15a67f5c215fb20e5736e49ba2e50670.ppt
- Количество слайдов: 45
 Lessons learnt from 1 st registration and how to prepare for 2013 REACH registration INT MARKT 24 – 25 Sep Cairo, Egypt Leo Heezen
Lessons learnt from 1 st registration and how to prepare for 2013 REACH registration INT MARKT 24 – 25 Sep Cairo, Egypt Leo Heezen
 Content » Working in SIEFs » Working in Consortia » Only Representative » Lessons learnt for next REACH registration deadline 2013 2
Content » Working in SIEFs » Working in Consortia » Only Representative » Lessons learnt for next REACH registration deadline 2013 2
 Cooperation under REACH • REACH requires working together: - Art 11: Joint submission Art 27: Sharing of data in case of registered substances Art 29: Substance Information Exchange Fora (SIEF) - To exchange info, to avoid animal tests and reduce costs To agree on C&L Art 30: Sharing of data involving tests 3
Cooperation under REACH • REACH requires working together: - Art 11: Joint submission Art 27: Sharing of data in case of registered substances Art 29: Substance Information Exchange Fora (SIEF) - To exchange info, to avoid animal tests and reduce costs To agree on C&L Art 30: Sharing of data involving tests 3
 Cooperation under REACH Forms of cooperation • Industry cooperation mandatory in particular in respect of SIEF obligations and joint submission of data • REACH Regulation does not organise the way SIEF participants should cooperate nor does regulate possible forms of co-operation • Potential registrants free to organise themselves in order to meet their SIEF obligations (data sharing and C&L) and the joint submission of data 4
Cooperation under REACH Forms of cooperation • Industry cooperation mandatory in particular in respect of SIEF obligations and joint submission of data • REACH Regulation does not organise the way SIEF participants should cooperate nor does regulate possible forms of co-operation • Potential registrants free to organise themselves in order to meet their SIEF obligations (data sharing and C&L) and the joint submission of data 4
 From Pre-registration to SIEF • • • Phase-in substance registered Registration deadline depends volume REACH-IT gives names of other potential registrants Pre-registration Substance sameness discussions between potential registrants • Agree same substance or • Split to form multiple SIEF or • Merge with other (pre-)SIEF Pre-SIEF Agreement by potential registrants that they intend to register the same substance SIEF 5
From Pre-registration to SIEF • • • Phase-in substance registered Registration deadline depends volume REACH-IT gives names of other potential registrants Pre-registration Substance sameness discussions between potential registrants • Agree same substance or • Split to form multiple SIEF or • Merge with other (pre-)SIEF Pre-SIEF Agreement by potential registrants that they intend to register the same substance SIEF 5
 Pre-registration process • Already have a strategy “in house” on type of cooperation you are willing to adopt (may be different according to the substance and thus the SIEFs) • • Lead Registrant Facilitator Reactive only Dormant SIEF / Consortia 6
Pre-registration process • Already have a strategy “in house” on type of cooperation you are willing to adopt (may be different according to the substance and thus the SIEFs) • • Lead Registrant Facilitator Reactive only Dormant SIEF / Consortia 6
 Substance Information Exchange Fora Company mandatory on pre-reg SIEF optional at any time Consortium ACTIONS üSubmit Pre-registration üDetermine SIEF and consortium strategy üUnderstand own data ownership üPrepare Chemical Safety Report (optional) üSubmit registration Obligations to 1. Share hazard data 2. Agree upon classification and labelling üAgreement on rules of data/cost sharing üPreparation of technical registration dossier üPreparation of Chemical Safety Report (optional)
Substance Information Exchange Fora Company mandatory on pre-reg SIEF optional at any time Consortium ACTIONS üSubmit Pre-registration üDetermine SIEF and consortium strategy üUnderstand own data ownership üPrepare Chemical Safety Report (optional) üSubmit registration Obligations to 1. Share hazard data 2. Agree upon classification and labelling üAgreement on rules of data/cost sharing üPreparation of technical registration dossier üPreparation of Chemical Safety Report (optional)
 Purpose of SIEFs Article 29 Ø Facilitate information exchange for registration purposes Ø Agree classification and labeling Separate Submission ü Identification of registrant & substance ü Info on manufacture & use ü Exposure info (1 -10 tpa) Joint Submission ü Classification & Labelling ü Test data summaries and robust study summaries ü Test proposals Joint or Separate Submission ü Guidance on safe use ü Chemical Safety Report Ø Provide other participants with existing studies Ø React to requests Ø Collectively identify needs for future studies and arrange for such study Ø Each SIEF shall be operational at least until 1 June 2018 8
