a2d9eca77f6ee6639dadee672aa9232b.ppt
- Количество слайдов: 41

Lessons From The DIOR DCB Clinical Trials Dr Ian B. A. Menown MD FRCP Director, Interventional Cardiology, Craigavon Cardiac Centre, N. Ireland Hon. Senior Lecturer, Queen’s University

Relevant disclosures • Clinical trialist: Biosensors, Biotronik, Boston Scientific, Capella, Eurocor, Medtronic, Orbus Neich, Terumo • Conference sponsorship: Abbott, Boston, Biosensors, Eurocor, Medtronic 2
DIORII technology Clinical trial overview Key lessons learned 3

DIOR-II Technology • Shellac coating - well established as a food coating, tablet coating and in cosmetics • CE marked; safety recognised by FDA (GRAS). • Coating method – micropipette administered 1: 1 mixture of Paclitaxel (Ph Eur. ) and Shellac (Ph Eur. ) which results in homogenous surface distribution (<10% variability) • Paclitaxel balloon surface concentration: 3 µg/mm² • Drug hidden within balloon folds – helps protect from guide/proximal vessel wash-off:

DIOR-II Technology Shellac: high optical refraction (smooth, shiny surface)
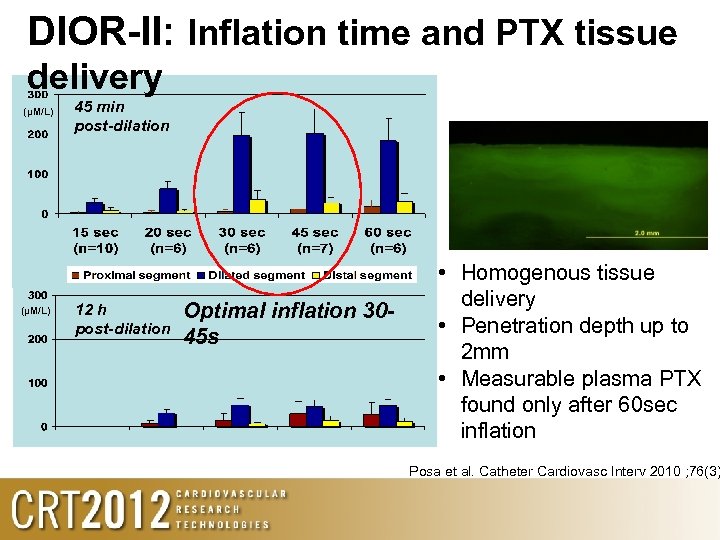
DIOR-II: Inflation time and PTX tissue delivery (µM/L) 45 min post-dilation 12 h post-dilation Optimal inflation 3045 s • Homogenous tissue delivery • Penetration depth up to 2 mm • Measurable plasma PTX found only after 60 sec inflation Posa et al. Catheter Cardiovasc Interv 2010 ; 76(3)
overview In-stent restenosis Small vessels Bifurcations AMI De-novo large vessels

