53f8604303611ec61c2444530b061986.ppt
- Количество слайдов: 26
 Lecture 3 QM/MM Applications
Lecture 3 QM/MM Applications
 Quantum Simulation in Industry Overview ¤ Objectives • Extend QM/MM Codes and port to HPC architectures • Incorporate QM/MM molecular dynamics for chemical reactions • Demonstrate the value of HPC simulations in industrial chemistry ¤ Consortium • Daresbury (Coordinator) • Academic (Zurich/Muelheim, Royal Institution) • Industrial (Norsk Hydro, BASF, ICI) ¤ Resources • Funded by the European Union (EU contribution of 1. 2 MECU) • 1998 -2001 http: //www. cse. clrc. ac. uk/Activity/QUASI
Quantum Simulation in Industry Overview ¤ Objectives • Extend QM/MM Codes and port to HPC architectures • Incorporate QM/MM molecular dynamics for chemical reactions • Demonstrate the value of HPC simulations in industrial chemistry ¤ Consortium • Daresbury (Coordinator) • Academic (Zurich/Muelheim, Royal Institution) • Industrial (Norsk Hydro, BASF, ICI) ¤ Resources • Funded by the European Union (EU contribution of 1. 2 MECU) • 1998 -2001 http: //www. cse. clrc. ac. uk/Activity/QUASI
 QUASI - Partners ¤ Drs Paul Sherwood, Martyn Guest (Daresbury Laboratory) • Coordinator • Ab-initio and HPC implementations • Chem. Shell software ¤ Prof Walter Thiel (MPI Muelheim) • Semi-emprical (MNDO 94), QM/MM coupling ¤ Prof Richard Catlow (Royal Insitution) • Classical simulation, shell model, force field derivation ¤ Dr Steve Rogers (ICI) • Methanol synthesis by metal oxide catalysts (with Royal Institution) ¤ Dr Ansgar Schaeffer (BASF) • Enzyme inhibitor simulation (with Zurich) ¤ Dr Klaus Schoeffel (Norsk Hydro) • Zeolite catalysis for N 2 O abatement (with Daresbury)
QUASI - Partners ¤ Drs Paul Sherwood, Martyn Guest (Daresbury Laboratory) • Coordinator • Ab-initio and HPC implementations • Chem. Shell software ¤ Prof Walter Thiel (MPI Muelheim) • Semi-emprical (MNDO 94), QM/MM coupling ¤ Prof Richard Catlow (Royal Insitution) • Classical simulation, shell model, force field derivation ¤ Dr Steve Rogers (ICI) • Methanol synthesis by metal oxide catalysts (with Royal Institution) ¤ Dr Ansgar Schaeffer (BASF) • Enzyme inhibitor simulation (with Zurich) ¤ Dr Klaus Schoeffel (Norsk Hydro) • Zeolite catalysis for N 2 O abatement (with Daresbury)
