 Lesson 12. 1 Gases
Lesson 12. 1 Gases
 Density • density pepsi vs diet pepsi 3. 5 min • volume of a marble • density animation
Density • density pepsi vs diet pepsi 3. 5 min • volume of a marble • density animation
 What are the main physical properties of gases? • Gases assume the volume and shape of the container. • Gases are most compressible of states of matter. • Gases will mix evenly and completely when confined to same container. • Gases have much lower densities than liquids and solids.
What are the main physical properties of gases? • Gases assume the volume and shape of the container. • Gases are most compressible of states of matter. • Gases will mix evenly and completely when confined to same container. • Gases have much lower densities than liquids and solids.
 List the Gases Periodic Table in back of textbook Why diatomic?
List the Gases Periodic Table in back of textbook Why diatomic?
 Noble Gases • Single atoms • Complete outer valance shell • Octet rule is satisfied
Noble Gases • Single atoms • Complete outer valance shell • Octet rule is satisfied
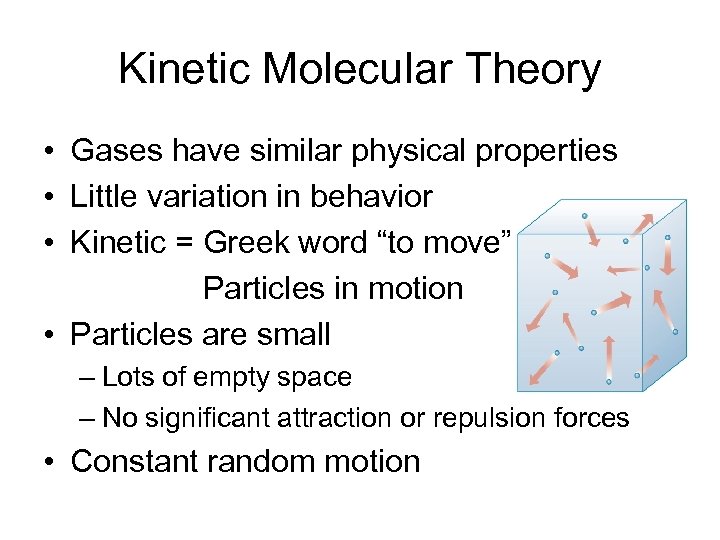 Kinetic Molecular Theory • Gases have similar physical properties • Little variation in behavior • Kinetic = Greek word “to move” Particles in motion • Particles are small – Lots of empty space – No significant attraction or repulsion forces • Constant random motion
Kinetic Molecular Theory • Gases have similar physical properties • Little variation in behavior • Kinetic = Greek word “to move” Particles in motion • Particles are small – Lots of empty space – No significant attraction or repulsion forces • Constant random motion