Purpose of SIEFs Article 29 Ø Facilitate information exchange for registration purposes Ø Agree classification and labeling Separate Submission ü Identification of registrant & substance ü Info on manufacture & use ü Exposure info (1 -10 tpa) Joint Submission ü Classification & Labelling ü Test data summaries and robust study summaries ü Test proposals Joint or Separate Submission ü Guidance on safe use ü Chemical Safety Report Ø Provide other participants with existing studies Ø React to requests Ø Collectively identify needs for future studies and arrange for such study Ø Each SIEF shall be operational at least until 1 June 2018 8
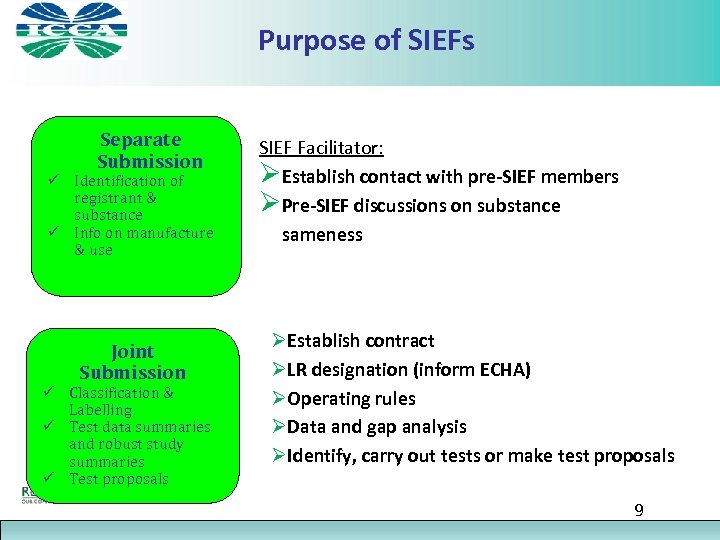 Purpose of SIEFs Separate Submission ü Identification of registrant & substance ü Info on manufacture & use Joint Submission ü Classification & Labelling ü Test data summaries and robust study summaries ü Test proposals SIEF Facilitator: ØEstablish contact with pre-SIEF members ØPre-SIEF discussions on substance sameness ØEstablish contract ØLR designation (inform ECHA) ØOperating rules ØData and gap analysis ØIdentify, carry out tests or make test proposals 9
Purpose of SIEFs Separate Submission ü Identification of registrant & substance ü Info on manufacture & use Joint Submission ü Classification & Labelling ü Test data summaries and robust study summaries ü Test proposals SIEF Facilitator: ØEstablish contact with pre-SIEF members ØPre-SIEF discussions on substance sameness ØEstablish contract ØLR designation (inform ECHA) ØOperating rules ØData and gap analysis ØIdentify, carry out tests or make test proposals 9
 Legal framework Cefic has developed model agreements for SIEFs: Consortium agreement Cooperation agreement SIEF agreement Data sharing agreement The Cefic model is available upon request for Cefic Members Agreement among Lead Members if no consortium Agreement between LR and all SIEF members who wish to participate in the Joint submission Agreement between LR and Data holders These model agreements are available on Cefic website. 10
Legal framework Cefic has developed model agreements for SIEFs: Consortium agreement Cooperation agreement SIEF agreement Data sharing agreement The Cefic model is available upon request for Cefic Members Agreement among Lead Members if no consortium Agreement between LR and all SIEF members who wish to participate in the Joint submission Agreement between LR and Data holders These model agreements are available on Cefic website. 10
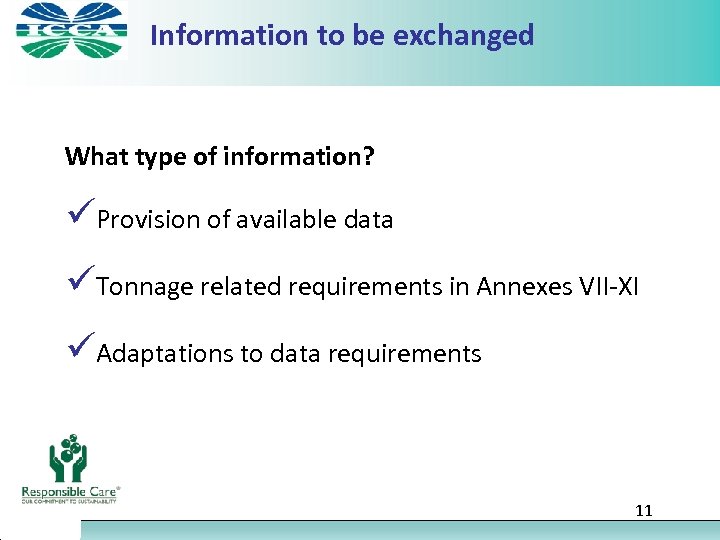 Information to be exchanged What type of information? üProvision of available data üTonnage related requirements in Annexes VII-XI üAdaptations to data requirements 11
Information to be exchanged What type of information? üProvision of available data üTonnage related requirements in Annexes VII-XI üAdaptations to data requirements 11
 Data sharing in SIEF Agree on cooperati on Gather data Share data ü Legal obligations ü Ownerships and rights Evaluate data Generate new data Or prepare test proposal Information requirem ents? Identify data gaps Share costs fair, transparent & nondiscriminatory Joint submission 12
Data sharing in SIEF Agree on cooperati on Gather data Share data ü Legal obligations ü Ownerships and rights Evaluate data Generate new data Or prepare test proposal Information requirem ents? Identify data gaps Share costs fair, transparent & nondiscriminatory Joint submission 12
 Consortium • Consortium: more formal and organised type of cooperation • Consortium may be created by signing an agreement, accepting operating rules by a decision in a meeting or deciding to refer to a commonly agreed set of rules • There is no requirement to organise a consortium as a separate legal entity • Consortium usually created as a « task force » , flexible and limited in time and scope 13
Consortium • Consortium: more formal and organised type of cooperation • Consortium may be created by signing an agreement, accepting operating rules by a decision in a meeting or deciding to refer to a commonly agreed set of rules • There is no requirement to organise a consortium as a separate legal entity • Consortium usually created as a « task force » , flexible and limited in time and scope 13
 Cooperation process SIEF (mandatory) Consortia (form of cooperation) • Company may always decide not to enter into consortia and simply “pay” for access data and co-operate via the SIEF for the joint submission • However, it may be an “appropriate” safeguard measure to have rules adopted still better to enter into Consortium • But, different membership categories may exist 14
Cooperation process SIEF (mandatory) Consortia (form of cooperation) • Company may always decide not to enter into consortia and simply “pay” for access data and co-operate via the SIEF for the joint submission • However, it may be an “appropriate” safeguard measure to have rules adopted still better to enter into Consortium • But, different membership categories may exist 14