 QUASI - Workplan • Design ¤ QM and MM validation ¤ QM/MM coupling approaches (Daresbury, Zurich) • Enhancements to QM/MM Methodology ¤ ¤ ¤ Geometry Optimisation for QM/MM Systems (Zurich/Daresbury) Classical Shell Model QM/MM (Royal Institution/Daresbury) Molecular Dynamics (DL/Royal Institution) GUI Development (BASF/Daresbury) Forcefield Development (Royal Institition) • Joint Academic/Industrial Applications ¤ Demonstration and Commercial Calculations ¤ Workshop 25 -27 September 2000, Muelheim, Germany
QUASI - Workplan • Design ¤ QM and MM validation ¤ QM/MM coupling approaches (Daresbury, Zurich) • Enhancements to QM/MM Methodology ¤ ¤ ¤ Geometry Optimisation for QM/MM Systems (Zurich/Daresbury) Classical Shell Model QM/MM (Royal Institution/Daresbury) Molecular Dynamics (DL/Royal Institution) GUI Development (BASF/Daresbury) Forcefield Development (Royal Institition) • Joint Academic/Industrial Applications ¤ Demonstration and Commercial Calculations ¤ Workshop 25 -27 September 2000, Muelheim, Germany
 Solvation studies using QM/MM
Solvation studies using QM/MM
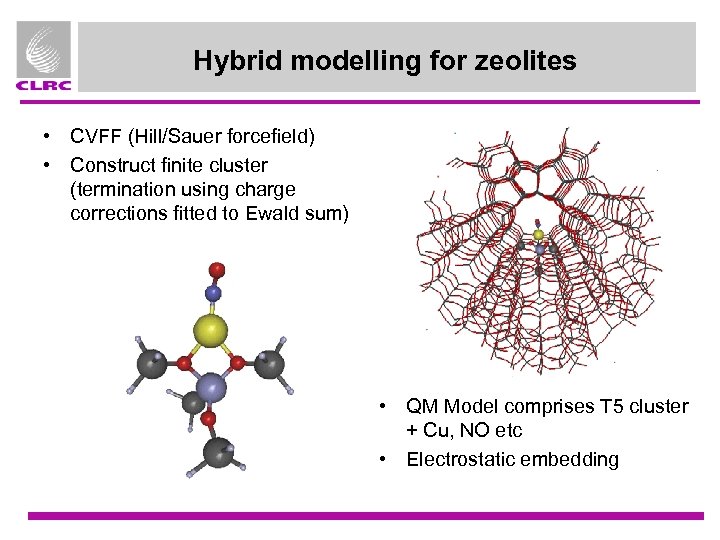 Hybrid modelling for zeolites • CVFF (Hill/Sauer forcefield) • Construct finite cluster (termination using charge corrections fitted to Ewald sum) • QM Model comprises T 5 cluster + Cu, NO etc • Electrostatic embedding
Hybrid modelling for zeolites • CVFF (Hill/Sauer forcefield) • Construct finite cluster (termination using charge corrections fitted to Ewald sum) • QM Model comprises T 5 cluster + Cu, NO etc • Electrostatic embedding
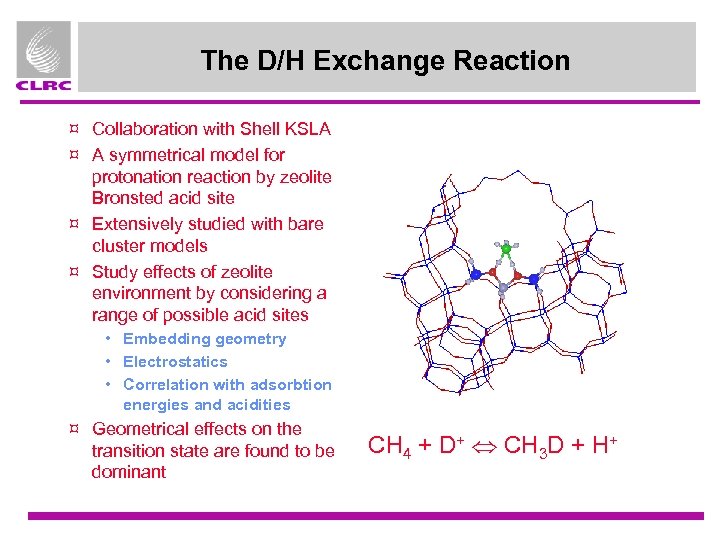 The D/H Exchange Reaction ¤ Collaboration with Shell KSLA ¤ A symmetrical model for protonation reaction by zeolite Bronsted acid site ¤ Extensively studied with bare cluster models ¤ Study effects of zeolite environment by considering a range of possible acid sites • Embedding geometry • Electrostatics • Correlation with adsorbtion energies and acidities ¤ Geometrical effects on the transition state are found to be dominant CH 4 + D+ CH 3 D + H+
The D/H Exchange Reaction ¤ Collaboration with Shell KSLA ¤ A symmetrical model for protonation reaction by zeolite Bronsted acid site ¤ Extensively studied with bare cluster models ¤ Study effects of zeolite environment by considering a range of possible acid sites • Embedding geometry • Electrostatics • Correlation with adsorbtion energies and acidities ¤ Geometrical effects on the transition state are found to be dominant CH 4 + D+ CH 3 D + H+
