36017ea2bc27fb398f9eb849120b69fb.ppt
- Количество слайдов: 39
 Lessons Learned in Integrating Risk Management and Process Validation Medical Device Congress Harvard – March 2007 Jim Handzo – Senior Manager QA Innovative Spinal Technologies Fran Akelewicz – Principal Practical Solutions
Lessons Learned in Integrating Risk Management and Process Validation Medical Device Congress Harvard – March 2007 Jim Handzo – Senior Manager QA Innovative Spinal Technologies Fran Akelewicz – Principal Practical Solutions
 Agenda Optimizing the validation activities sequence using risk management n Determining the statistical approach and acceptance criteria based on risk n Maximizing test plans and resources n
Agenda Optimizing the validation activities sequence using risk management n Determining the statistical approach and acceptance criteria based on risk n Maximizing test plans and resources n
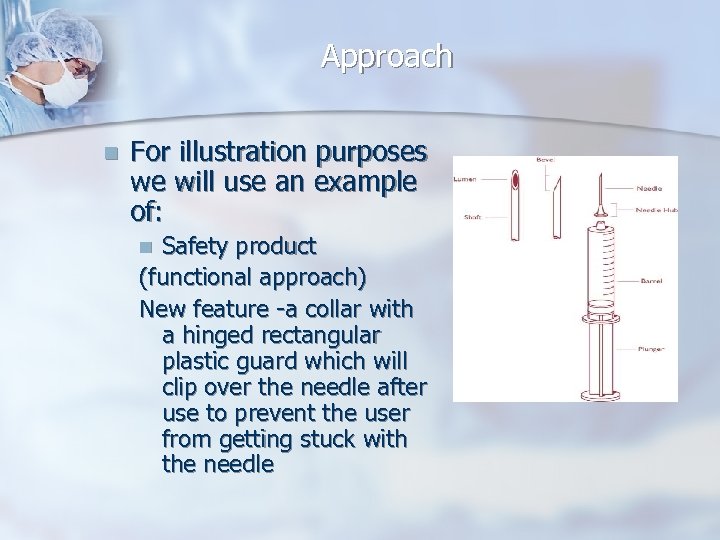 Approach n For illustration purposes we will use an example of: Safety product (functional approach) New feature -a collar with a hinged rectangular plastic guard which will clip over the needle after use to prevent the user from getting stuck with the needle n
Approach n For illustration purposes we will use an example of: Safety product (functional approach) New feature -a collar with a hinged rectangular plastic guard which will clip over the needle after use to prevent the user from getting stuck with the needle n
 Optimizing the validation activities sequence
Optimizing the validation activities sequence
 Key questions for process validation n n n What are the important design characteristics? Where and how are they impacted in the process? What resources do I need? What do I do first? How should I conduct the validation? What do I do if I don’t like the answer?
Key questions for process validation n n n What are the important design characteristics? Where and how are they impacted in the process? What resources do I need? What do I do first? How should I conduct the validation? What do I do if I don’t like the answer?
 What are the important design characteristics? n Product inputs to process validation n Key design features Residual risk profile (d. FMEA) Controls for the risk-prioritized design features
What are the important design characteristics? n Product inputs to process validation n Key design features Residual risk profile (d. FMEA) Controls for the risk-prioritized design features