 SIEF and Consortia Competition law • Competition law applies to REACH activities => REACH actors should ensure that their activities comply with competition law irrespective of their form of cooperation • REACH actors should restrict the scope of their exchange of information strictly to what is required under REACH => in order not to enable competitors to align their market behaviour • REACH actors should use a trustee/ independent third party where necessary 15
SIEF and Consortia Competition law • Competition law applies to REACH activities => REACH actors should ensure that their activities comply with competition law irrespective of their form of cooperation • REACH actors should restrict the scope of their exchange of information strictly to what is required under REACH => in order not to enable competitors to align their market behaviour • REACH actors should use a trustee/ independent third party where necessary 15
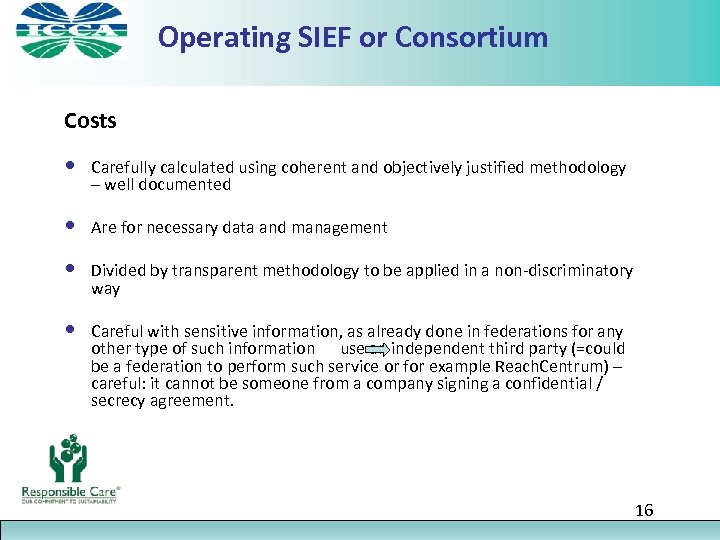 Operating SIEF or Consortium Costs • Carefully calculated using coherent and objectively justified methodology – well documented • Are for necessary data and management • Divided by transparent methodology to be applied in a non-discriminatory way • Careful with sensitive information, as already done in federations for any other type of such information use an independent third party (=could be a federation to perform such service or for example Reach. Centrum) – careful: it cannot be someone from a company signing a confidential / secrecy agreement. 16
Operating SIEF or Consortium Costs • Carefully calculated using coherent and objectively justified methodology – well documented • Are for necessary data and management • Divided by transparent methodology to be applied in a non-discriminatory way • Careful with sensitive information, as already done in federations for any other type of such information use an independent third party (=could be a federation to perform such service or for example Reach. Centrum) – careful: it cannot be someone from a company signing a confidential / secrecy agreement. 16
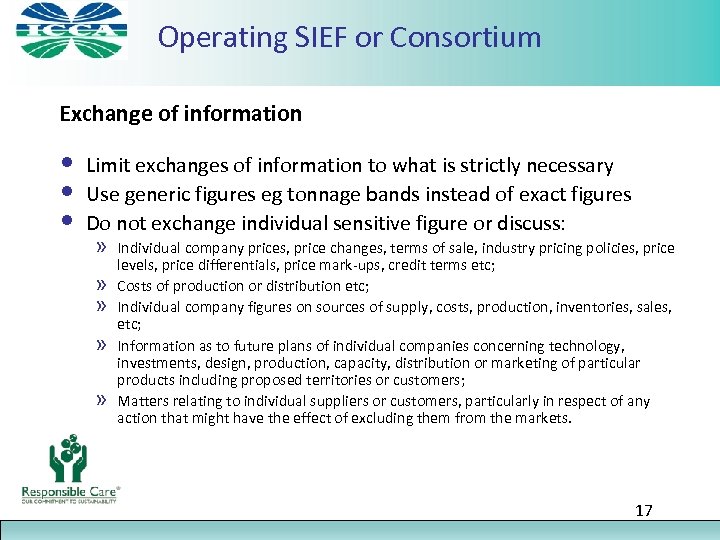 Operating SIEF or Consortium Exchange of information • • • Limit exchanges of information to what is strictly necessary Use generic figures eg tonnage bands instead of exact figures Do not exchange individual sensitive figure or discuss: » Individual company prices, price changes, terms of sale, industry pricing policies, price » » levels, price differentials, price mark-ups, credit terms etc; Costs of production or distribution etc; Individual company figures on sources of supply, costs, production, inventories, sales, etc; Information as to future plans of individual companies concerning technology, investments, design, production, capacity, distribution or marketing of particular products including proposed territories or customers; Matters relating to individual suppliers or customers, particularly in respect of any action that might have the effect of excluding them from the markets. 17
Operating SIEF or Consortium Exchange of information • • • Limit exchanges of information to what is strictly necessary Use generic figures eg tonnage bands instead of exact figures Do not exchange individual sensitive figure or discuss: » Individual company prices, price changes, terms of sale, industry pricing policies, price » » levels, price differentials, price mark-ups, credit terms etc; Costs of production or distribution etc; Individual company figures on sources of supply, costs, production, inventories, sales, etc; Information as to future plans of individual companies concerning technology, investments, design, production, capacity, distribution or marketing of particular products including proposed territories or customers; Matters relating to individual suppliers or customers, particularly in respect of any action that might have the effect of excluding them from the markets. 17
 Operating SIEF or Consortium Discussion business related issues • • Be very careful on this – Do not for example discuss collectively changes in sales, supply, purchasing and marketing strategy including business plans Limit to what is necessary to REACH • No “collective deselection” – This should be an individual decision from each company not to for example to pre- register, or register, or pursue an use in particular • Do not exchange individual sensitive figure or discuss as mentioned already 18
Operating SIEF or Consortium Discussion business related issues • • Be very careful on this – Do not for example discuss collectively changes in sales, supply, purchasing and marketing strategy including business plans Limit to what is necessary to REACH • No “collective deselection” – This should be an individual decision from each company not to for example to pre- register, or register, or pursue an use in particular • Do not exchange individual sensitive figure or discuss as mentioned already 18
