e6ed02de339896a564812fa646fddae5.ppt
- Количество слайдов: 43

Lecture Power. Point to accompany Molecular Biology Fourth Edition Robert F. Weaver Chapter 17 The Mechanism of Translation I: Initiation Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.
17. 1 Initiation of Translation in Bacteria • Two important events must occur before translation initiation can take place – Generate a supply of aminoacyl-t. RNAs • Amino acids must be covalently bound to t. RNAs • Process of bonding t. RNA to amino acid is called t. RNA charging – Dissociation of ribosomes into their two subunits • The cell assembles the initiation complex on the small ribosomal subunit • The two subunits must separate to make assembly possible 2
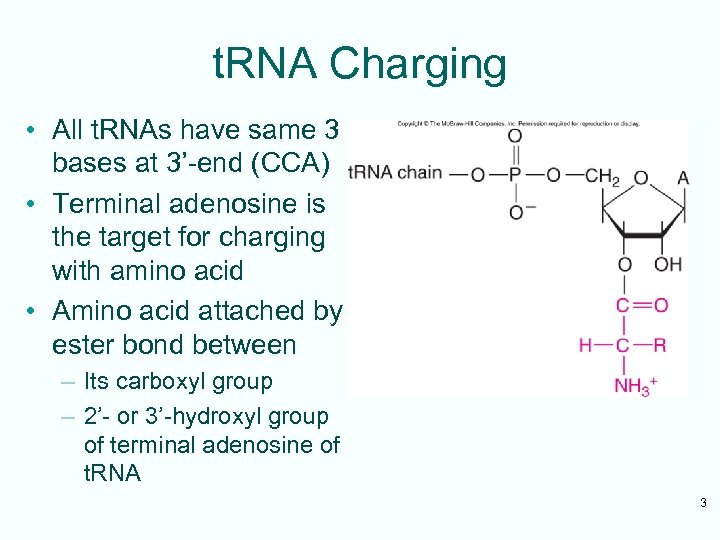
t. RNA Charging • All t. RNAs have same 3 bases at 3’-end (CCA) • Terminal adenosine is the target for charging with amino acid • Amino acid attached by ester bond between – Its carboxyl group – 2’- or 3’-hydroxyl group of terminal adenosine of t. RNA 3
Two-Step Charging • Aminoacyl-t. RNA synthetases join amino acids to their cognate t. RNAs • This is done in a two-step reaction: – Begins with activation of the amino acid with AMP derived from ATP – In the second step, the energy from the aminoacyl-AMP is used to transfer the amino acid to the t. RNA 4
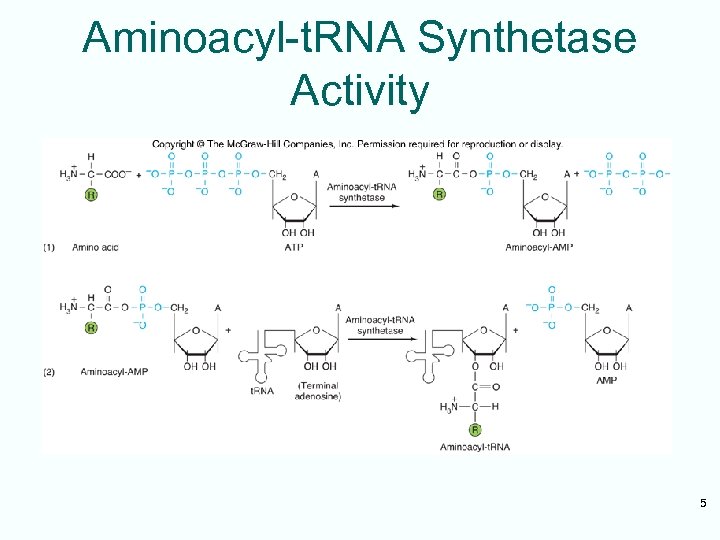
Aminoacyl-t. RNA Synthetase Activity 5
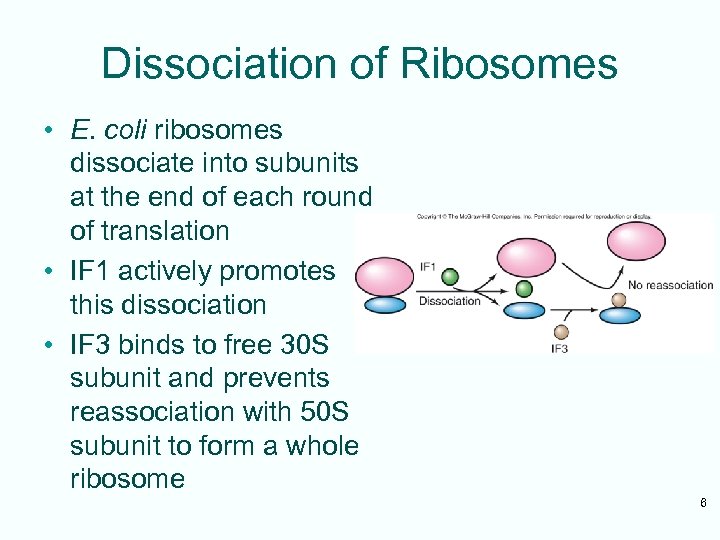
Dissociation of Ribosomes • E. coli ribosomes dissociate into subunits at the end of each round of translation • IF 1 actively promotes this dissociation • IF 3 binds to free 30 S subunit and prevents reassociation with 50 S subunit to form a whole ribosome 6
