924e08e20beb9c784bfa1c058e03717a.ppt
- Количество слайдов: 58
 Legionella Risk Management Drew Industrial Division Ashland Canada Corp
Legionella Risk Management Drew Industrial Division Ashland Canada Corp
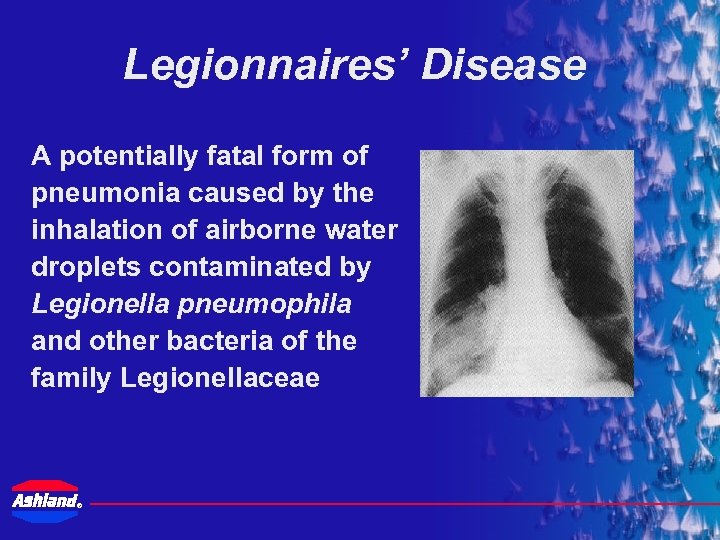 Legionnaires’ Disease A potentially fatal form of pneumonia caused by the inhalation of airborne water droplets contaminated by Legionella pneumophila and other bacteria of the family Legionellaceae ®
Legionnaires’ Disease A potentially fatal form of pneumonia caused by the inhalation of airborne water droplets contaminated by Legionella pneumophila and other bacteria of the family Legionellaceae ®
 Facts About Legionnaires’ Disease USA n n n First cases in 1976 at Bellevue Stratford Hotel, PA; 221 people contracted LD, 34 died Severe respiratory infection simulating pneumonia An estimated 10, 000 to 50, 000 cases per year An estimated 1 percent of those exposed contract Legionnaires’ disease Estimated fatality rate is 15 -20 percent ®
Facts About Legionnaires’ Disease USA n n n First cases in 1976 at Bellevue Stratford Hotel, PA; 221 people contracted LD, 34 died Severe respiratory infection simulating pneumonia An estimated 10, 000 to 50, 000 cases per year An estimated 1 percent of those exposed contract Legionnaires’ disease Estimated fatality rate is 15 -20 percent ®
 Legionella Regulations n Legionella legislation in Australia, NZ and UK forces customers to treat properly: – Prosecution and fines for noncompliance – Can shut down the system ®
Legionella Regulations n Legionella legislation in Australia, NZ and UK forces customers to treat properly: – Prosecution and fines for noncompliance – Can shut down the system ®
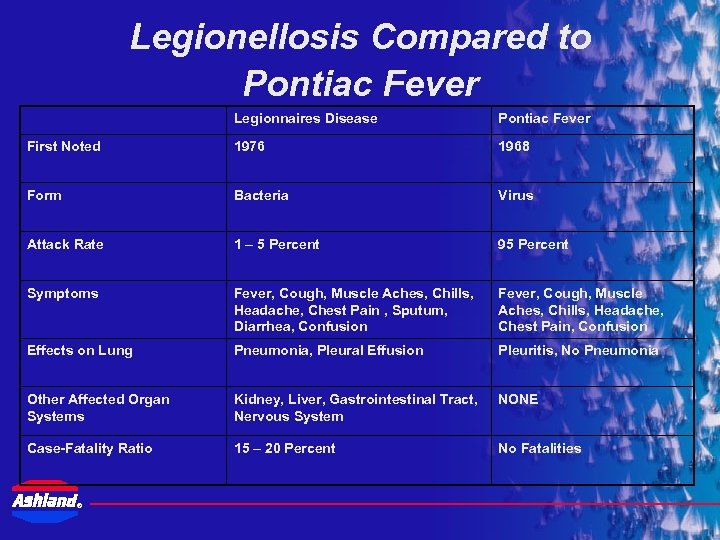 Legionellosis Compared to Pontiac Fever Legionnaires Disease Pontiac Fever First Noted 1976 1968 Form Bacteria Virus Attack Rate 1 – 5 Percent 95 Percent Symptoms Fever, Cough, Muscle Aches, Chills, Headache, Chest Pain , Sputum, Diarrhea, Confusion Fever, Cough, Muscle Aches, Chills, Headache, Chest Pain, Confusion Effects on Lung Pneumonia, Pleural Effusion Pleuritis, No Pneumonia Other Affected Organ Systems Kidney, Liver, Gastrointestinal Tract, Nervous System NONE Case-Fatality Ratio 15 – 20 Percent No Fatalities ®
Legionellosis Compared to Pontiac Fever Legionnaires Disease Pontiac Fever First Noted 1976 1968 Form Bacteria Virus Attack Rate 1 – 5 Percent 95 Percent Symptoms Fever, Cough, Muscle Aches, Chills, Headache, Chest Pain , Sputum, Diarrhea, Confusion Fever, Cough, Muscle Aches, Chills, Headache, Chest Pain, Confusion Effects on Lung Pneumonia, Pleural Effusion Pleuritis, No Pneumonia Other Affected Organ Systems Kidney, Liver, Gastrointestinal Tract, Nervous System NONE Case-Fatality Ratio 15 – 20 Percent No Fatalities ®
 Susceptability to Legionnaires’ Disease n n n n Age – The very young and 40 – 70 year olds Gender – Males are twice as likely to contract the disease than females Heavy Smoker Heavy Drinker Individuals with weakend immune systems – Cancer, AIDS, HIV positive Chronic Medical Problems – respiratory, diabetes, asthma, renal dialysis Certain Drug Therapies – corticasteroids or other immunosuppressive therapies Organ Transplants ®
Susceptability to Legionnaires’ Disease n n n n Age – The very young and 40 – 70 year olds Gender – Males are twice as likely to contract the disease than females Heavy Smoker Heavy Drinker Individuals with weakend immune systems – Cancer, AIDS, HIV positive Chronic Medical Problems – respiratory, diabetes, asthma, renal dialysis Certain Drug Therapies – corticasteroids or other immunosuppressive therapies Organ Transplants ®
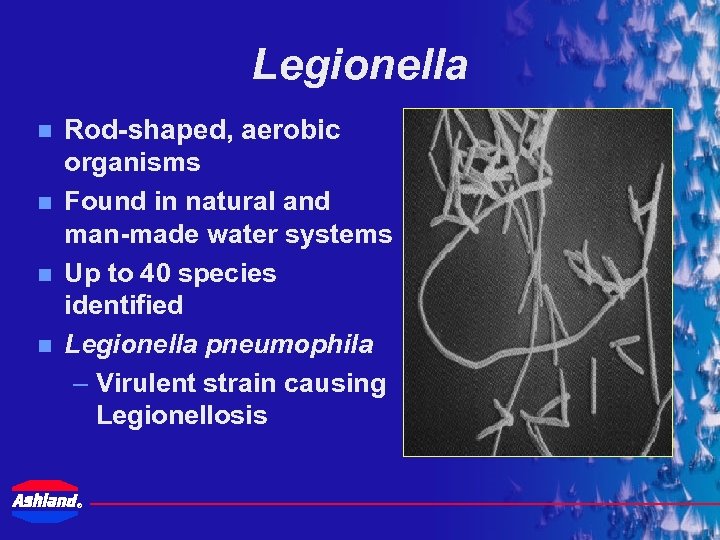 Legionella n n Rod-shaped, aerobic organisms Found in natural and man-made water systems Up to 40 species identified Legionella pneumophila – Virulent strain causing Legionellosis ®
Legionella n n Rod-shaped, aerobic organisms Found in natural and man-made water systems Up to 40 species identified Legionella pneumophila – Virulent strain causing Legionellosis ®
 Factors Determining the Risk of Contracting the Disease n n Favorable growth conditions n Aqueous aerosol n Sufficient organisms to cause infection n ® A source of Legionella Susceptible individual