 Kinetic Energy = ½ mv 2 • Molecules travel in a straight line • Hit a wall or another molecule then Energy is transferred – no energy is lost • m is mass • v is velocity (speed and direction)
Kinetic Energy = ½ mv 2 • Molecules travel in a straight line • Hit a wall or another molecule then Energy is transferred – no energy is lost • m is mass • v is velocity (speed and direction)
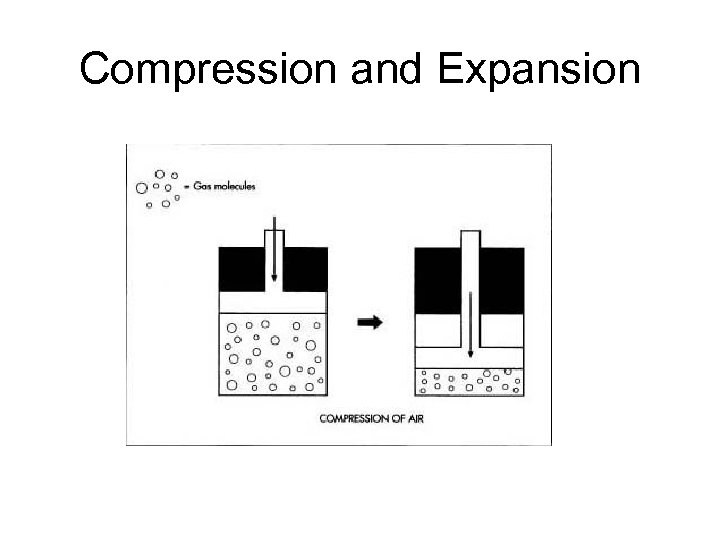 Compression and Expansion
Compression and Expansion
 Diffusion and Effusion • Diffusion – smell cooking throughout the house • Effusion – gas escapes from a small hole different rates depends on size • Smaller particles travel faster
Diffusion and Effusion • Diffusion – smell cooking throughout the house • Effusion – gas escapes from a small hole different rates depends on size • Smaller particles travel faster
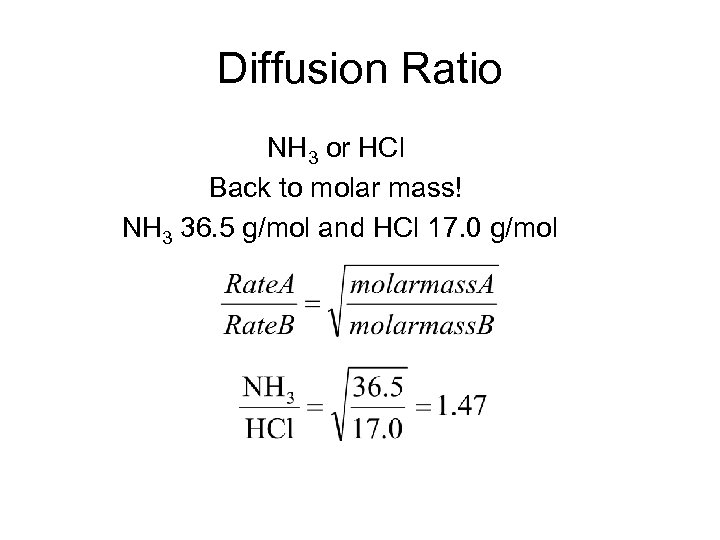 Diffusion Ratio NH 3 or HCl Back to molar mass! NH 3 36. 5 g/mol and HCl 17. 0 g/mol
Diffusion Ratio NH 3 or HCl Back to molar mass! NH 3 36. 5 g/mol and HCl 17. 0 g/mol
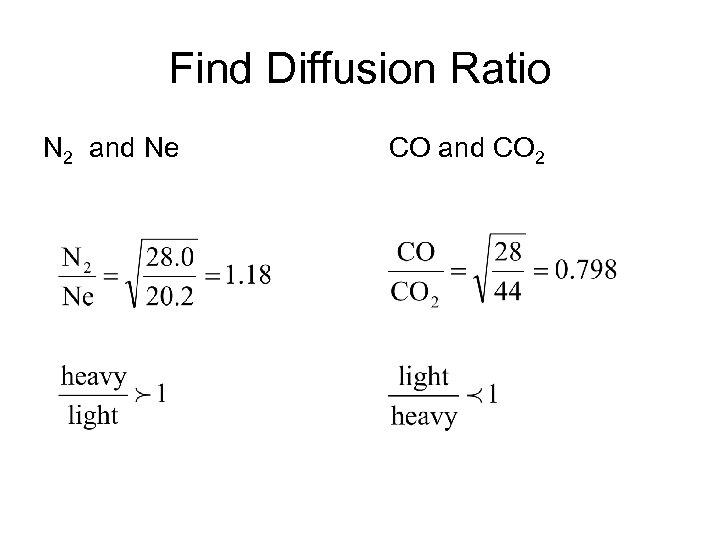 Find Diffusion Ratio N 2 and Ne CO and CO 2
Find Diffusion Ratio N 2 and Ne CO and CO 2
 Pressure Think about it Walk on deep snow with boots, snow shoes, skis. Which sink the most? Why?
Pressure Think about it Walk on deep snow with boots, snow shoes, skis. Which sink the most? Why?
 Pressure = force / unit of area The pressure in car or bicycle tires is measured in pounds per square inches. Car tires 26 -30 lb/sq. in. Bicycle tires 40 -60/sq. in.