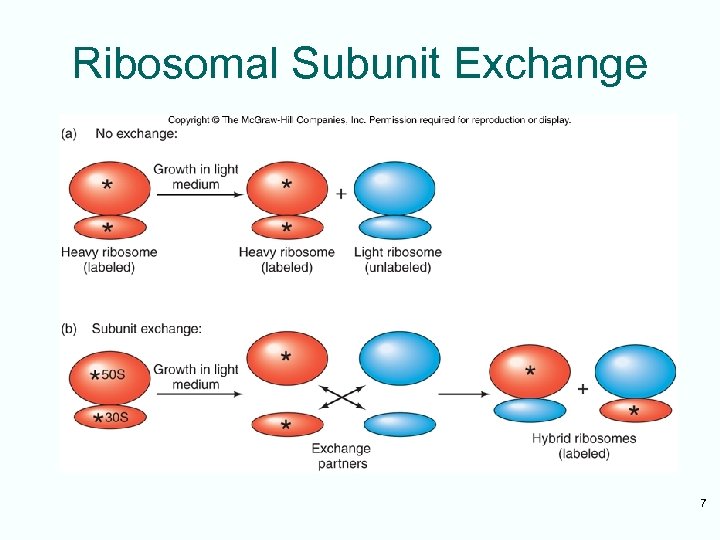
Ribosomal Subunit Exchange 7
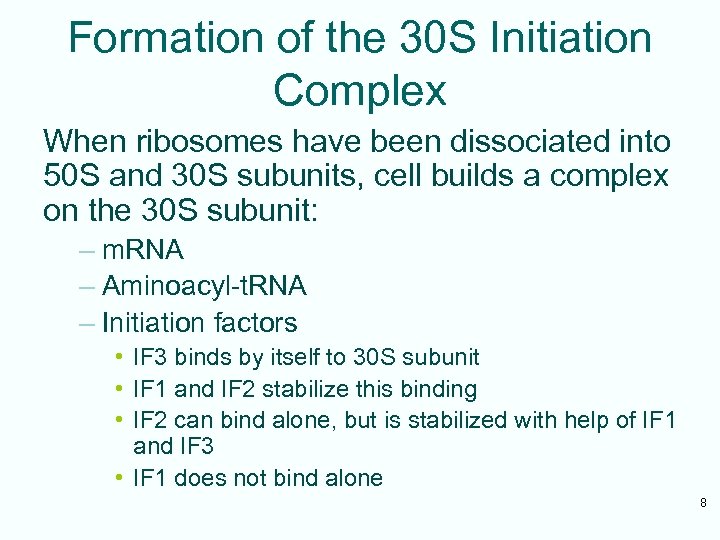
Formation of the 30 S Initiation Complex When ribosomes have been dissociated into 50 S and 30 S subunits, cell builds a complex on the 30 S subunit: – m. RNA – Aminoacyl-t. RNA – Initiation factors • IF 3 binds by itself to 30 S subunit • IF 1 and IF 2 stabilize this binding • IF 2 can bind alone, but is stabilized with help of IF 1 and IF 3 • IF 1 does not bind alone 8
First Codon and the First Aminoacyl-t. RNA • Prokaryotic initiation codon is: – Usually AUG – Can be GUG – Rarely UUG • Initiating aminoacyl-t. RNA is N-formylmethionyl-t. RNA • N-formyl-methionine (f. Met) is the first amino acid incorporated into a polypeptide • This amino acid is frequently removed from the protein during maturation 9
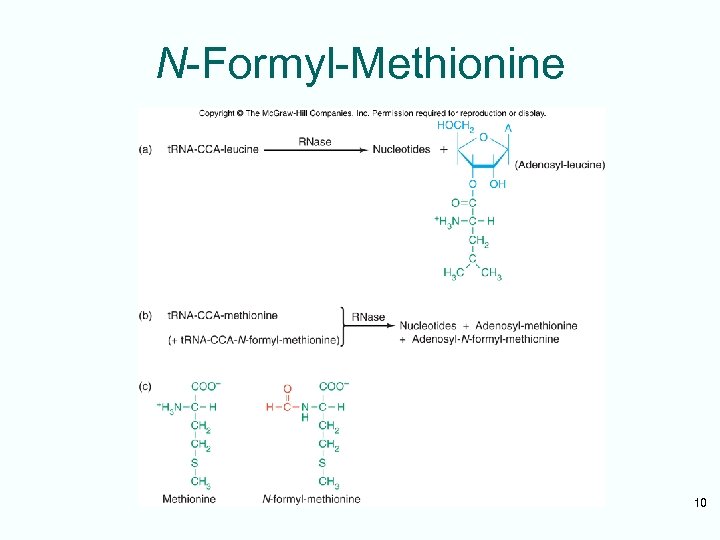
N-Formyl-Methionine 10
Binding m. RNA to the 30 S Ribosomal Subunit • The 30 S initiation complex is formed from a free 30 S ribosomal subunit plus m. RNA and f. Met-t. RNA • Binding between the 30 S prokaryotic ribosomal subunit and the initiation site of a message depends on base pairing between – Short RNA sequence • Shine-Dalgarno sequence • Upstream of initiation codon – Complementary sequence • 3’-end of 16 S RNA 11
Initiation Factors and 30 S Subunit • Binding of the Shine-Dalgarno sequence with the complementary sequence of the 16 S r. RNA is mediated by IF 3 – Assisted by IF 1 and IF 2 – All 3 initiation factors have bound to the 30 S subunit at this time 12
