Lectures 21 and 22: The Cytoskeleton: Microfilaments Essential


38594-2017_lectures_21_and_223_actin_cytoskeleton_and_cell_motility.ppt
- Количество слайдов: 31
 Lectures 21 and 22: The Cytoskeleton: Microfilaments Essential Cell Biology Third Edition Chapter 17
Lectures 21 and 22: The Cytoskeleton: Microfilaments Essential Cell Biology Third Edition Chapter 17
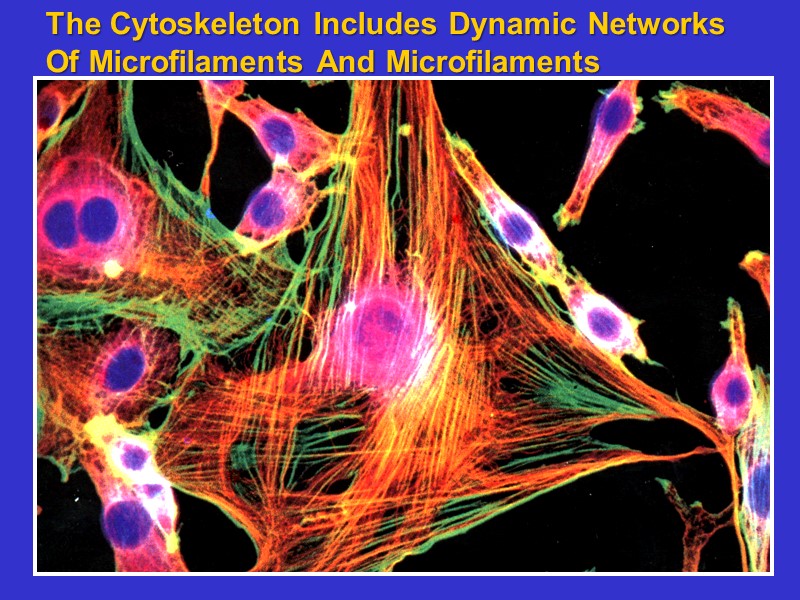
 The Cytoskeleton Includes Dynamic Networks Of Microfilaments And Microfilaments
The Cytoskeleton Includes Dynamic Networks Of Microfilaments And Microfilaments
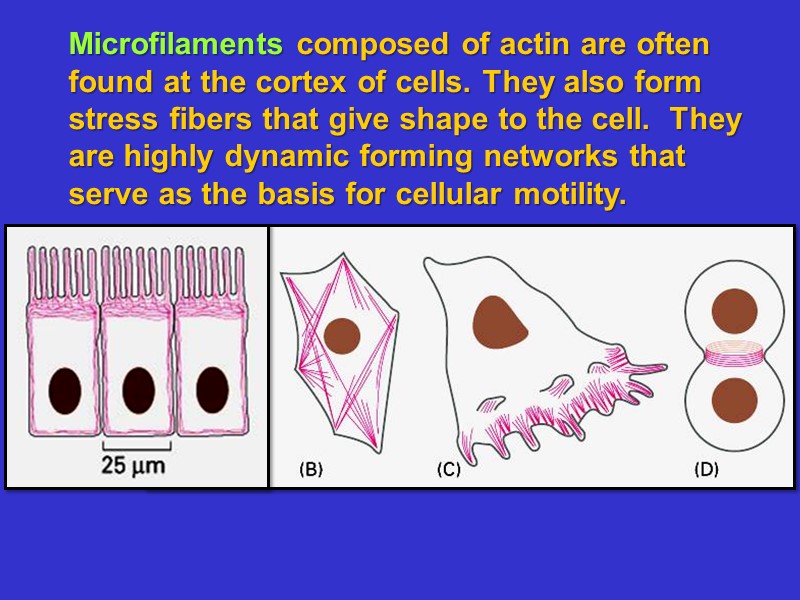
 Microfilaments composed of actin are often found at the cortex of cells. They also form stress fibers that give shape to the cell. They are highly dynamic forming networks that serve as the basis for cellular motility.
Microfilaments composed of actin are often found at the cortex of cells. They also form stress fibers that give shape to the cell. They are highly dynamic forming networks that serve as the basis for cellular motility.
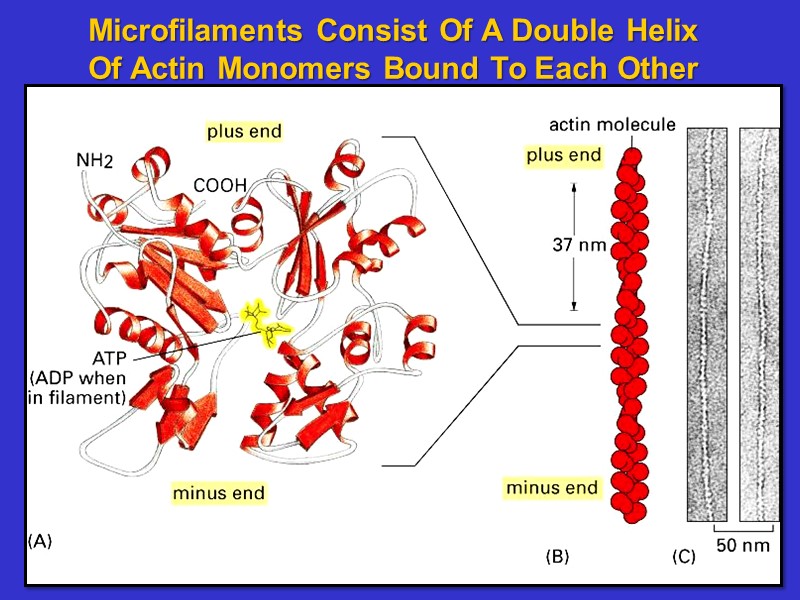
 Microfilaments Consist Of A Double Helix Of Actin Monomers Bound To Each Other
Microfilaments Consist Of A Double Helix Of Actin Monomers Bound To Each Other
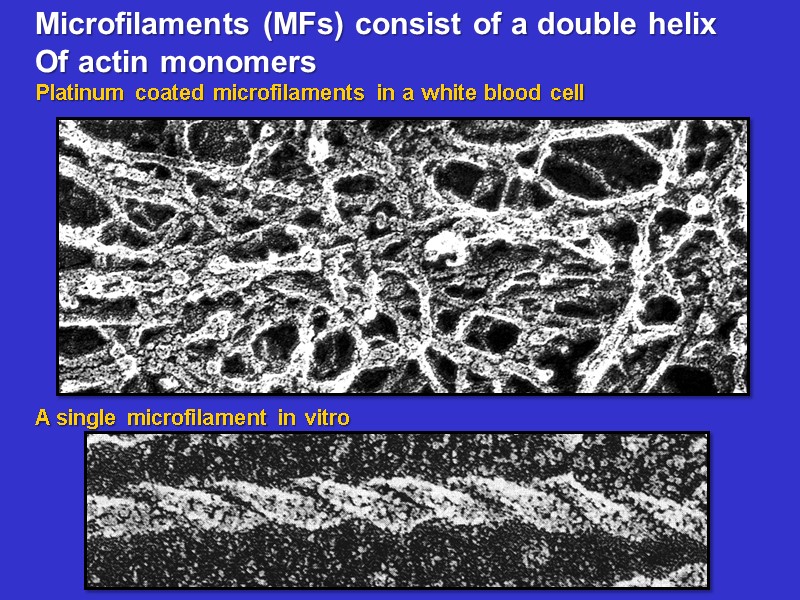
 Microfilaments (MFs) consist of a double helix Of actin monomers Platinum coated microfilaments in a white blood cell A single microfilament in vitro
Microfilaments (MFs) consist of a double helix Of actin monomers Platinum coated microfilaments in a white blood cell A single microfilament in vitro
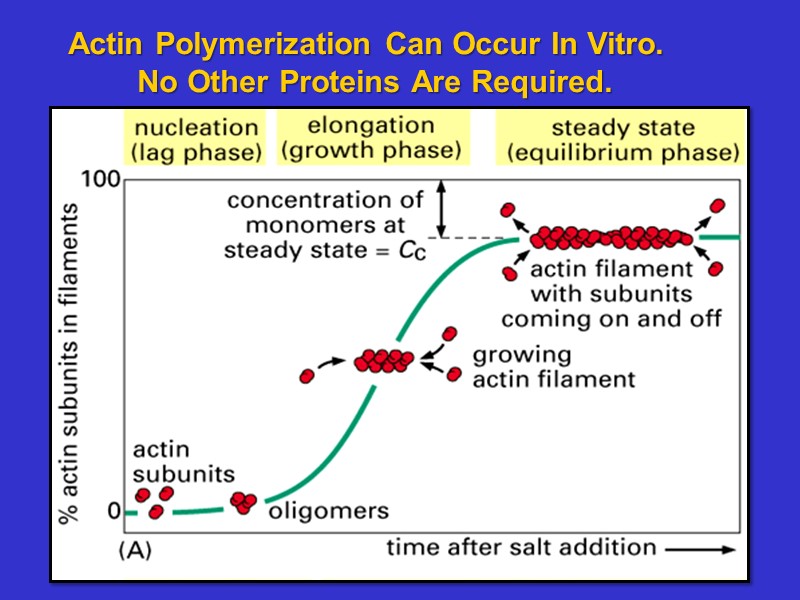
 Actin Polymerization Can Occur In Vitro. No Other Proteins Are Required.