 What are the important design characteristics? n Product Inputs (User and Patient) n Key Design Features n Tools n Customer Requirements Matrix n Design Requirements Matrix
What are the important design characteristics? n Product Inputs (User and Patient) n Key Design Features n Tools n Customer Requirements Matrix n Design Requirements Matrix
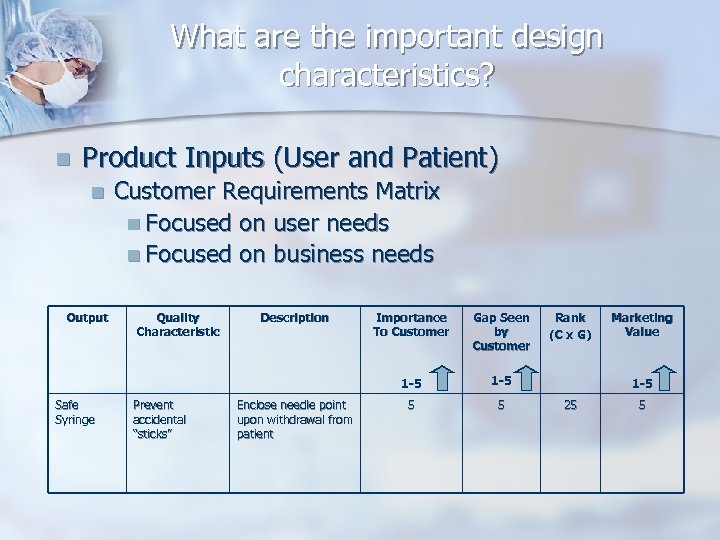 What are the important design characteristics? n Product Inputs (User and Patient) n Output Customer Requirements Matrix n Focused on user needs n Focused on business needs Prevent accidental “sticks” Description Enclose needle point upon withdrawal from patient Importance To Customer Gap Seen by Customer 1 -5 Safe Syringe Quality Characteristic 1 -5 5 5 Rank (C x G) Marketing Value 1 -5 25 5
What are the important design characteristics? n Product Inputs (User and Patient) n Output Customer Requirements Matrix n Focused on user needs n Focused on business needs Prevent accidental “sticks” Description Enclose needle point upon withdrawal from patient Importance To Customer Gap Seen by Customer 1 -5 Safe Syringe Quality Characteristic 1 -5 5 5 Rank (C x G) Marketing Value 1 -5 25 5
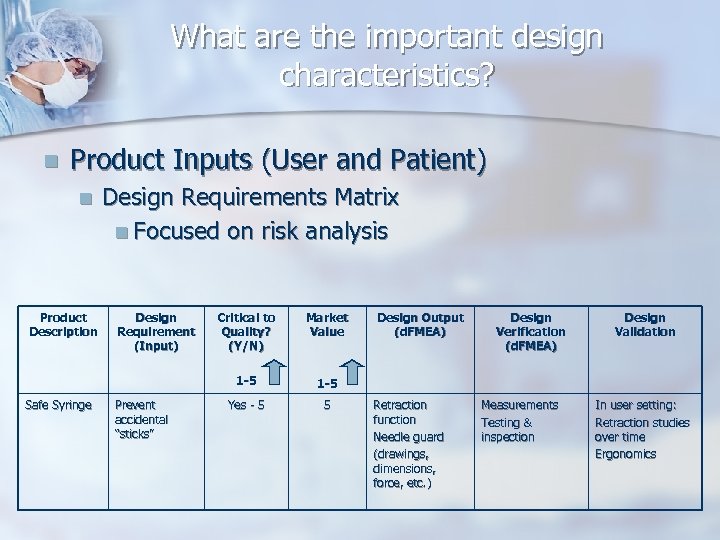 What are the important design characteristics? n Product Inputs (User and Patient) n Product Description Design Requirements Matrix n Focused on risk analysis Prevent accidental “sticks” Critical to Quality? (Y/N) Market Value 1 -5 Safe Syringe Design Requirement (Input) 1 -5 Yes - 5 5 Design Output (d. FMEA) Retraction function Needle guard (drawings, dimensions, force, etc. ) Design Verification (d. FMEA) Measurements Testing & inspection Design Validation In user setting: Retraction studies over time Ergonomics
What are the important design characteristics? n Product Inputs (User and Patient) n Product Description Design Requirements Matrix n Focused on risk analysis Prevent accidental “sticks” Critical to Quality? (Y/N) Market Value 1 -5 Safe Syringe Design Requirement (Input) 1 -5 Yes - 5 5 Design Output (d. FMEA) Retraction function Needle guard (drawings, dimensions, force, etc. ) Design Verification (d. FMEA) Measurements Testing & inspection Design Validation In user setting: Retraction studies over time Ergonomics
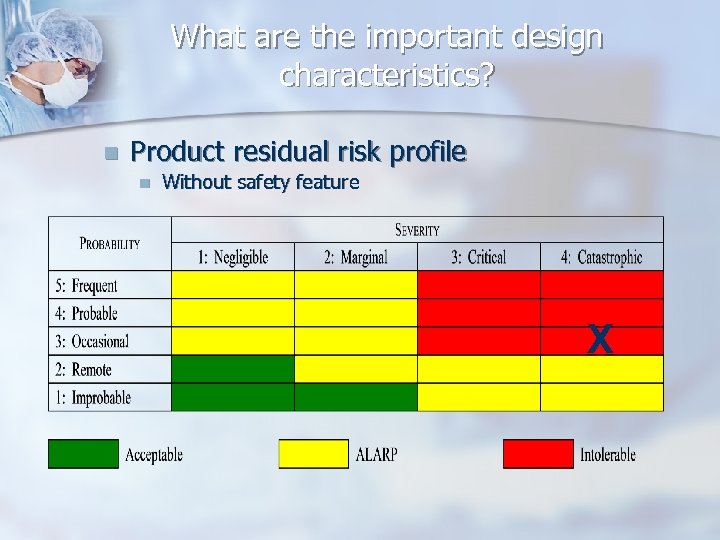 What are the important design characteristics? n Product residual risk profile n Without safety feature X
What are the important design characteristics? n Product residual risk profile n Without safety feature X