Binding of f. Met-t. RNA to the 30 S Initiation Complex • IF 2 is the major factor promoting binding of f. Met-t. RNA to the 30 S initiation complex • Two other initiation factors also play an important supporting role • GTP is also required for IF 2 binding at physiological IF 2 concentrations • The GTP is not hydrolyzed in the process 13

30 S Initiation Complex The complete 30 S initiation complex contains one each: – 30 S ribosomal subunit – m. RNA – f. Met-t. RNA – GTP – Factors IF 1, IF 2, IF 3 14
Formation of the 70 S Initiation Complex • GTP is hydrolyzed after the 50 S subunit joins the 30 S complex to form the 70 S initiation complex • This GTP hydrolysis is carried out by IF 2 in conjunction with the 50 S ribosomal subunit • Hydrolysis purpose is to release IF 2 and GTP from the complex so polypeptide chain elongation can begin 15
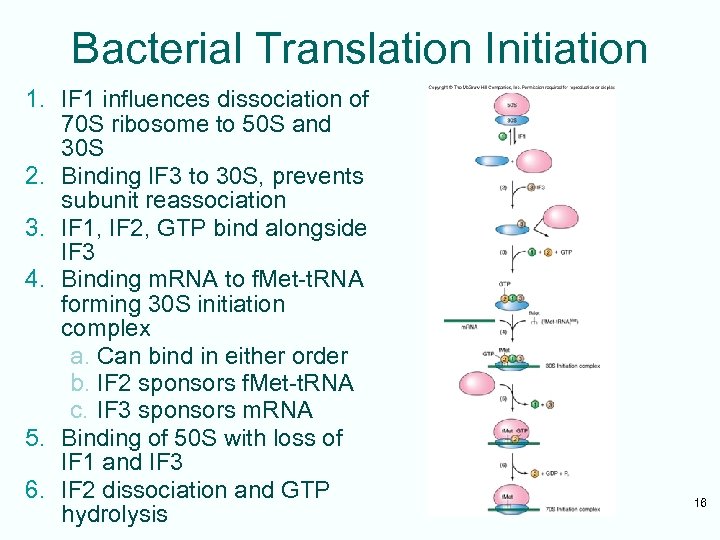
Bacterial Translation Initiation 1. IF 1 influences dissociation of 70 S ribosome to 50 S and 30 S 2. Binding IF 3 to 30 S, prevents subunit reassociation 3. IF 1, IF 2, GTP bind alongside IF 3 4. Binding m. RNA to f. Met-t. RNA forming 30 S initiation complex a. Can bind in either order b. IF 2 sponsors f. Met-t. RNA c. IF 3 sponsors m. RNA 5. Binding of 50 S with loss of IF 1 and IF 3 6. IF 2 dissociation and GTP hydrolysis 16
17. 2 Initiation in Eukaryotes • Eukaryotic – Begins with methionine – Initiating t. RNA not same as t. RNA for interior – No Shine-Dalgarno – m. RNA have caps at 5’end • Bacterial – N-formyl-methionine – Shine-Dalgarno sequence to show ribosomes where to start 17
Scanning Model of Initiation • Eukaryotic 40 S ribosomal subunits locate start codon by binding to 5’-cap and scanning downstream to find the 1 st AUG in a favorable context • Kozak’s Rules are a set of requirements • Best context uses A of ACCAUGG as +1: – Purine in -3 position – G in +4 position • 5 -10% cases ribosomal subunits bypass 1 st AUG scanning for more favorable one 18
