83dace7a954d02b7bdabb21e72031a18.ppt
- Количество слайдов: 36
 Lecture Contents -- Unit 3 • Drug Discovery – – – Basic objectives and problems Screening approach vs. rational design Phytopharmacology Databases, QSAR, and Co. MFA “Pharmacogenomics” and “proteomics” Case study: GV 150526 A
Lecture Contents -- Unit 3 • Drug Discovery – – – Basic objectives and problems Screening approach vs. rational design Phytopharmacology Databases, QSAR, and Co. MFA “Pharmacogenomics” and “proteomics” Case study: GV 150526 A
 Basic Facts About Drug Discovery • Almost any metabolic pathway with all it’s adjuncts (receptors, enzymes, genes therefor, and regulatory elements) is a potential drug target • During the past century, pharmacology has identified some 400 such targets; the human genome project confirms that thousands must exist • Independent of this, the present rate of drug discovery is insufficient; new strategies are required
Basic Facts About Drug Discovery • Almost any metabolic pathway with all it’s adjuncts (receptors, enzymes, genes therefor, and regulatory elements) is a potential drug target • During the past century, pharmacology has identified some 400 such targets; the human genome project confirms that thousands must exist • Independent of this, the present rate of drug discovery is insufficient; new strategies are required
 Some Companies Specialize in Drug Discovery
Some Companies Specialize in Drug Discovery
 Drug Discovery Strategies • Screening-based: – Traditional medicine – Bioprospecting – Mass screening of microbial strains – Combinatorial chemistry • Rational Drug Design – Target interaction analysis and molecular modeling
Drug Discovery Strategies • Screening-based: – Traditional medicine – Bioprospecting – Mass screening of microbial strains – Combinatorial chemistry • Rational Drug Design – Target interaction analysis and molecular modeling
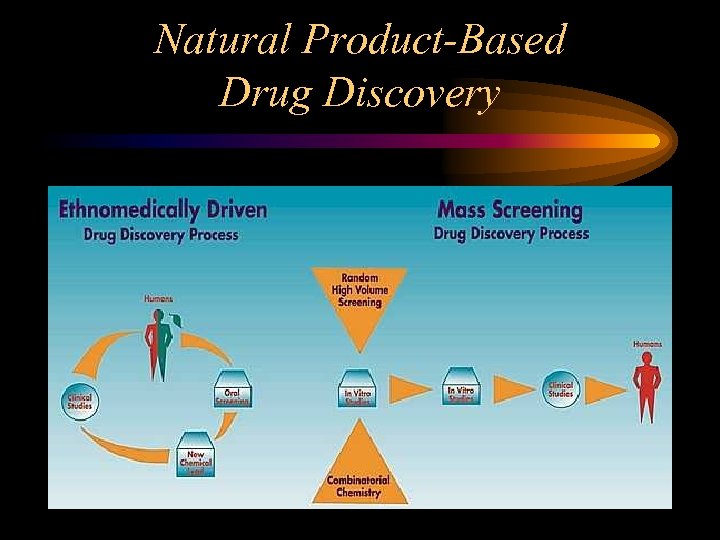 Natural Product-Based Drug Discovery
Natural Product-Based Drug Discovery