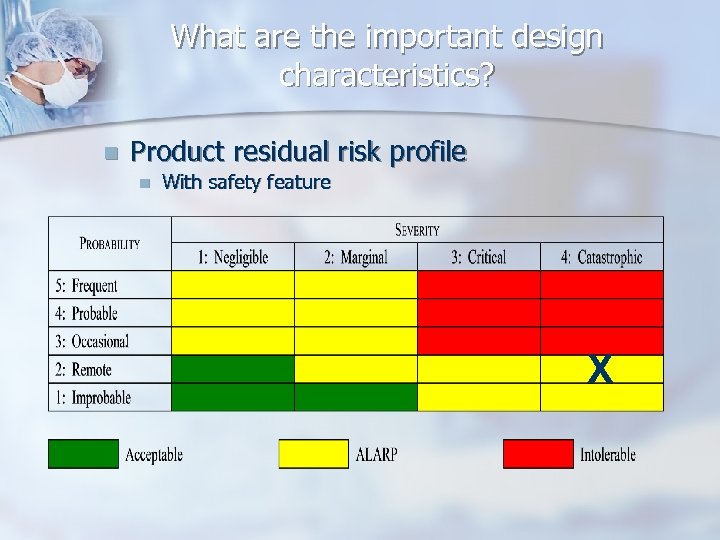 What are the important design characteristics? n Product residual risk profile n With safety feature X
What are the important design characteristics? n Product residual risk profile n With safety feature X
 Where and how are key design features impacted in the process? n Process Inputs n Determine process control points that potentially impact the design features n Are process control points One to one? n One to many? n Many to one? n n Determine residual risk impact (p. FMEA, FTA, etc. )
Where and how are key design features impacted in the process? n Process Inputs n Determine process control points that potentially impact the design features n Are process control points One to one? n One to many? n Many to one? n n Determine residual risk impact (p. FMEA, FTA, etc. )
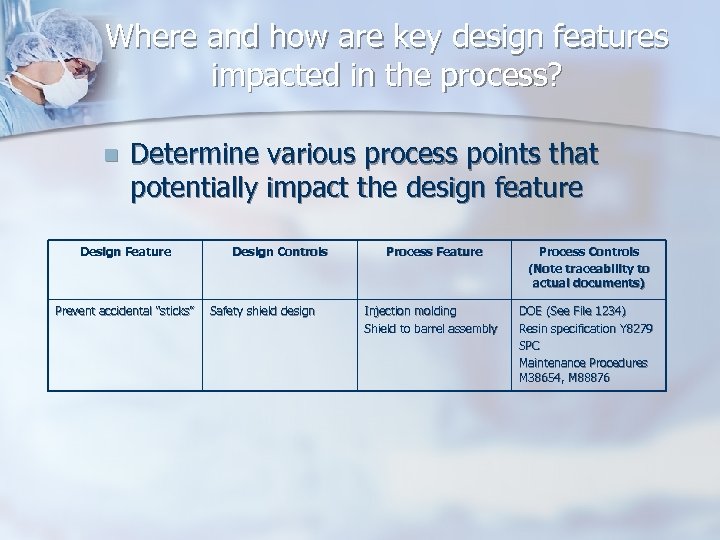 Where and how are key design features impacted in the process? n Determine various process points that potentially impact the design feature Design Feature Prevent accidental “sticks” Design Controls Safety shield design Process Feature Injection molding Shield to barrel assembly Process Controls (Note traceability to actual documents) DOE (See File 1234) Resin specification Y 8279 SPC Maintenance Procedures M 38654, M 88876
Where and how are key design features impacted in the process? n Determine various process points that potentially impact the design feature Design Feature Prevent accidental “sticks” Design Controls Safety shield design Process Feature Injection molding Shield to barrel assembly Process Controls (Note traceability to actual documents) DOE (See File 1234) Resin specification Y 8279 SPC Maintenance Procedures M 38654, M 88876
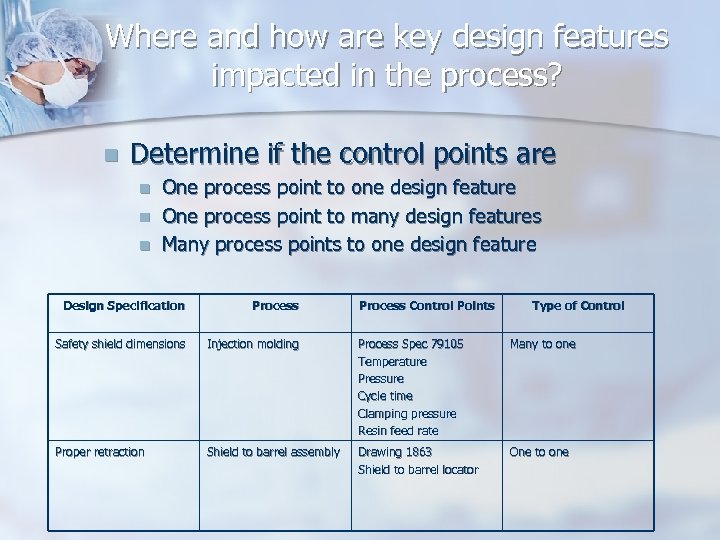 Where and how are key design features impacted in the process? n Determine if the control points are n n n One process point to one design feature One process point to many design features Many process points to one design feature Design Specification Process Control Points Type of Control Safety shield dimensions Injection molding Process Spec 79105 Temperature Pressure Cycle time Clamping pressure Resin feed rate Many to one Proper retraction Shield to barrel assembly Drawing 1863 Shield to barrel locator One to one