Recommended PCI technique • Initial pre-dilatation balloon: – balloon: artery ratio 1: 1 – length 5 mm shorter than that of the DIOR balloon • DIOR dilatation (used to deliver PTX to the vessel wall): – balloon: artery ratio 1: 1 – dilatation pressure just above nominal – Inflation time 30 - 45 seconds (DIOR-II) • Angiographic success: – final residual lesion stenosis <30% in the target lesion 8
Valentines trial I: DIOR in ISR • Multi-centre, international registry study • 276 patients (244 with follow up), 96 centres, 26 countries, 50% on site monitoring • To assess the efficacy of Dior®-II for ISR following BMS or DES ISR. • “Snapshot enrollment” during 9 days (starting Valentine’s day, 14 th-23 rd Feb 2010 - CRT) • Online-CRF, data management and statistical analysis performed by MEDSTAR
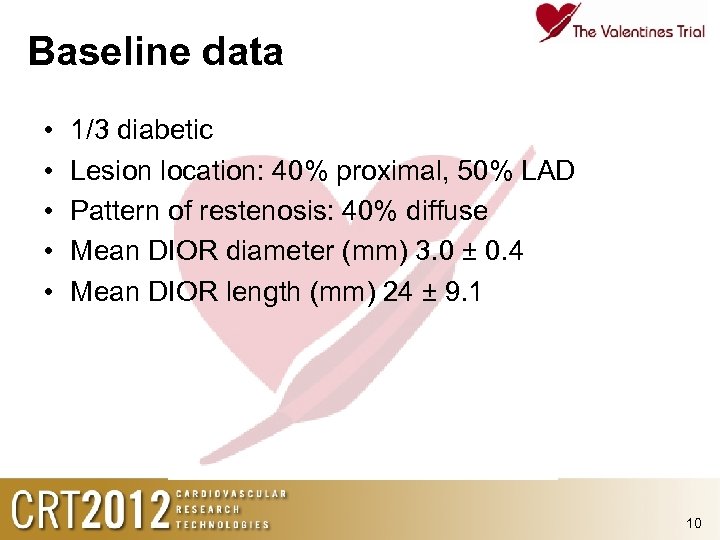
Baseline data • • • 1/3 diabetic Lesion location: 40% proximal, 50% LAD Pattern of restenosis: 40% diffuse Mean DIOR diameter (mm) 3. 0 ± 0. 4 Mean DIOR length (mm) 24 ± 9. 1 10
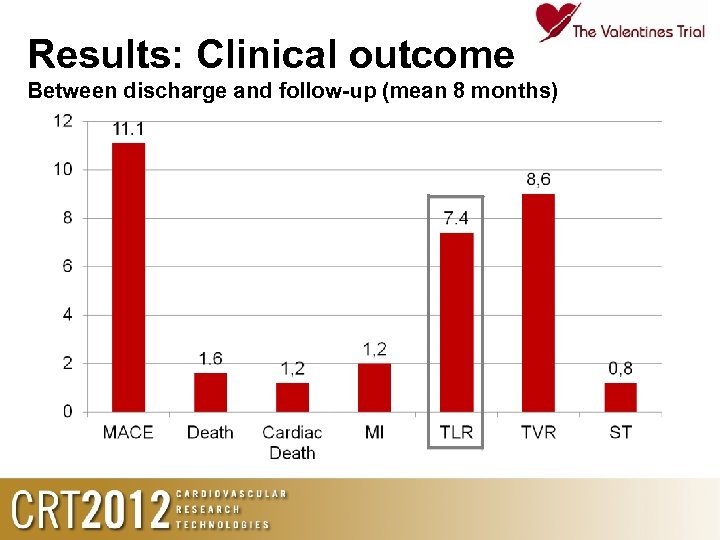
Results: Clinical outcome Between discharge and follow-up (mean 8 months)
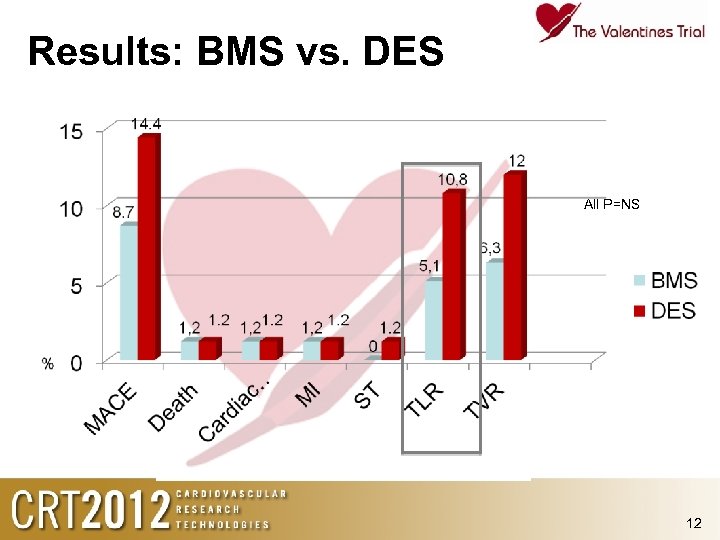
Results: BMS vs. DES All P=NS 12

Spanish DIOR Registry: n=250 In-stent restenosis (126 pts) De novo lesions in small vessels (<2. 5 mm) (103 pts ) Bifurcations excluding ISR or small vessels (21 pts) Vaquerizo B, Serra A et al. J Interv Cardiol. 2011; 24(6): 518 -28. Vaquerizo B, Serra A et al. In preparation 2012.
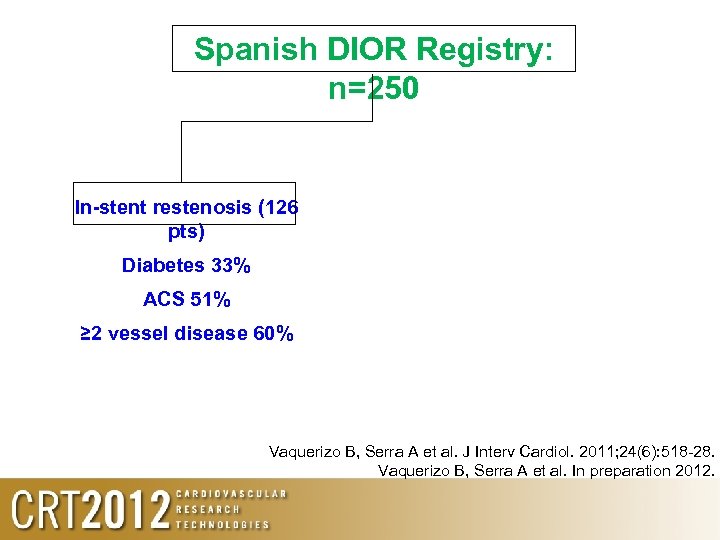
Spanish DIOR Registry: n=250 In-stent restenosis (126 pts) Diabetes 33% ACS 51% ≥ 2 vessel disease 60% Vaquerizo B, Serra A et al. J Interv Cardiol. 2011; 24(6): 518 -28. Vaquerizo B, Serra A et al. In preparation 2012.