 QUASI - Applications Focus ¤ Norsk Hydro / Daresbury • Zeolites systems with adsorbed Cu species, decomposition of N 2 O and NOx • Based on CFF forcefield, GAMESS-UK+DL_POLY ¤ BASF / Muelheim • Enzyme inhibitor binding (thrombin and anticoagulant drug candidates) • Enzyme reactivity modelling (Triose Phosphate Isomerase) • Using MNDO/TURBOMOLE with CHARMM forcefield (DL_POLY) ¤ ICI/ Royal Institution • Modelling surface catalysis, methanol synthesis reaction • Using GULP shell model potentials and GAMESS-UK DFT
QUASI - Applications Focus ¤ Norsk Hydro / Daresbury • Zeolites systems with adsorbed Cu species, decomposition of N 2 O and NOx • Based on CFF forcefield, GAMESS-UK+DL_POLY ¤ BASF / Muelheim • Enzyme inhibitor binding (thrombin and anticoagulant drug candidates) • Enzyme reactivity modelling (Triose Phosphate Isomerase) • Using MNDO/TURBOMOLE with CHARMM forcefield (DL_POLY) ¤ ICI/ Royal Institution • Modelling surface catalysis, methanol synthesis reaction • Using GULP shell model potentials and GAMESS-UK DFT
 Embedded cluster and QM/MM Applications • Proton transfer (ZOH+ + NH 3 -> ZO- + NH 4+) ¤ S. P. Greatbanks, I. H. Hillier and P. Sherwood, J. Comp. Chem. , 18, 562, 1997. • Methyl shift reaction of propenium ion ¤ P. Sherwood, A. H. de Vries, S. J. Collins, S. P. Greatbanks, N. A. Burton, M. A. Vincent and I. H. Hillier, Faraday Discuss. , 106, 1997 • Alkene chemisorption ¤ P. E. Sinclair, A. H. de Vries, P. Sherwood, C. R. A. Catlow and R. A. Van Santen, J. Chem. Soc. , Faraday Trans. , 94, 3401, 1998 • D/H exchange reaction for methane ¤ A. H. de Vries, P. Sherwood, S. J. Collins, A. M. Rigby, M. Rigutto and G. J. Kramer, J. Phys. Chem. B, 103, 6133 (1999)
Embedded cluster and QM/MM Applications • Proton transfer (ZOH+ + NH 3 -> ZO- + NH 4+) ¤ S. P. Greatbanks, I. H. Hillier and P. Sherwood, J. Comp. Chem. , 18, 562, 1997. • Methyl shift reaction of propenium ion ¤ P. Sherwood, A. H. de Vries, S. J. Collins, S. P. Greatbanks, N. A. Burton, M. A. Vincent and I. H. Hillier, Faraday Discuss. , 106, 1997 • Alkene chemisorption ¤ P. E. Sinclair, A. H. de Vries, P. Sherwood, C. R. A. Catlow and R. A. Van Santen, J. Chem. Soc. , Faraday Trans. , 94, 3401, 1998 • D/H exchange reaction for methane ¤ A. H. de Vries, P. Sherwood, S. J. Collins, A. M. Rigby, M. Rigutto and G. J. Kramer, J. Phys. Chem. B, 103, 6133 (1999)
