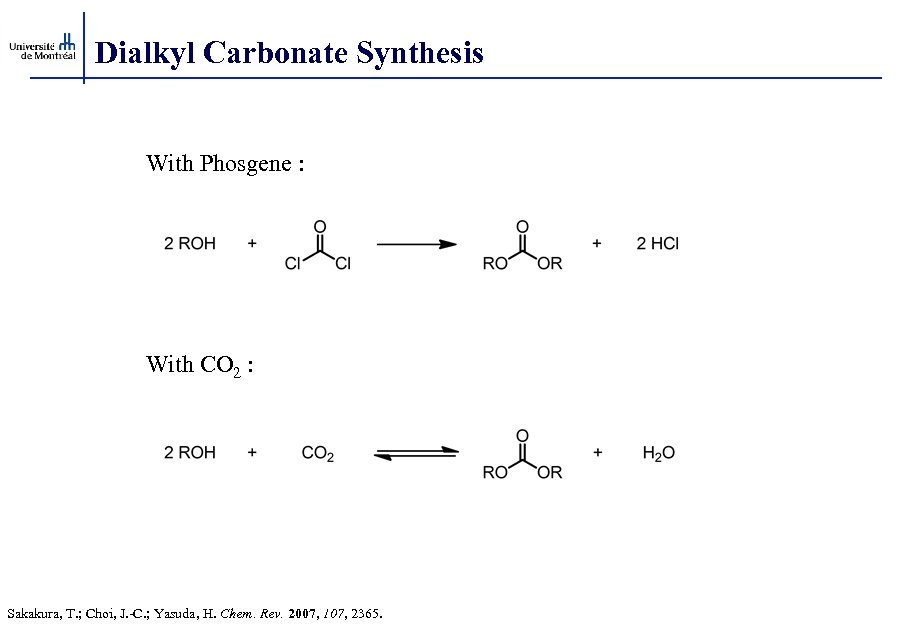 Dialkyl Carbonate Synthesis With Phosgene : With CO 2 : Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
Dialkyl Carbonate Synthesis With Phosgene : With CO 2 : Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
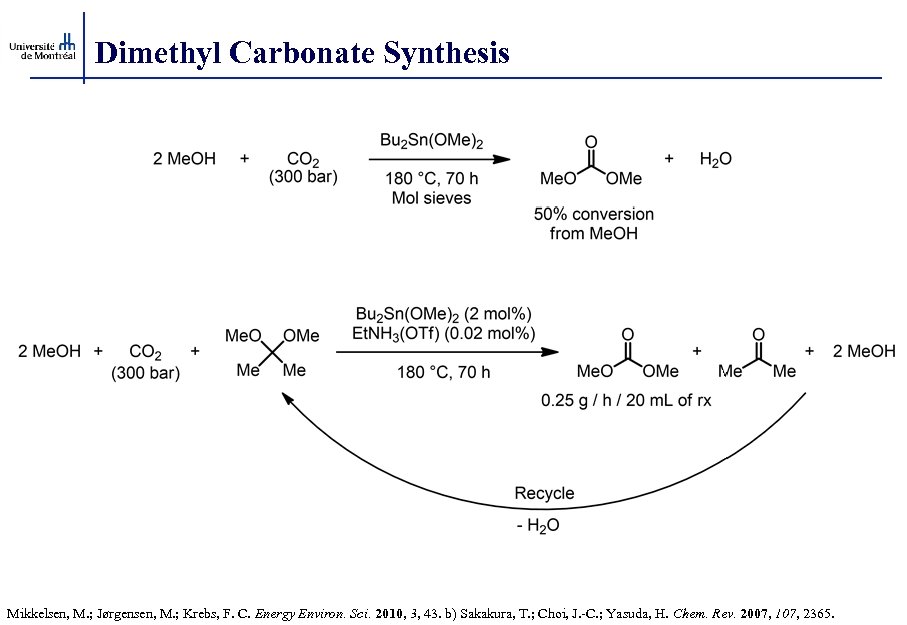 Dimethyl Carbonate Synthesis Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
Dimethyl Carbonate Synthesis Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
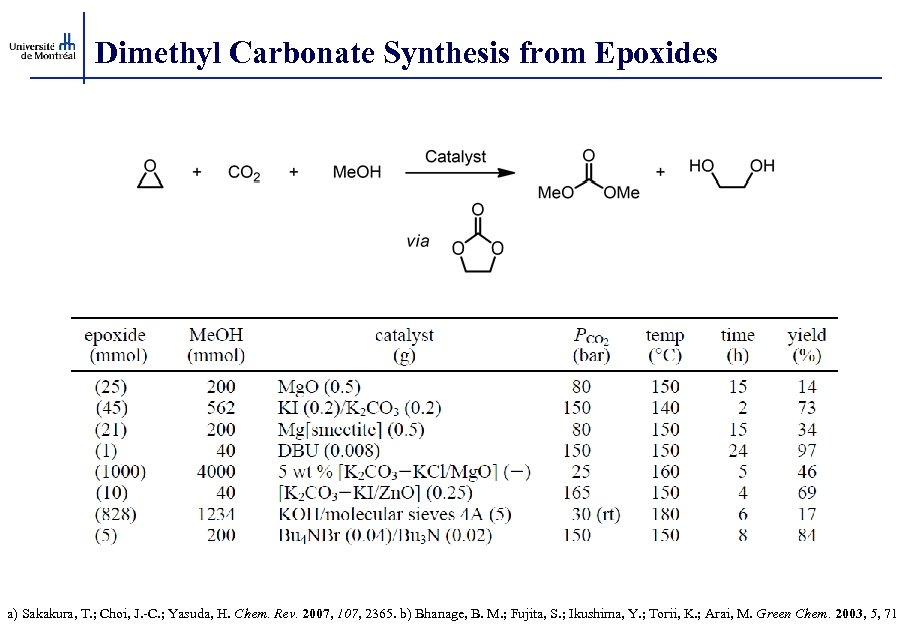 Dimethyl Carbonate Synthesis from Epoxides a) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365. b) Bhanage, B. M. ; Fujita, S. ; Ikushima, Y. ; Torii, K. ; Arai, M. Green Chem. 2003, 5, 71