Translation With a Short ORF • Ribosomes can use a short upstream open reading frame: – Initiate at an upstream AUG – Translate a short Open Reading Frame – Continue scanning – Reinitiate at a downstream AUG 19
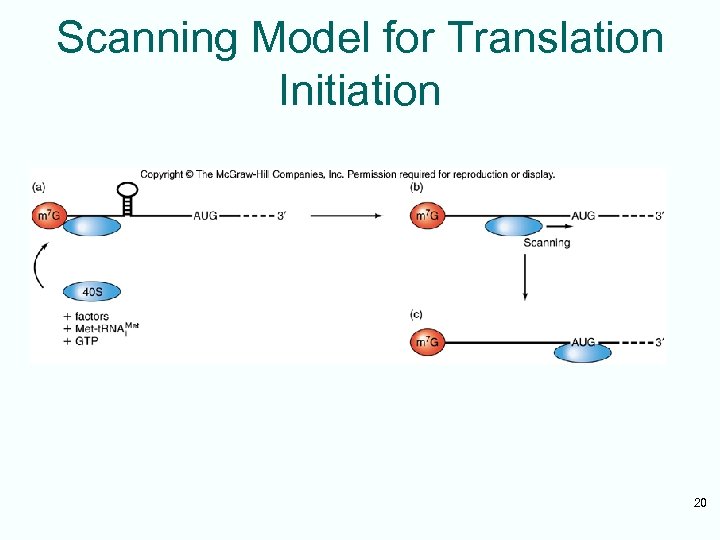
Scanning Model for Translation Initiation 20
Effects of m. RNA Secondary Structure • Secondary structure near the 5’-end of an m. RNA can have either positive or negative effects • Hairpin just past an AUG can force a pause by ribosomal subunit and stimulate translation • Very stable stem loop between cap and initiation site can block scanning and inhibit translation 21

Eukaryotic Initiation Factors • Bacterial translation initiation requires initiation factors as does eukaryotic initiation of translation • Eukaryotic system is more complex than bacterial – Scanning process – Factors to recognize the 5’-end cap 22
Translation Initiation in Eukaryotes Eukaryotic initiation factors and general functions: • e. IF 2 binds Met-t. RNA to ribosomes • e. IF 2 B activates e. IF 2 replacing its GDP with GTP • e. IF 1 and e. IF 1 A aid in scanning to initiation codon • e. IF 3 binds to 40 S ribosomal subunit, inhibits reassociation with 60 S subunit • e. IF 4 is a cap-binding protein allowing 40 S subunit to bind 5’-end of m. RNA • e. IF 5 encourages association between 60 S ribosome subunit and 48 S complex • e. IF 6 binds to 60 S subunit, blocks reassociation with 40 S subunit 23
Function of e. IF 4 • e. IF 4 is a cap-binding protein • This protein is composed of 3 parts: – e. IF 4 E, 24 -k. D, has actual cap binding activity – e. IF 4 A, a 50 -k. D polypeptide – e. IF 4 G is a 220 -k. D polypeptide • The complex of the three polypeptides together is called e. IF 4 F 24