Actin Polymerization Can Occur In Vitro. No Other Proteins Are Required.
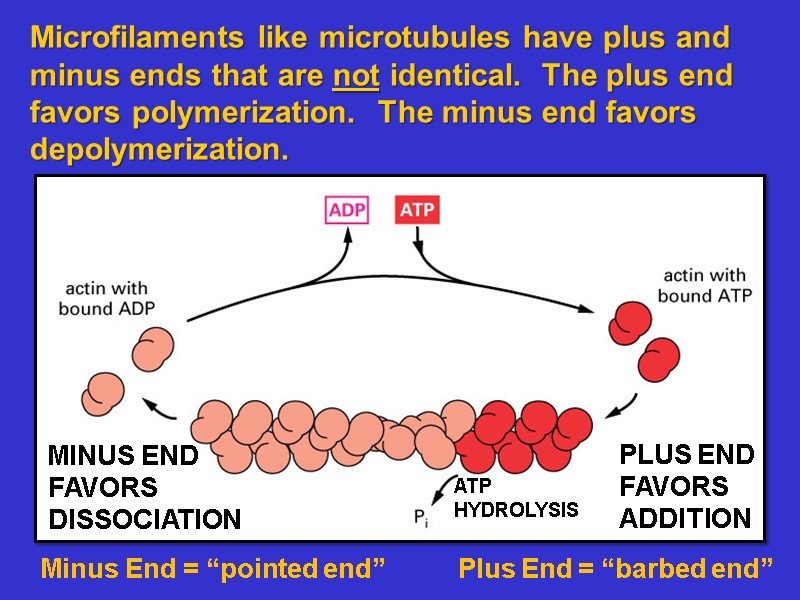
 Microfilaments like microtubules have plus and minus ends that are not identical. The plus end favors polymerization. The minus end favors depolymerization. Minus End = “pointed end” Plus End = “barbed end”
Microfilaments like microtubules have plus and minus ends that are not identical. The plus end favors polymerization. The minus end favors depolymerization. Minus End = “pointed end” Plus End = “barbed end”
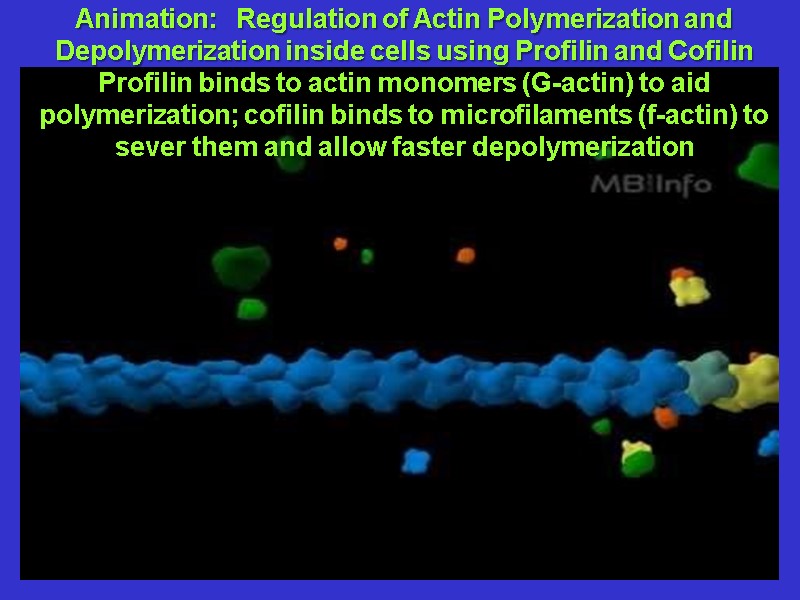
 Animation: Regulation of Actin Polymerization and Depolymerization inside cells using Profilin and Cofilin Profilin binds to actin monomers (G-actin) to aid polymerization; cofilin binds to microfilaments (f-actin) to sever them and allow faster depolymerization
Animation: Regulation of Actin Polymerization and Depolymerization inside cells using Profilin and Cofilin Profilin binds to actin monomers (G-actin) to aid polymerization; cofilin binds to microfilaments (f-actin) to sever them and allow faster depolymerization
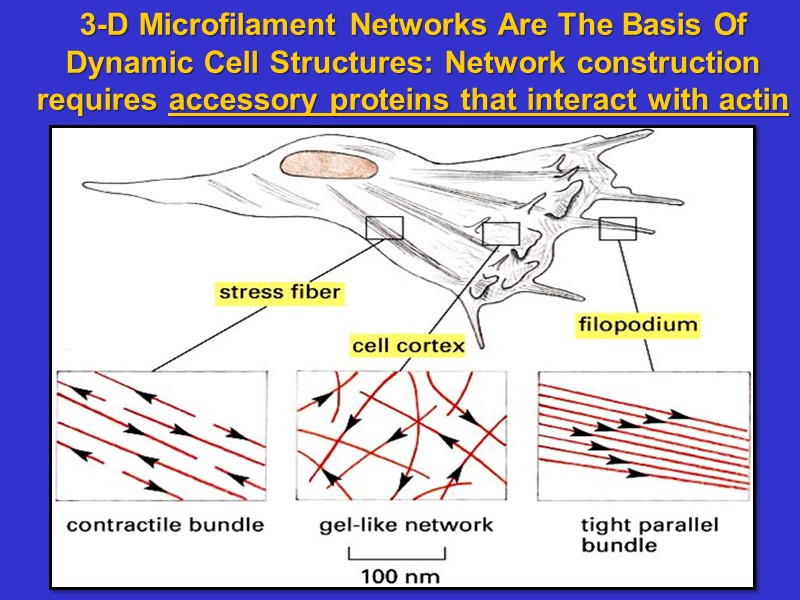