Dimethyl Carbonate Synthesis from Epoxides a) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365. b) Bhanage, B. M. ; Fujita, S. ; Ikushima, Y. ; Torii, K. ; Arai, M. Green Chem. 2003, 5, 71
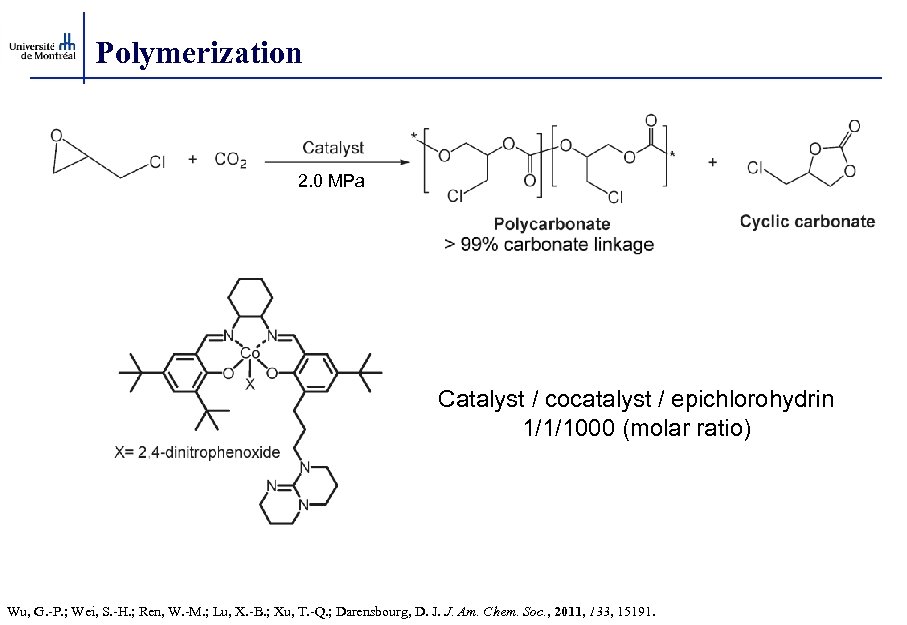 Polymerization 2. 0 MPa Catalyst / cocatalyst / epichlorohydrin 1/1/1000 (molar ratio) Wu, G. -P. ; Wei, S. -H. ; Ren, W. -M. ; Lu, X. -B. ; Xu, T. -Q. ; Darensbourg, D. J. J. Am. Chem. Soc. , 2011, 133, 15191.
Polymerization 2. 0 MPa Catalyst / cocatalyst / epichlorohydrin 1/1/1000 (molar ratio) Wu, G. -P. ; Wei, S. -H. ; Ren, W. -M. ; Lu, X. -B. ; Xu, T. -Q. ; Darensbourg, D. J. J. Am. Chem. Soc. , 2011, 133, 15191.