Function of e. IF 4 A and e. IF 4 B • e. IF 4 A – Has an RNA helicase activity – This activity unwinds hairpins found in the 5’leaders of eukaryotic m. RNA – Unwinding activity is ATP dependent • e. IF 4 B – Has an RNA-binding domain – Can stimulate the binding of e. IF 4 A to m. RNA 25
Function of e. IF 4 G • e. IF 4 G is an adaptor protein capable of binding to other proteins including: – e. IF 4 E, cap-binding protein – e. IF 3, 40 S ribosomal subunit-binding protein – Pab 1 p, a poly[A]-binding protein • Interacting with these proteins lets e. IF 4 G recruit 40 S ribosomal subunits to m. RNA and stimulate translation 26
Structure and Function of e. IF 3 • e. IF 3 is a 5 -lobed protein that binds at the same site to: – e. IF 4 G – Prominent part of viral IRES • This explains how the IRES can substitute for 40 S ribosomal subunit to m. RNA • Cryo-EM studies have produced a model for the e. IF 3 -IRES-40 S complex explaining how e. IF 3 prevents premature 40 S-60 S association 27
Prevention of Premature 40 S 60 S Association • e. IF 3 blocks key contact point between subunits 40 S and 60 S • e. IF 4 G, so also e. IF 4 E, locate close to the cap on an m. RNA bound to 40 S ribosomal particle • e. IF 4 would be in position to cap-bind 28
Functions of e. IF 1 and e. IF 1 A • e. IF 1 and e. IF 1 A act synergistically to promote formation of a stable 48 S complex involving: – Initiation factors – Met-t. RNA – 40 S ribosomal subunits bound at initiation codon of m. RNA • e. IF 1 and e. IF 1 A act by – Dissociating improper complexes between 40 S subunits and m. RNA – Encouraging formation of stable 48 S complexes 29