Where and how are key design features impacted in the process? n Determine if the control points are n n n One process point to one design feature One process point to many design features Many process points to one design feature Design Specification Process Control Points Type of Control Safety shield dimensions Injection molding Process Spec 79105 Temperature Pressure Cycle time Clamping pressure Resin feed rate Many to one Proper retraction Shield to barrel assembly Drawing 1863 Shield to barrel locator One to one
 Where and how are key design features impacted in the process? n Use tools to characterize process outputs vs. design features n n n Design of Experiments (DOE) Edge of Failure (EOF) Studies Ranging Studies Mean Time between Failures (MTBF) Cpk vs. Ppk Determine process residual risk n n Process Map & p. FMEA FTA Waterfall with Feedback Loop EN 60601 -1 -4 PEMS “V” Diagram
Where and how are key design features impacted in the process? n Use tools to characterize process outputs vs. design features n n n Design of Experiments (DOE) Edge of Failure (EOF) Studies Ranging Studies Mean Time between Failures (MTBF) Cpk vs. Ppk Determine process residual risk n n Process Map & p. FMEA FTA Waterfall with Feedback Loop EN 60601 -1 -4 PEMS “V” Diagram
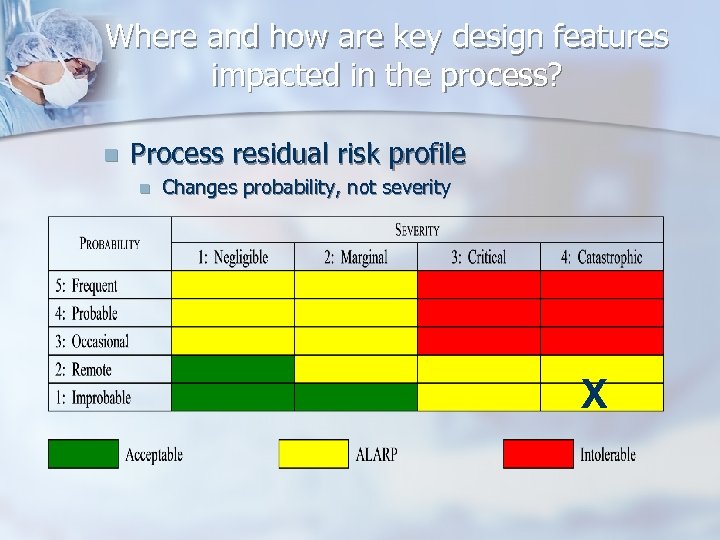 Where and how are key design features impacted in the process? n Process residual risk profile n Changes probability, not severity X
Where and how are key design features impacted in the process? n Process residual risk profile n Changes probability, not severity X
 What resources do I need? n n Dependent on type of product and/or process being evaluated Electrical Engineer n n Software Engineer Reliability Engineer Quality Engineer Commensurate with the degree of residual risk
What resources do I need? n n Dependent on type of product and/or process being evaluated Electrical Engineer n n Software Engineer Reliability Engineer Quality Engineer Commensurate with the degree of residual risk
 What do I do first? n Develop Process Validation Priority Risk Chart n n Similar methodology to Product Risk Chart Process effects on product are rated based on their impact Safety n Performance n Acceptance n Compliance n Verification n n Can also be used to develop a company Validation Master Plan
What do I do first? n Develop Process Validation Priority Risk Chart n n Similar methodology to Product Risk Chart Process effects on product are rated based on their impact Safety n Performance n Acceptance n Compliance n Verification n n Can also be used to develop a company Validation Master Plan