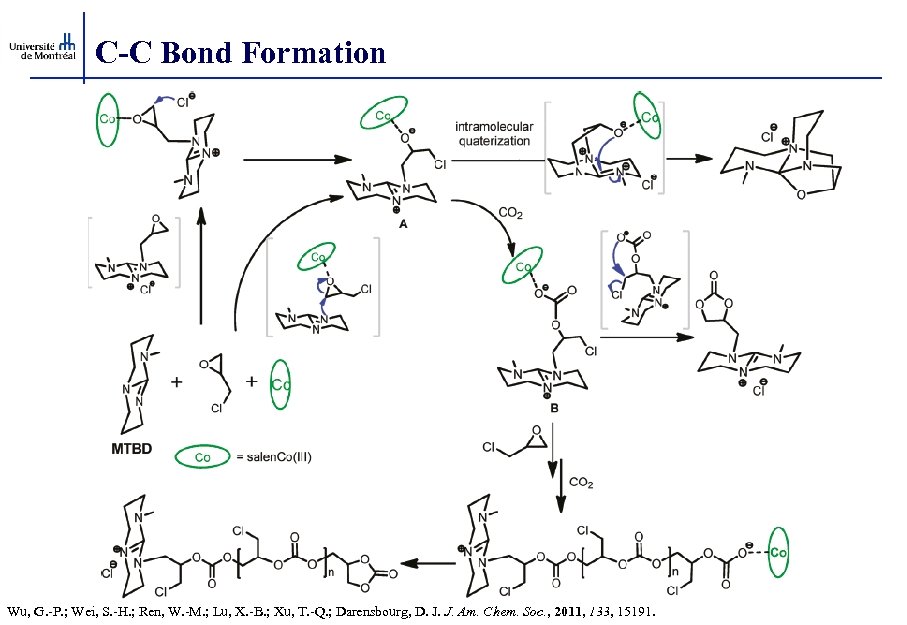 C-C Bond Formation Wu, G. -P. ; Wei, S. -H. ; Ren, W. -M. ; Lu, X. -B. ; Xu, T. -Q. ; Darensbourg, D. J. J. Am. Chem. Soc. , 2011, 133, 15191.
C-C Bond Formation Wu, G. -P. ; Wei, S. -H. ; Ren, W. -M. ; Lu, X. -B. ; Xu, T. -Q. ; Darensbourg, D. J. J. Am. Chem. Soc. , 2011, 133, 15191.
 Synthesis of a Cyclic Carbonate from an Oxirane a) Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43. b) Baba, A. ; Kashiwagi, H. ; Matsuda, H. Organometallics 1987, 6, 137. c) Tian, J. S. ; Wang, J. Q. ; Chen, J. Y. ; Fan, J. G. ; Cai, F. ; He, L. N. Appl. Catal. , A 2006, 301, 215.
Synthesis of a Cyclic Carbonate from an Oxirane a) Mikkelsen, M. ; Jørgensen, M. ; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43. b) Baba, A. ; Kashiwagi, H. ; Matsuda, H. Organometallics 1987, 6, 137. c) Tian, J. S. ; Wang, J. Q. ; Chen, J. Y. ; Fan, J. G. ; Cai, F. ; He, L. N. Appl. Catal. , A 2006, 301, 215.
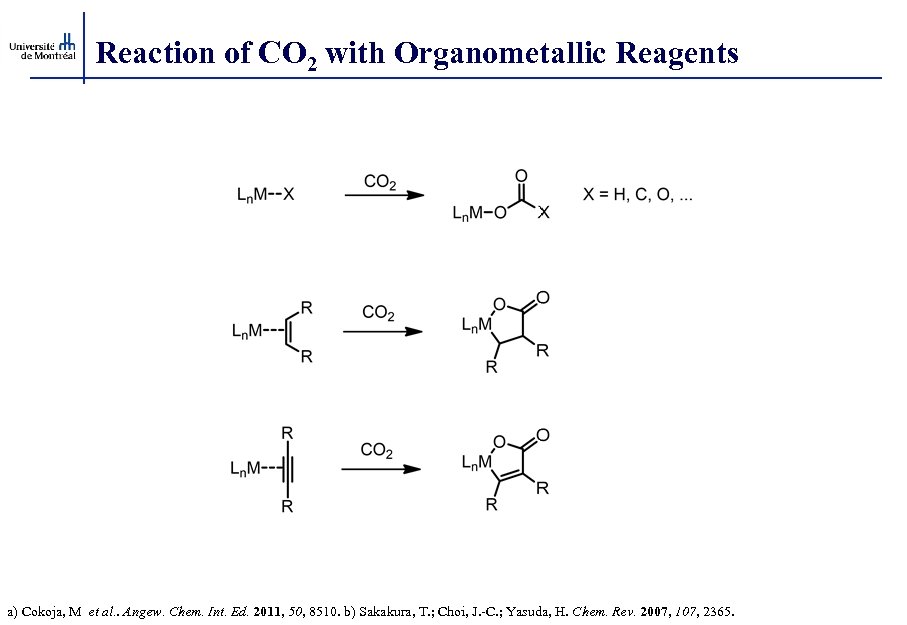 Reaction of CO 2 with Organometallic Reagents a) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
Reaction of CO 2 with Organometallic Reagents a) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
 Possible Catalytic Synthesis of Acrylic Acid “b-H elimination is not favored for steric reasons: the rigid five membered ring does not allow the b-H atoms to come close to the nickel center. ” a) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365. c) Bruckmeier, C. ; Lehenmeier, M. W. ; Reichhardt, R. ; Vagin, S. ; Rieger, B. Organometallics 2010, 29, 2199.