 Natural Product Success Stories • Microorganisms: Antibiotics • Plants: – Taxoids for cancer – Artemisinin for malaria – Huperzine A and galanthamine for Alzheimer • Animals: Conotoxins as ultra-high potency analgetics
Natural Product Success Stories • Microorganisms: Antibiotics • Plants: – Taxoids for cancer – Artemisinin for malaria – Huperzine A and galanthamine for Alzheimer • Animals: Conotoxins as ultra-high potency analgetics
 Phytopharmacology: Decision Tree
Phytopharmacology: Decision Tree
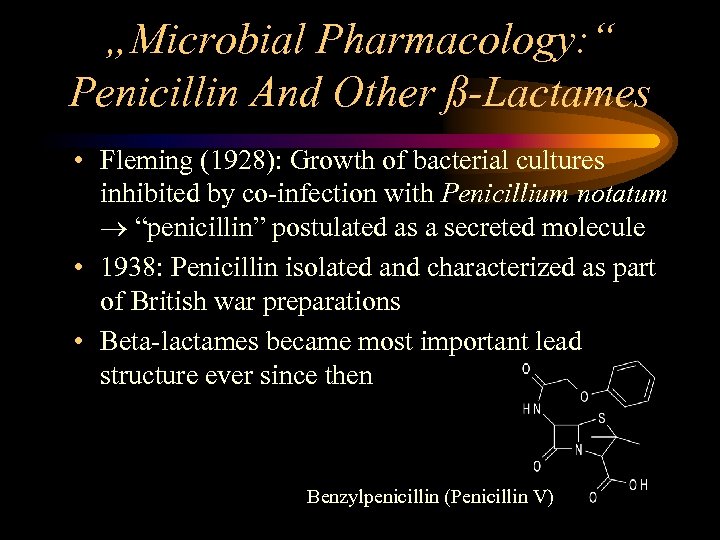 „Microbial Pharmacology: “ Penicillin And Other ß-Lactames • Fleming (1928): Growth of bacterial cultures inhibited by co-infection with Penicillium notatum “penicillin” postulated as a secreted molecule • 1938: Penicillin isolated and characterized as part of British war preparations • Beta-lactames became most important lead structure ever since then Benzylpenicillin (Penicillin V)
„Microbial Pharmacology: “ Penicillin And Other ß-Lactames • Fleming (1928): Growth of bacterial cultures inhibited by co-infection with Penicillium notatum “penicillin” postulated as a secreted molecule • 1938: Penicillin isolated and characterized as part of British war preparations • Beta-lactames became most important lead structure ever since then Benzylpenicillin (Penicillin V)
 Phytopharmacology: Taxoids • Diterpene from Taxus brevifolia • Most significant anticancer agent developed in the past two decades (“mitotic poison”)
Phytopharmacology: Taxoids • Diterpene from Taxus brevifolia • Most significant anticancer agent developed in the past two decades (“mitotic poison”)
 Phytopharmacology: Artemisinin • Unusual sesquiterpene endoperoxide from Artemisia annua (Quinghaosu in Chinese traditional medicine) • Lead compound for new generation of malaria therapeutics (including chloroquineresistant and cerebral malaria) C 15 H 22 O 5 MW = 282. 3