Pressure = force / unit of area The pressure in car or bicycle tires is measured in pounds per square inches. Car tires 26 -30 lb/sq. in. Bicycle tires 40 -60/sq. in.
 Who will wear out the carpet first? 125 lbs woman wearing high heels? Or, same woman wearing flats? Why?
Who will wear out the carpet first? 125 lbs woman wearing high heels? Or, same woman wearing flats? Why?
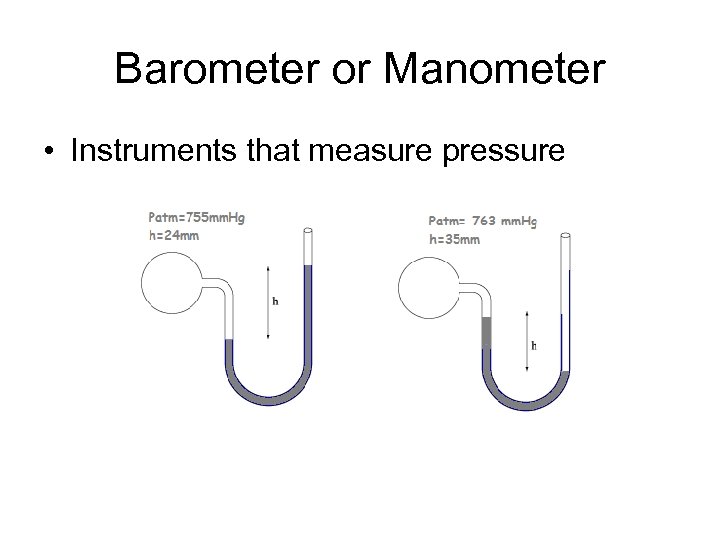 Barometer or Manometer • Instruments that measure pressure
Barometer or Manometer • Instruments that measure pressure
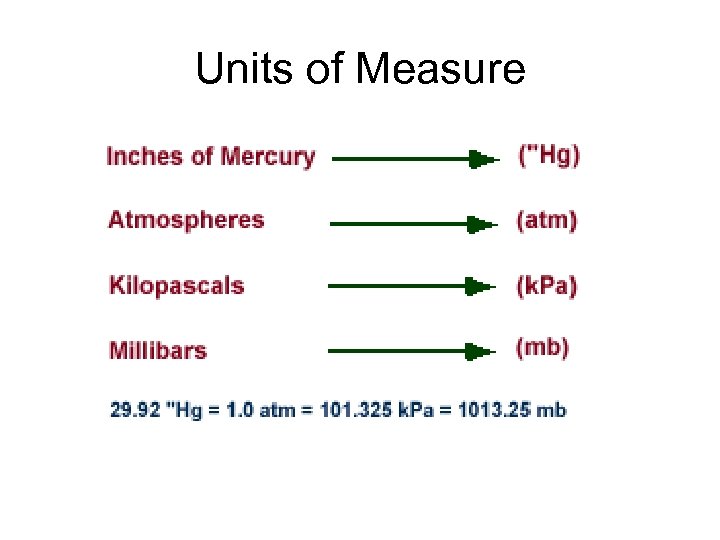 Units of Measure
Units of Measure