Possible Catalytic Synthesis of Acrylic Acid “b-H elimination is not favored for steric reasons: the rigid five membered ring does not allow the b-H atoms to come close to the nickel center. ” a) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365. c) Bruckmeier, C. ; Lehenmeier, M. W. ; Reichhardt, R. ; Vagin, S. ; Rieger, B. Organometallics 2010, 29, 2199.
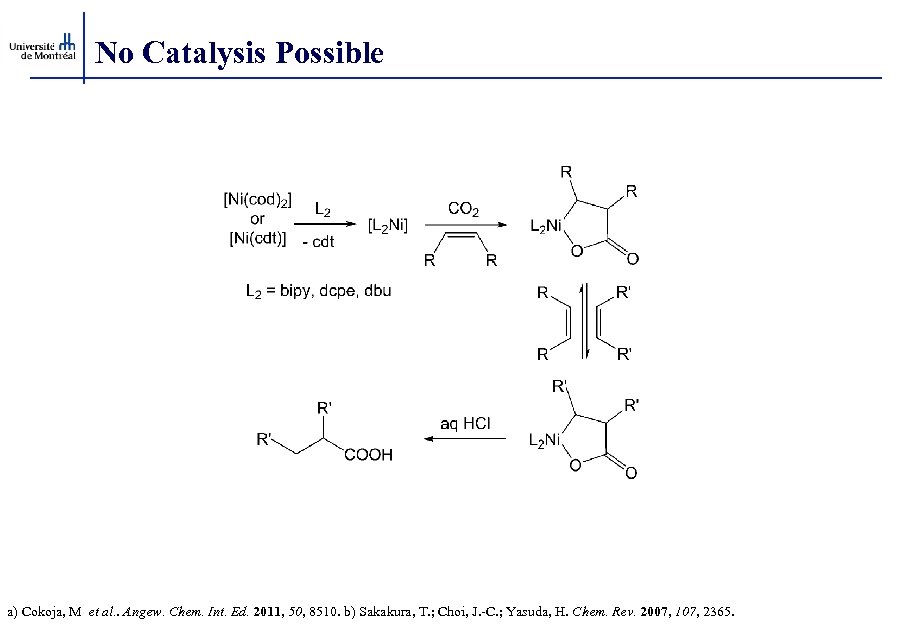 No Catalysis Possible a) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
No Catalysis Possible a) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
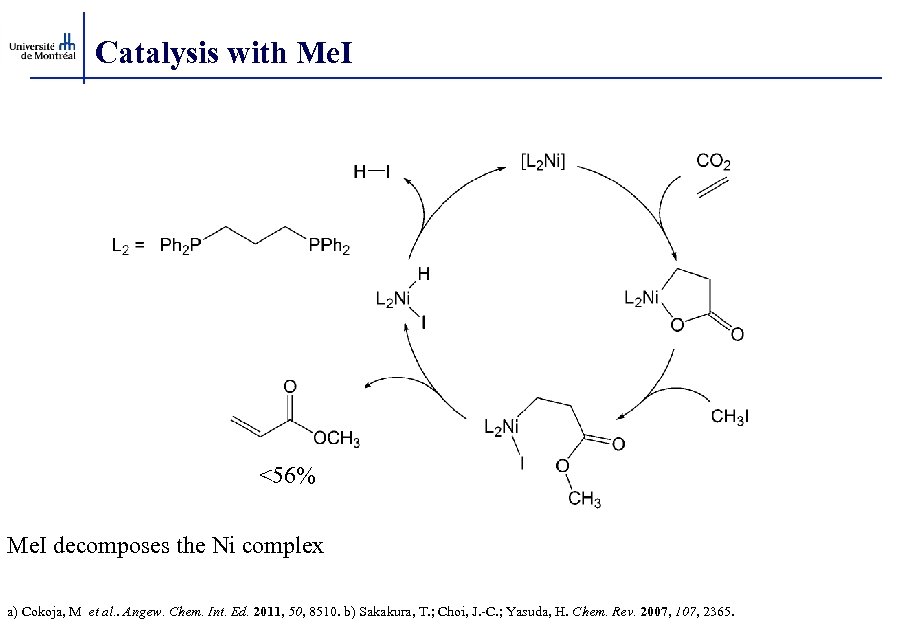 Catalysis with Me. I <56% Me. I decomposes the Ni complex a) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
Catalysis with Me. I <56% Me. I decomposes the Ni complex a) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
 Ni-Catalyzed Stereoselective Ring-Closing Carboxylation a) Takimoto, M. ; Nakamura, Y. ; Kimura, K. ; Mori, M. J. Am. Chem. Soc. 2004, 126, 5956. a) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
Ni-Catalyzed Stereoselective Ring-Closing Carboxylation a) Takimoto, M. ; Nakamura, Y. ; Kimura, K. ; Mori, M. J. Am. Chem. Soc. 2004, 126, 5956. a) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