Factors Determining the Risk of Contracting the Disease n n Favorable growth conditions n Aqueous aerosol n Sufficient organisms to cause infection n ® A source of Legionella Susceptible individual
 Legionella Bacteria n Source of Legionella – Pervasive organism n Conditions for growth – 68 - 122 F (20 - 50 C) – p. H 6 -8 – Stagnant waters – A nutrient source n n ® Biofilms, organics Sediments, deposits
Legionella Bacteria n Source of Legionella – Pervasive organism n Conditions for growth – 68 - 122 F (20 - 50 C) – p. H 6 -8 – Stagnant waters – A nutrient source n n ® Biofilms, organics Sediments, deposits
 Legionnella Bacteria n n Soil Derived Spore Former , Facultative Anaerobe (Maturing Biofilm) Iron and Amino Acids are Food Sources “CDC” Legionella Pnemophila – – ® 90% of All Outbreaks 82% are from Serogroup 1 Others from Serogroup 4 and 6 Outbreak Potential at ≥ 1000 CFU/ML
Legionnella Bacteria n n Soil Derived Spore Former , Facultative Anaerobe (Maturing Biofilm) Iron and Amino Acids are Food Sources “CDC” Legionella Pnemophila – – ® 90% of All Outbreaks 82% are from Serogroup 1 Others from Serogroup 4 and 6 Outbreak Potential at ≥ 1000 CFU/ML
 ®
®
 Systems Promoting Growth n n n n Cooling towers Evaporative condensers Hot and cold water systems Taps and showerheads Humidifiers and air washers Spa and whirlpool baths Decorative fountains ®
Systems Promoting Growth n n n n Cooling towers Evaporative condensers Hot and cold water systems Taps and showerheads Humidifiers and air washers Spa and whirlpool baths Decorative fountains ®
 Hot Water Requirements for Superheating of potable water systems; If the water is heated to at least 149 degrees F. Legionella die rapidly at 131 F and killed immediately at temperatures over 140 F. Water outlets are flushed for at least 30 minutes (Pittsburgh) or 5 min (CDC). It is recommended that the Hot water stored above 140 F circulation with the minimum return of 124 F. n n ®
Hot Water Requirements for Superheating of potable water systems; If the water is heated to at least 149 degrees F. Legionella die rapidly at 131 F and killed immediately at temperatures over 140 F. Water outlets are flushed for at least 30 minutes (Pittsburgh) or 5 min (CDC). It is recommended that the Hot water stored above 140 F circulation with the minimum return of 124 F. n n ®
 Cold Water For cold water, the same as above can be done with heater or shock hyperchlorination ( >10 ppm) of chlorine in water and flush for at least 5 minutes. n n Additional protocols available and may be required on a local basis. ®
Cold Water For cold water, the same as above can be done with heater or shock hyperchlorination ( >10 ppm) of chlorine in water and flush for at least 5 minutes. n n Additional protocols available and may be required on a local basis. ®
 Legionella Testing n n n General consensus is that testing is not effective Money better spent on biofilm control Many professional organizations provide guidance such as CTI, ASHRAE, etc. ®
Legionella Testing n n n General consensus is that testing is not effective Money better spent on biofilm control Many professional organizations provide guidance such as CTI, ASHRAE, etc. ®
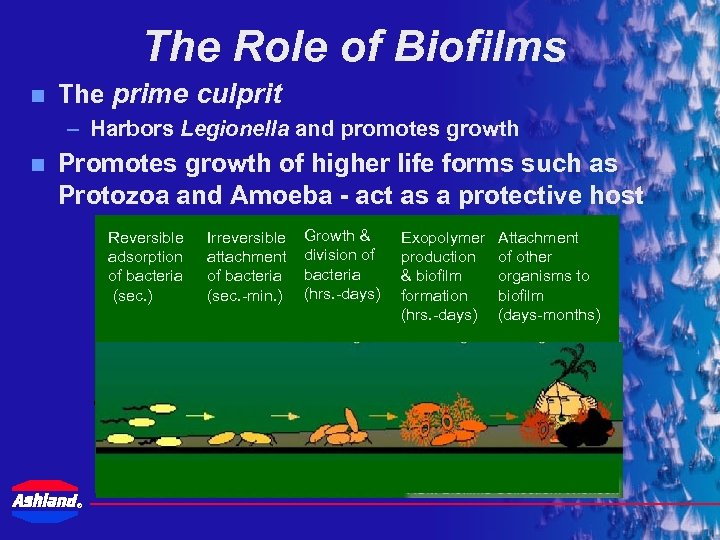 The Role of Biofilms n The prime culprit – Harbors Legionella and promotes growth n Promotes growth of higher life forms such as Protozoa and Amoeba - act as a protective host Reversible adsorption of bacteria (sec. ) Irreversible Growth & attachment division of Water Flow of bacteria (sec. -min. ) (hrs. -days) trapped particles providing nutrients Exopolymer production & biofilm formation embedded bacterial cells (hrs. -days) Attachment Planktonic of other organisms to biofilm (days-months) Sessile Surface ®
The Role of Biofilms n The prime culprit – Harbors Legionella and promotes growth n Promotes growth of higher life forms such as Protozoa and Amoeba - act as a protective host Reversible adsorption of bacteria (sec. ) Irreversible Growth & attachment division of Water Flow of bacteria (sec. -min. ) (hrs. -days) trapped particles providing nutrients Exopolymer production & biofilm formation embedded bacterial cells (hrs. -days) Attachment Planktonic of other organisms to biofilm (days-months) Sessile Surface ®