 Operating SIEF or Consortium DO and DON’T • REACH activities and EC Competition law DO NOT presume that because you are strictly applying REACH, EC competition law will not apply • Organisation of activities DO NOT work in a disorganised way. If you have rules or sign an agreement apply these in full and ensure they are followed • Type of activities DO NOT engage in prohibited activities during social gatherings incidental to your lawful activities or otherwise; EC competition law rules will equally apply to these • Oversight & supervision DO NOT apply EC competition law compliance guidance infrequently but instead, apply it in your day-to-day activities in order for it to become routine good practice + apply meetings checklist 19
Operating SIEF or Consortium DO and DON’T • REACH activities and EC Competition law DO NOT presume that because you are strictly applying REACH, EC competition law will not apply • Organisation of activities DO NOT work in a disorganised way. If you have rules or sign an agreement apply these in full and ensure they are followed • Type of activities DO NOT engage in prohibited activities during social gatherings incidental to your lawful activities or otherwise; EC competition law rules will equally apply to these • Oversight & supervision DO NOT apply EC competition law compliance guidance infrequently but instead, apply it in your day-to-day activities in order for it to become routine good practice + apply meetings checklist 19
 Only Representative (OR) • Article 8(1): A natural or legal person established outside the • Article 8(2): “The representative […. ] shall keep available and up-to-date • Article 8(3): If a representative is appointed (…)the non-Community [……. ] a legal person established in the Community to fulfill, as his only representative, the obligations on importers under this Title. information on quantities imported and customers sold to, as well as information on the supply of the latest update of the safety data sheet referred to in Article 31” manufacturer shall inform the importer(s) within the same supply chain of the appointment. These importers shall be regarded as downstream users for the purposes of this Regulation. 20
Only Representative (OR) • Article 8(1): A natural or legal person established outside the • Article 8(2): “The representative […. ] shall keep available and up-to-date • Article 8(3): If a representative is appointed (…)the non-Community [……. ] a legal person established in the Community to fulfill, as his only representative, the obligations on importers under this Title. information on quantities imported and customers sold to, as well as information on the supply of the latest update of the safety data sheet referred to in Article 31” manufacturer shall inform the importer(s) within the same supply chain of the appointment. These importers shall be regarded as downstream users for the purposes of this Regulation. 20
 Who can appoint/be an OR? Ø Who can appoint an OR? » “non-Community” manufacturers (non-EU substance manufacturers, formulators, producers of articles) » but not distributors (as they are not mentioned) Ø Who can be an OR? » legal entity established in the EU which has sufficient background in the practical handling of substances and the information related to them 21 21
Who can appoint/be an OR? Ø Who can appoint an OR? » “non-Community” manufacturers (non-EU substance manufacturers, formulators, producers of articles) » but not distributors (as they are not mentioned) Ø Who can be an OR? » legal entity established in the EU which has sufficient background in the practical handling of substances and the information related to them 21 21
 Only Representative (status) The Only Representative (OR) ØMust have sufficient background in the practical handling of substances and the information related to them ØNeeds a written confirmation from his non-EU manufacturer (to be inserted in IUCLID in section 1. 7 Suppliers) ØAppointed after 1 December 2008 can pre-register the substance in accordance with Article 28(6) until 12 months before the relevant registration deadline, provided that the substance originating from the non-EU manufacturer was not placed on the market previously in a quantity at or above 1 tonne/year after 1 June 2008 ØCan represent several non-EU manufactures, but has to submit separate (pre-)registrations for each of these manufacturers 22
Only Representative (status) The Only Representative (OR) ØMust have sufficient background in the practical handling of substances and the information related to them ØNeeds a written confirmation from his non-EU manufacturer (to be inserted in IUCLID in section 1. 7 Suppliers) ØAppointed after 1 December 2008 can pre-register the substance in accordance with Article 28(6) until 12 months before the relevant registration deadline, provided that the substance originating from the non-EU manufacturer was not placed on the market previously in a quantity at or above 1 tonne/year after 1 June 2008 ØCan represent several non-EU manufactures, but has to submit separate (pre-)registrations for each of these manufacturers 22