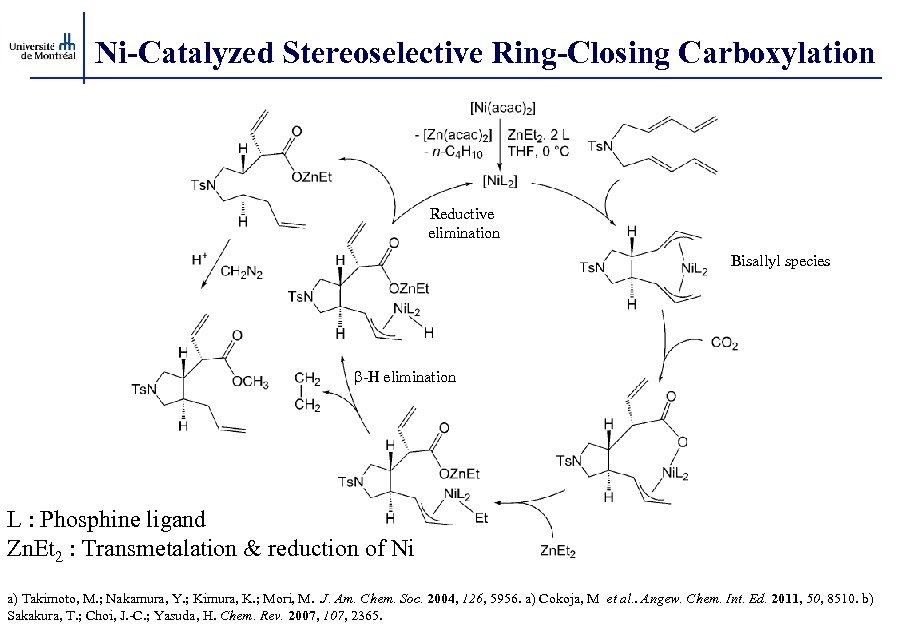 Ni-Catalyzed Stereoselective Ring-Closing Carboxylation Reductive elimination Bisallyl species b-H elimination L : Phosphine ligand Zn. Et 2 : Transmetalation & reduction of Ni a) Takimoto, M. ; Nakamura, Y. ; Kimura, K. ; Mori, M. J. Am. Chem. Soc. 2004, 126, 5956. a) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
Ni-Catalyzed Stereoselective Ring-Closing Carboxylation Reductive elimination Bisallyl species b-H elimination L : Phosphine ligand Zn. Et 2 : Transmetalation & reduction of Ni a) Takimoto, M. ; Nakamura, Y. ; Kimura, K. ; Mori, M. J. Am. Chem. Soc. 2004, 126, 5956. a) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. b) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
 Coupling of CO 2 and Alkynes + + <10% a) Inoue, Y. ; Itoh, Y. ; Hashimoto, H. Chem. Lett. 1977, 85. b) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. c) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
Coupling of CO 2 and Alkynes + + <10% a) Inoue, Y. ; Itoh, Y. ; Hashimoto, H. Chem. Lett. 1977, 85. b) Cokoja, M et al. . Angew. Chem. Int. Ed. 2011, 50, 8510. c) Sakakura, T. ; Choi, J. -C. ; Yasuda, H. Chem. Rev. 2007, 107, 2365.