Phytopharmacology: Artemisinin • Unusual sesquiterpene endoperoxide from Artemisia annua (Quinghaosu in Chinese traditional medicine) • Lead compound for new generation of malaria therapeutics (including chloroquineresistant and cerebral malaria) C 15 H 22 O 5 MW = 282. 3
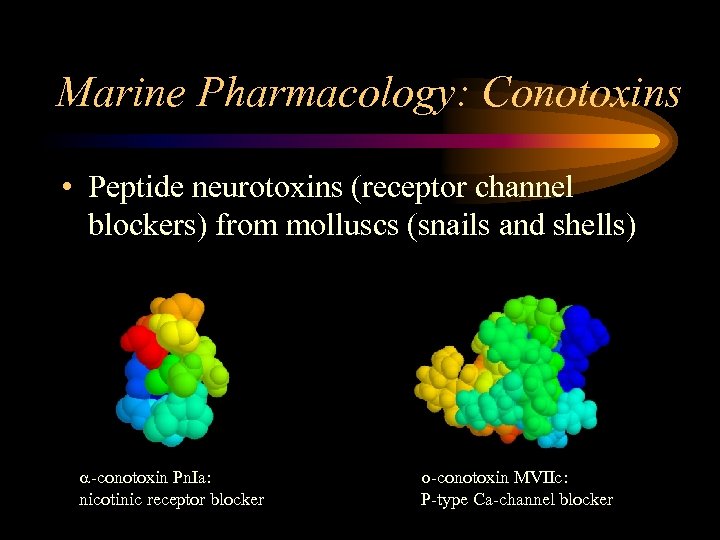 Marine Pharmacology: Conotoxins • Peptide neurotoxins (receptor channel blockers) from molluscs (snails and shells) -conotoxin Pn. Ia: nicotinic receptor blocker -conotoxin MVIIc: P-type Ca-channel blocker
Marine Pharmacology: Conotoxins • Peptide neurotoxins (receptor channel blockers) from molluscs (snails and shells) -conotoxin Pn. Ia: nicotinic receptor blocker -conotoxin MVIIc: P-type Ca-channel blocker
 The Ideal Combinatorial Library Made by forming all possible combinations of a series of sets of precursor molecules, and applying the same sequence of reactions to each combination
The Ideal Combinatorial Library Made by forming all possible combinations of a series of sets of precursor molecules, and applying the same sequence of reactions to each combination
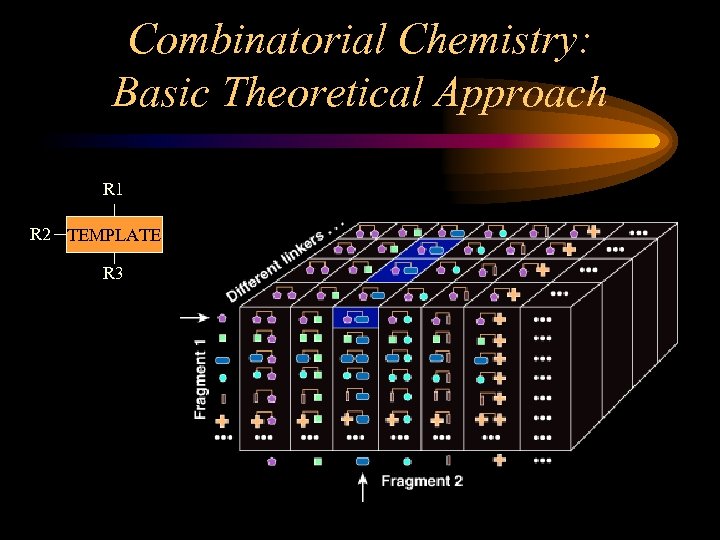 Combinatorial Chemistry: Basic Theoretical Approach R 1 R 2 TEMPLATE R 3
Combinatorial Chemistry: Basic Theoretical Approach R 1 R 2 TEMPLATE R 3
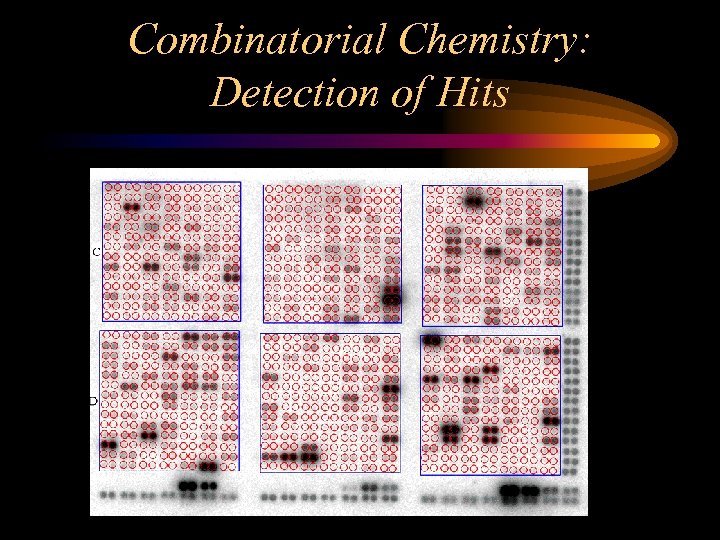 Combinatorial Chemistry: Detection of Hits
Combinatorial Chemistry: Detection of Hits
 Obstacles to Combinatorial Chemistry • Restricted and specialized chemistry, needs training • Not yet suitable for large molecules • Automated synthesis needs to be installed and integrated with the laboratory workflow • Equipment AND organization must be tightly integrated with a tailored data management infrastructure
Obstacles to Combinatorial Chemistry • Restricted and specialized chemistry, needs training • Not yet suitable for large molecules • Automated synthesis needs to be installed and integrated with the laboratory workflow • Equipment AND organization must be tightly integrated with a tailored data management infrastructure