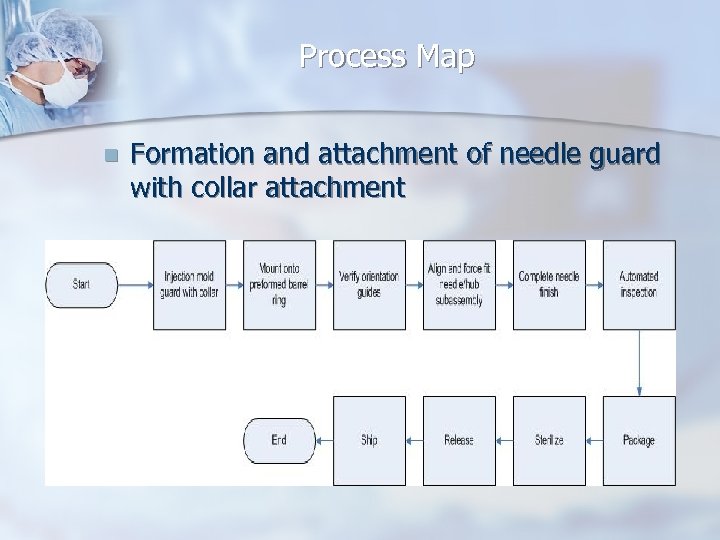 Process Map n Formation and attachment of needle guard with collar attachment
Process Map n Formation and attachment of needle guard with collar attachment
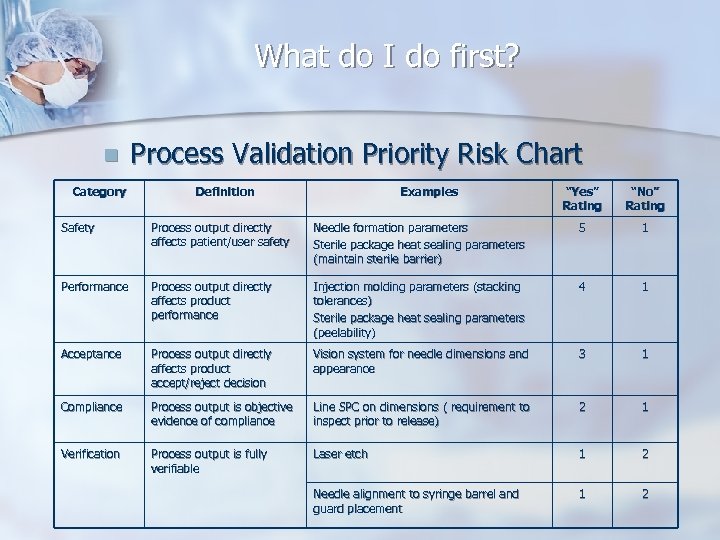 What do I do first? n Category Process Validation Priority Risk Chart Definition Examples “Yes” Rating “No” Rating Safety Process output directly affects patient/user safety Needle formation parameters Sterile package heat sealing parameters (maintain sterile barrier) 5 1 Performance Process output directly affects product performance Injection molding parameters (stacking tolerances) Sterile package heat sealing parameters (peelability) 4 1 Acceptance Process output directly affects product accept/reject decision Vision system for needle dimensions and appearance 3 1 Compliance Process output is objective evidence of compliance Line SPC on dimensions ( requirement to inspect prior to release) 2 1 Verification Process output is fully verifiable Laser etch 1 2 Needle alignment to syringe barrel and guard placement 1 2
What do I do first? n Category Process Validation Priority Risk Chart Definition Examples “Yes” Rating “No” Rating Safety Process output directly affects patient/user safety Needle formation parameters Sterile package heat sealing parameters (maintain sterile barrier) 5 1 Performance Process output directly affects product performance Injection molding parameters (stacking tolerances) Sterile package heat sealing parameters (peelability) 4 1 Acceptance Process output directly affects product accept/reject decision Vision system for needle dimensions and appearance 3 1 Compliance Process output is objective evidence of compliance Line SPC on dimensions ( requirement to inspect prior to release) 2 1 Verification Process output is fully verifiable Laser etch 1 2 Needle alignment to syringe barrel and guard placement 1 2
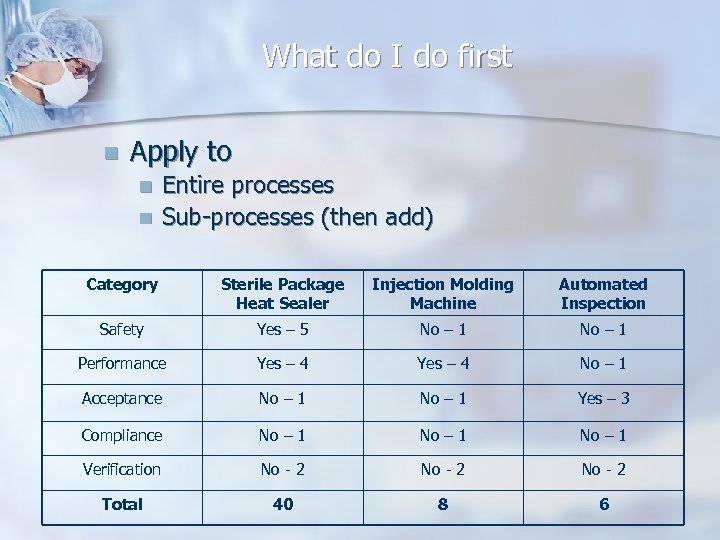 What do I do first n Apply to n n Entire processes Sub-processes (then add) Category Sterile Package Heat Sealer Injection Molding Machine Automated Inspection Safety Yes – 5 No – 1 Performance Yes – 4 No – 1 Acceptance No – 1 Yes – 3 Compliance No – 1 Verification No - 2 Total 40 8 6
What do I do first n Apply to n n Entire processes Sub-processes (then add) Category Sterile Package Heat Sealer Injection Molding Machine Automated Inspection Safety Yes – 5 No – 1 Performance Yes – 4 No – 1 Acceptance No – 1 Yes – 3 Compliance No – 1 Verification No - 2 Total 40 8 6