 3-D Microfilament Networks Are The Basis Of Dynamic Cell Structures: Network construction requires accessory proteins that interact with actin
3-D Microfilament Networks Are The Basis Of Dynamic Cell Structures: Network construction requires accessory proteins that interact with actin
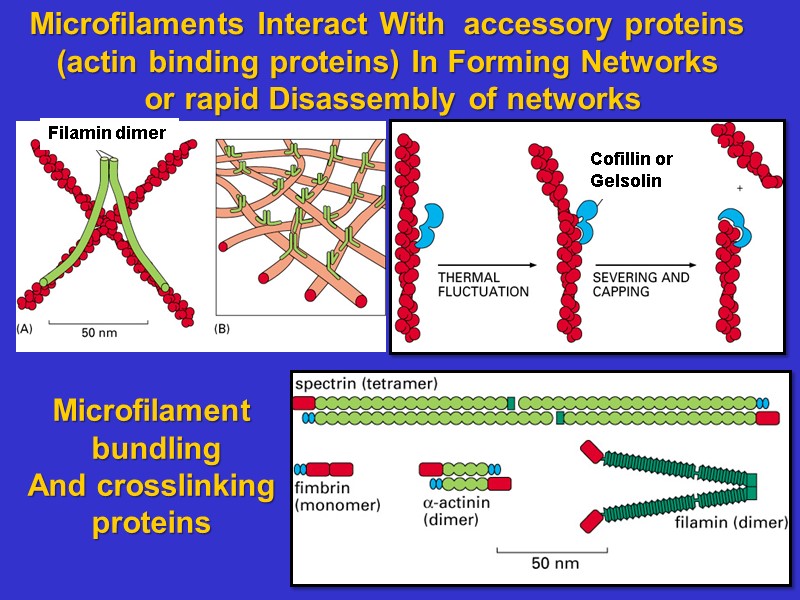
 Microfilaments Interact With accessory proteins (actin binding proteins) In Forming Networks or rapid Disassembly of networks Microfilament bundling And crosslinking proteins
Microfilaments Interact With accessory proteins (actin binding proteins) In Forming Networks or rapid Disassembly of networks Microfilament bundling And crosslinking proteins
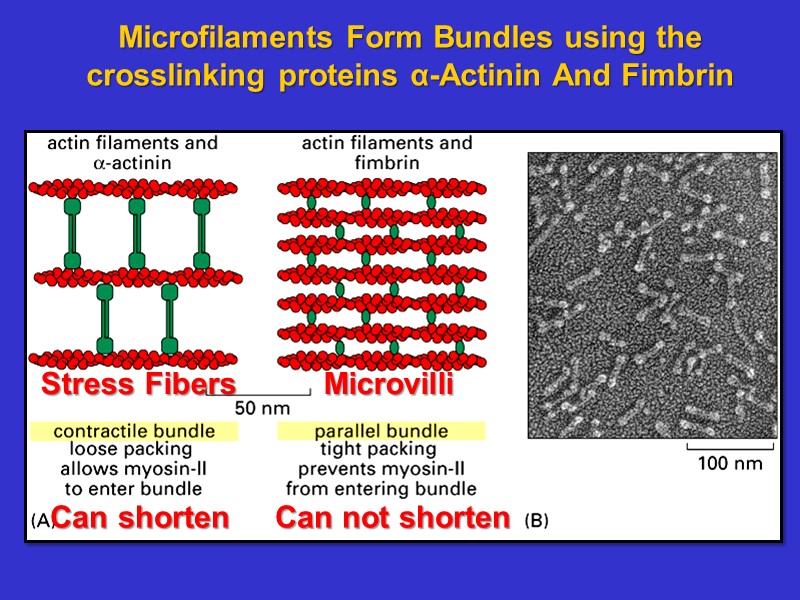
 Microfilaments Form Bundles using the crosslinking proteins α-Actinin And Fimbrin Stress Fibers Microvilli Can shorten Can not shorten
Microfilaments Form Bundles using the crosslinking proteins α-Actinin And Fimbrin Stress Fibers Microvilli Can shorten Can not shorten
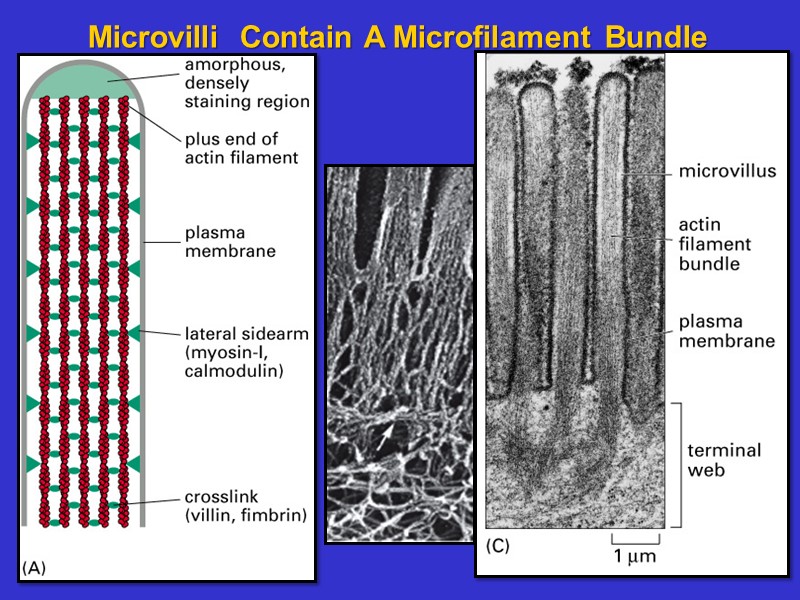
 Microvilli Contain A Microfilament Bundle
Microvilli Contain A Microfilament Bundle
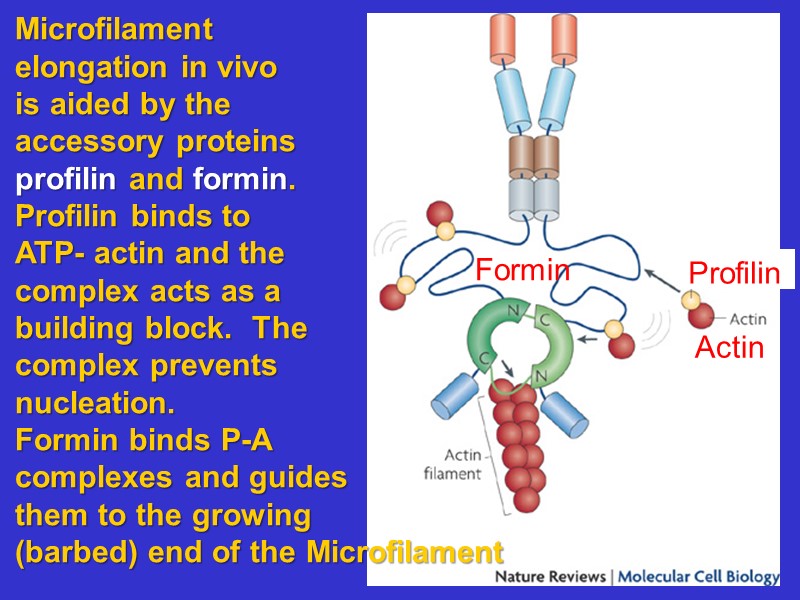
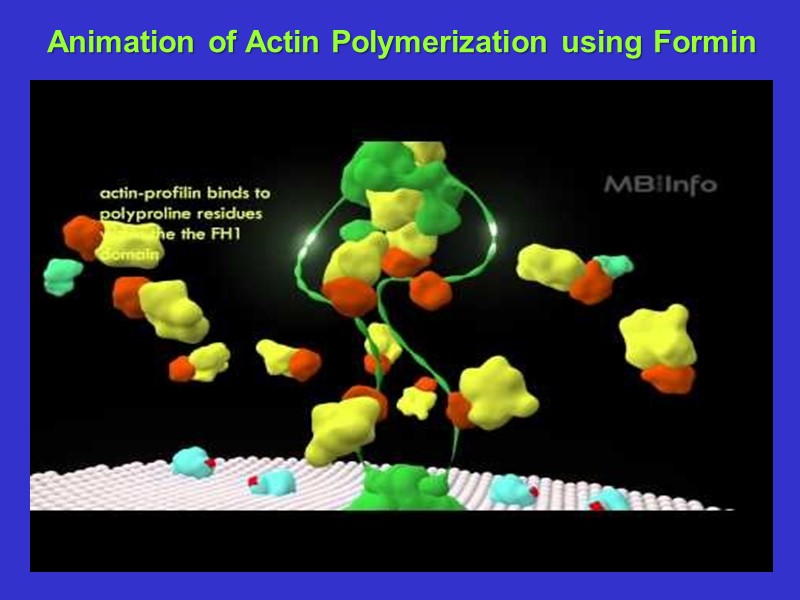