• Pre-dilatation (plain balloon) Diameter, mm (mean SD) Length, mm (mean SD) • Dior Balloon Diameter, mm (mean SD) Length, mm (mean SD) Main balloon pressure, mm. Hg, (mean SD) 1 st Generation of Dior Unless specified otherwise, values are % and (n) of patients 100% 2. 6+/-0. 5 14. 3 +/-4. 5 2. 9+/-0. 4 19. 2+/-5. 5 14. 6+/-3. 4 69. 0 (87)
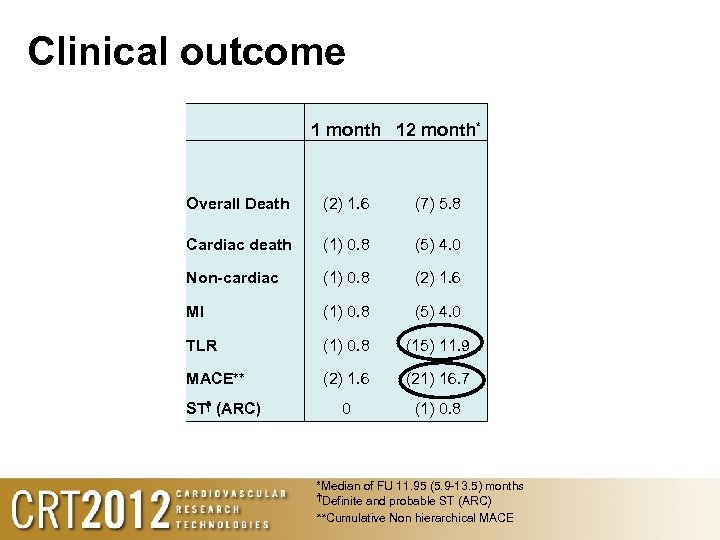
Clinical outcome 1 month 12 month* Overall Death (2) 1. 6 (7) 5. 8 Cardiac death (1) 0. 8 (5) 4. 0 Non-cardiac (1) 0. 8 (2) 1. 6 MI (1) 0. 8 (5) 4. 0 TLR (1) 0. 8 (15) 11. 9 MACE** (2) 1. 6 (21) 16. 7 0 (1) 0. 8 ST† (ARC) *Median of FU 11. 95 (5. 9 -13. 5) months †Definite and probable ST (ARC) **Cumulative Non hierarchical MACE
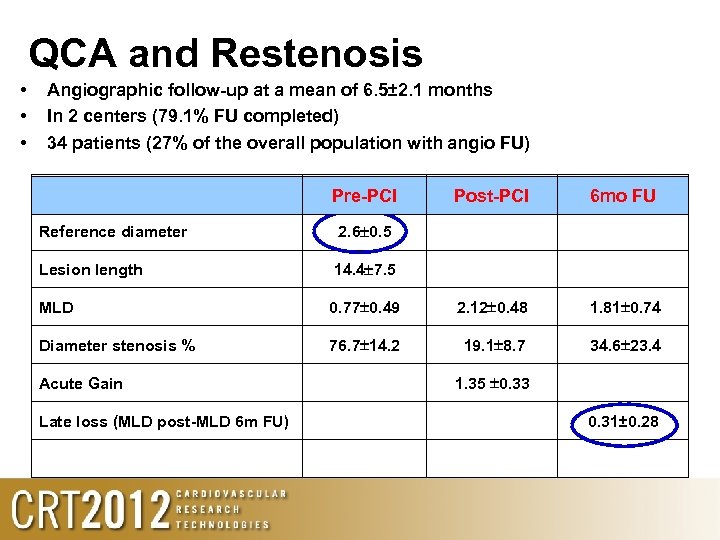
QCA and Restenosis • • • Angiographic follow-up at a mean of 6. 5± 2. 1 months In 2 centers (79. 1% FU completed) 34 patients (27% of the overall population with angio FU) Pre-PCI Post-PCI 6 mo FU Reference diameter 2. 6 0. 5 Lesion length 14. 4 7. 5 MLD 0. 77 0. 49 2. 12 0. 48 1. 81 0. 74 Diameter stenosis % 76. 7 14. 2 19. 1 8. 7 34. 6 23. 4 Acute Gain Late loss (MLD post-MLD 6 m FU) 1. 35 0. 33 0. 31± 0. 28
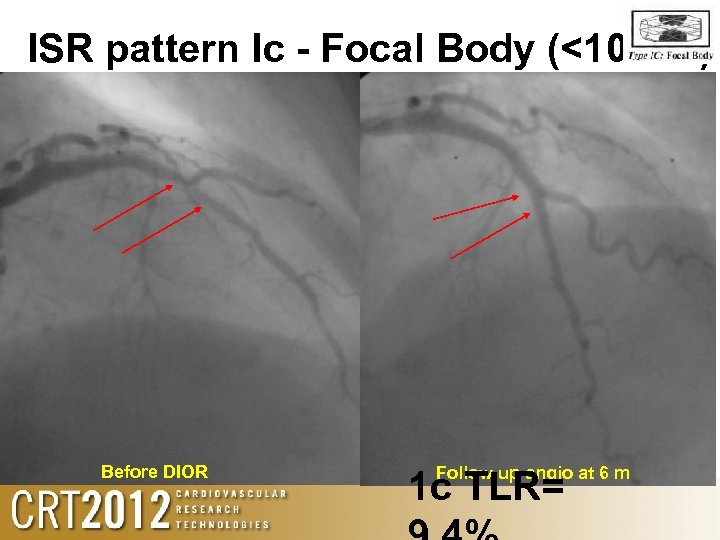
ISR pattern Ic - Focal Body (<10 mm). Before DIOR Follow up angio at 6 m 1 c TLR=

Spanish DIOR Registry: n=250 De novo lesions in small vessels (<2. 5 mm) (103 pts ) Vaquerizo B, Serra A et al. J Interv Cardiol. 2011; 24(6): 518 -28. Vaquerizo B, Serra A et al. In preparation 2012.