 A Well-Designed Library Can Mean BIG Money. . . • 1995: Schering-Plough pays $3 million for access to certain parts of the Neurogen compound library • Payment estimates for unrestricted access to targeted libraries run up to $15 million • Construction of large (diverse or targeted) combinatorial libraries) has become a significant outsourcing business
A Well-Designed Library Can Mean BIG Money. . . • 1995: Schering-Plough pays $3 million for access to certain parts of the Neurogen compound library • Payment estimates for unrestricted access to targeted libraries run up to $15 million • Construction of large (diverse or targeted) combinatorial libraries) has become a significant outsourcing business
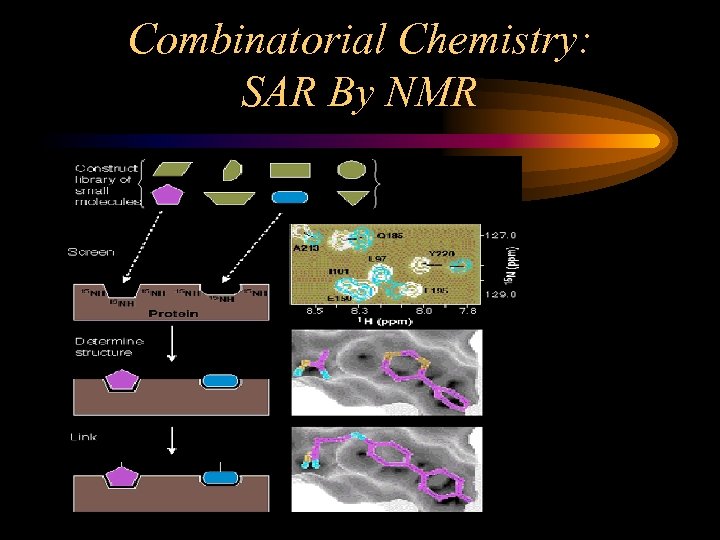 Combinatorial Chemistry: SAR By NMR
Combinatorial Chemistry: SAR By NMR
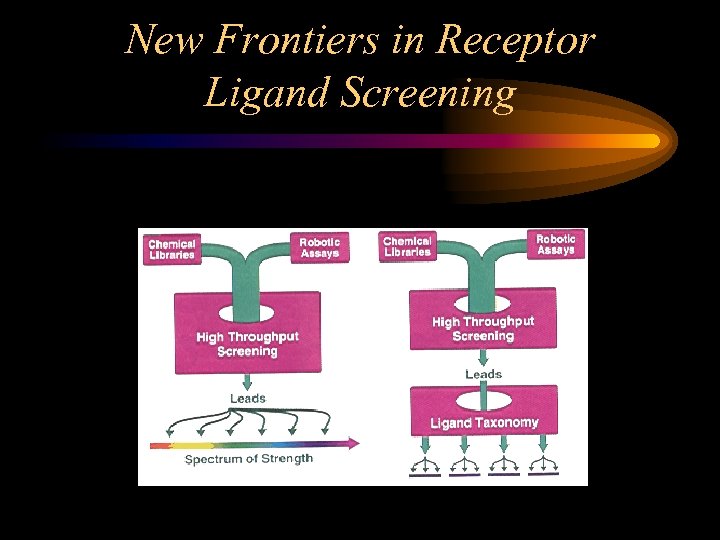 New Frontiers in Receptor Ligand Screening
New Frontiers in Receptor Ligand Screening
 Databases In Drug Discovery • Employ advanced search algorithms including artificial intelligence (AI) systems • “Data Mining” -- knowledge discovery in databases: – Fuzzy logic -- “soft” search criteria – Structural similarity searches – Retrieve implicit information – Link structural information with bio-informatics
Databases In Drug Discovery • Employ advanced search algorithms including artificial intelligence (AI) systems • “Data Mining” -- knowledge discovery in databases: – Fuzzy logic -- “soft” search criteria – Structural similarity searches – Retrieve implicit information – Link structural information with bio-informatics
 Tools for Rational Drug Design • (Q)SAR: (Quantitative) Structure-Activity Relationships • SAFIR: Structure-Affinity Relationships • SPAS: Structure-Property/Affinity Studies • Co. MFA: Comparative Molecular Field Analysis
Tools for Rational Drug Design • (Q)SAR: (Quantitative) Structure-Activity Relationships • SAFIR: Structure-Affinity Relationships • SPAS: Structure-Property/Affinity Studies • Co. MFA: Comparative Molecular Field Analysis
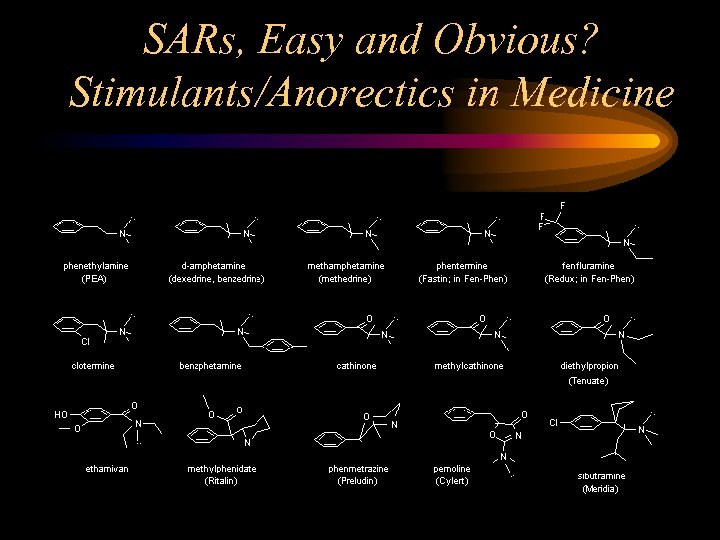 SARs, Easy and Obvious? Stimulants/Anorectics in Medicine