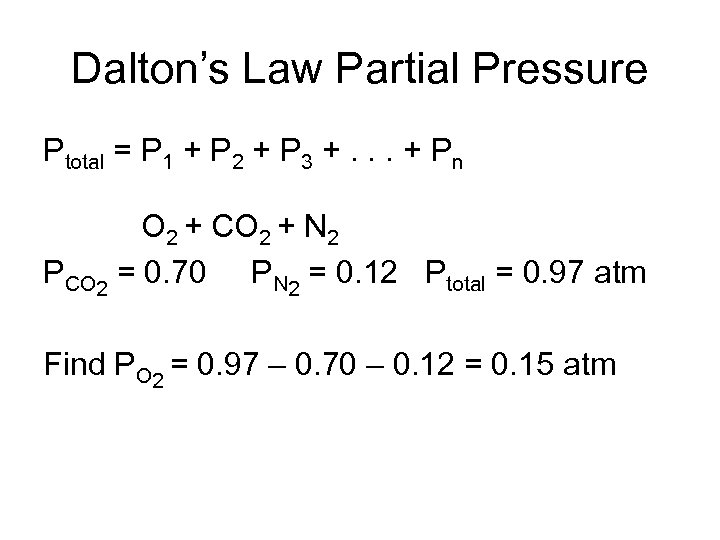 Dalton’s Law Partial Pressure Ptotal = P 1 + P 2 + P 3 +. . . + Pn O 2 + CO 2 + N 2 PCO 2 = 0. 70 PN 2 = 0. 12 Ptotal = 0. 97 atm Find PO 2 = 0. 97 – 0. 70 – 0. 12 = 0. 15 atm
Dalton’s Law Partial Pressure Ptotal = P 1 + P 2 + P 3 +. . . + Pn O 2 + CO 2 + N 2 PCO 2 = 0. 70 PN 2 = 0. 12 Ptotal = 0. 97 atm Find PO 2 = 0. 97 – 0. 70 – 0. 12 = 0. 15 atm
 Textbook page 409 # 4, 5, 6
Textbook page 409 # 4, 5, 6
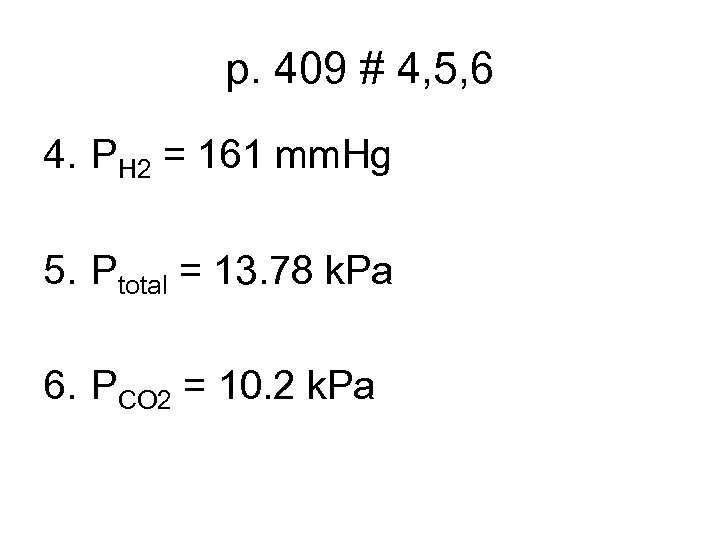 p. 409 # 4, 5, 6 4. PH 2 = 161 mm. Hg 5. Ptotal = 13. 78 k. Pa 6. PCO 2 = 10. 2 k. Pa
p. 409 # 4, 5, 6 4. PH 2 = 161 mm. Hg 5. Ptotal = 13. 78 k. Pa 6. PCO 2 = 10. 2 k. Pa
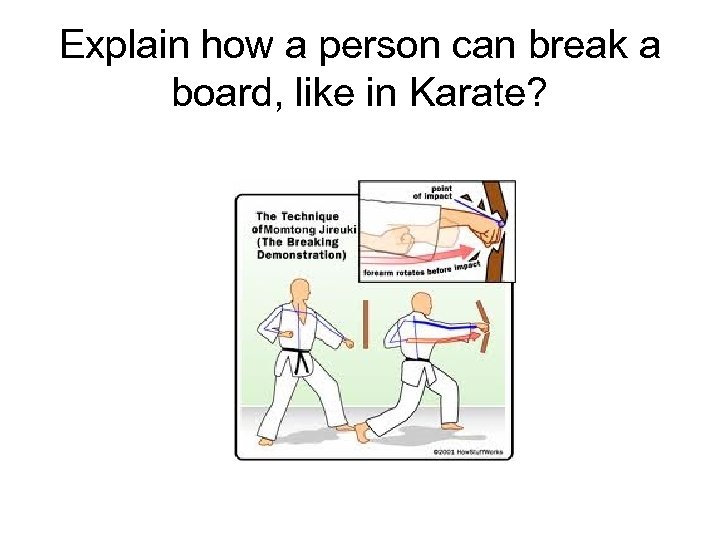 Explain how a person can break a board, like in Karate?
Explain how a person can break a board, like in Karate?