 We know that… In order to minimize Legionella growth: n n n Chemical treatment alone is not effective Minimization is dependent upon design, maintenance, contaminants, awareness and consistent implementation Effective Legionella management requires a “best practices” approach: A system that is properly treated, serviced and supervised ®
We know that… In order to minimize Legionella growth: n n n Chemical treatment alone is not effective Minimization is dependent upon design, maintenance, contaminants, awareness and consistent implementation Effective Legionella management requires a “best practices” approach: A system that is properly treated, serviced and supervised ®
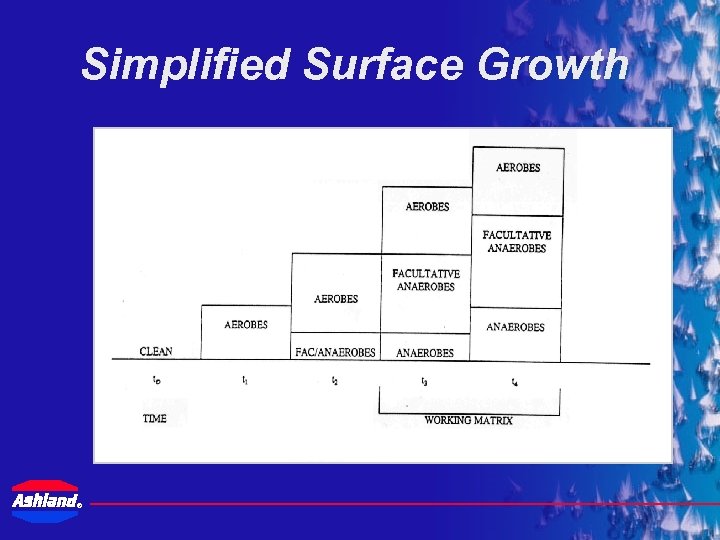 Simplified Surface Growth ®
Simplified Surface Growth ®
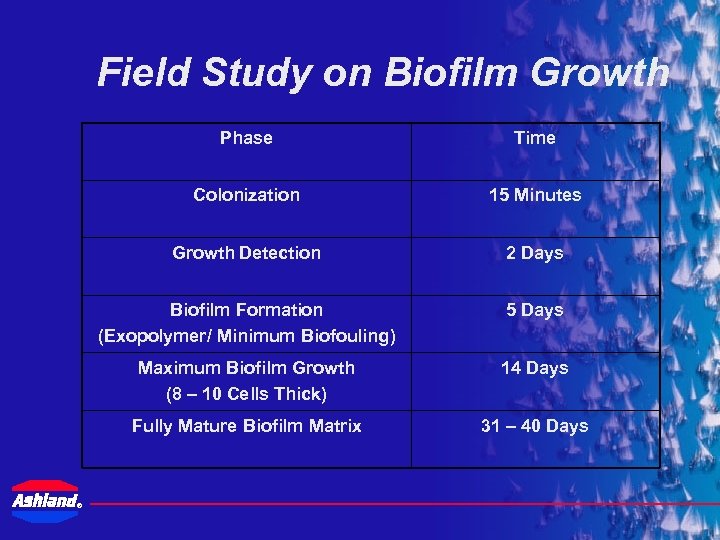 Field Study on Biofilm Growth Phase Colonization 15 Minutes Growth Detection 2 Days Biofilm Formation (Exopolymer/ Minimum Biofouling) 5 Days Maximum Biofilm Growth (8 – 10 Cells Thick) 14 Days Fully Mature Biofilm Matrix ® Time 31 – 40 Days
Field Study on Biofilm Growth Phase Colonization 15 Minutes Growth Detection 2 Days Biofilm Formation (Exopolymer/ Minimum Biofouling) 5 Days Maximum Biofilm Growth (8 – 10 Cells Thick) 14 Days Fully Mature Biofilm Matrix ® Time 31 – 40 Days
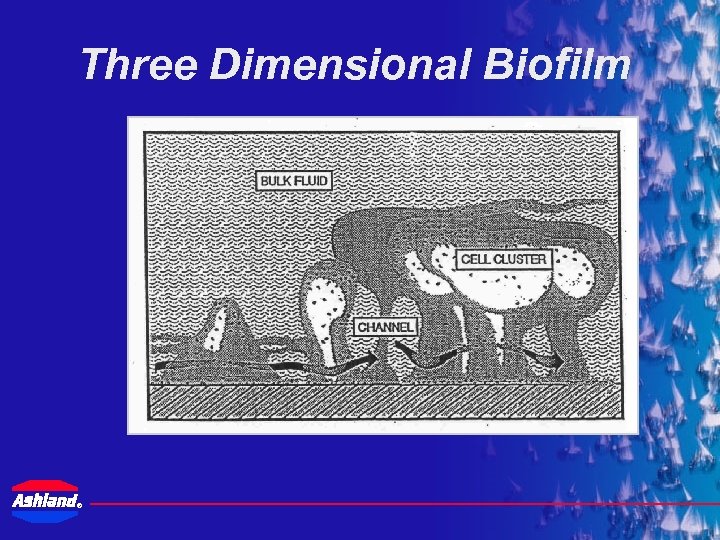 Three Dimensional Biofilm ®
Three Dimensional Biofilm ®
 We know that… Legionella risk minimization depends on the ability to control: Growth ® Dissemination Transmission
We know that… Legionella risk minimization depends on the ability to control: Growth ® Dissemination Transmission
 Areas Promoting Growth Biofilm Algae Biofilm Debris ®
Areas Promoting Growth Biofilm Algae Biofilm Debris ®
 CTI Protocol Establishes Baseline Program n Legionella Guidelines February, 2000 position paper – Background summary and guide – Platform for developing a more comprehensive, definitive program n Guidelines were based on industry consensus prior to ACOP 2001 (UK-Regulated Approved Code of Practice) CTI position paper is an industry-developed consensus for Legionella best practices protocol. However, program implementation is subject to facility interpretation. ®
CTI Protocol Establishes Baseline Program n Legionella Guidelines February, 2000 position paper – Background summary and guide – Platform for developing a more comprehensive, definitive program n Guidelines were based on industry consensus prior to ACOP 2001 (UK-Regulated Approved Code of Practice) CTI position paper is an industry-developed consensus for Legionella best practices protocol. However, program implementation is subject to facility interpretation. ®
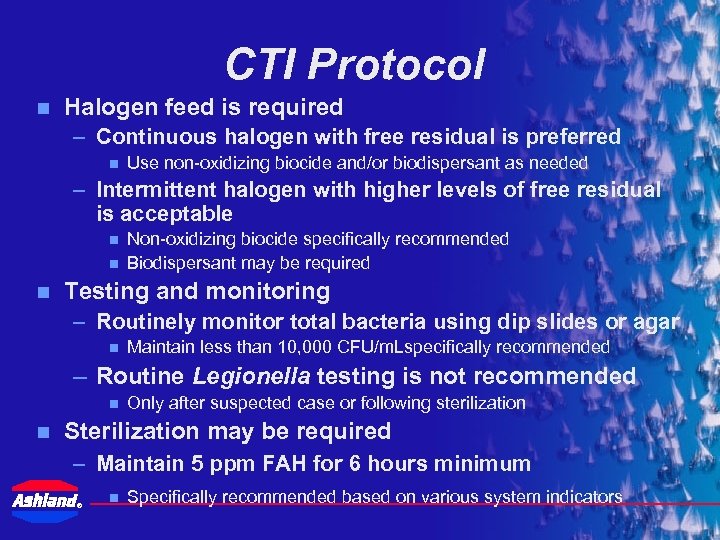 CTI Protocol n Halogen feed is required – Continuous halogen with free residual is preferred n Use non-oxidizing biocide and/or biodispersant as needed – Intermittent halogen with higher levels of free residual is acceptable n n n Non-oxidizing biocide specifically recommended Biodispersant may be required Testing and monitoring – Routinely monitor total bacteria using dip slides or agar n Maintain less than 10, 000 CFU/m. Lspecifically recommended – Routine Legionella testing is not recommended n n Only after suspected case or following sterilization Sterilization may be required – Maintain 5 ppm FAH for 6 hours minimum ® n Specifically recommended based on various system indicators
CTI Protocol n Halogen feed is required – Continuous halogen with free residual is preferred n Use non-oxidizing biocide and/or biodispersant as needed – Intermittent halogen with higher levels of free residual is acceptable n n n Non-oxidizing biocide specifically recommended Biodispersant may be required Testing and monitoring – Routinely monitor total bacteria using dip slides or agar n Maintain less than 10, 000 CFU/m. Lspecifically recommended – Routine Legionella testing is not recommended n n Only after suspected case or following sterilization Sterilization may be required – Maintain 5 ppm FAH for 6 hours minimum ® n Specifically recommended based on various system indicators