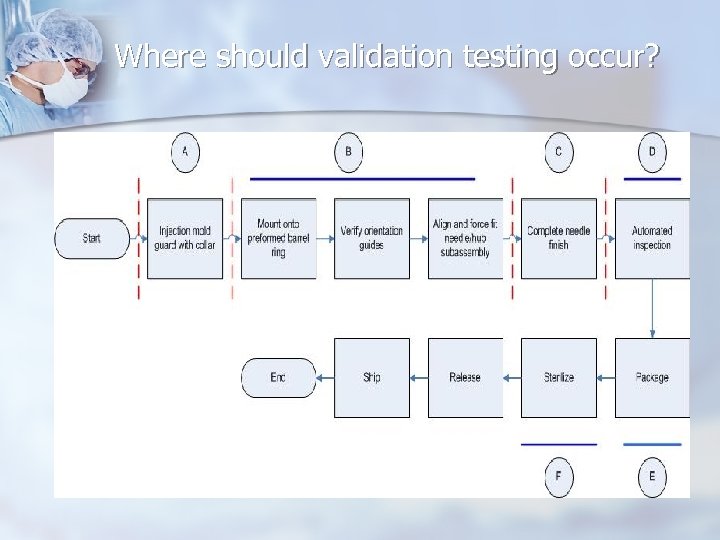 Where should validation testing occur?
Where should validation testing occur?
 What do I do if I don’t like the answer? n Have a “Plan B” n n Identify assignable causes n Justify discounting results with great care Revisit assumptions made during validation setup Revisit probabilities assigned to key design features n But you cannot change severity unless you change the design Define contingencies in acceptance criteria
What do I do if I don’t like the answer? n Have a “Plan B” n n Identify assignable causes n Justify discounting results with great care Revisit assumptions made during validation setup Revisit probabilities assigned to key design features n But you cannot change severity unless you change the design Define contingencies in acceptance criteria
 Determining the statistical approach and acceptance criteria based on risk
Determining the statistical approach and acceptance criteria based on risk
 Determining acceptance criteria n Document in company policy n n Similar to product risk chart Key elements derived from product risk chart n n Severity drives confidence levels Confidence levels and probability drive sampling plans Qualitative probabilities (Frequent, Probable, etc. ) must be expressed quantitatively n Sampling plans should be discriminating enough to detect defects that relate to high severity/frequently occurring hazards n
Determining acceptance criteria n Document in company policy n n Similar to product risk chart Key elements derived from product risk chart n n Severity drives confidence levels Confidence levels and probability drive sampling plans Qualitative probabilities (Frequent, Probable, etc. ) must be expressed quantitatively n Sampling plans should be discriminating enough to detect defects that relate to high severity/frequently occurring hazards n
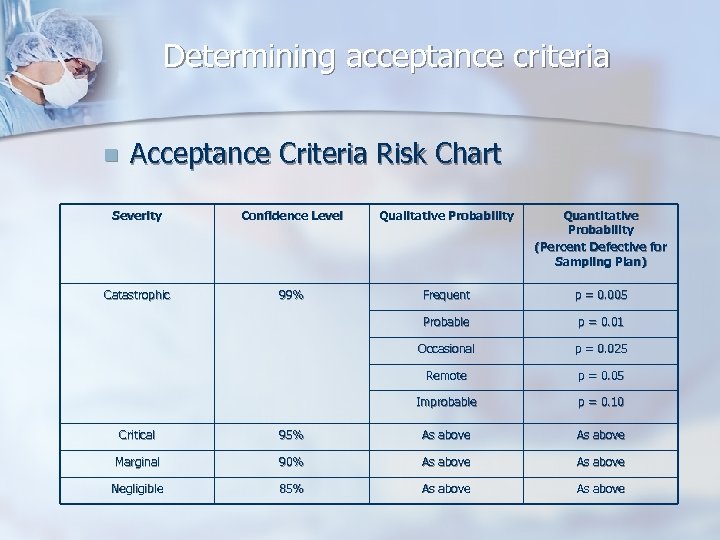 Determining acceptance criteria n Acceptance Criteria Risk Chart Severity Confidence Level Qualitative Probability Quantitative Probability (Percent Defective for Sampling Plan) Catastrophic 99% Frequent p = 0. 005 Probable p = 0. 01 Occasional p = 0. 025 Remote p = 0. 05 Improbable p = 0. 10 Critical 95% As above Marginal 90% As above Negligible 85% As above
Determining acceptance criteria n Acceptance Criteria Risk Chart Severity Confidence Level Qualitative Probability Quantitative Probability (Percent Defective for Sampling Plan) Catastrophic 99% Frequent p = 0. 005 Probable p = 0. 01 Occasional p = 0. 025 Remote p = 0. 05 Improbable p = 0. 10 Critical 95% As above Marginal 90% As above Negligible 85% As above