 Ni- Catalyzed Organozinc Coupling with CO 2 Yeung, C. S. ; Dong, V. M. J. Am. Chem. Soc. 2008, 130, 7826.
Ni- Catalyzed Organozinc Coupling with CO 2 Yeung, C. S. ; Dong, V. M. J. Am. Chem. Soc. 2008, 130, 7826.
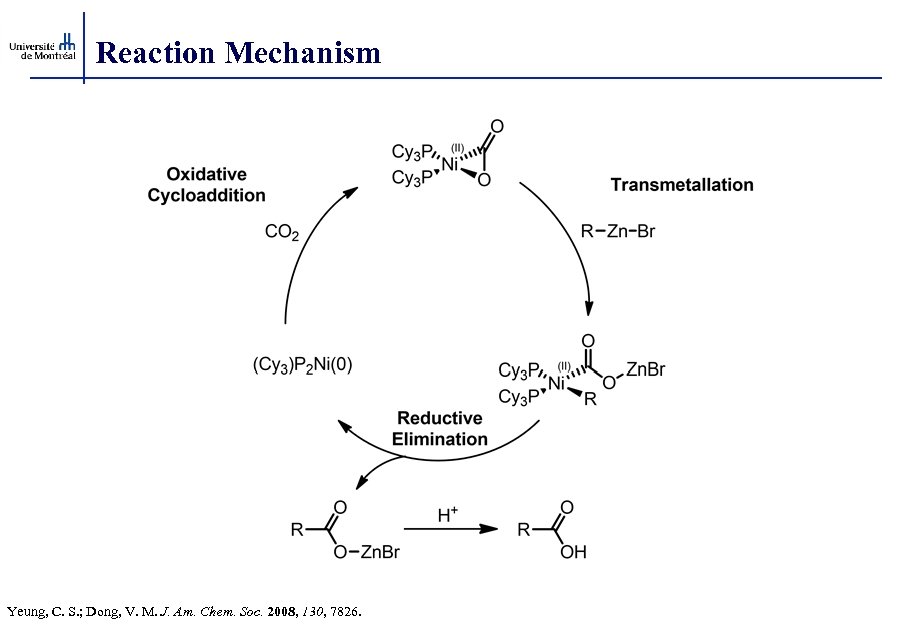 Reaction Mechanism Yeung, C. S. ; Dong, V. M. J. Am. Chem. Soc. 2008, 130, 7826.
Reaction Mechanism Yeung, C. S. ; Dong, V. M. J. Am. Chem. Soc. 2008, 130, 7826.
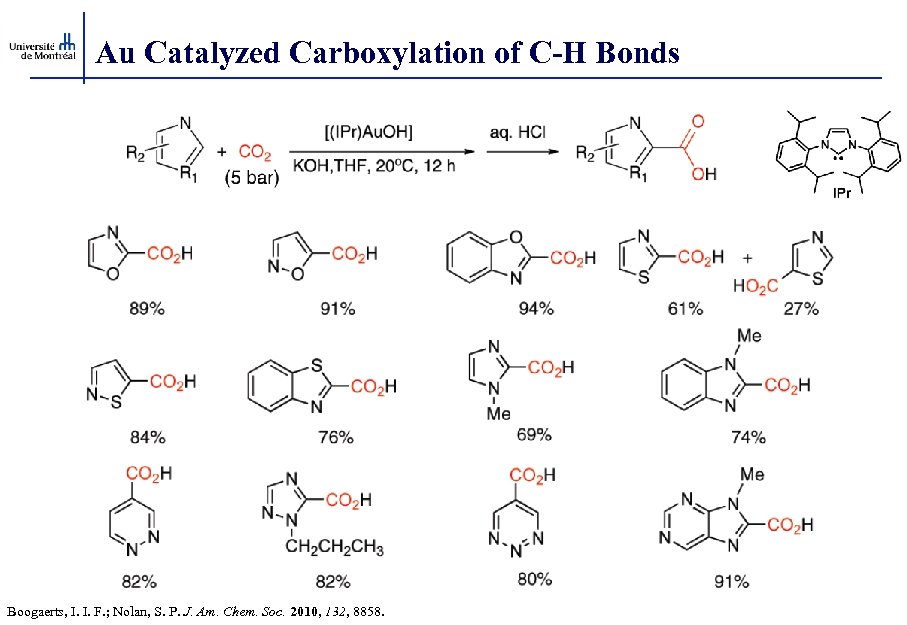 Au Catalyzed Carboxylation of C-H Bonds Boogaerts, I. I. F. ; Nolan, S. P. J. Am. Chem. Soc. 2010, 132, 8858.