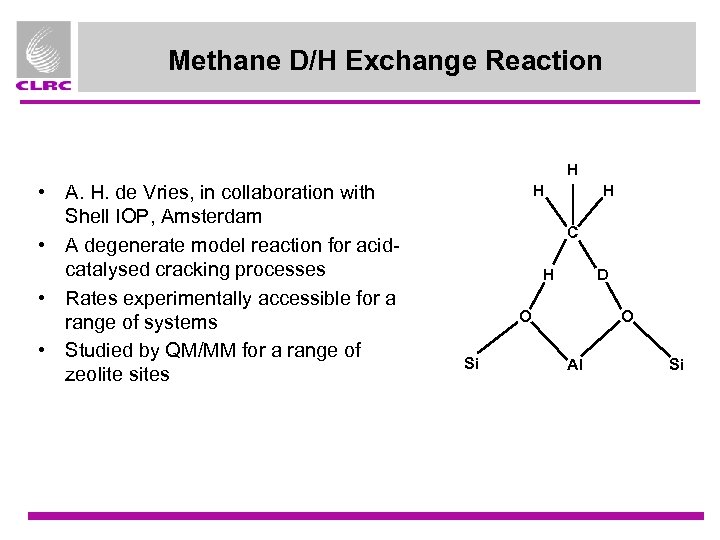 Methane D/H Exchange Reaction H • A. H. de Vries, in collaboration with Shell IOP, Amsterdam • A degenerate model reaction for acidcatalysed cracking processes • Rates experimentally accessible for a range of systems • Studied by QM/MM for a range of zeolite sites H H C H D O Si O Al Si
Methane D/H Exchange Reaction H • A. H. de Vries, in collaboration with Shell IOP, Amsterdam • A degenerate model reaction for acidcatalysed cracking processes • Rates experimentally accessible for a range of systems • Studied by QM/MM for a range of zeolite sites H H C H D O Si O Al Si
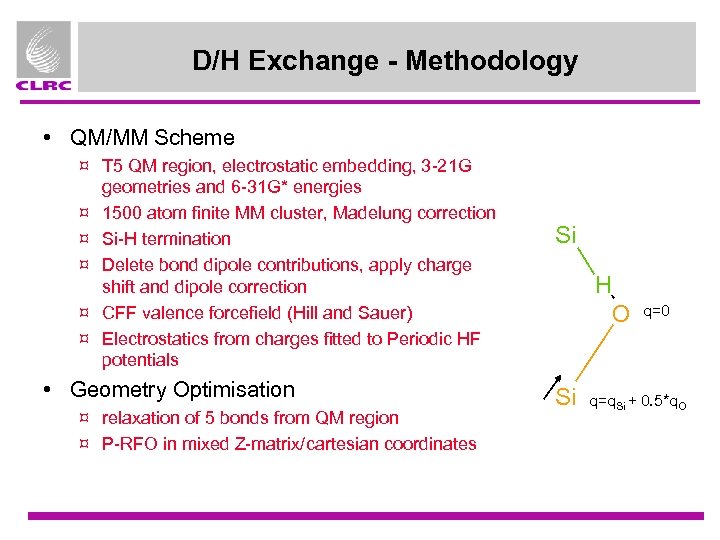 D/H Exchange - Methodology • QM/MM Scheme ¤ T 5 QM region, electrostatic embedding, 3 -21 G geometries and 6 -31 G* energies ¤ 1500 atom finite MM cluster, Madelung correction ¤ Si-H termination ¤ Delete bond dipole contributions, apply charge shift and dipole correction ¤ CFF valence forcefield (Hill and Sauer) ¤ Electrostatics from charges fitted to Periodic HF potentials • Geometry Optimisation ¤ relaxation of 5 bonds from QM region ¤ P-RFO in mixed Z-matrix/cartesian coordinates Si H O Si q=0 q=q. Si + 0. 5*q. O
D/H Exchange - Methodology • QM/MM Scheme ¤ T 5 QM region, electrostatic embedding, 3 -21 G geometries and 6 -31 G* energies ¤ 1500 atom finite MM cluster, Madelung correction ¤ Si-H termination ¤ Delete bond dipole contributions, apply charge shift and dipole correction ¤ CFF valence forcefield (Hill and Sauer) ¤ Electrostatics from charges fitted to Periodic HF potentials • Geometry Optimisation ¤ relaxation of 5 bonds from QM region ¤ P-RFO in mixed Z-matrix/cartesian coordinates Si H O Si q=0 q=q. Si + 0. 5*q. O
 D/H Exchange Reaction - Results • Relaxation and TS searching for embedded models now practical • Can differentiate of protonation energies for the 4 distinct oxygen sites (FAU) ¤ correctly predict protonation at O 3 (at 6 -31 G*), with O 1 site slightly (1 k. J/mol) higher • Results emphasise importance of mechanical constraints ¤ Highest activation energies can be identified with sites with non-planar Si. O-Al-O-Si fragments ¤ For remaining structures, a strong correlation seen between activation energy of D/H exchange with the chemisorption energy of ammonium (analogous bidentate structures) • Absolute values of D/H exchange activation energies too high (single point MP 2 correction based on HF structures) ¤ 160 (computed) vs 109 +/- 15 k. J/mol (MFI) ¤ 175 (computed) vs 129 +/- 20 k. J/mol (FAU)