 Microfilament elongation in vivo is aided by the accessory proteins profilin and formin. Profilin binds to ATP- actin and the complex acts as a building block. The complex prevents nucleation. Formin binds P-A complexes and guides them to the growing (barbed) end of the Microfilament Formin Profilin Actin
Microfilament elongation in vivo is aided by the accessory proteins profilin and formin. Profilin binds to ATP- actin and the complex acts as a building block. The complex prevents nucleation. Formin binds P-A complexes and guides them to the growing (barbed) end of the Microfilament Formin Profilin Actin
 Animation of Actin Polymerization using Formin
Animation of Actin Polymerization using Formin
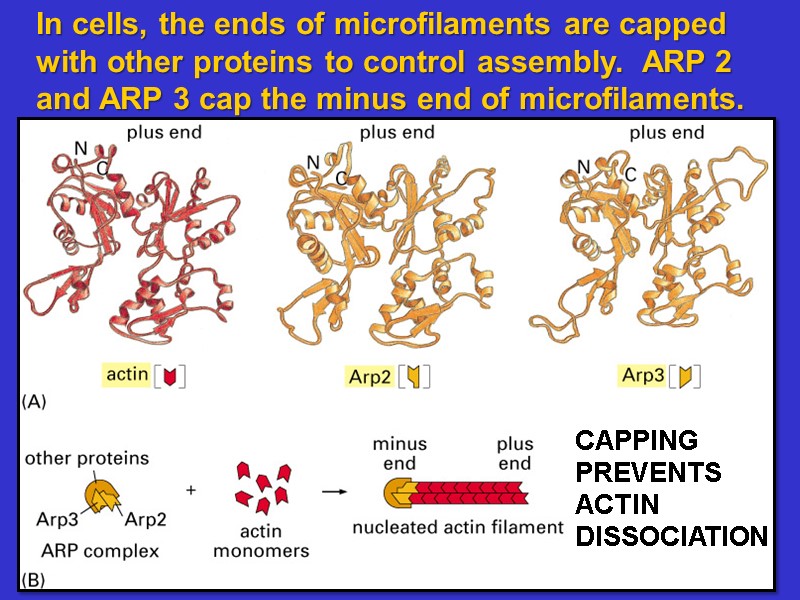
 In cells, the ends of microfilaments are capped with other proteins to control assembly. ARP 2 and ARP 3 cap the minus end of microfilaments.
In cells, the ends of microfilaments are capped with other proteins to control assembly. ARP 2 and ARP 3 cap the minus end of microfilaments.
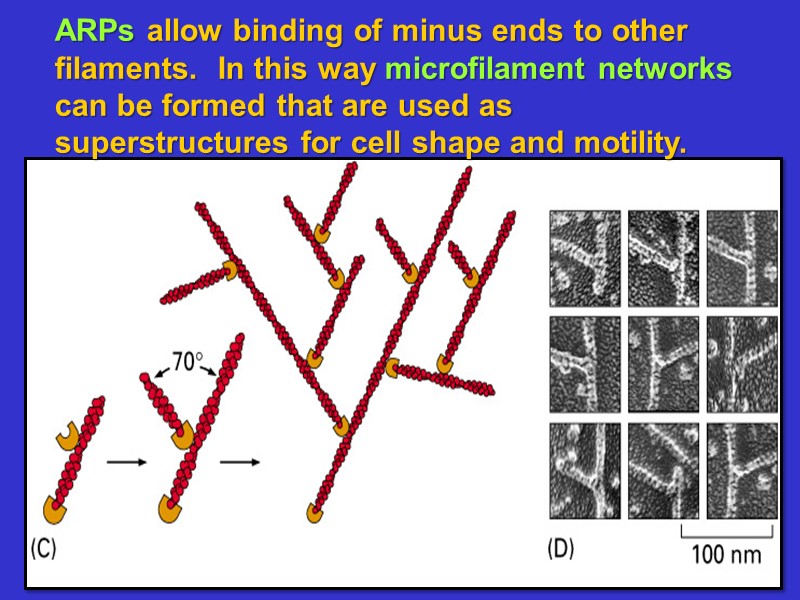
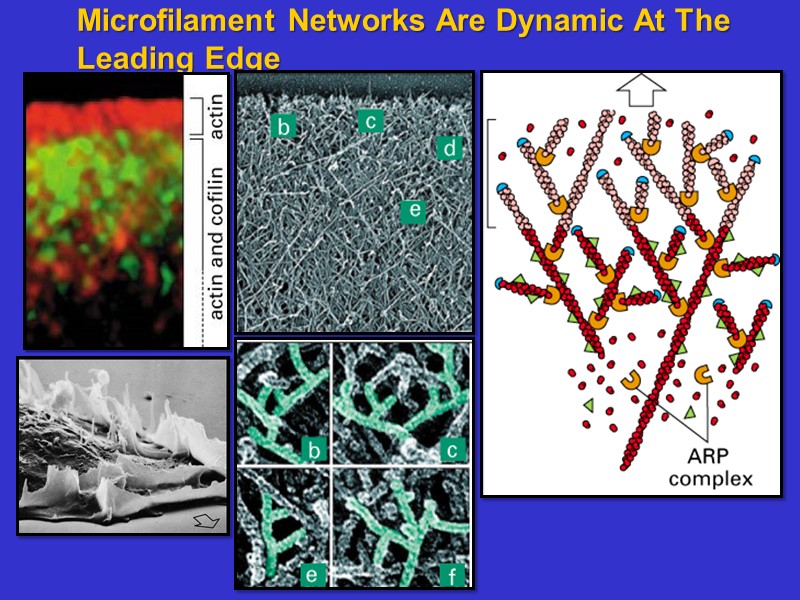
 ARPs allow binding of minus ends to other filaments. In this way microfilament networks can be formed that are used as superstructures for cell shape and motility.