Au Catalyzed Carboxylation of C-H Bonds Boogaerts, I. I. F. ; Nolan, S. P. J. Am. Chem. Soc. 2010, 132, 8858.
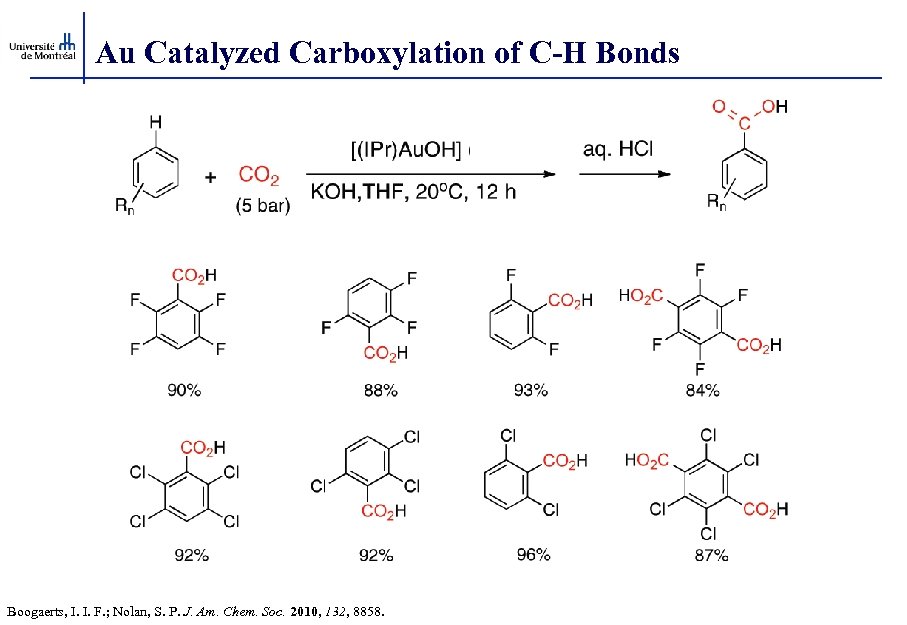 Au Catalyzed Carboxylation of C-H Bonds Boogaerts, I. I. F. ; Nolan, S. P. J. Am. Chem. Soc. 2010, 132, 8858.
Au Catalyzed Carboxylation of C-H Bonds Boogaerts, I. I. F. ; Nolan, S. P. J. Am. Chem. Soc. 2010, 132, 8858.
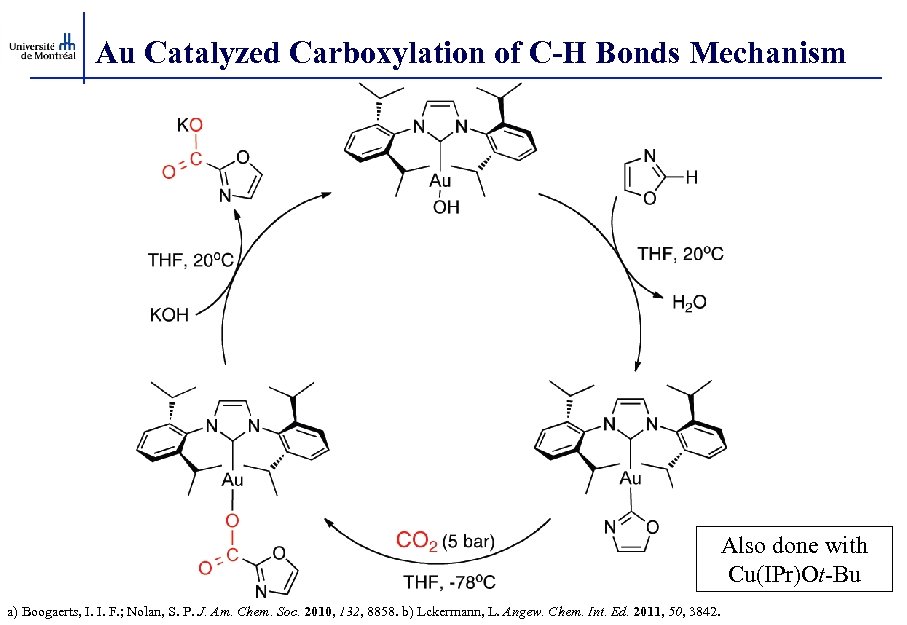 Au Catalyzed Carboxylation of C-H Bonds Mechanism Also done with Cu(IPr)Ot-Bu a) Boogaerts, I. I. F. ; Nolan, S. P. J. Am. Chem. Soc. 2010, 132, 8858. b) Lckermann, L. Angew. Chem. Int. Ed. 2011, 50, 3842.