D/H Exchange Reaction - Results • Relaxation and TS searching for embedded models now practical • Can differentiate of protonation energies for the 4 distinct oxygen sites (FAU) ¤ correctly predict protonation at O 3 (at 6 -31 G*), with O 1 site slightly (1 k. J/mol) higher • Results emphasise importance of mechanical constraints ¤ Highest activation energies can be identified with sites with non-planar Si. O-Al-O-Si fragments ¤ For remaining structures, a strong correlation seen between activation energy of D/H exchange with the chemisorption energy of ammonium (analogous bidentate structures) • Absolute values of D/H exchange activation energies too high (single point MP 2 correction based on HF structures) ¤ 160 (computed) vs 109 +/- 15 k. J/mol (MFI) ¤ 175 (computed) vs 129 +/- 20 k. J/mol (FAU)
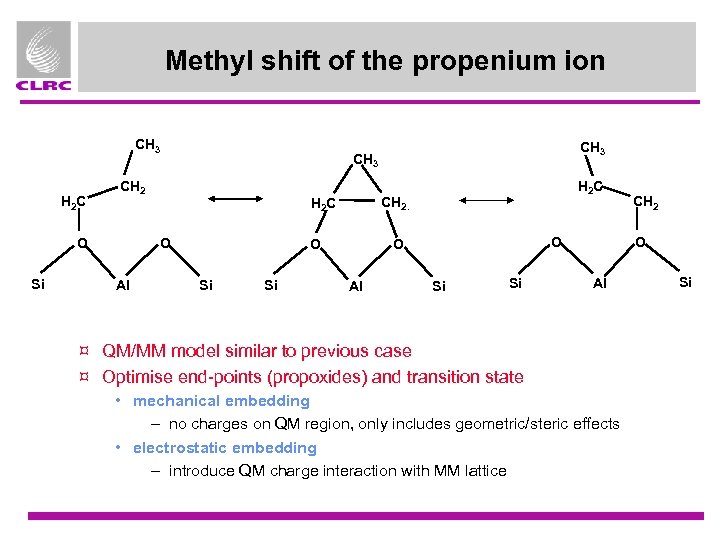 Methyl shift of the propenium ion CH 3 H 2 C CH 2 O Si Si H 2 C CH 2. H 2 C O Al CH 3 O O Al Si Si CH 2 O Al ¤ QM/MM model similar to previous case ¤ Optimise end-points (propoxides) and transition state • mechanical embedding – no charges on QM region, only includes geometric/steric effects • electrostatic embedding – introduce QM charge interaction with MM lattice Si
Methyl shift of the propenium ion CH 3 H 2 C CH 2 O Si Si H 2 C CH 2. H 2 C O Al CH 3 O O Al Si Si CH 2 O Al ¤ QM/MM model similar to previous case ¤ Optimise end-points (propoxides) and transition state • mechanical embedding – no charges on QM region, only includes geometric/steric effects • electrostatic embedding – introduce QM charge interaction with MM lattice Si
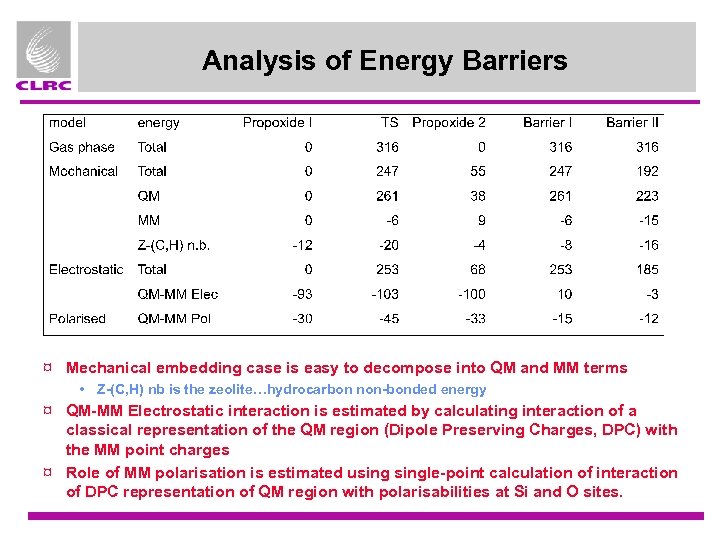 Analysis of Energy Barriers ¤ Mechanical embedding case is easy to decompose into QM and MM terms • Z-(C, H) nb is the zeolite…hydrocarbon non-bonded energy ¤ QM-MM Electrostatic interaction is estimated by calculating interaction of a classical representation of the QM region (Dipole Preserving Charges, DPC) with the MM point charges ¤ Role of MM polarisation is estimated usingle-point calculation of interaction of DPC representation of QM region with polarisabilities at Si and O sites.