 Drew Industrial’s Best Practices Legionella Risk Management Program ®
Drew Industrial’s Best Practices Legionella Risk Management Program ®
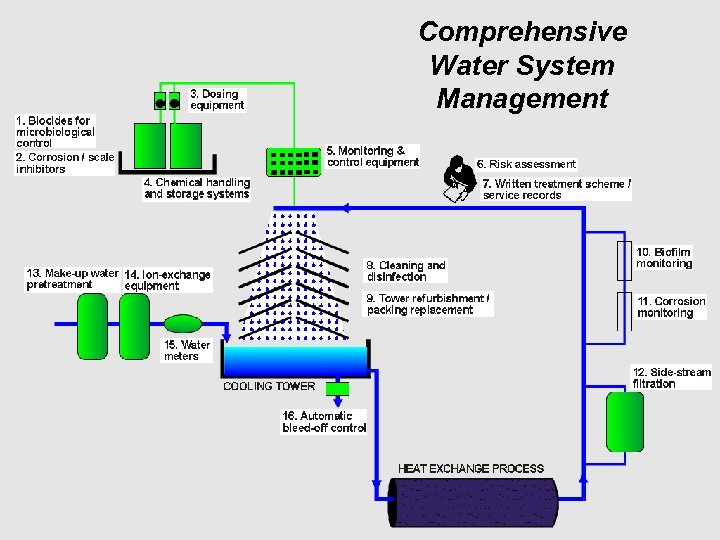
 Total System Approach: Five Areas of Activity and Performance Comprehensive system assessment Intensive microbiological treatment program Sterilization and cleaning Monitoring and control Documentation ®
Total System Approach: Five Areas of Activity and Performance Comprehensive system assessment Intensive microbiological treatment program Sterilization and cleaning Monitoring and control Documentation ®
 Comprehensive Water System Management ®
Comprehensive Water System Management ®
 System Assessment n System survey – In-depth survey of system mechanical design and operating conditions – Utilizes established protocol, ex BSRIABuilding Services Research Institute Assoc. (UK) n Identify, evaluate and rank specific factors associated with potential for microbiological growth and Legionella – Mechanical and chemical n Determine risk minimization action plans ®
System Assessment n System survey – In-depth survey of system mechanical design and operating conditions – Utilizes established protocol, ex BSRIABuilding Services Research Institute Assoc. (UK) n Identify, evaluate and rank specific factors associated with potential for microbiological growth and Legionella – Mechanical and chemical n Determine risk minimization action plans ®
 Survey Provides Plan to Reduce Growth 1. Address non-chemical ways to improve microbiological control n Remove piping dead legs n Revise operating procedures n Rotate idle equipment n Use side stream filtration 2. Identify operating procedures, mechanical design and other factors that contribute to growth of Legionella ®
Survey Provides Plan to Reduce Growth 1. Address non-chemical ways to improve microbiological control n Remove piping dead legs n Revise operating procedures n Rotate idle equipment n Use side stream filtration 2. Identify operating procedures, mechanical design and other factors that contribute to growth of Legionella ®
 Identify areas that promote growth or dissemmination of Legionella
Identify areas that promote growth or dissemmination of Legionella
 Critical Factors Stagnant conditions Nutrients and conditions for growth Water and chemical treatment quality Water system mechanical conditions Location and exposure risk n n n ®
Critical Factors Stagnant conditions Nutrients and conditions for growth Water and chemical treatment quality Water system mechanical conditions Location and exposure risk n n n ®
 “Ideal System” n n n n Water flow is continuous No dead legs or stagnant conditions Basin and deck protected from sun No evidence of sludge, debris, algae Drift eliminators installed, functioning No evidence of aerosols, drift System not near health care, aged, residential facility ® n n n Low number of people potentially exposed Halogen used Biodispersant/biodispersing biocide used Comprehensive water treatment program Automated biocide and chemical dosing Continuous automated monitoring, control
“Ideal System” n n n n Water flow is continuous No dead legs or stagnant conditions Basin and deck protected from sun No evidence of sludge, debris, algae Drift eliminators installed, functioning No evidence of aerosols, drift System not near health care, aged, residential facility ® n n n Low number of people potentially exposed Halogen used Biodispersant/biodispersing biocide used Comprehensive water treatment program Automated biocide and chemical dosing Continuous automated monitoring, control
 Intensive Microbiological Treatment Program Drew Industrial’s Best Practices Legionella risk management program requires an effective system approach that incorporates an intensive microbiological control program along with additional risk management actions ®
Intensive Microbiological Treatment Program Drew Industrial’s Best Practices Legionella risk management program requires an effective system approach that incorporates an intensive microbiological control program along with additional risk management actions ®