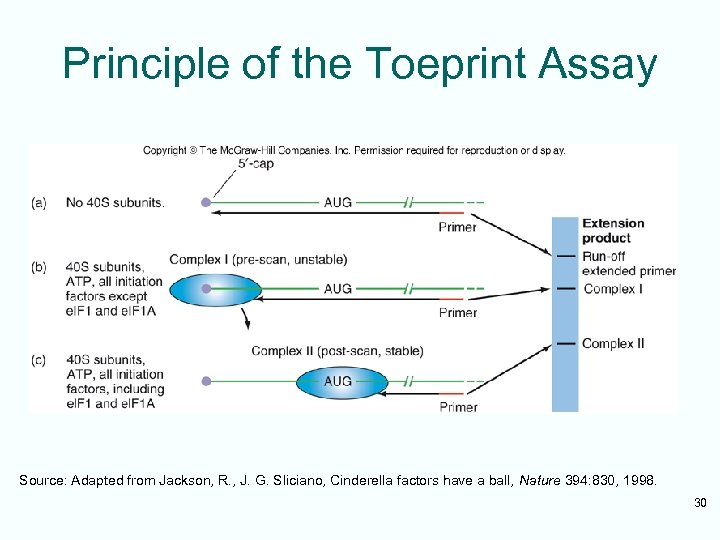
Principle of the Toeprint Assay Source: Adapted from Jackson, R. , J. G. Sliciano, Cinderella factors have a ball, Nature 394: 830, 1998. 30
Functions of e. IF 5 and e. IF 5 B • e. IF 5 B is homologous to prokaryotic factor IF 2 – Binds GTP • Uses GTP hydrolysis to promote its own dissociation from ribosome • Permits protein synthesis to begin – Stimulates association of 2 ribosomal subunits • Differs from IF 2 as e. IF 5 B cannot stimulate binding of initiating aminoacyl-t. RNA to small ribosomal subunit • e. IF 5 B works with e. IF 5 31
17. 3 Control of Initiation • Given the amount of control at the transcriptional and posttranscriptional levels, why control gene expression at translational level? • Major advantage = speed – New gene products can be produced quickly – Simply turn on translation of preexisting m. RNA • Valuable in eukaryotes • Transcripts are relatively long • Take correspondingly long time to make – Most control of translation happens at the initiation step 32
Bacterial Translational Control • Most bacterial gene expression is controlled at transcription level • Majority of bacterial m. RNA has a very short lifetime – Only 1 to 3 minutes – Allows bacteria to respond quickly to changing circumstances • Different cistrons on a polycistronic transcript can be translated better than others 33
Shifts in m. RNA Secondary Structure • m. RNA secondary structure can govern translation initiation – Replicase gene of the MS 2 class of phages • Initiation codon is buried in secondary structure until ribosomes translating the coat gene open up the structure – Heat shock sigma factor, s 32 of E. coli • Repressed by secondary structure that is relaxed by heating • Heat can cause an immediate unmasking of initiation codons and burst of synthesis 34