Analysis of Energy Barriers ¤ Mechanical embedding case is easy to decompose into QM and MM terms • Z-(C, H) nb is the zeolite…hydrocarbon non-bonded energy ¤ QM-MM Electrostatic interaction is estimated by calculating interaction of a classical representation of the QM region (Dipole Preserving Charges, DPC) with the MM point charges ¤ Role of MM polarisation is estimated usingle-point calculation of interaction of DPC representation of QM region with polarisabilities at Si and O sites.
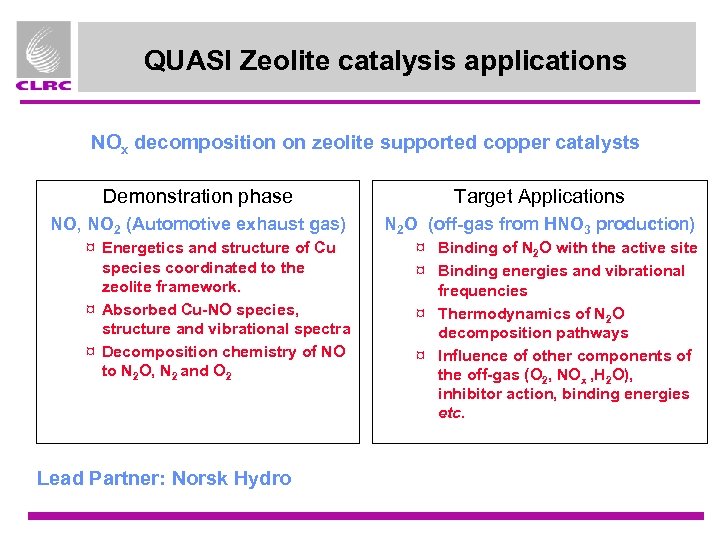 QUASI Zeolite catalysis applications NOx decomposition on zeolite supported copper catalysts Demonstration phase Target Applications NO, NO 2 (Automotive exhaust gas) N 2 O (off-gas from HNO 3 production) ¤ Energetics and structure of Cu species coordinated to the zeolite framework. ¤ Absorbed Cu-NO species, structure and vibrational spectra ¤ Decomposition chemistry of NO to N 2 O, N 2 and O 2 Lead Partner: Norsk Hydro ¤ Binding of N 2 O with the active site ¤ Binding energies and vibrational frequencies ¤ Thermodynamics of N 2 O decomposition pathways ¤ Influence of other components of the off-gas (O 2, NOx , H 2 O), inhibitor action, binding energies etc.
QUASI Zeolite catalysis applications NOx decomposition on zeolite supported copper catalysts Demonstration phase Target Applications NO, NO 2 (Automotive exhaust gas) N 2 O (off-gas from HNO 3 production) ¤ Energetics and structure of Cu species coordinated to the zeolite framework. ¤ Absorbed Cu-NO species, structure and vibrational spectra ¤ Decomposition chemistry of NO to N 2 O, N 2 and O 2 Lead Partner: Norsk Hydro ¤ Binding of N 2 O with the active site ¤ Binding energies and vibrational frequencies ¤ Thermodynamics of N 2 O decomposition pathways ¤ Influence of other components of the off-gas (O 2, NOx , H 2 O), inhibitor action, binding energies etc.