SARs, Easy and Obvious? Stimulants/Anorectics in Medicine
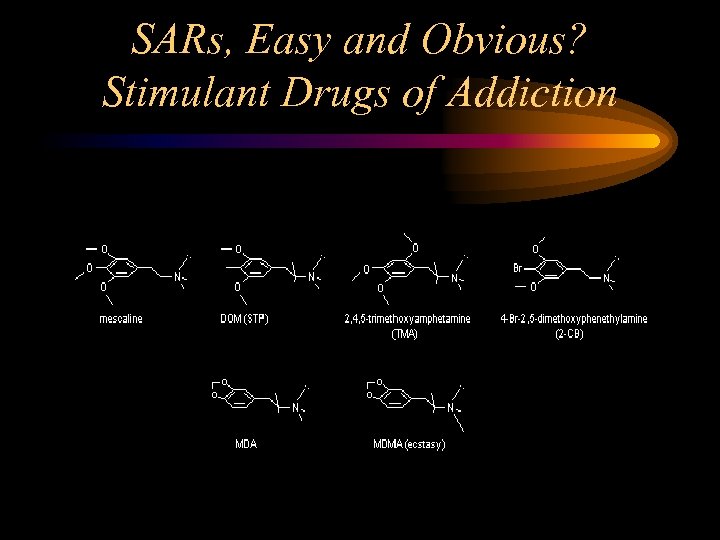 SARs, Easy and Obvious? Stimulant Drugs of Addiction
SARs, Easy and Obvious? Stimulant Drugs of Addiction
 Can „Drug-Like“ Structures Be Predicted? • Only 32 basic templates describe half of all known drugs (Bemis et al. 1996) • Medicinal chemists essentially use their intuition (“expert rules”) to gauge drug structures emulation by trainable (and self-entraining) neuronal networks working from relatively few molecular descriptors • If “drug-likeness” can be quantified targeted design of combinatorial libraries
Can „Drug-Like“ Structures Be Predicted? • Only 32 basic templates describe half of all known drugs (Bemis et al. 1996) • Medicinal chemists essentially use their intuition (“expert rules”) to gauge drug structures emulation by trainable (and self-entraining) neuronal networks working from relatively few molecular descriptors • If “drug-likeness” can be quantified targeted design of combinatorial libraries
 Comparative Molecular Field Analysis • Co. MFA: Method to analyze and predict structure-activity relationships (Cramer 1988) • Based on superimposition techniques: – Steric overlap (“distance geometry”) – Crystallographic data – Pharmacophore theory – Steric and electrostatic alignment algorithms – „Automated field fit“ Further reading: http: //www. netsci. org/Science/Compchem/feature 11. html ; http: //cmcind. far. ruu. nl/webcmc/camd/3 dqsar. html
Comparative Molecular Field Analysis • Co. MFA: Method to analyze and predict structure-activity relationships (Cramer 1988) • Based on superimposition techniques: – Steric overlap (“distance geometry”) – Crystallographic data – Pharmacophore theory – Steric and electrostatic alignment algorithms – „Automated field fit“ Further reading: http: //www. netsci. org/Science/Compchem/feature 11. html ; http: //cmcind. far. ruu. nl/webcmc/camd/3 dqsar. html