 Intensive Microbiological Treatment Program n Cooling Technology Institute (CTI) position paper – Basic program approach n Drew Industrial’s recommended intensive microbiological treatment program – Comprehensive treatment program incorporating the CTI-recommended actions plus several additional practices ®
Intensive Microbiological Treatment Program n Cooling Technology Institute (CTI) position paper – Basic program approach n Drew Industrial’s recommended intensive microbiological treatment program – Comprehensive treatment program incorporating the CTI-recommended actions plus several additional practices ®
 Intensive Microbiological Treatment Program n n Drew Industrial’s program recommendations for minimizing the potential for growth of Legionella bacteria in cooling systems A comprehensive microbiological control program n Includes all recommended CTI actions n Includes CTI optional recommendations n Provides more definitive guidelines n Four protocols based on halogen feed ®
Intensive Microbiological Treatment Program n n Drew Industrial’s program recommendations for minimizing the potential for growth of Legionella bacteria in cooling systems A comprehensive microbiological control program n Includes all recommended CTI actions n Includes CTI optional recommendations n Provides more definitive guidelines n Four protocols based on halogen feed ®
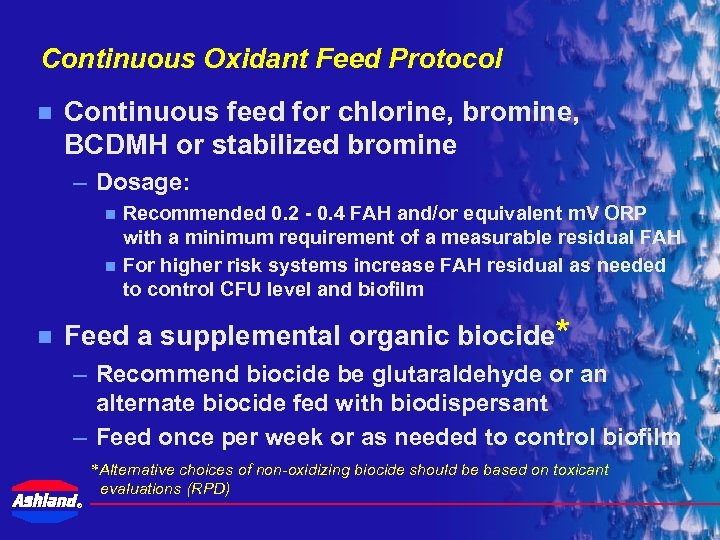 Continuous Oxidant Feed Protocol n Continuous feed for chlorine, bromine, BCDMH or stabilized bromine – Dosage: n n n Recommended 0. 2 - 0. 4 FAH and/or equivalent m. V ORP with a minimum requirement of a measurable residual FAH For higher risk systems increase FAH residual as needed to control CFU level and biofilm Feed a supplemental organic biocide* – Recommend biocide be glutaraldehyde or an alternate biocide fed with biodispersant – Feed once per week or as needed to control biofilm *Alternative choices of non-oxidizing biocide should be based on toxicant evaluations (RPD) ®
Continuous Oxidant Feed Protocol n Continuous feed for chlorine, bromine, BCDMH or stabilized bromine – Dosage: n n n Recommended 0. 2 - 0. 4 FAH and/or equivalent m. V ORP with a minimum requirement of a measurable residual FAH For higher risk systems increase FAH residual as needed to control CFU level and biofilm Feed a supplemental organic biocide* – Recommend biocide be glutaraldehyde or an alternate biocide fed with biodispersant – Feed once per week or as needed to control biofilm *Alternative choices of non-oxidizing biocide should be based on toxicant evaluations (RPD) ®
 Total System Approach: Five Areas of Activity and Performance Comprehensive system assessment Intensive microbiological treatment program Sterilization and cleaning Monitoring and control Documentation ®
Total System Approach: Five Areas of Activity and Performance Comprehensive system assessment Intensive microbiological treatment program Sterilization and cleaning Monitoring and control Documentation ®
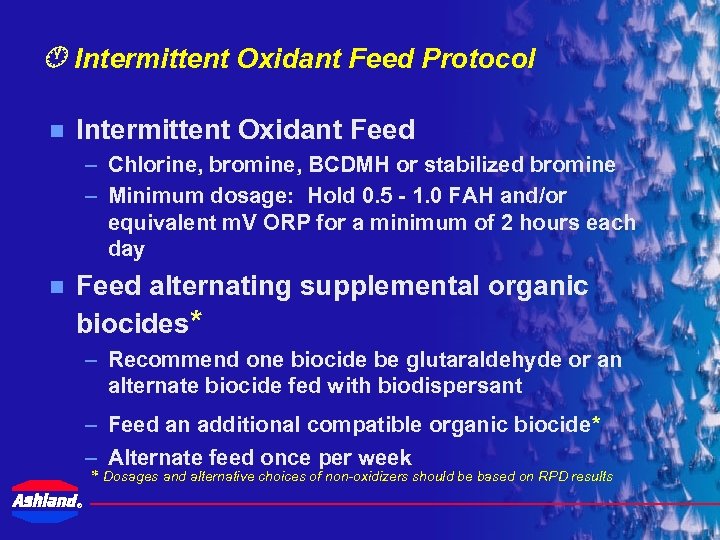 Intermittent Oxidant Feed Protocol n Intermittent Oxidant Feed – Chlorine, bromine, BCDMH or stabilized bromine – Minimum dosage: Hold 0. 5 - 1. 0 FAH and/or equivalent m. V ORP for a minimum of 2 hours each day n Feed alternating supplemental organic biocides* – Recommend one biocide be glutaraldehyde or an alternate biocide fed with biodispersant – Feed an additional compatible organic biocide* – Alternate feed once per week * Dosages and alternative choices of non-oxidizers should be based on RPD results ®
Intermittent Oxidant Feed Protocol n Intermittent Oxidant Feed – Chlorine, bromine, BCDMH or stabilized bromine – Minimum dosage: Hold 0. 5 - 1. 0 FAH and/or equivalent m. V ORP for a minimum of 2 hours each day n Feed alternating supplemental organic biocides* – Recommend one biocide be glutaraldehyde or an alternate biocide fed with biodispersant – Feed an additional compatible organic biocide* – Alternate feed once per week * Dosages and alternative choices of non-oxidizers should be based on RPD results ®