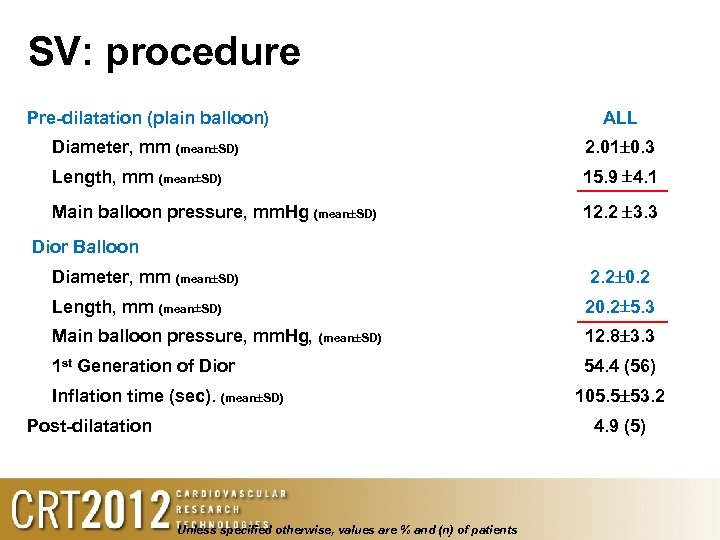
SV: procedure Pre-dilatation (plain balloon) ALL Diameter, mm (mean SD) 2. 01 0. 3 Length, mm (mean SD) 15. 9 4. 1 Main balloon pressure, mm. Hg (mean SD) 12. 2 3. 3 Dior Balloon Diameter, mm (mean SD) 2. 2 0. 2 Length, mm (mean SD) 20. 2 5. 3 Main balloon pressure, mm. Hg, (mean SD) 12. 8 3. 3 1 st Generation of Dior 54. 4 (56) Inflation time (sec). (mean SD) Post-dilatation 105. 5 53. 2 4. 9 (5) Unless specified otherwise, values are % and (n) of patients
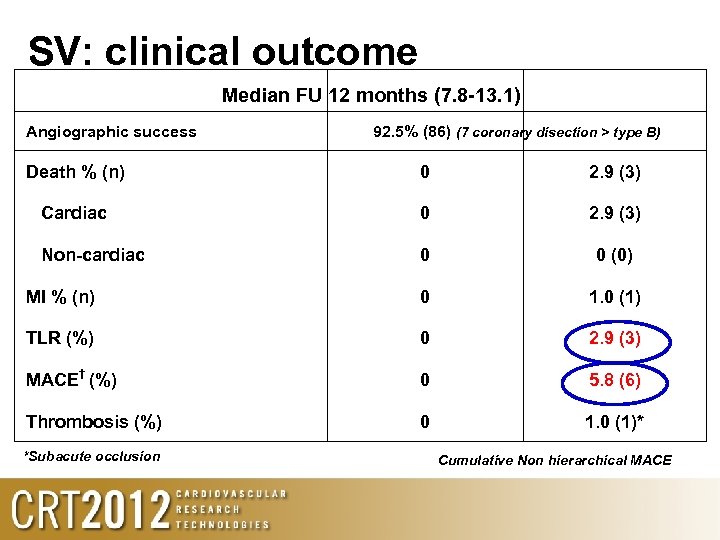
SV: clinical outcome Median FU 12 months (7. 8 -13. 1) Angiographic success 92. 5% (86) (7 coronary disection > type B) Death % (n) 0 2. 9 (3) Cardiac 0 2. 9 (3) Non-cardiac 0 0 (0) MI % (n) 0 1. 0 (1) TLR (%) 0 2. 9 (3) MACE† (%) 0 5. 8 (6) Thrombosis (%) 0 1. 0 (1)* *Subacute occlusion Cumulative Non hierarchical MACE
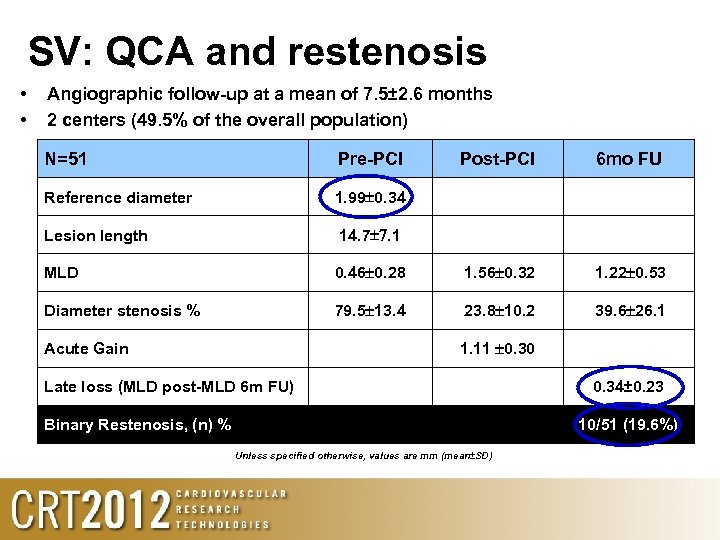
SV: QCA and restenosis • • Angiographic follow-up at a mean of 7. 5± 2. 6 months 2 centers (49. 5% of the overall population) N=51 Pre-PCI Reference diameter 1. 99 0. 34 Lesion length Post-PCI 6 mo FU 14. 7 7. 1 MLD 0. 46 0. 28 1. 56 0. 32 1. 22 0. 53 Diameter stenosis % 79. 5 13. 4 23. 8 10. 2 39. 6 26. 1 1. 11 0. 30 Acute Gain Late loss (MLD post-MLD 6 m FU) Binary Restenosis, (n) % 0. 34± 0. 23 10/51 (19. 6%) Unless specified otherwise, values are mm (mean±SD)