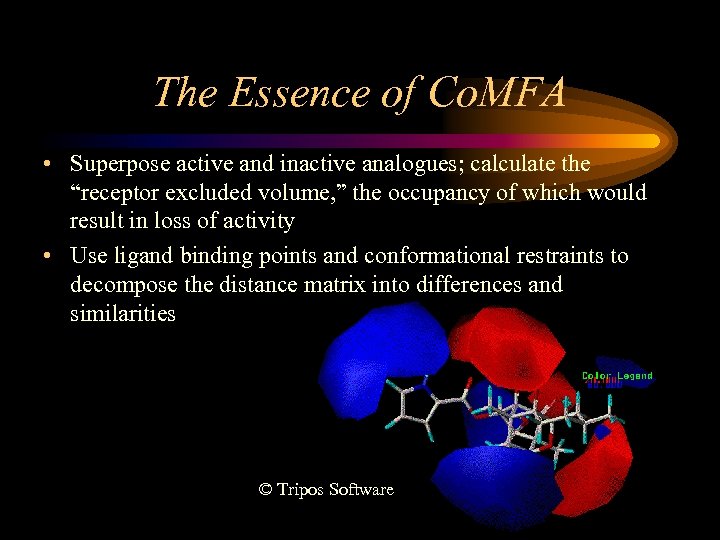 The Essence of Co. MFA • Superpose active and inactive analogues; calculate the “receptor excluded volume, ” the occupancy of which would result in loss of activity • Use ligand binding points and conformational restraints to decompose the distance matrix into differences and similarities © Tripos Software
The Essence of Co. MFA • Superpose active and inactive analogues; calculate the “receptor excluded volume, ” the occupancy of which would result in loss of activity • Use ligand binding points and conformational restraints to decompose the distance matrix into differences and similarities © Tripos Software
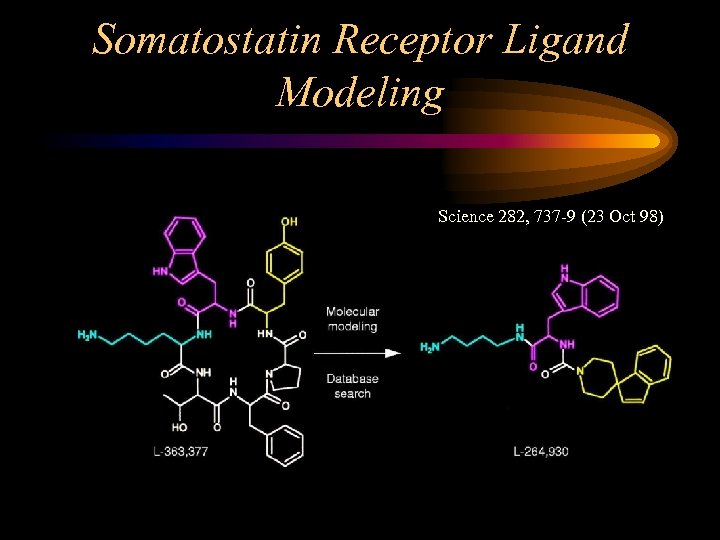 Somatostatin Receptor Ligand Modeling Science 282, 737 -9 (23 Oct 98)
Somatostatin Receptor Ligand Modeling Science 282, 737 -9 (23 Oct 98)
 New Buzzwords in Drug Discovery
New Buzzwords in Drug Discovery
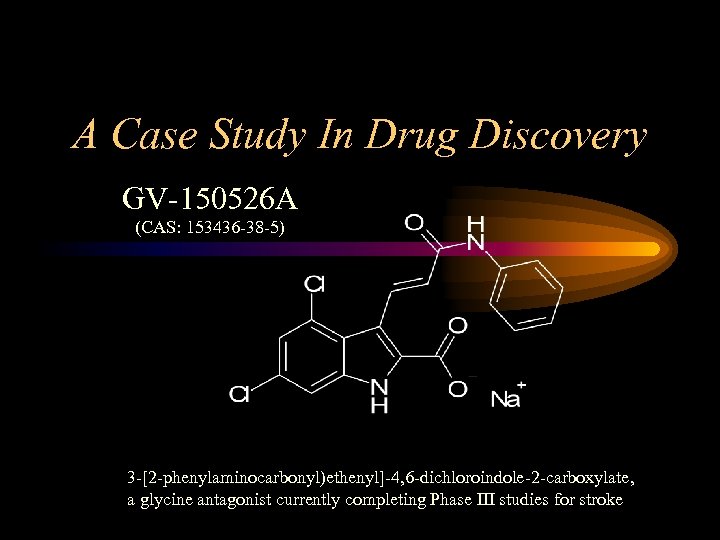 A Case Study In Drug Discovery GV-150526 A (CAS: 153436 -38 -5) 3 -[2 -phenylaminocarbonyl)ethenyl]-4, 6 -dichloroindole-2 -carboxylate, a glycine antagonist currently completing Phase III studies for stroke
A Case Study In Drug Discovery GV-150526 A (CAS: 153436 -38 -5) 3 -[2 -phenylaminocarbonyl)ethenyl]-4, 6 -dichloroindole-2 -carboxylate, a glycine antagonist currently completing Phase III studies for stroke
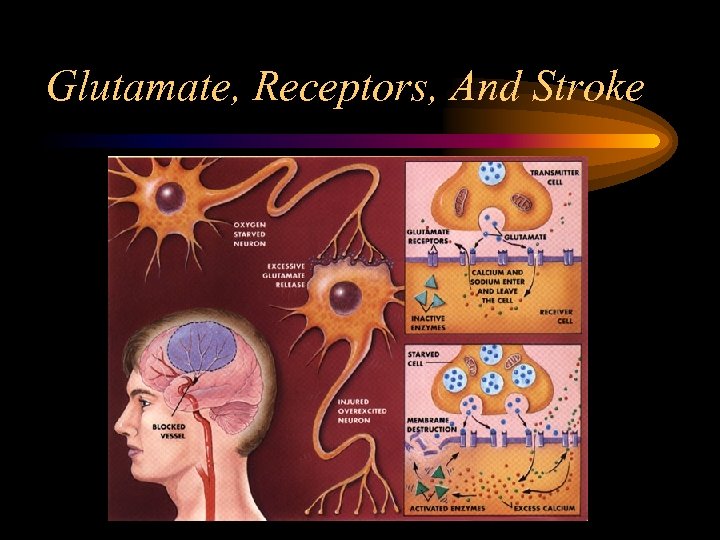 Glutamate, Receptors, And Stroke
Glutamate, Receptors, And Stroke
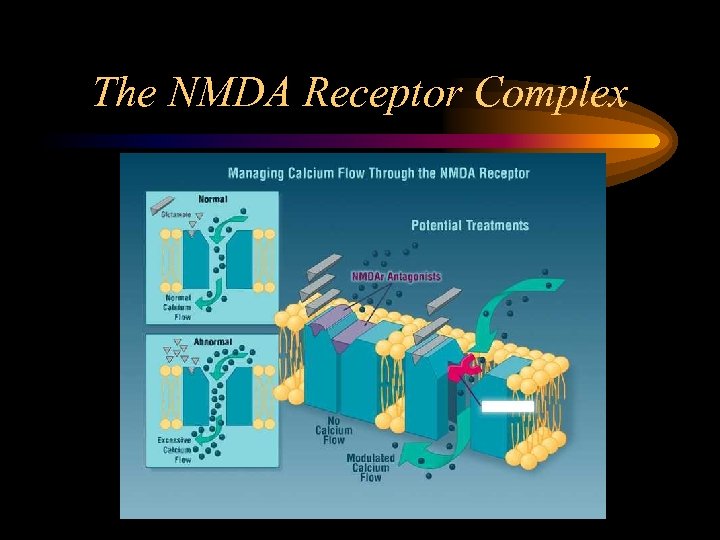 The NMDA Receptor Complex
The NMDA Receptor Complex
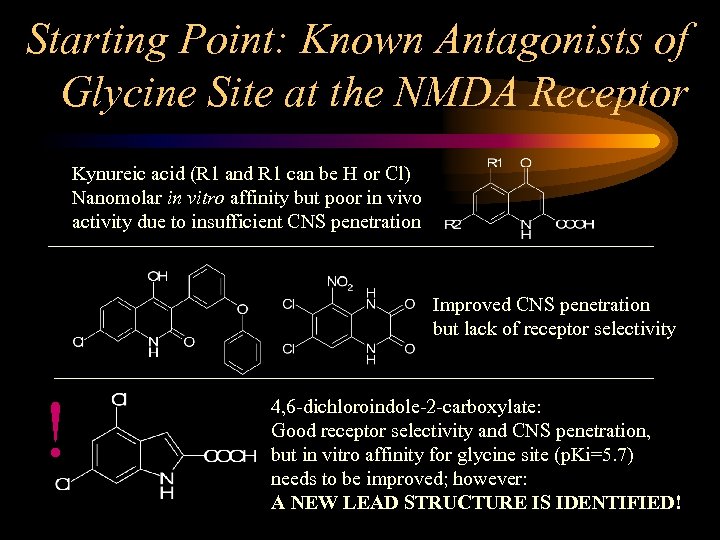 Starting Point: Known Antagonists of Glycine Site at the NMDA Receptor Kynureic acid (R 1 and R 1 can be H or Cl) Nanomolar in vitro affinity but poor in vivo activity due to insufficient CNS penetration Improved CNS penetration but lack of receptor selectivity ! 4, 6 -dichloroindole-2 -carboxylate: Good receptor selectivity and CNS penetration, but in vitro affinity for glycine site (p. Ki=5. 7) needs to be improved; however: A NEW LEAD STRUCTURE IS IDENTIFIED!