 Chlorine Dioxide - A Selective Oxidant n n n ® Oxidation potential not affected by p. H Selective oxidant No dissociation; does not react with water Does not react with amines, nitrogen compounds Highly effective against biofilm
Chlorine Dioxide - A Selective Oxidant n n n ® Oxidation potential not affected by p. H Selective oxidant No dissociation; does not react with water Does not react with amines, nitrogen compounds Highly effective against biofilm
 Continuous Chlorine Dioxide Feed Protocol n Continuous chlorine dioxide feed – Minimum dosage: 0. 1 ppm residual Cl. O 2 or equivalent m. V ORP n Feed a supplemental organic biocide as needed based on biofilm control – Recommend biocide is glutaraldehyde or an alternate biocide fed with biodispersant* n Feed once per week or as needed to control biofilm *Alternative choices of non-oxidizing biocide should be based on RPD results ®
Continuous Chlorine Dioxide Feed Protocol n Continuous chlorine dioxide feed – Minimum dosage: 0. 1 ppm residual Cl. O 2 or equivalent m. V ORP n Feed a supplemental organic biocide as needed based on biofilm control – Recommend biocide is glutaraldehyde or an alternate biocide fed with biodispersant* n Feed once per week or as needed to control biofilm *Alternative choices of non-oxidizing biocide should be based on RPD results ®
 Intermittent Chlorine Dioxide Feed Protocol n Intermittent chlorine dioxide feed – Minimum dosage: 0. 5 ppm residual Cl. O 2 and/or equivalent m. V ORP for a minimum of 2 hours per day n Feed alternating supplemental organic biocides* – Recommend one biocide be glutaraldehyde or an alternate biocide fed with biodispersant – Feed an additional compatible organic biocide* – Alternate feed once per week Chlorine dioxide is also an effective supplemental biocide for process cooling systems where contaminants that increase bacterial growth are present. *Alternative choices of non-oxidizing biocide should be based on RPD results ®
Intermittent Chlorine Dioxide Feed Protocol n Intermittent chlorine dioxide feed – Minimum dosage: 0. 5 ppm residual Cl. O 2 and/or equivalent m. V ORP for a minimum of 2 hours per day n Feed alternating supplemental organic biocides* – Recommend one biocide be glutaraldehyde or an alternate biocide fed with biodispersant – Feed an additional compatible organic biocide* – Alternate feed once per week Chlorine dioxide is also an effective supplemental biocide for process cooling systems where contaminants that increase bacterial growth are present. *Alternative choices of non-oxidizing biocide should be based on RPD results ®
 Sterilization n n Drew Industrial’s Best Practices program recommendation includes annual sterilization regardless of overall system indicators Procedure is per the CTI protocol – Annual on-line system hyperhalogenation n Maintain 5 ppm FAH for 6 hours minimum, per the CTI process – Annual full system cleaning highly recommended n n ® Perform six months following hyperhalogenation If an off-line cleaning can not be performed, clean as possible on-line and follow with a second hyperhalogenation
Sterilization n n Drew Industrial’s Best Practices program recommendation includes annual sterilization regardless of overall system indicators Procedure is per the CTI protocol – Annual on-line system hyperhalogenation n Maintain 5 ppm FAH for 6 hours minimum, per the CTI process – Annual full system cleaning highly recommended n n ® Perform six months following hyperhalogenation If an off-line cleaning can not be performed, clean as possible on-line and follow with a second hyperhalogenation
 Monitoring & Control Program monitoring n n FAH and/or ORP Maintain Total Bacteria Counts below 10, 000 CFU/m. L – Frequency as required to maintain performance Treatment levels, system parameters, corrosion, fouling, etc. Legionella testing n n n Random Legionella testing is not recommended Testing is recommended only after sterilization Control schemes n n n ® Automation improves performance and efficiencies Wide range of controllers available
Monitoring & Control Program monitoring n n FAH and/or ORP Maintain Total Bacteria Counts below 10, 000 CFU/m. L – Frequency as required to maintain performance Treatment levels, system parameters, corrosion, fouling, etc. Legionella testing n n n Random Legionella testing is not recommended Testing is recommended only after sterilization Control schemes n n n ® Automation improves performance and efficiencies Wide range of controllers available