 Only Representative (status) According to Article 8, the OR is responsible for Ø(Pre-)registration (incl. keeping the registration dossier upto-date) ØCommunication in the supply chain ØNotification of SVHC ØC&L ØObligations resulting from authorisations and/or restrictions ØUp-to-date information on quantities imported and EU importers This information must kept available for enforcement authorities upon request 23
Only Representative (status) According to Article 8, the OR is responsible for Ø(Pre-)registration (incl. keeping the registration dossier upto-date) ØCommunication in the supply chain ØNotification of SVHC ØC&L ØObligations resulting from authorisations and/or restrictions ØUp-to-date information on quantities imported and EU importers This information must kept available for enforcement authorities upon request 23
 Obligations of OR’s (1) v OR is fully liable for fulfilling importer obligations under REACH vnot only registration but also pre-registration, communication in the supply chain, notification of SVHC, C&L, authorisation and restrictions v OR must keep up-to-date list of EU importers, incl. : vtonnage covered for each of the importers vinformation on the supply of the latest SDS ðthis information must be presented to enforcement authorities upon request 24 24
Obligations of OR’s (1) v OR is fully liable for fulfilling importer obligations under REACH vnot only registration but also pre-registration, communication in the supply chain, notification of SVHC, C&L, authorisation and restrictions v OR must keep up-to-date list of EU importers, incl. : vtonnage covered for each of the importers vinformation on the supply of the latest SDS ðthis information must be presented to enforcement authorities upon request 24 24
 Obligations of OR’s (2) Ø No distinction between different types of imports vi. e. OR of non-EU substance manufacturer may cover imports into the EU even if: – they are traded in a supply chain outside the EU or – integrated into a formulation before import vhowever, there is a need for clear documentation which imports are covered ðdocumentation must be presented to enforcement authorities upon request 3/19/2018 25 25
Obligations of OR’s (2) Ø No distinction between different types of imports vi. e. OR of non-EU substance manufacturer may cover imports into the EU even if: – they are traded in a supply chain outside the EU or – integrated into a formulation before import vhowever, there is a need for clear documentation which imports are covered ðdocumentation must be presented to enforcement authorities upon request 3/19/2018 25 25
 Obligations of OR’s (3) Ø Change of OR vcan be done via an update under the condition that the earlier OR agrees (needs to be documented in update) vit is recommended to include a clause on the eventuality of a later change into the OR contracts ðin the absence of an agreement, a new registration dossier must be submitted 3/19/2018 26 26
Obligations of OR’s (3) Ø Change of OR vcan be done via an update under the condition that the earlier OR agrees (needs to be documented in update) vit is recommended to include a clause on the eventuality of a later change into the OR contracts ðin the absence of an agreement, a new registration dossier must be submitted 3/19/2018 26 26
 Obligations of OR’s (4) Ø Tonnage aggregation v. An only representative acting on behalf of several “non-Community manufacturers” must submit a separate registration for each of these manufacturers 3/19/2018 27 27
Obligations of OR’s (4) Ø Tonnage aggregation v. An only representative acting on behalf of several “non-Community manufacturers” must submit a separate registration for each of these manufacturers 3/19/2018 27 27
 Other involved Actors Ø When appointed an OR Ø Present Importers: » will be regarded as downstream users » need to establish clearly whether the quantity he receives is covered by OR registration • otherwise he may remain subject to registration obligations • written confirmation recommended » can prepare a DU CSA 3/19/2018 28 28
Other involved Actors Ø When appointed an OR Ø Present Importers: » will be regarded as downstream users » need to establish clearly whether the quantity he receives is covered by OR registration • otherwise he may remain subject to registration obligations • written confirmation recommended » can prepare a DU CSA 3/19/2018 28 28
 Terms and conditions OR (Principal) • Pay attention in the selection of a qualified OR to • Ownership • ØExpertise ØRemuneration ØVulnerability/risk factor Ø(Pre-)registration ØData Secure ownership by contract CBI ØProtection of CBI in case of an external OR? Secure CBI by contract ØJV agreements on CBI in case of a intracompany OR? Read your JV contract on CBI 29