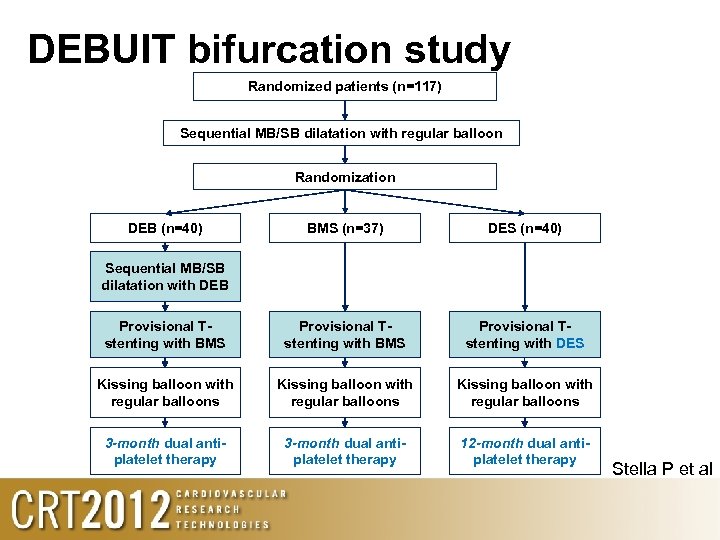
DEBUIT bifurcation study Randomized patients (n=117) Sequential MB/SB dilatation with regular balloon Randomization DEB (n=40) BMS (n=37) DES (n=40) Provisional Tstenting with BMS Provisional Tstenting with DES Kissing balloon with regular balloons 3 -month dual antiplatelet therapy 12 -month dual antiplatelet therapy Sequential MB/SB dilatation with DEB Stella P et al
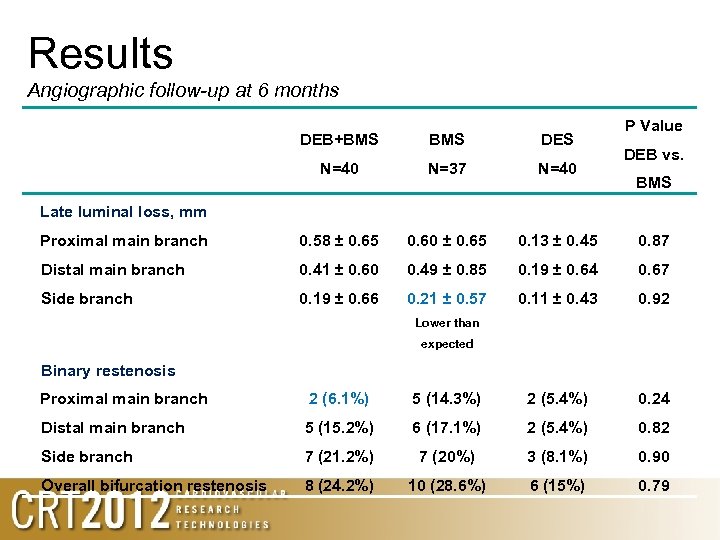
Results Angiographic follow-up at 6 months P Value DEB+BMS DES N=40 N=37 N=40 Proximal main branch 0. 58 ± 0. 65 0. 60 ± 0. 65 0. 13 ± 0. 45 0. 87 Distal main branch 0. 41 ± 0. 60 0. 49 ± 0. 85 0. 19 ± 0. 64 0. 67 Side branch 0. 19 ± 0. 66 0. 21 ± 0. 57 0. 11 ± 0. 43 0. 92 DEB vs. BMS Late luminal loss, mm Lower than expected Binary restenosis Proximal main branch 2 (6. 1%) 5 (14. 3%) 2 (5. 4%) 0. 24 Distal main branch 5 (15. 2%) 6 (17. 1%) 2 (5. 4%) 0. 82 Side branch 7 (21. 2%) 7 (20%) 3 (8. 1%) 0. 90 Overall bifurcation restenosis 8 (24. 2%) 10 (28. 6%) 6 (15%) 0. 79
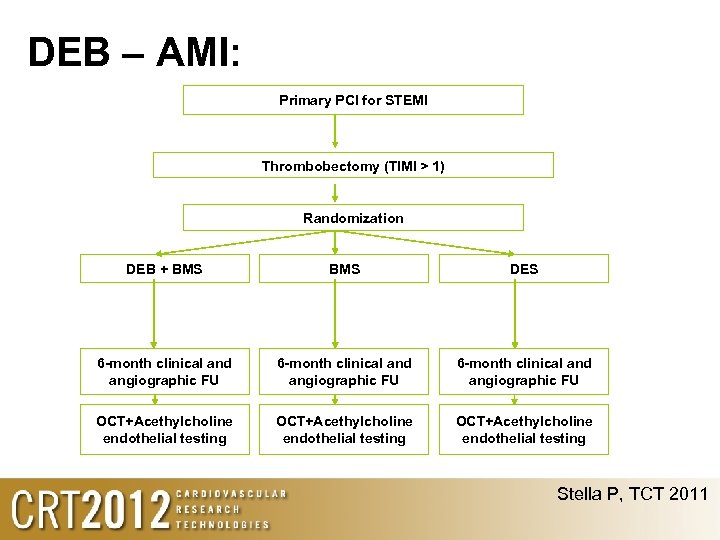
DEB – AMI: Primary PCI for STEMI Thrombobectomy (TIMI > 1) Randomization DEB + BMS DES 6 -month clinical and angiographic FU OCT+Acethylcholine endothelial testing Stella P, TCT 2011
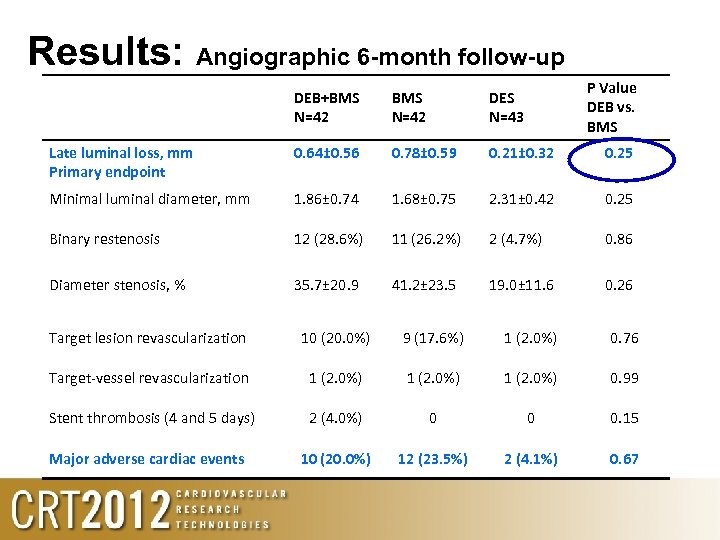