ARPs allow binding of minus ends to other filaments. In this way microfilament networks can be formed that are used as superstructures for cell shape and motility.
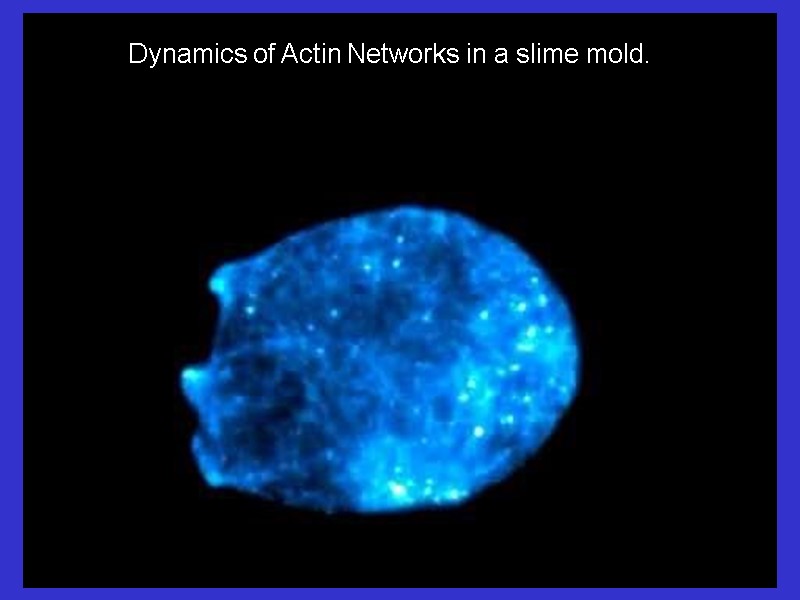
 Dynamics of Actin Networks in a slime mold.
Dynamics of Actin Networks in a slime mold.
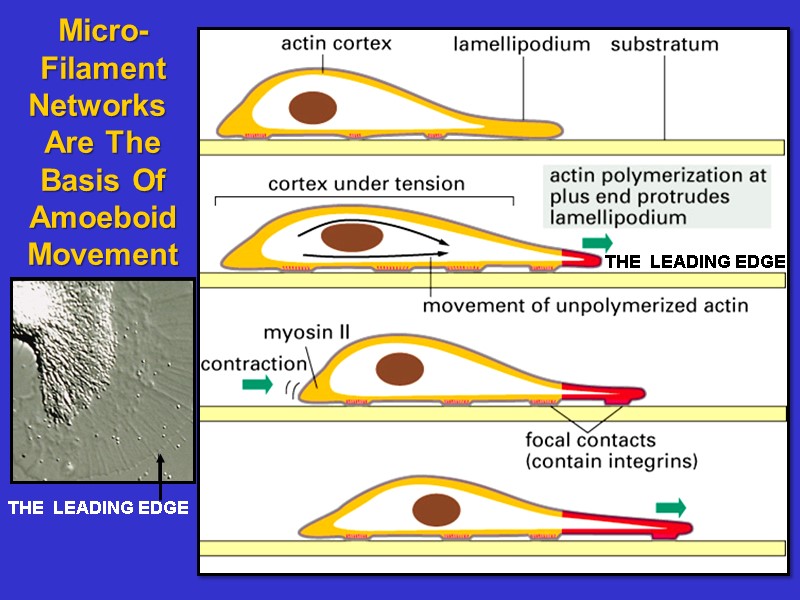
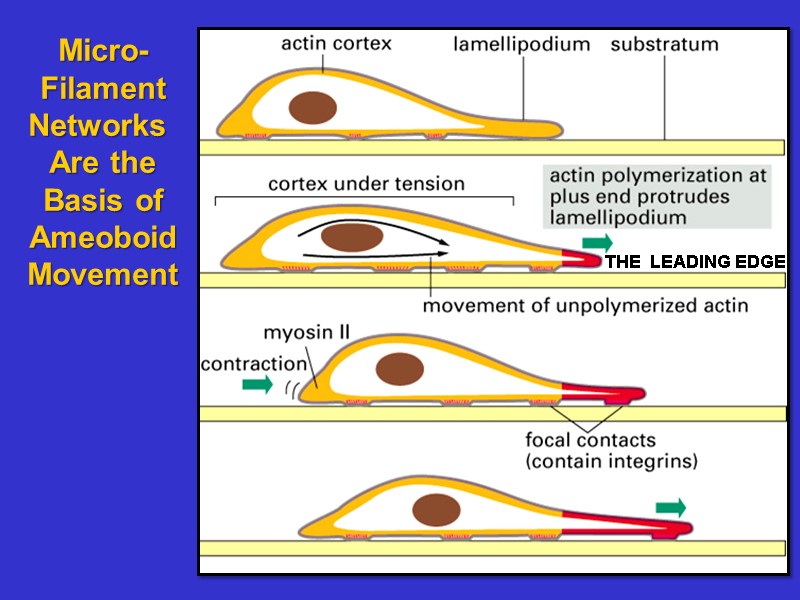
 Micro- Filament Networks Are The Basis Of Amoeboid Movement THE LEADING EDGE THE LEADING EDGE
Micro- Filament Networks Are The Basis Of Amoeboid Movement THE LEADING EDGE THE LEADING EDGE
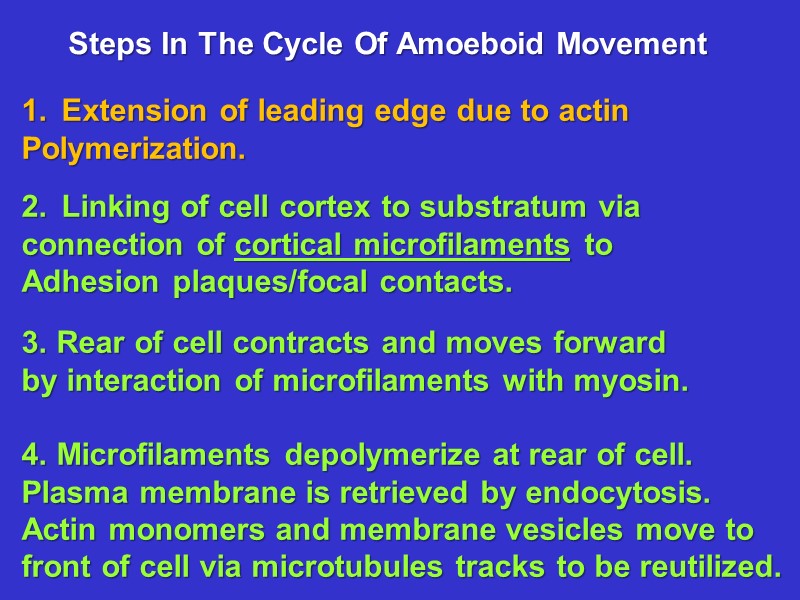
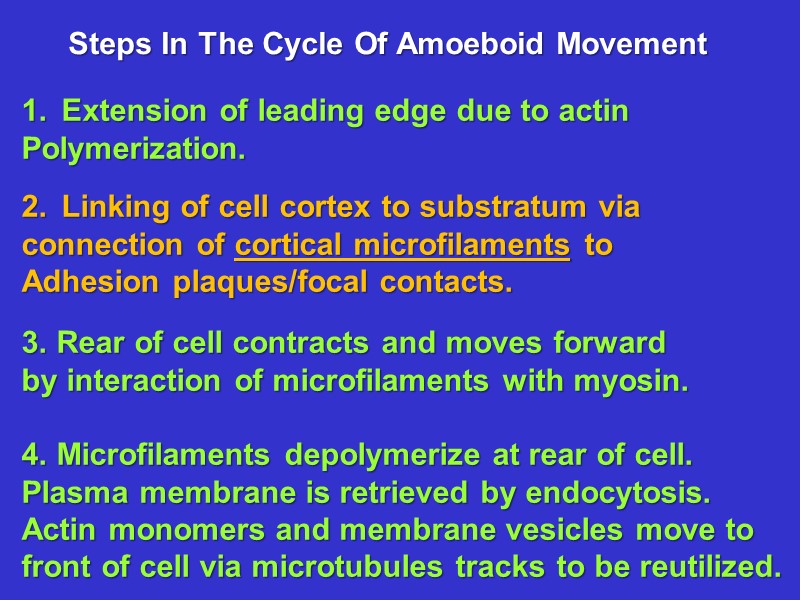
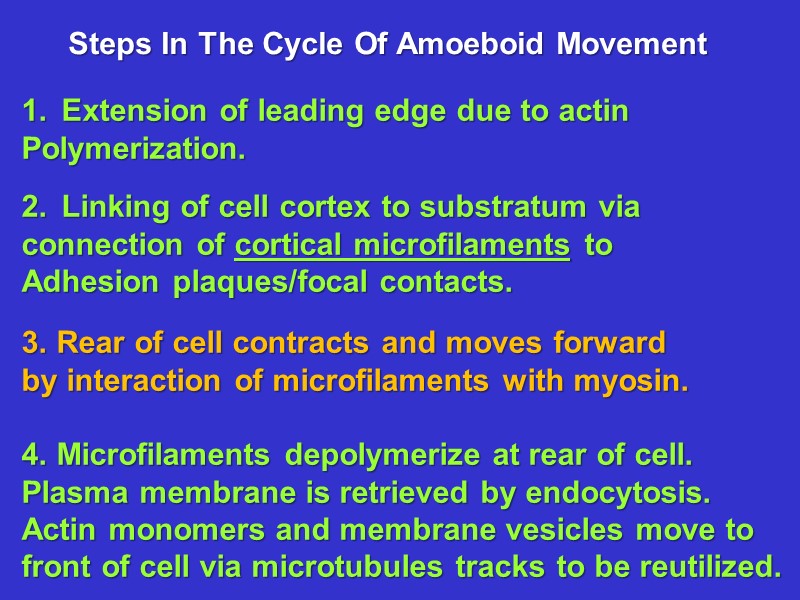
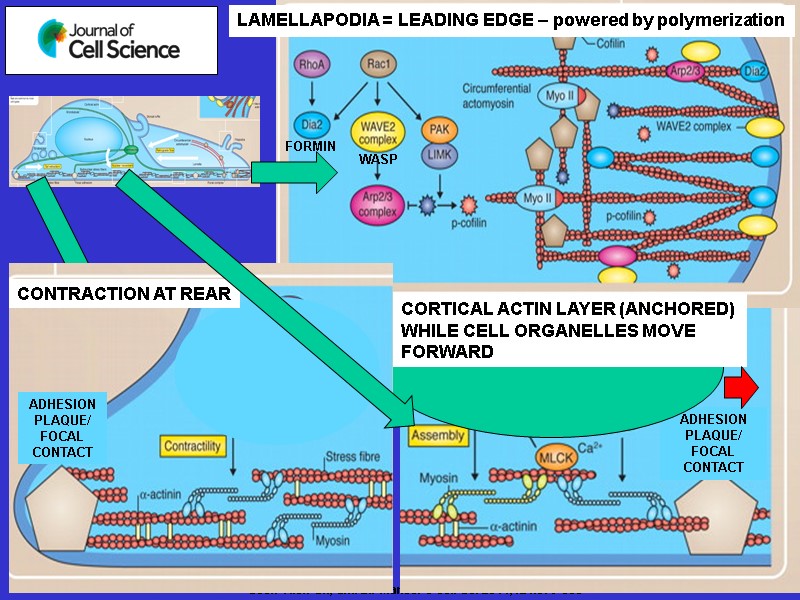
 Steps In The Cycle Of Amoeboid Movement Extension of leading edge due to actin Polymerization. Linking of cell cortex to substratum via connection of cortical microfilaments to Adhesion plaques/focal contacts. 3. Rear of cell contracts and moves forward by interaction of microfilaments with myosin. 4. Microfilaments depolymerize at rear of cell. Plasma membrane is retrieved by endocytosis. Actin monomers and membrane vesicles move to front of cell via microtubules tracks to be reutilized.
Steps In The Cycle Of Amoeboid Movement Extension of leading edge due to actin Polymerization. Linking of cell cortex to substratum via connection of cortical microfilaments to Adhesion plaques/focal contacts. 3. Rear of cell contracts and moves forward by interaction of microfilaments with myosin. 4. Microfilaments depolymerize at rear of cell. Plasma membrane is retrieved by endocytosis. Actin monomers and membrane vesicles move to front of cell via microtubules tracks to be reutilized.