Terms and conditions OR (Principal) • Pay attention in the selection of a qualified OR to • Ownership • ØExpertise ØRemuneration ØVulnerability/risk factor Ø(Pre-)registration ØData Secure ownership by contract CBI ØProtection of CBI in case of an external OR? Secure CBI by contract ØJV agreements on CBI in case of a intracompany OR? Read your JV contract on CBI 29
 • Contract or service level agreement Ø Ø • Service level agreement could be sufficient in case of an intracompany OR Contract is strictly needed in case of an external OR Duration of the cooperation Ø Ø Ø Consult your legal department Terms and conditions Forever? Agreed period? Termination If needed include a clause in the contract on the eventuality of a later change of the OR, otherwise the successor will have to submit a new registration dossier 30
• Contract or service level agreement Ø Ø • Service level agreement could be sufficient in case of an intracompany OR Contract is strictly needed in case of an external OR Duration of the cooperation Ø Ø Ø Consult your legal department Terms and conditions Forever? Agreed period? Termination If needed include a clause in the contract on the eventuality of a later change of the OR, otherwise the successor will have to submit a new registration dossier 30
 Terms and conditions (OR) Liability and Warranty ØOR is fully responsible for the (pre-)registration ØNon-EU company is responsible for the business ØOR is fully dependent on correct and up-to-date information of his principal ØIs the OR indemnified against any claims/penalties? ØOthers Make a contract with your principal 31
Terms and conditions (OR) Liability and Warranty ØOR is fully responsible for the (pre-)registration ØNon-EU company is responsible for the business ØOR is fully dependent on correct and up-to-date information of his principal ØIs the OR indemnified against any claims/penalties? ØOthers Make a contract with your principal 31
 Importer under REACH ü REACH requires each legal entity that is a Manufacturer or/and Importer to submit individually some parts of registration dossier, regardless of it being part of the group or a head office and its subsidiaries. REACH Importer: • Established in EU • Responsible for physical introduction » There is no provision in REACH to link importer for customs purposes and REACH • Companies have to assess their obligations • It is up to companies to determine which LE bears the REACH importers responsibility
Importer under REACH ü REACH requires each legal entity that is a Manufacturer or/and Importer to submit individually some parts of registration dossier, regardless of it being part of the group or a head office and its subsidiaries. REACH Importer: • Established in EU • Responsible for physical introduction » There is no provision in REACH to link importer for customs purposes and REACH • Companies have to assess their obligations • It is up to companies to determine which LE bears the REACH importers responsibility
 Third Party Representative • EU Manufacturer, Only Representative or Importer may appoint TPR to represent in SIEF, to prevent disclosure of company name
Third Party Representative • EU Manufacturer, Only Representative or Importer may appoint TPR to represent in SIEF, to prevent disclosure of company name
 How can Non –EU Manufacturer manage registrations? - - Non-EU manufacturer may choose: - Each Importer to register Appoint OR Importer - Pushes responsibility downstream Allows importers to source from different manufacturers Importer needs formulation information (CBI) Only Representative - Pushes responsibilty upstream Allows multiple routes to EU Importer becomes DU and cannot buy freely, only from registered sources CBI can remain with manufacturer 34
How can Non –EU Manufacturer manage registrations? - - Non-EU manufacturer may choose: - Each Importer to register Appoint OR Importer - Pushes responsibility downstream Allows importers to source from different manufacturers Importer needs formulation information (CBI) Only Representative - Pushes responsibilty upstream Allows multiple routes to EU Importer becomes DU and cannot buy freely, only from registered sources CBI can remain with manufacturer 34
 Next deadline 2013 Early estimates for 2013 registrations: • 3 500 substances – Number of substances revised downwards compared to 2007 estimates – Lessons from 2010 registration: less substances than initially estimated, especially intermediates – Same ratio applied to 2013 estimates • 13 300 new dossiers expected 35
Next deadline 2013 Early estimates for 2013 registrations: • 3 500 substances – Number of substances revised downwards compared to 2007 estimates – Lessons from 2010 registration: less substances than initially estimated, especially intermediates – Same ratio applied to 2013 estimates • 13 300 new dossiers expected 35
 Working is SIEFs • • • From pre-SIEF to SIEF (2010 experience) Difficult to form a SIEF and get the work started » » Use Substance Identity Profile (SIP) template Guidance on SIEF formation SFF not filling in its role » Recommendations to bypass Companies reluctant to take the LR role » » » Cefic guidance for LR Checklist SIEF tasks Obligations and liabilities of LR What are intentions of SIEF members? » Use SIEF codes: Leading, Involved, Passive and Dormant Consortia formation did help » Cefic model REACH SIEF agreement 36