Results: Angiographic 6 -month follow-up P Value DEB vs. BMS DEB+BMS N=42 DES N=43 Late luminal loss, mm Primary endpoint 0. 64± 0. 56 0. 78± 0. 59 0. 21± 0. 32 0. 25 Minimal luminal diameter, mm 1. 86± 0. 74 1. 68± 0. 75 2. 31± 0. 42 0. 25 Binary restenosis 12 (28. 6%) 11 (26. 2%) 2 (4. 7%) 0. 86 Diameter stenosis, % 35. 7± 20. 9 41. 2± 23. 5 19. 0± 11. 6 0. 26 Target lesion revascularization 10 (20. 0%) 9 (17. 6%) 1 (2. 0%) 0. 76 Target-vessel revascularization 1 (2. 0%) 0. 99 Stent thrombosis (4 and 5 days) 2 (4. 0%) 0 0 0. 15 10 (20. 0%) 12 (23. 5%) 2 (4. 1%) 0. 67 Major adverse cardiac events
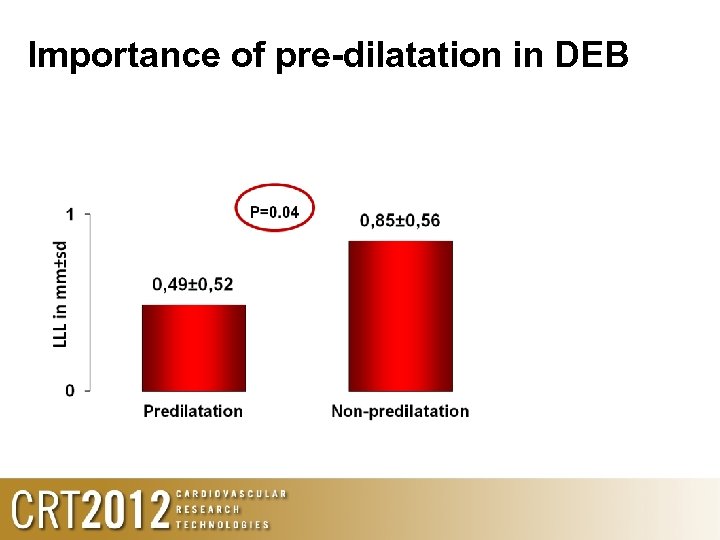
Importance of pre-dilatation in DEB
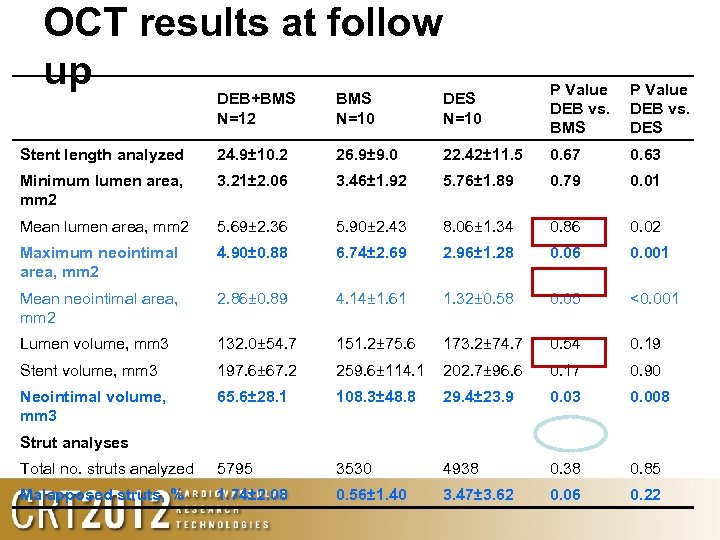
OCT results at follow up DEB+BMS N=12 BMS N=10 DES N=10 P Value DEB vs. BMS P Value DEB vs. DES Stent length analyzed 24. 9± 10. 2 26. 9± 9. 0 22. 42± 11. 5 0. 67 0. 63 Minimum lumen area, mm 2 3. 21± 2. 06 3. 46± 1. 92 5. 76± 1. 89 0. 79 0. 01 Mean lumen area, mm 2 5. 69± 2. 36 5. 90± 2. 43 8. 06± 1. 34 0. 86 0. 02 Maximum neointimal area, mm 2 4. 90± 0. 88 6. 74± 2. 69 2. 96± 1. 28 0. 06 0. 001 Mean neointimal area, mm 2 2. 86± 0. 89 4. 14± 1. 61 1. 32± 0. 58 0. 05 <0. 001 Lumen volume, mm 3 132. 0± 54. 7 151. 2± 75. 6 173. 2± 74. 7 0. 54 0. 19 Stent volume, mm 3 197. 6± 67. 2 259. 6± 114. 1 202. 7± 96. 6 0. 17 0. 90 Neointimal volume, mm 3 65. 6± 28. 1 108. 3± 48. 8 29. 4± 23. 9 0. 03 0. 008 Strut analyses Total no. struts analyzed 5795 3530 4938 0. 85 Malapposed struts, % 1. 74± 2. 08 0. 56± 1. 40 3. 47± 3. 62 0. 06 0. 22