Proteins/m. RNAs Induce m. RNA Secondary Structure Shifts • Small RNAs with proteins can affect m. RNA 2° structure to control translation initiation • Riboswitches can be used to control translation initiation via m. RNA 2° structure – 5’-untranslated region of E. coli thi. M m. RNA contain a riboswitch – This includes an aptamer that binds thiamine and its metabolite • Thiamine phosphate • Thiamine pyrophosphate (TTP) 35
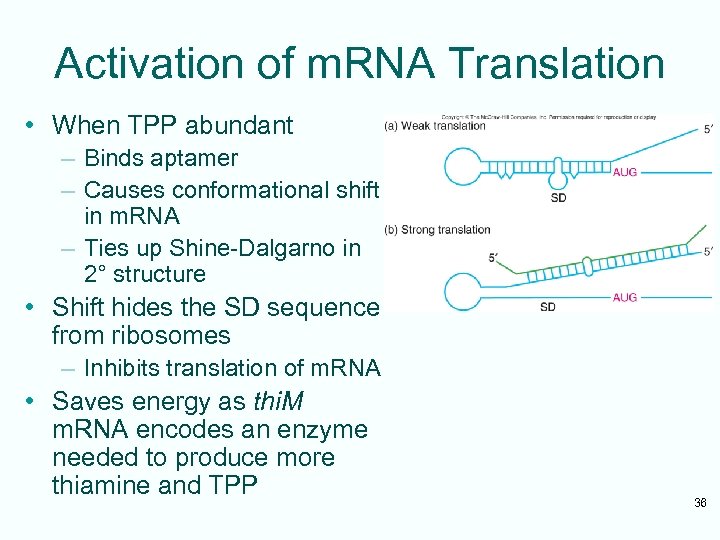
Activation of m. RNA Translation • When TPP abundant – Binds aptamer – Causes conformational shift in m. RNA – Ties up Shine-Dalgarno in 2° structure • Shift hides the SD sequence from ribosomes – Inhibits translation of m. RNA • Saves energy as thi. M m. RNA encodes an enzyme needed to produce more thiamine and TPP 36
Eukaryotic Translational Control • Eukaryotic m. RNA lifetimes are relatively long, so there is more opportunity for translation control than in bacteria • e. IF 2 a-subunit is a favorite target for translation control – Heme-starved reticulocytes activate HCR (hemecontrolled repressor) • Phosphorylates e. IF 2 a • Inhibit initiation – Virus-infected cells have another kinase, DAI – Phosphorylates e. IF 2 a – Inhibits translation initiation 37
Phosphorylation of an e. IF 4 EBinding Protein • Insulin and a number of growth factors stimulate a pathway involving a protein kinase known as m. TOR • m. TOR kinase’s target protein – PHAS-1 (rat) – 4 E-BP 1 (human) • Once phosphorylated by m. TOR – This protein dissociates from e. IF 4 E – Releases it to participate in active translation initiation 38