 Sampling plan determination n Determine the operating characteristic (OC) curve of selected sampling plan n n Incorporate quantitative probabilities Determine acceptable errors n Type I (Producer’s Risk) – we say it’s bad when it’s not n Type II (Consumer Risk) – we say it’s good when it’s not n Does it match residual risk and probability?
Sampling plan determination n Determine the operating characteristic (OC) curve of selected sampling plan n n Incorporate quantitative probabilities Determine acceptable errors n Type I (Producer’s Risk) – we say it’s bad when it’s not n Type II (Consumer Risk) – we say it’s good when it’s not n Does it match residual risk and probability?
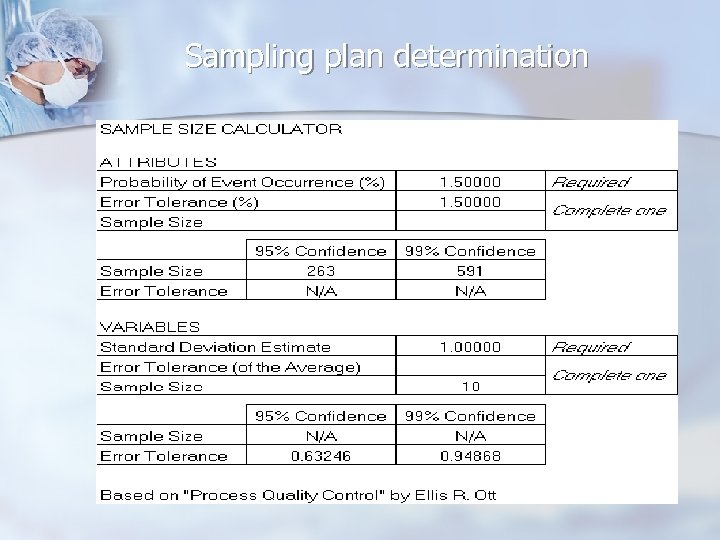
 Sampling plan determination n Determine how much risk (error) you can tolerate in your estimates n n n Attributes n Percent defective error tolerance Variables n Error tolerance of the average Must constantly weigh sample size vs. estimate risk (error) n Economics vs. certainty
Sampling plan determination n Determine how much risk (error) you can tolerate in your estimates n n n Attributes n Percent defective error tolerance Variables n Error tolerance of the average Must constantly weigh sample size vs. estimate risk (error) n Economics vs. certainty
 Sampling plan determination
Sampling plan determination
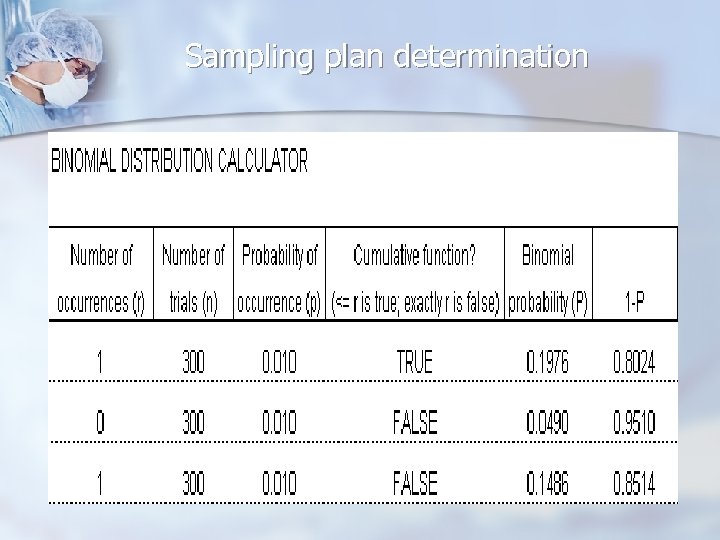
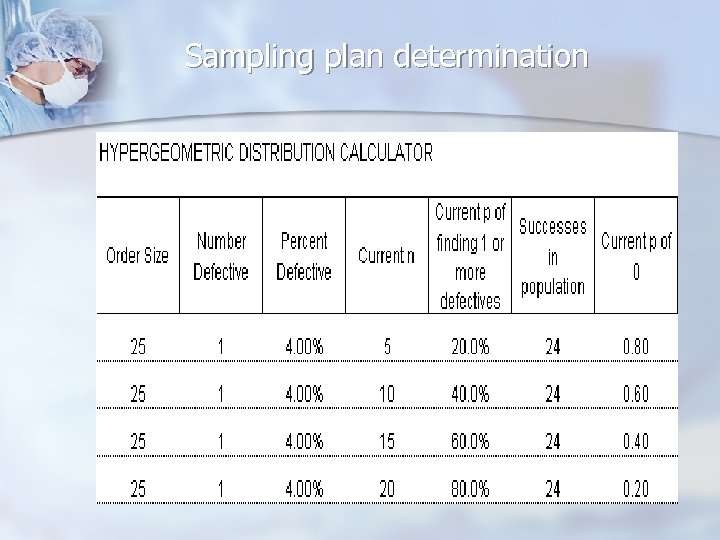
 Sampling plan determination n Use appropriate probability distributions when determining sampling plans n n Binomial n Large populations n Sampling with replacement Hypergeometric n Small populations n Sampling without replacement
Sampling plan determination n Use appropriate probability distributions when determining sampling plans n n Binomial n Large populations n Sampling with replacement Hypergeometric n Small populations n Sampling without replacement
 Sampling plan determination
Sampling plan determination
 Sampling plan determination
Sampling plan determination
 Sampling plan determination Sampling Calculators available for download at: www. devicecongress. com/agenda/day 2. html n (Once on the website, go to Track A, 4: 10 pm session, for links to presentation materials. Calculators are in the excel document. )