Starting Point: Known Antagonists of Glycine Site at the NMDA Receptor Kynureic acid (R 1 and R 1 can be H or Cl) Nanomolar in vitro affinity but poor in vivo activity due to insufficient CNS penetration Improved CNS penetration but lack of receptor selectivity ! 4, 6 -dichloroindole-2 -carboxylate: Good receptor selectivity and CNS penetration, but in vitro affinity for glycine site (p. Ki=5. 7) needs to be improved; however: A NEW LEAD STRUCTURE IS IDENTIFIED!
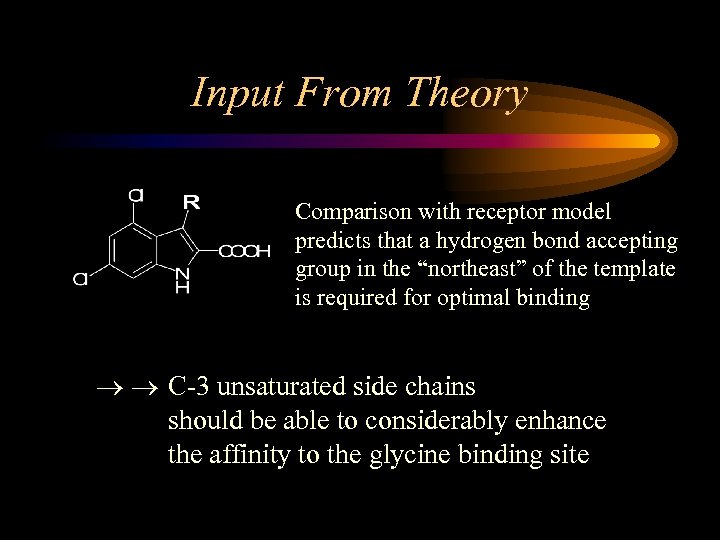 Input From Theory Comparison with receptor model predicts that a hydrogen bond accepting group in the “northeast” of the template is required for optimal binding C-3 unsaturated side chains should be able to considerably enhance the affinity to the glycine binding site
Input From Theory Comparison with receptor model predicts that a hydrogen bond accepting group in the “northeast” of the template is required for optimal binding C-3 unsaturated side chains should be able to considerably enhance the affinity to the glycine binding site
![Template Derivatization At C-3 PRIMARY SCREENING SYSTEM: In vitro binding inhibition of [3 H]-glycine Template Derivatization At C-3 PRIMARY SCREENING SYSTEM: In vitro binding inhibition of [3 H]-glycine](https://present5.com/presentation/83dace7a954d02b7bdabb21e72031a18/image-33.jpg) Template Derivatization At C-3 PRIMARY SCREENING SYSTEM: In vitro binding inhibition of [3 H]-glycine to crude synaptic membrane preparations from adult rat cerebral cortex