 Total System Approach: Five Areas of Activity and Performance Comprehensive system assessment Intensive microbiological treatment program Sterilization and cleaning Monitoring and control Documentation ®
Total System Approach: Five Areas of Activity and Performance Comprehensive system assessment Intensive microbiological treatment program Sterilization and cleaning Monitoring and control Documentation ®
 Use of Proper Monitoring & Control Equipment is Critical! Microbiological Residuals, Corrosion, Scale & Biofouling On-line
Use of Proper Monitoring & Control Equipment is Critical! Microbiological Residuals, Corrosion, Scale & Biofouling On-line
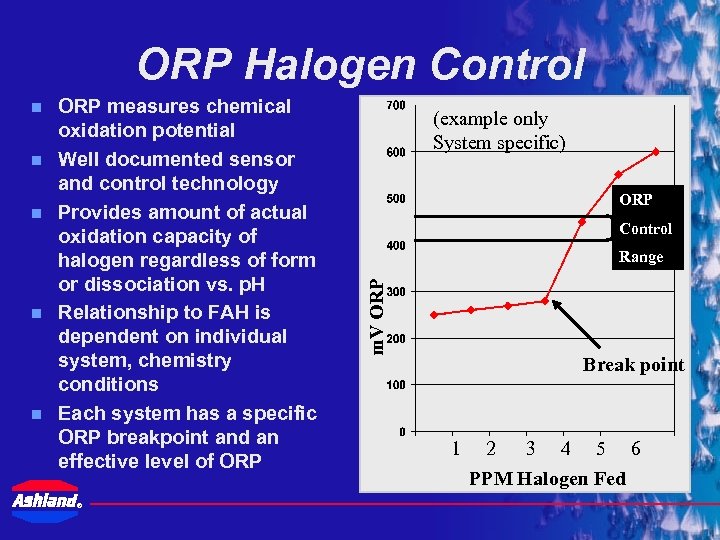 ORP Halogen Control n n ORP measures chemical oxidation potential Well documented sensor and control technology Provides amount of actual oxidation capacity of halogen regardless of form or dissociation vs. p. H Relationship to FAH is dependent on individual system, chemistry conditions Each system has a specific ORP breakpoint and an effective level of ORP ® (example only System specific) ORP Control Range m. V ORP n Break point 1 2 3 4 5 6 PPM Halogen Fed
ORP Halogen Control n n ORP measures chemical oxidation potential Well documented sensor and control technology Provides amount of actual oxidation capacity of halogen regardless of form or dissociation vs. p. H Relationship to FAH is dependent on individual system, chemistry conditions Each system has a specific ORP breakpoint and an effective level of ORP ® (example only System specific) ORP Control Range m. V ORP n Break point 1 2 3 4 5 6 PPM Halogen Fed
 Total System Approach: Five Areas of Activity and Performance Comprehensive system assessment Intensive microbiological treatment program Sterilization and cleaning Monitoring and control Documentation ®
Total System Approach: Five Areas of Activity and Performance Comprehensive system assessment Intensive microbiological treatment program Sterilization and cleaning Monitoring and control Documentation ®
 Documentation n Maintain complete, accurate logs – – – n n n Biocide usage Halogen tests and/or ORP log Bacteria counts Start-up and shut-down log Log of operating procedures Monthly service inspection reports System disinfection logs Contingency plans ®
Documentation n Maintain complete, accurate logs – – – n n n Biocide usage Halogen tests and/or ORP log Bacteria counts Start-up and shut-down log Log of operating procedures Monthly service inspection reports System disinfection logs Contingency plans ®
 Problem Systems? Despite good control, monitoring and response, some systems remain problematic. Why? n Cooling systems are dynamic n Biofilms DO exist within system n System contamination DOES exist n Some system designs present obstacles to effective Legionella control ®
Problem Systems? Despite good control, monitoring and response, some systems remain problematic. Why? n Cooling systems are dynamic n Biofilms DO exist within system n System contamination DOES exist n Some system designs present obstacles to effective Legionella control ®
 Problematic Systems For these systems… – Maintain an environment hostile to bacteria at all times – Continuously halogenate – Continuously feed biodispersant – Utilize reliable automation – Utilize ORP control – Regularly clean and disinfect – Rigorously inspect and maintain ®
Problematic Systems For these systems… – Maintain an environment hostile to bacteria at all times – Continuously halogenate – Continuously feed biodispersant – Utilize reliable automation – Utilize ORP control – Regularly clean and disinfect – Rigorously inspect and maintain ®
 Survey of Field Test Results n n In general it has been reported that 40 – 60% of Cooling Towers tested positive for Legionnella 794 evaluations performed 277 (35%) tested positive for Legionella Ranged from 1 to >9, 000 CFU/ml ®