DEAR Registry - Flow April 2009 to March 2011 1. 528 patients underwent PCI in 3 centres from Argentina, of whom 275 (18%) were diabetics PI: Alfredo Rodrigues (Buenos Aires, Argentina) Diabetics, de novo 92 pts consented Inability to cross lesion in one pt 91 pts (33. 1%) included 106 lesions; 60% LAD Mean DIOR 2. 5 mm diameter, length 24. 8 mm DIOR+BMS =96%; DIOR=4%
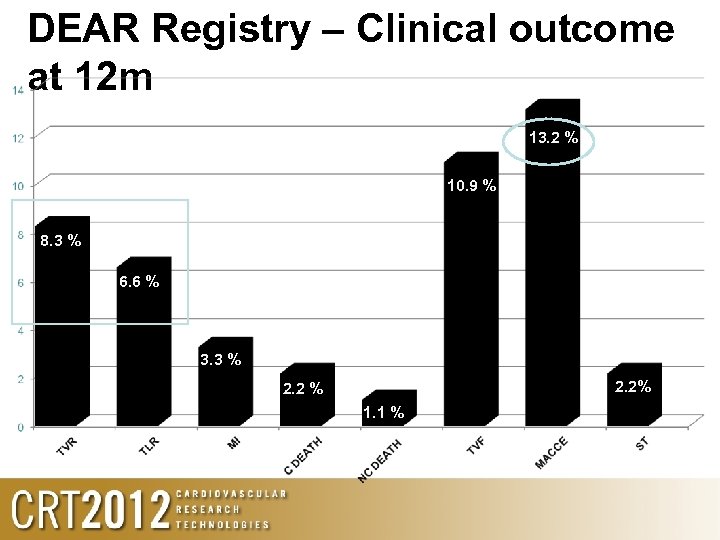
DEAR Registry – Clinical outcome at 12 m 13. 2 % 10. 9 % 8. 3 % 6. 6 % 3. 3 % 2. 2 % 1. 1 %
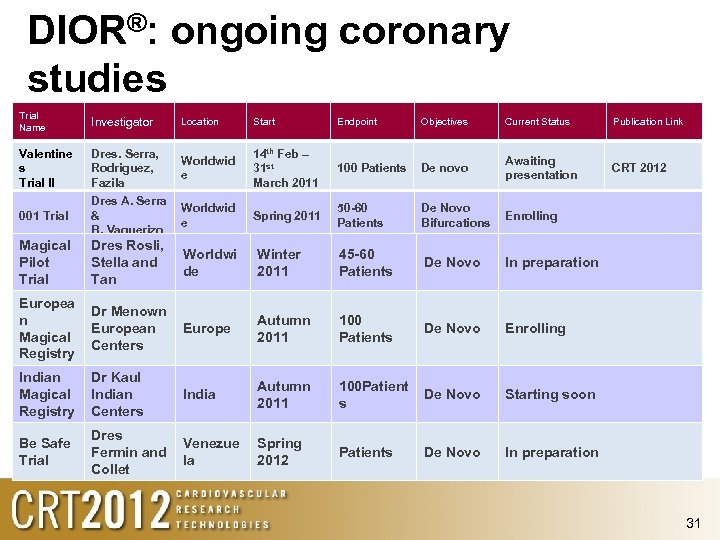
DIOR®: ongoing coronary studies Trial Name Investigator Location Start Endpoint Objectives Current Status Publication Link Valentine s Trial II Dres. Serra, Rodriguez, Fazila Worldwid e 14 th Feb – 31 st March 2011 100 Patients De novo Awaiting presentation CRT 2012 001 Trial Dres A. Serra & B. Vaquerizo Worldwid e Spring 2011 50 -60 Patients De Novo Bifurcations Enrolling Magical Pilot Trial Dres Rosli, Stella and Tan Worldwi de Winter 2011 45 -60 Patients De Novo In preparation Europea n Magical Registry Dr Menown European Centers Europe Autumn 2011 100 Patients De Novo Enrolling Indian Magical Registry Dr Kaul Indian Centers India Autumn 2011 100 Patient s De Novo Starting soon Be Safe Trial Dres Fermin and Collet Venezue la Spring 2012 Patients De Novo In preparation 31