Template Derivatization At C-3 PRIMARY SCREENING SYSTEM: In vitro binding inhibition of [3 H]-glycine to crude synaptic membrane preparations from adult rat cerebral cortex
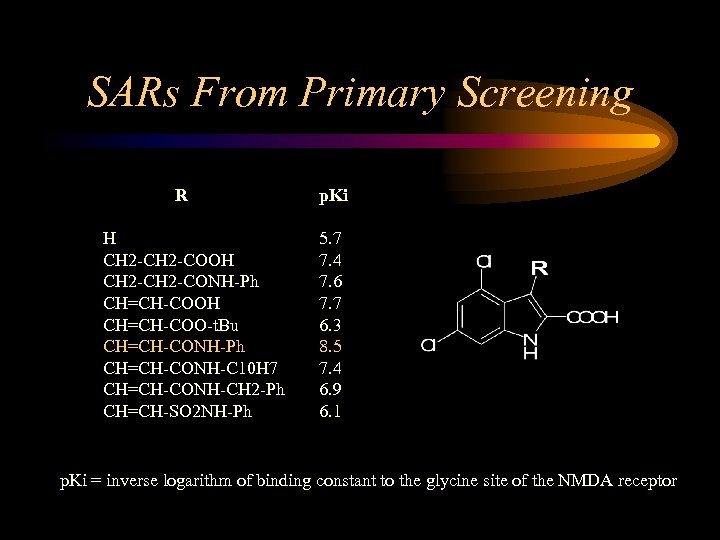 SARs From Primary Screening R H CH 2 -COOH CH 2 -CONH-Ph CH=CH-COOH CH=CH-COO-t. Bu CH=CH-CONH-Ph CH=CH-CONH-C 10 H 7 CH=CH-CONH-CH 2 -Ph CH=CH-SO 2 NH-Ph p. Ki 5. 7 7. 4 7. 6 7. 7 6. 3 8. 5 7. 4 6. 9 6. 1 p. Ki = inverse logarithm of binding constant to the glycine site of the NMDA receptor
SARs From Primary Screening R H CH 2 -COOH CH 2 -CONH-Ph CH=CH-COOH CH=CH-COO-t. Bu CH=CH-CONH-Ph CH=CH-CONH-C 10 H 7 CH=CH-CONH-CH 2 -Ph CH=CH-SO 2 NH-Ph p. Ki 5. 7 7. 4 7. 6 7. 7 6. 3 8. 5 7. 4 6. 9 6. 1 p. Ki = inverse logarithm of binding constant to the glycine site of the NMDA receptor
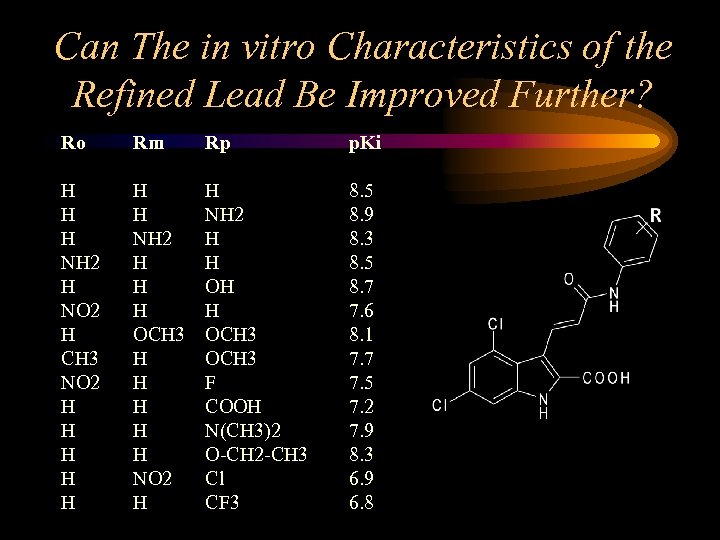 Can The in vitro Characteristics of the Refined Lead Be Improved Further? Ro Rm Rp p. Ki H H H NH 2 H NO 2 H CH 3 NO 2 H H H H NH 2 H H H OCH 3 H H H NO 2 H H NH 2 H H OCH 3 F COOH N(CH 3)2 O-CH 2 -CH 3 Cl CF 3 8. 5 8. 9 8. 3 8. 5 8. 7 7. 6 8. 1 7. 7 7. 5 7. 2 7. 9 8. 3 6. 9 6. 8
Can The in vitro Characteristics of the Refined Lead Be Improved Further? Ro Rm Rp p. Ki H H H NH 2 H NO 2 H CH 3 NO 2 H H H H NH 2 H H H OCH 3 H H H NO 2 H H NH 2 H H OCH 3 F COOH N(CH 3)2 O-CH 2 -CH 3 Cl CF 3 8. 5 8. 9 8. 3 8. 5 8. 7 7. 6 8. 1 7. 7 7. 5 7. 2 7. 9 8. 3 6. 9 6. 8
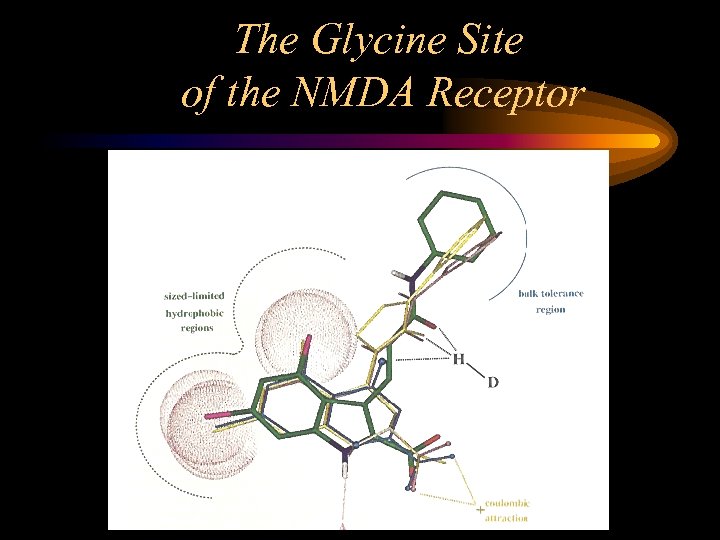 The Glycine Site of the NMDA Receptor
The Glycine Site of the NMDA Receptor