Working is SIEFs • • • From pre-SIEF to SIEF (2010 experience) Difficult to form a SIEF and get the work started » » Use Substance Identity Profile (SIP) template Guidance on SIEF formation SFF not filling in its role » Recommendations to bypass Companies reluctant to take the LR role » » » Cefic guidance for LR Checklist SIEF tasks Obligations and liabilities of LR What are intentions of SIEF members? » Use SIEF codes: Leading, Involved, Passive and Dormant Consortia formation did help » Cefic model REACH SIEF agreement 36
 SIEFs: main challenges and learning's • • Need for an efficient SIEF management process Early, clear, transparent and regular communication to all SIEF members Determine substance sameness and communicate to all SIEF members C&L process Data availability check Agreement on LR and notification to ECHA » Cefic REACH model cooperation agreement Report progress status Joint/separate CSA/CSR 37
SIEFs: main challenges and learning's • • Need for an efficient SIEF management process Early, clear, transparent and regular communication to all SIEF members Determine substance sameness and communicate to all SIEF members C&L process Data availability check Agreement on LR and notification to ECHA » Cefic REACH model cooperation agreement Report progress status Joint/separate CSA/CSR 37
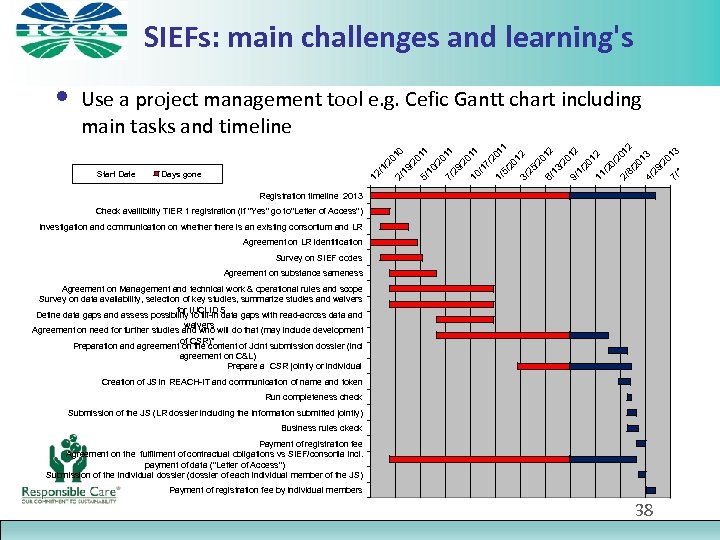 SIEFs: main challenges and learning's 01 1 20 12 3/ 25 /2 01 6/ 13 2 /2 01 9/ 2 1/ 20 12 11 /2 0/ 20 2/ 12 8/ 20 13 4/ 29 /2 01 7/ 18 3 /2 01 3 5/ 1/ 17 /2 20 11 10 / 29 / 20 11 7/ 10 / 20 11 19 / /2 Days gone 2/ 12 /1 Start Date 5/ Use a project management tool e. g. Cefic Gantt chart including main tasks and timeline 01 0 • Registration timeline 2013 Check availibility TIER 1 registration (if "Yes" go to"Letter of Access") Investigation and communication on whethere is an existing consortium and LR Agreement on LR identification Survey on SIEF codes Agreement on substance sameness Agreement on Management and technical work & operational rules and scope Survey on data availability, selection of key studies, summarize studies and waivers for IUCLID 5 Define data gaps and assess possibility to fill-in data gaps with read-across data and waivers Agreement on need for further studies and who will do that (may include development of CSR)* Preparation and agreement on the content of Joint submission dossier (incl agreement on C&L) Prepare a CSR jointly or individual Creation of JS in REACH-IT and communication of name and token Run completeness check Submission of the JS (LR dossier including the information submitted jointly) Business rules ckeck Payment of registration fee Agreement on the fulfilment of contractual obligations vs SIEF/consortia incl. payment of data ("Letter of Access") Submission of the individual dossier (dossier of each individual member of the JS) Payment of registration fee by individual members 38
SIEFs: main challenges and learning's 01 1 20 12 3/ 25 /2 01 6/ 13 2 /2 01 9/ 2 1/ 20 12 11 /2 0/ 20 2/ 12 8/ 20 13 4/ 29 /2 01 7/ 18 3 /2 01 3 5/ 1/ 17 /2 20 11 10 / 29 / 20 11 7/ 10 / 20 11 19 / /2 Days gone 2/ 12 /1 Start Date 5/ Use a project management tool e. g. Cefic Gantt chart including main tasks and timeline 01 0 • Registration timeline 2013 Check availibility TIER 1 registration (if "Yes" go to"Letter of Access") Investigation and communication on whethere is an existing consortium and LR Agreement on LR identification Survey on SIEF codes Agreement on substance sameness Agreement on Management and technical work & operational rules and scope Survey on data availability, selection of key studies, summarize studies and waivers for IUCLID 5 Define data gaps and assess possibility to fill-in data gaps with read-across data and waivers Agreement on need for further studies and who will do that (may include development of CSR)* Preparation and agreement on the content of Joint submission dossier (incl agreement on C&L) Prepare a CSR jointly or individual Creation of JS in REACH-IT and communication of name and token Run completeness check Submission of the JS (LR dossier including the information submitted jointly) Business rules ckeck Payment of registration fee Agreement on the fulfilment of contractual obligations vs SIEF/consortia incl. payment of data ("Letter of Access") Submission of the individual dossier (dossier of each individual member of the JS) Payment of registration fee by individual members 38
 SIEF: main challenges and learning’s Consistent and efficient communication » SIEF Management can be done by LR or outsourced to service providers e. g. Reach. Centrum » SIEF communication platform is key e. g. : LINKin. SIEF • New tool based on experience made during the previous phase • Focus on practicability and user friendliness • For LR: Flexible surveys and centralized document storage. Support for compliance/liabilities • For SIEF members: Free; one single point for access SIEF information in a simple & secured way 39