Valentine II case: Initial
DIOR II (after predilatation)
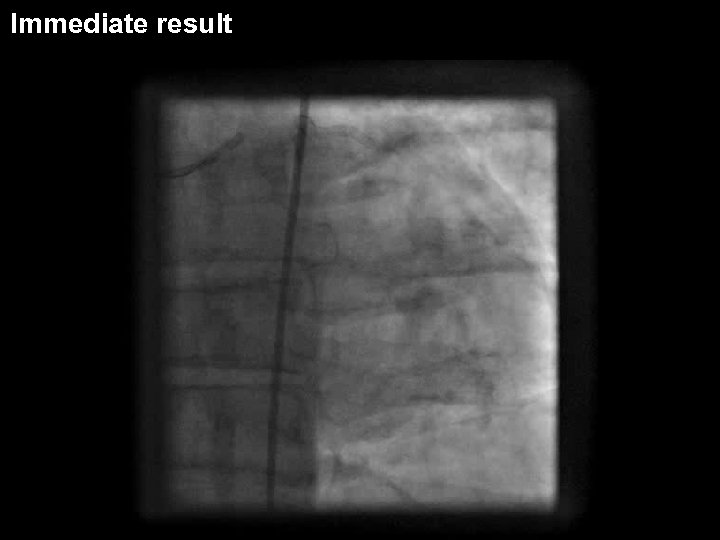
Immediate result
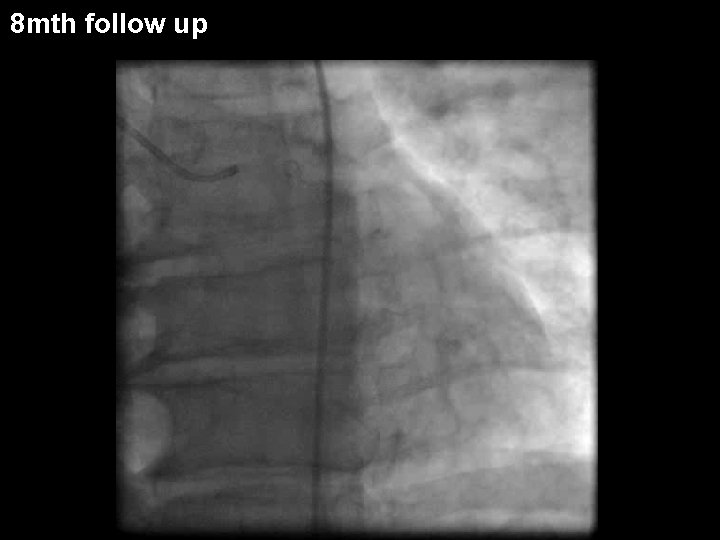
8 mth follow up
DIOR-II Key lessons learned 36

Lessons learned 1. Adequate lesion preparation (predilatation) before DIOR important to optimise results 2. Avoid geographic miss – aim to use a DIOR >5 mm longer than the pre-dilated segment 3. New DCB (DEB) designs/coatings/excipients can markedly improve the speed and volume of PTX delivery to the vessel wall. Optimum vessel

Lessons learned 4. Clinical indications Probably useful: – ISR, small vessel Possibly useful: bifurcation, AMI Larger vessel de-novo: under investigation



Potential advantages of DCB (DEB) 1. Short lived homogeneous local drug delivery: – highest drug concentrations in vessel wall at time of injury – Near complete absence of drug in vessel wall by ~30 days helps re-endothelialization and reduces required duration of antiplatelet therapy 2. Absence of permanent polymer: avoids chronic inflammation 3. Absence of a permanent stent: – preserves original vessel anatomy (e. g. bifurcation, tortuosity) – avoid double/triple metal layers (e. g. ISR or bifurcation) 1. easier initial device tracking and easier re-cross 2. avoids risk of stent fracture (e. g. at tortuosity) 41
a2d9eca77f6ee6639dadee672aa9232b.ppt