 Microfilament Networks Are Dynamic At The Leading Edge
Microfilament Networks Are Dynamic At The Leading Edge
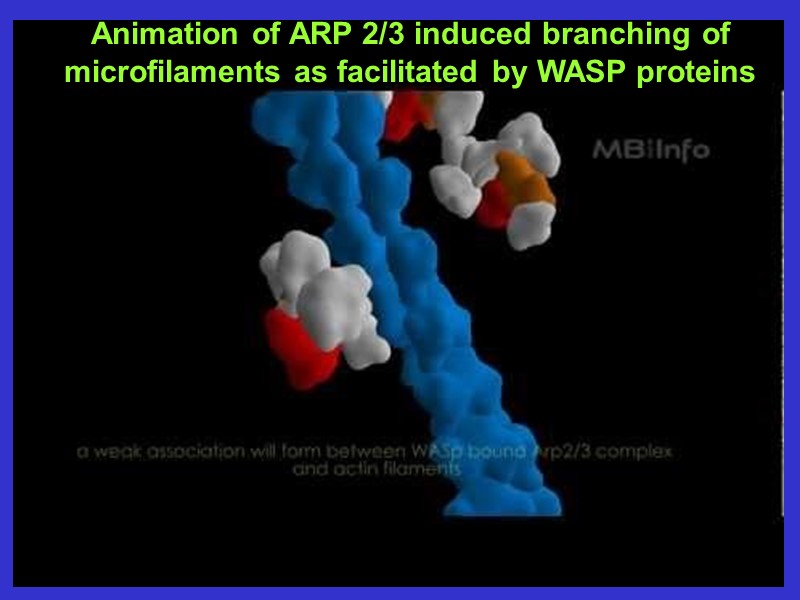
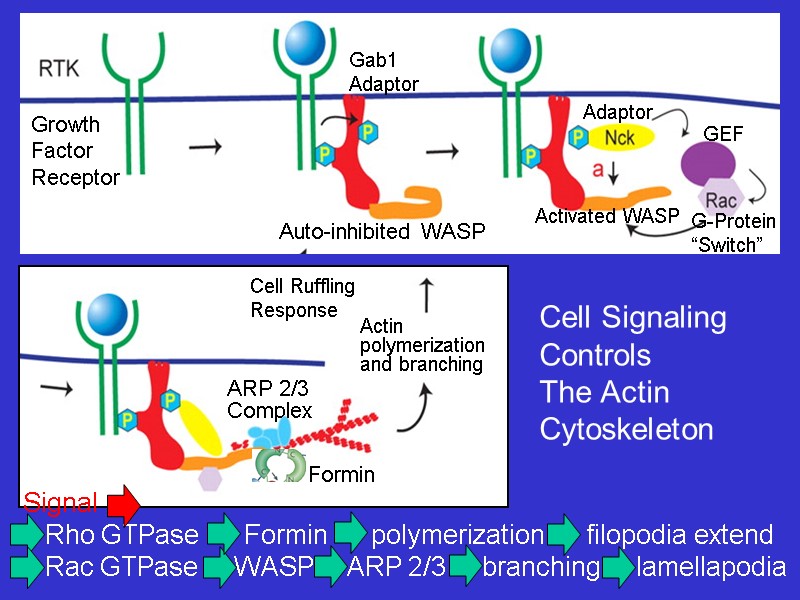
 Animation of ARP 2/3 induced branching of microfilaments as facilitated by WASP proteins
Animation of ARP 2/3 induced branching of microfilaments as facilitated by WASP proteins
 Cell Signaling Controls The Actin Cytoskeleton Formin Actin polymerization and branching
Cell Signaling Controls The Actin Cytoskeleton Formin Actin polymerization and branching
 Micro- Filament Networks Are the Basis of Ameoboid Movement THE LEADING EDGE
Micro- Filament Networks Are the Basis of Ameoboid Movement THE LEADING EDGE
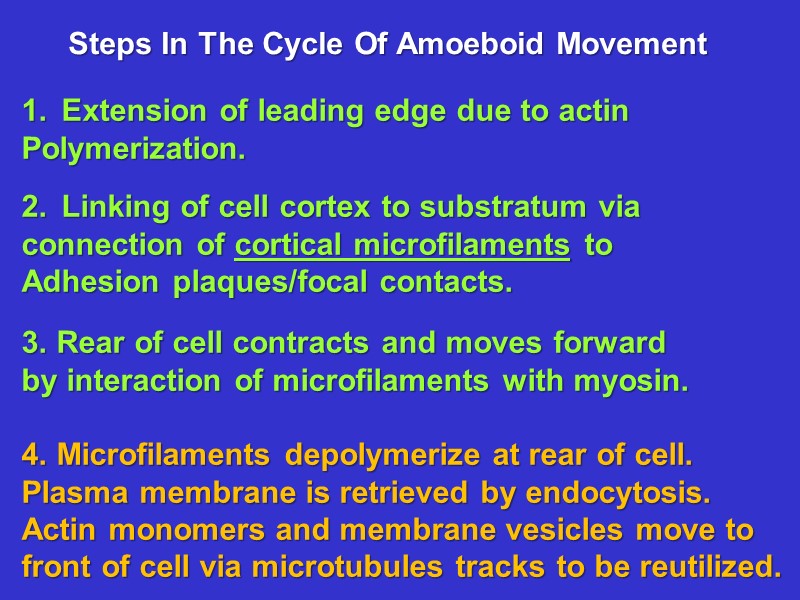
 Steps In The Cycle Of Amoeboid Movement Extension of leading edge due to actin Polymerization. Linking of cell cortex to substratum via connection of cortical microfilaments to Adhesion plaques/focal contacts. 3. Rear of cell contracts and moves forward by interaction of microfilaments with myosin. 4. Microfilaments depolymerize at rear of cell. Plasma membrane is retrieved by endocytosis. Actin monomers and membrane vesicles move to front of cell via microtubules tracks to be reutilized.
Steps In The Cycle Of Amoeboid Movement Extension of leading edge due to actin Polymerization. Linking of cell cortex to substratum via connection of cortical microfilaments to Adhesion plaques/focal contacts. 3. Rear of cell contracts and moves forward by interaction of microfilaments with myosin. 4. Microfilaments depolymerize at rear of cell. Plasma membrane is retrieved by endocytosis. Actin monomers and membrane vesicles move to front of cell via microtubules tracks to be reutilized.
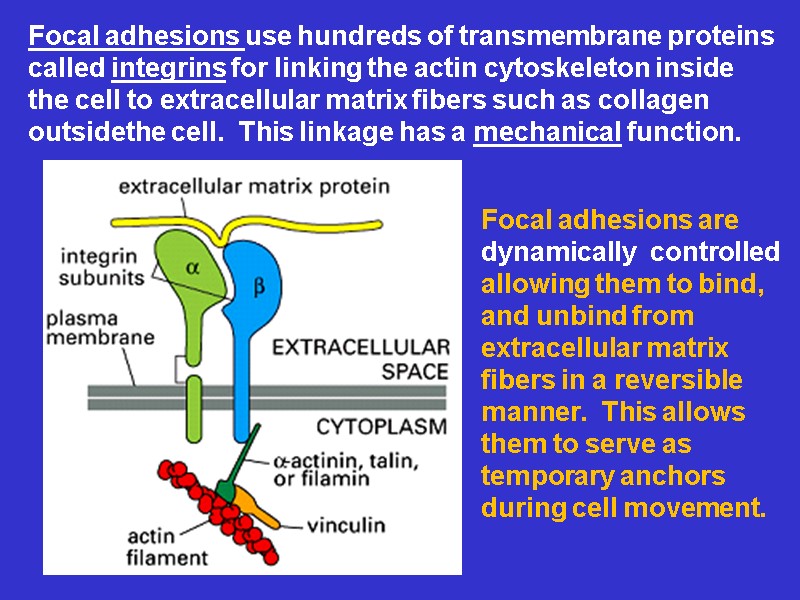
 Focal adhesions use hundreds of transmembrane proteins called integrins for linking the actin cytoskeleton inside the cell to extracellular matrix fibers such as collagen outsidethe cell. This linkage has a mechanical function. Focal adhesions are dynamically controlled allowing them to bind, and unbind from extracellular matrix fibers in a reversible manner. This allows them to serve as temporary anchors during cell movement.
Focal adhesions use hundreds of transmembrane proteins called integrins for linking the actin cytoskeleton inside the cell to extracellular matrix fibers such as collagen outsidethe cell. This linkage has a mechanical function. Focal adhesions are dynamically controlled allowing them to bind, and unbind from extracellular matrix fibers in a reversible manner. This allows them to serve as temporary anchors during cell movement.
 Steps In The Cycle Of Amoeboid Movement Extension of leading edge due to actin Polymerization. Linking of cell cortex to substratum via connection of cortical microfilaments to Adhesion plaques/focal contacts. 3. Rear of cell contracts and moves forward by interaction of microfilaments with myosin. 4. Microfilaments depolymerize at rear of cell. Plasma membrane is retrieved by endocytosis. Actin monomers and membrane vesicles move to front of cell via microtubules tracks to be reutilized.
Steps In The Cycle Of Amoeboid Movement Extension of leading edge due to actin Polymerization. Linking of cell cortex to substratum via connection of cortical microfilaments to Adhesion plaques/focal contacts. 3. Rear of cell contracts and moves forward by interaction of microfilaments with myosin. 4. Microfilaments depolymerize at rear of cell. Plasma membrane is retrieved by endocytosis. Actin monomers and membrane vesicles move to front of cell via microtubules tracks to be reutilized.