Au Catalyzed Carboxylation of C-H Bonds Mechanism Also done with Cu(IPr)Ot-Bu a) Boogaerts, I. I. F. ; Nolan, S. P. J. Am. Chem. Soc. 2010, 132, 8858. b) Lckermann, L. Angew. Chem. Int. Ed. 2011, 50, 3842.
 Biomass Synthesis Algae + CO 2 + H 2 O + hn = O 2 + Biomass (Biofuel) = CO 2 RWE's Algae Project, The Niederaussem Coal Innovation Centre, http: //www. rwe. com/web/cms/en/213188/rwe-power-ag/innovations/coal-innovationcentre/rwes-algae-project/
Biomass Synthesis Algae + CO 2 + H 2 O + hn = O 2 + Biomass (Biofuel) = CO 2 RWE's Algae Project, The Niederaussem Coal Innovation Centre, http: //www. rwe. com/web/cms/en/213188/rwe-power-ag/innovations/coal-innovationcentre/rwes-algae-project/
 Conclusion A lot of work has been done for CO 2 recycling and still a lot of work will have to be done to lower CO 2 emissions. - Elucidate mechanisms - Find more cost-effective methods - Incorporate renewable source of energy. ex. solar, etc. - Perform cyclic reactions where CO 2 is formed and reduced in one reactor providing clean energy. Renewable Energy Fuels Reduction Combustion Energy - Why not directly invest in renewable energy? ? ?
Conclusion A lot of work has been done for CO 2 recycling and still a lot of work will have to be done to lower CO 2 emissions. - Elucidate mechanisms - Find more cost-effective methods - Incorporate renewable source of energy. ex. solar, etc. - Perform cyclic reactions where CO 2 is formed and reduced in one reactor providing clean energy. Renewable Energy Fuels Reduction Combustion Energy - Why not directly invest in renewable energy? ? ?
 Consolidating Phase for the Pharma - Astra. Zeneca announced it is buying Ardea for $1 billion. - Watson Pharmaceuticals announced it is buying Actavis for $5. 6 billion. - J&J stated being days away from closing on its $21 billion acquisition of Synthes. - Glaxo got rebuffed from Human Genome Sciences in a $2. 6 billion bid. - Pfizer announced the $12 billion divestiture of its infant nutritional business to Nestlé. Why? - Blockbusters going off patent - Fewer drug approvals Consequences : - Buy companies with solid pipelines that will deliver growth - Layoff - More partnerships to save $ : ex. Merck : 75 partnerships, Lilly : > 100 partnerships, etc One biotech CEO who had sold his first company for several hundred million dollars, who is now on his second, put it this way to me: “Large pharma can’t develop drugs any more. They are too slow. They make decisions for political reasons. Their hurdles are too high. They have to keep buying companies like us just to stay innovative. ” What's Really Driving The Pharma M&A Frenzy, Forbes, http: //www. forbes. com/sites/davidmaris/2012/04/27/pharma-feeding-frenzy/
Consolidating Phase for the Pharma - Astra. Zeneca announced it is buying Ardea for $1 billion. - Watson Pharmaceuticals announced it is buying Actavis for $5. 6 billion. - J&J stated being days away from closing on its $21 billion acquisition of Synthes. - Glaxo got rebuffed from Human Genome Sciences in a $2. 6 billion bid. - Pfizer announced the $12 billion divestiture of its infant nutritional business to Nestlé. Why? - Blockbusters going off patent - Fewer drug approvals Consequences : - Buy companies with solid pipelines that will deliver growth - Layoff - More partnerships to save $ : ex. Merck : 75 partnerships, Lilly : > 100 partnerships, etc One biotech CEO who had sold his first company for several hundred million dollars, who is now on his second, put it this way to me: “Large pharma can’t develop drugs any more. They are too slow. They make decisions for political reasons. Their hurdles are too high. They have to keep buying companies like us just to stay innovative. ” What's Really Driving The Pharma M&A Frenzy, Forbes, http: //www. forbes. com/sites/davidmaris/2012/04/27/pharma-feeding-frenzy/