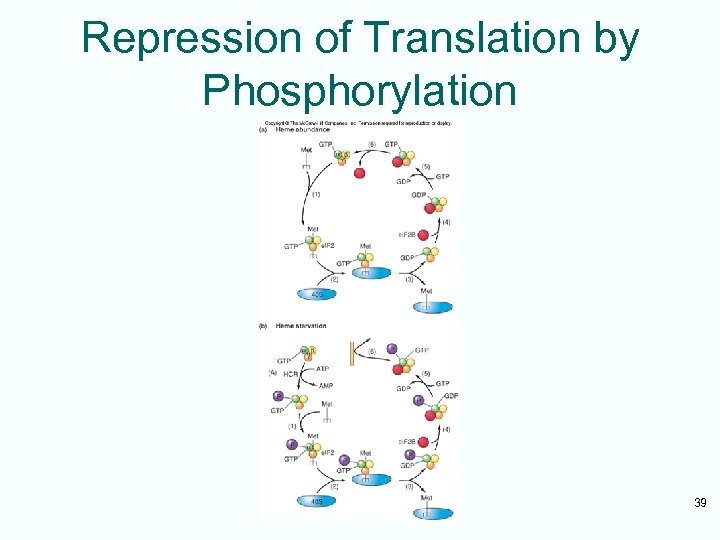
Repression of Translation by Phosphorylation 39
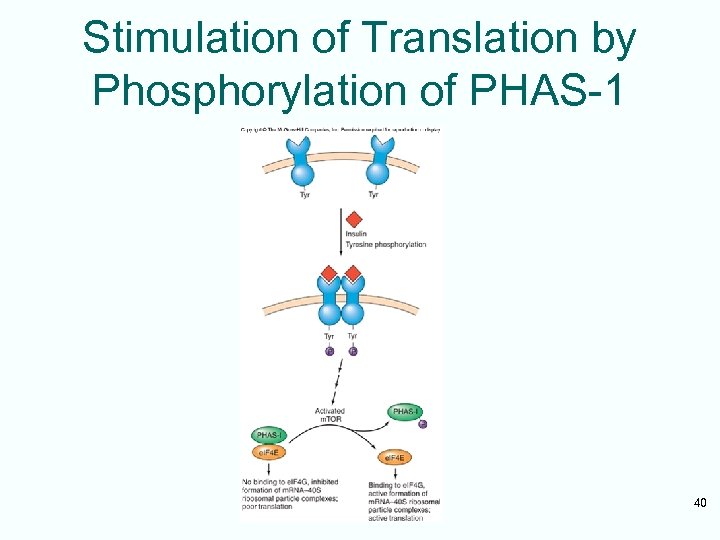
Stimulation of Translation by Phosphorylation of PHAS-1 40
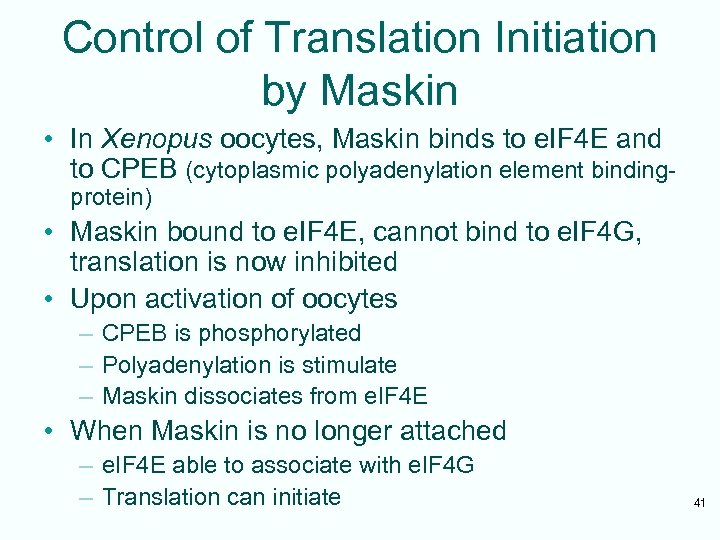
Control of Translation Initiation by Maskin • In Xenopus oocytes, Maskin binds to e. IF 4 E and to CPEB (cytoplasmic polyadenylation element bindingprotein) • Maskin bound to e. IF 4 E, cannot bind to e. IF 4 G, translation is now inhibited • Upon activation of oocytes – CPEB is phosphorylated – Polyadenylation is stimulate – Maskin dissociates from e. IF 4 E • When Maskin is no longer attached – e. IF 4 E able to associate with e. IF 4 G – Translation can initiate 41
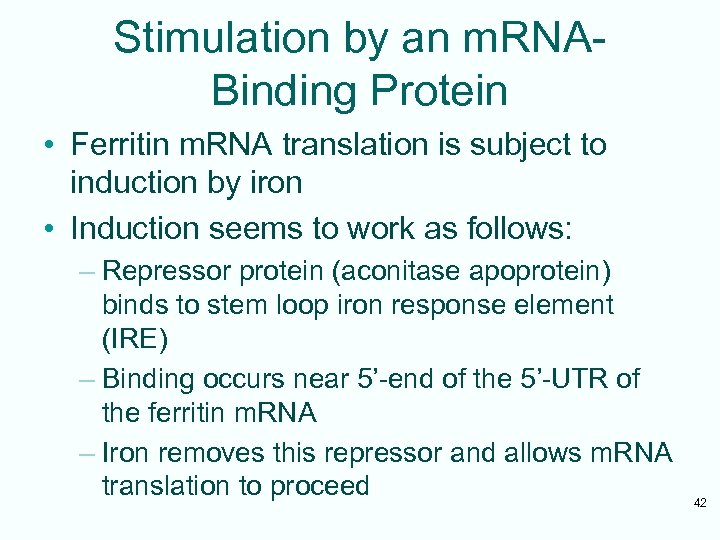
Stimulation by an m. RNABinding Protein • Ferritin m. RNA translation is subject to induction by iron • Induction seems to work as follows: – Repressor protein (aconitase apoprotein) binds to stem loop iron response element (IRE) – Binding occurs near 5’-end of the 5’-UTR of the ferritin m. RNA – Iron removes this repressor and allows m. RNA translation to proceed 42
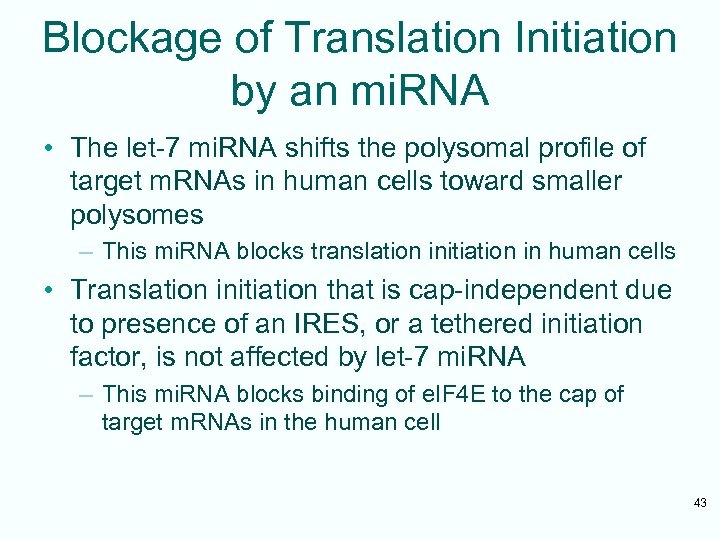
Blockage of Translation Initiation by an mi. RNA • The let-7 mi. RNA shifts the polysomal profile of target m. RNAs in human cells toward smaller polysomes – This mi. RNA blocks translation initiation in human cells • Translation initiation that is cap-independent due to presence of an IRES, or a tethered initiation factor, is not affected by let-7 mi. RNA – This mi. RNA blocks binding of e. IF 4 E to the cap of target m. RNAs in the human cell 43
e6ed02de339896a564812fa646fddae5.ppt