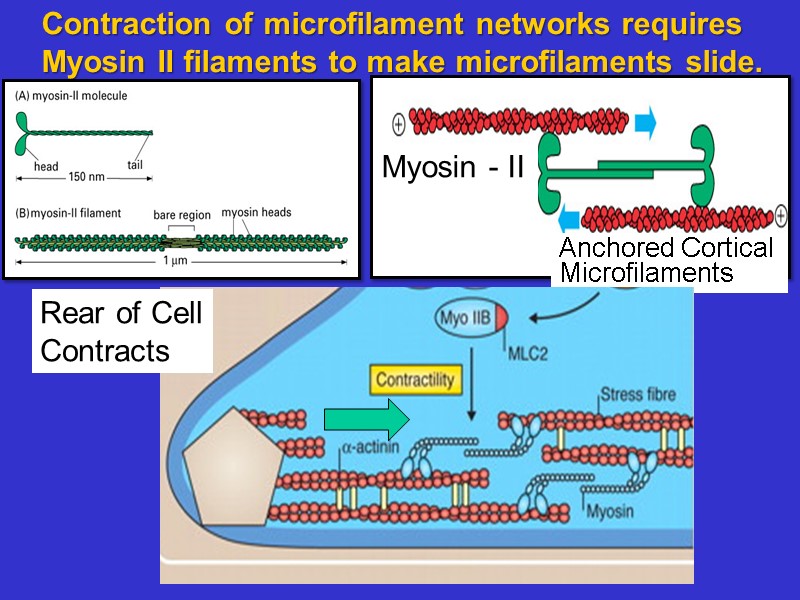
 Contraction of microfilament networks requires Myosin II filaments to make microfilaments slide. Rear of Cell Contracts
Contraction of microfilament networks requires Myosin II filaments to make microfilaments slide. Rear of Cell Contracts
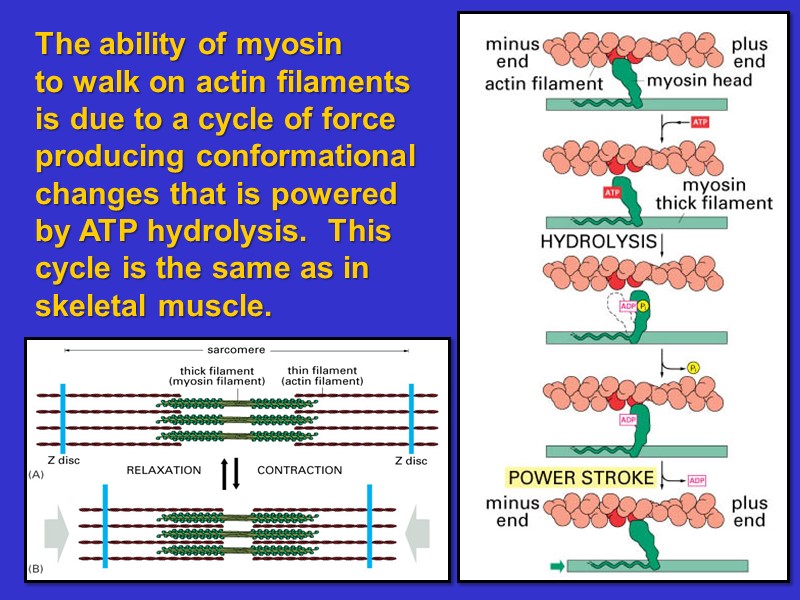
 The ability of myosin to walk on actin filaments is due to a cycle of force producing conformational changes that is powered by ATP hydrolysis. This cycle is the same as in skeletal muscle.
The ability of myosin to walk on actin filaments is due to a cycle of force producing conformational changes that is powered by ATP hydrolysis. This cycle is the same as in skeletal muscle.
 Steps In The Cycle Of Amoeboid Movement Extension of leading edge due to actin Polymerization. Linking of cell cortex to substratum via connection of cortical microfilaments to Adhesion plaques/focal contacts. 3. Rear of cell contracts and moves forward by interaction of microfilaments with myosin. 4. Microfilaments depolymerize at rear of cell. Plasma membrane is retrieved by endocytosis. Actin monomers and membrane vesicles move to front of cell via microtubules tracks to be reutilized.
Steps In The Cycle Of Amoeboid Movement Extension of leading edge due to actin Polymerization. Linking of cell cortex to substratum via connection of cortical microfilaments to Adhesion plaques/focal contacts. 3. Rear of cell contracts and moves forward by interaction of microfilaments with myosin. 4. Microfilaments depolymerize at rear of cell. Plasma membrane is retrieved by endocytosis. Actin monomers and membrane vesicles move to front of cell via microtubules tracks to be reutilized.
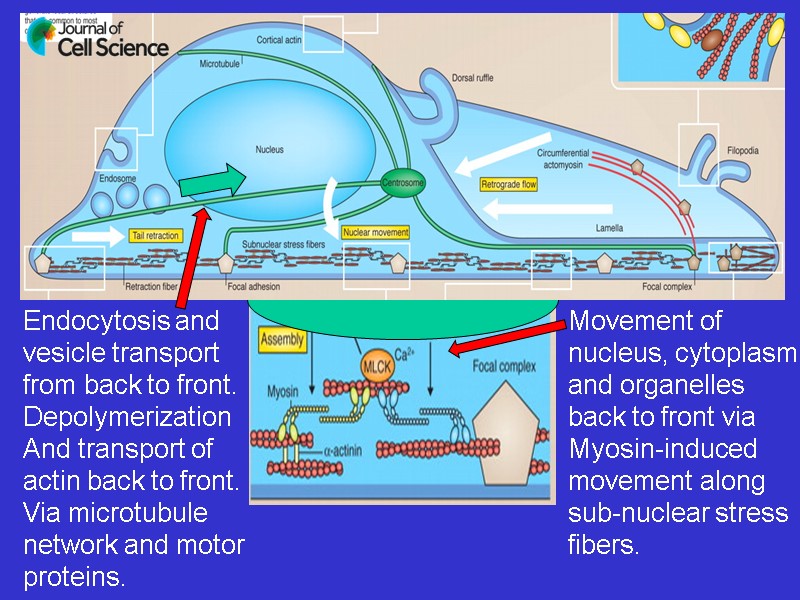
 Endocytosis and vesicle transport from back to front. Depolymerization And transport of actin back to front. Via microtubule network and motor proteins. Movement of nucleus, cytoplasm and organelles back to front via Myosin-induced movement along sub-nuclear stress fibers.
Endocytosis and vesicle transport from back to front. Depolymerization And transport of actin back to front. Via microtubule network and motor proteins. Movement of nucleus, cytoplasm and organelles back to front via Myosin-induced movement along sub-nuclear stress fibers.
 Soon-Tuck Sit, and Ed Manser J Cell Sci 2011;124:679-683 ADHESION PLAQUE/ FOCAL CONTACT CORTICAL ACTIN LAYER (ANCHORED) WHILE CELL ORGANELLES MOVE FORWARD
Soon-Tuck Sit, and Ed Manser J Cell Sci 2011;124:679-683 ADHESION PLAQUE/ FOCAL CONTACT CORTICAL ACTIN LAYER (ANCHORED) WHILE CELL ORGANELLES MOVE FORWARD