Sampling plan determination Sampling Calculators available for download at: www. devicecongress. com/agenda/day 2. html n (Once on the website, go to Track A, 4: 10 pm session, for links to presentation materials. Calculators are in the excel document. )
 Maximizing test plans and resources
Maximizing test plans and resources
 Maximizing Test Plans and Resources n n Identify resources based on risk Prioritize the process validation activities based on risk Choose the acceptance criteria Choose the appropriate sample methodology to minimize the business risk
Maximizing Test Plans and Resources n n Identify resources based on risk Prioritize the process validation activities based on risk Choose the acceptance criteria Choose the appropriate sample methodology to minimize the business risk
 Change Control n n Once process validation is completed you have established a “state of control” Any changes made should consider: n n n Impact to product design risk (design verification testing; design validation testing) Impact to process design risk (equipment design decisions) Impact to control of risk within process
Change Control n n Once process validation is completed you have established a “state of control” Any changes made should consider: n n n Impact to product design risk (design verification testing; design validation testing) Impact to process design risk (equipment design decisions) Impact to control of risk within process
 Conclusions n Risk management in process validation: n n n Provides the opportunity to: n challenge existing assumptions n “think out of the box” Acknowledges the risks that we have taken (product and business) Provides a common language Provides a framework for consistency Formalizes what we have done Contributes to safer products
Conclusions n Risk management in process validation: n n n Provides the opportunity to: n challenge existing assumptions n “think out of the box” Acknowledges the risks that we have taken (product and business) Provides a common language Provides a framework for consistency Formalizes what we have done Contributes to safer products
 Questions
Questions
 Thank You ! Jim Handzo Innovative Spinal Technologies +1 508 452 -3517 jhandzo@istspine. com www. istspine. com Fran Akelewicz Practical Solutions +1 215 337 -9238 franakelewicz@comcast. net www. practicalsolns. net
Thank You ! Jim Handzo Innovative Spinal Technologies +1 508 452 -3517 jhandzo@istspine. com www. istspine. com Fran Akelewicz Practical Solutions +1 215 337 -9238 franakelewicz@comcast. net www. practicalsolns. net