SIEF: main challenges and learning’s Consistent and efficient communication » SIEF Management can be done by LR or outsourced to service providers e. g. Reach. Centrum » SIEF communication platform is key e. g. : LINKin. SIEF • New tool based on experience made during the previous phase • Focus on practicability and user friendliness • For LR: Flexible surveys and centralized document storage. Support for compliance/liabilities • For SIEF members: Free; one single point for access SIEF information in a simple & secured way 39
 SIEF: main challenges and learning’s Cost sharing • • Should be fair, transparent and non-discriminatory » » Pay for the data you need(tonnage band, intermediate…) Explanation of cost sharing system e. g. coverletter + early estimate if possible When will SIEF members get the data? Lo. A procedure Costs may include: • SIEF management • Preparation of IUCLID dossier, RSS, CSR • Generation of invoices, letters of access, etc. • Provision for reimbursement (with a threshold? ) Consult Cefic notes on cost sharing 40
SIEF: main challenges and learning’s Cost sharing • • Should be fair, transparent and non-discriminatory » » Pay for the data you need(tonnage band, intermediate…) Explanation of cost sharing system e. g. coverletter + early estimate if possible When will SIEF members get the data? Lo. A procedure Costs may include: • SIEF management • Preparation of IUCLID dossier, RSS, CSR • Generation of invoices, letters of access, etc. • Provision for reimbursement (with a threshold? ) Consult Cefic notes on cost sharing 40
 SIEF: main challenges and learning’s • • Handling new registrants in SIEF’s SIEF that have submitted dossier in 2010: » LR sends out note with procedure to follow and timing to SIEF members » LR to assure the latest SIEF membership from REACH IT • Transparent SIEF communication including • SIEF agreement remains key! » clear cost sharing rules » Scope of LR dossier (CSR jointly prepared? ) » Model agreement on Cefic web 41
SIEF: main challenges and learning’s • • Handling new registrants in SIEF’s SIEF that have submitted dossier in 2010: » LR sends out note with procedure to follow and timing to SIEF members » LR to assure the latest SIEF membership from REACH IT • Transparent SIEF communication including • SIEF agreement remains key! » clear cost sharing rules » Scope of LR dossier (CSR jointly prepared? ) » Model agreement on Cefic web 41
 2013 registration: challenges New SIEFs for 2013 will look different: ü Expect fewer substances will be handled in consortia ü Smaller companies will require registrations ü less experienced? ü less resources? ü SIEF management challenges will become even more important ü Substances are likely to be less data rich ü Average cost per dossier could go up ü Less experienced Lead Registrants to manage SIEFs 42
2013 registration: challenges New SIEFs for 2013 will look different: ü Expect fewer substances will be handled in consortia ü Smaller companies will require registrations ü less experienced? ü less resources? ü SIEF management challenges will become even more important ü Substances are likely to be less data rich ü Average cost per dossier could go up ü Less experienced Lead Registrants to manage SIEFs 42
 2013 registration: challenges New registrants: üCheck if substance has been registered and contact LR/consortium üIf not registered yet, consider forming SIEF leadership team üIf needed, do careful selection of service provider üDo pre-SIEF steps as soon as possible. Don’t loose time with SIP and agreement üUse documents and tools on Cefic website http: //www. cefic. org/Industry-support/Implementing-reach/ üUse best practices and tools, don’t invent the wheel again! 43
2013 registration: challenges New registrants: üCheck if substance has been registered and contact LR/consortium üIf not registered yet, consider forming SIEF leadership team üIf needed, do careful selection of service provider üDo pre-SIEF steps as soon as possible. Don’t loose time with SIP and agreement üUse documents and tools on Cefic website http: //www. cefic. org/Industry-support/Implementing-reach/ üUse best practices and tools, don’t invent the wheel again! 43
 For further information • ECHA Guidance on data sharing: • http: //reach. jrc. it/docs/guidance_document/data _sharing_en. pdf • Cefic Guidance’s: • http: //www. cefic. org/Templates/shw. Story. asp? NI D=494&HID=645&PHID=643&PPHID=494 44
For further information • ECHA Guidance on data sharing: • http: //reach. jrc. it/docs/guidance_document/data _sharing_en. pdf • Cefic Guidance’s: • http: //www. cefic. org/Templates/shw. Story. asp? NI D=494&HID=645&PHID=643&PPHID=494 44
 Thank You! Leo Heezen (reachheezen@caiway. net) 45
Thank You! Leo Heezen (reachheezen@caiway. net) 45