Survey of Field Test Results n n In general it has been reported that 40 – 60% of Cooling Towers tested positive for Legionnella 794 evaluations performed 277 (35%) tested positive for Legionella Ranged from 1 to >9, 000 CFU/ml ®
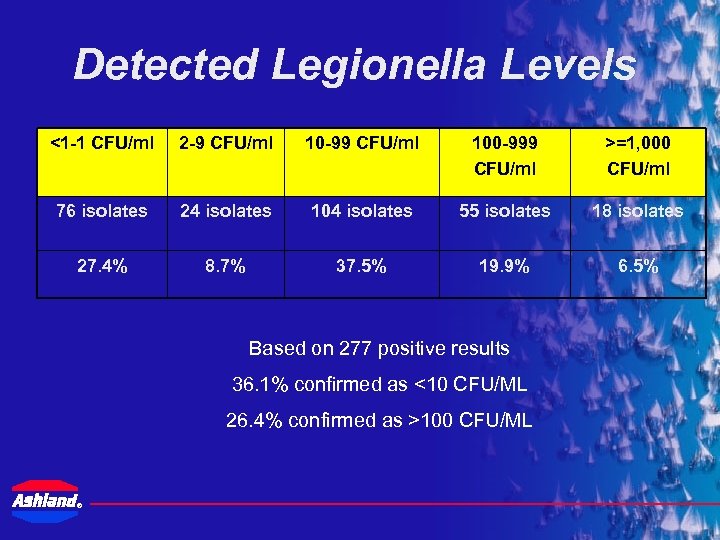 Detected Legionella Levels <1 -1 CFU/ml 2 -9 CFU/ml 10 -99 CFU/ml 100 -999 CFU/ml >=1, 000 CFU/ml 76 isolates 24 isolates 104 isolates 55 isolates 18 isolates 27. 4% 8. 7% 37. 5% 19. 9% 6. 5% Based on 277 positive results 36. 1% confirmed as <10 CFU/ML 26. 4% confirmed as >100 CFU/ML ®
Detected Legionella Levels <1 -1 CFU/ml 2 -9 CFU/ml 10 -99 CFU/ml 100 -999 CFU/ml >=1, 000 CFU/ml 76 isolates 24 isolates 104 isolates 55 isolates 18 isolates 27. 4% 8. 7% 37. 5% 19. 9% 6. 5% Based on 277 positive results 36. 1% confirmed as <10 CFU/ML 26. 4% confirmed as >100 CFU/ML ®
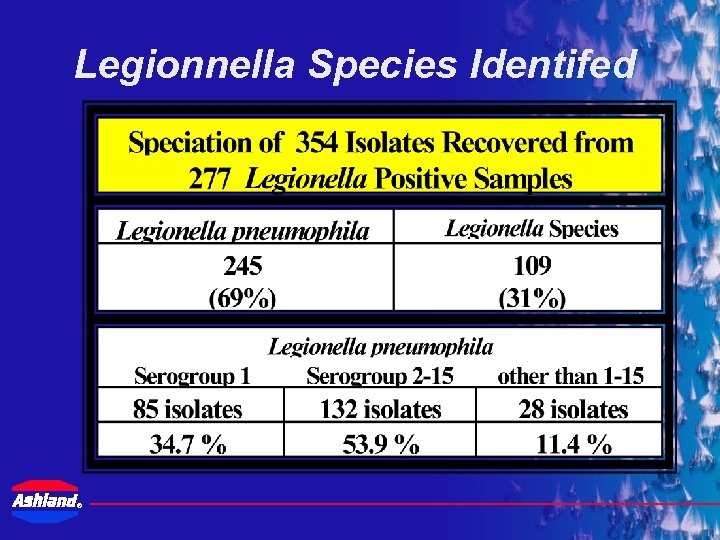 Legionnella Species Identifed ®
Legionnella Species Identifed ®
 Response to a Positive Legionella Test Result n Action Level 1 - (Detectable - <1 CFU/ML) – Risk Level - (Extremely Low) – Confirm current treatment program n Action Level 2 - (1 - 9 CFU/ML) – Risk Level - (Low) – Increase frequency of current treatment to one additional dose of biocide for two weeks ®
Response to a Positive Legionella Test Result n Action Level 1 - (Detectable - <1 CFU/ML) – Risk Level - (Extremely Low) – Confirm current treatment program n Action Level 2 - (1 - 9 CFU/ML) – Risk Level - (Low) – Increase frequency of current treatment to one additional dose of biocide for two weeks ®
 Response to a Positive Legionella Test Result n Action Level 3 - (10 - 99 CFU/ML) – Risk Level - (Low to Moderate) – Increase treatment concentration and frequency to two additional doses of biocide weekly for two weeks n Action Level 4 - (100 - 499 CFU/ML) – Risk Level - (Moderate) – Increase treatment concentration and frequency to two additional doses of biocide weekly for 3 to 4 weeks – On-line halogen addition / additional organic dispersant maybe required ®
Response to a Positive Legionella Test Result n Action Level 3 - (10 - 99 CFU/ML) – Risk Level - (Low to Moderate) – Increase treatment concentration and frequency to two additional doses of biocide weekly for two weeks n Action Level 4 - (100 - 499 CFU/ML) – Risk Level - (Moderate) – Increase treatment concentration and frequency to two additional doses of biocide weekly for 3 to 4 weeks – On-line halogen addition / additional organic dispersant maybe required ®
 Response to a Positive Legionella Test Result n Action Level 5 - (500 - 999 CFU/ML) – Risk Level - (Moderate to High) – Hyperchlorination required possibly off-line – Use Halogen at 0. 2 – 0. 4 ppm FAH continuous or daily shocks of 0. 5 – 1 ppm FAH for 2 to 4 hours n Action Level 6 - (>1, 000 CFU/ML) – Risk Level - (High) – Use halogen continuously at 1 ppm FAH for 6 hours, then 0. 2 – 0. 4 ppm FAH plus feed non-oxidizer every apparent retention time for 1 week will be required – Off-line sterilization using halogens at 5 -10 ppm FAH for 6 to 24 hours, organics, and biodispersants may be required – Retest in 1 week ®
Response to a Positive Legionella Test Result n Action Level 5 - (500 - 999 CFU/ML) – Risk Level - (Moderate to High) – Hyperchlorination required possibly off-line – Use Halogen at 0. 2 – 0. 4 ppm FAH continuous or daily shocks of 0. 5 – 1 ppm FAH for 2 to 4 hours n Action Level 6 - (>1, 000 CFU/ML) – Risk Level - (High) – Use halogen continuously at 1 ppm FAH for 6 hours, then 0. 2 – 0. 4 ppm FAH plus feed non-oxidizer every apparent retention time for 1 week will be required – Off-line sterilization using halogens at 5 -10 ppm FAH for 6 to 24 hours, organics, and biodispersants may be required – Retest in 1 week ®
 Action Levels n Immediate response to positive test results n On-line treatment requires a minimum of 14 days to produce results n Unrealistic a system could be totally Legionella free ®
Action Levels n Immediate response to positive test results n On-line treatment requires a minimum of 14 days to produce results n Unrealistic a system could be totally Legionella free ®
 Thank You ® Registered trademark and ™ trademark of Ashland Inc. © 2001 ®
Thank You ® Registered trademark and ™ trademark of Ashland Inc. © 2001 ®