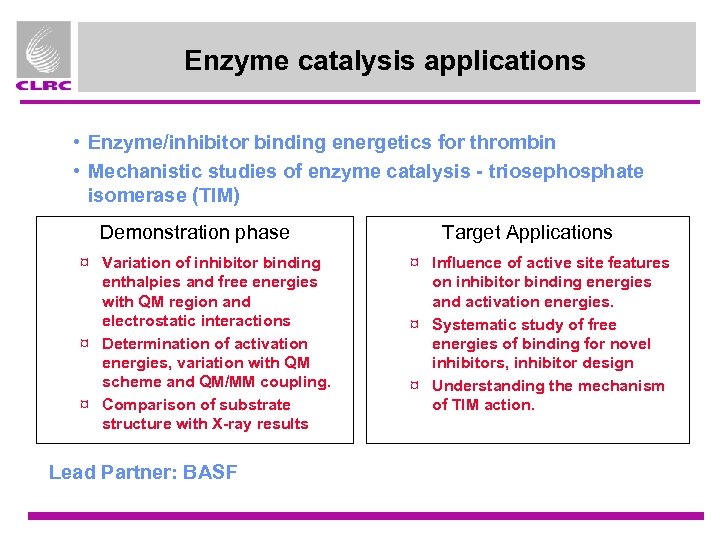 Enzyme catalysis applications • Enzyme/inhibitor binding energetics for thrombin • Mechanistic studies of enzyme catalysis - triosephosphate isomerase (TIM) Demonstration phase ¤ Variation of inhibitor binding enthalpies and free energies with QM region and electrostatic interactions ¤ Determination of activation energies, variation with QM scheme and QM/MM coupling. ¤ Comparison of substrate structure with X-ray results Lead Partner: BASF Target Applications ¤ Influence of active site features on inhibitor binding energies and activation energies. ¤ Systematic study of free energies of binding for novel inhibitors, inhibitor design ¤ Understanding the mechanism of TIM action.
Enzyme catalysis applications • Enzyme/inhibitor binding energetics for thrombin • Mechanistic studies of enzyme catalysis - triosephosphate isomerase (TIM) Demonstration phase ¤ Variation of inhibitor binding enthalpies and free energies with QM region and electrostatic interactions ¤ Determination of activation energies, variation with QM scheme and QM/MM coupling. ¤ Comparison of substrate structure with X-ray results Lead Partner: BASF Target Applications ¤ Influence of active site features on inhibitor binding energies and activation energies. ¤ Systematic study of free energies of binding for novel inhibitors, inhibitor design ¤ Understanding the mechanism of TIM action.
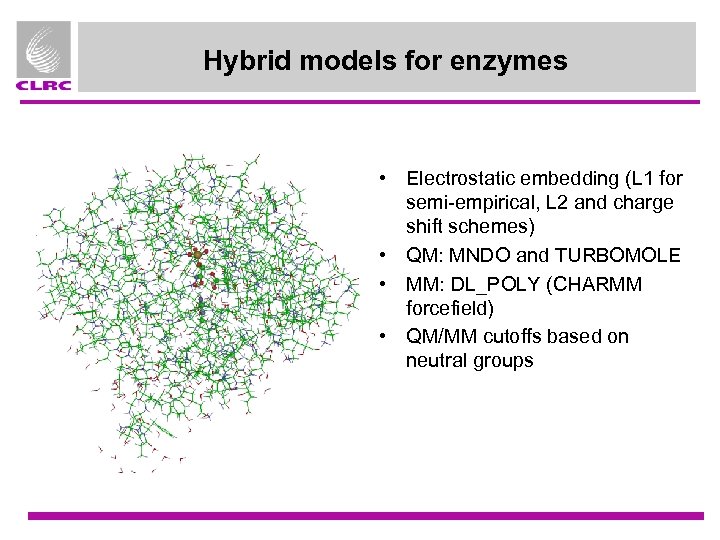 Hybrid models for enzymes • Electrostatic embedding (L 1 for semi-empirical, L 2 and charge shift schemes) • QM: MNDO and TURBOMOLE • MM: DL_POLY (CHARMM forcefield) • QM/MM cutoffs based on neutral groups
Hybrid models for enzymes • Electrostatic embedding (L 1 for semi-empirical, L 2 and charge shift schemes) • QM: MNDO and TURBOMOLE • MM: DL_POLY (CHARMM forcefield) • QM/MM cutoffs based on neutral groups
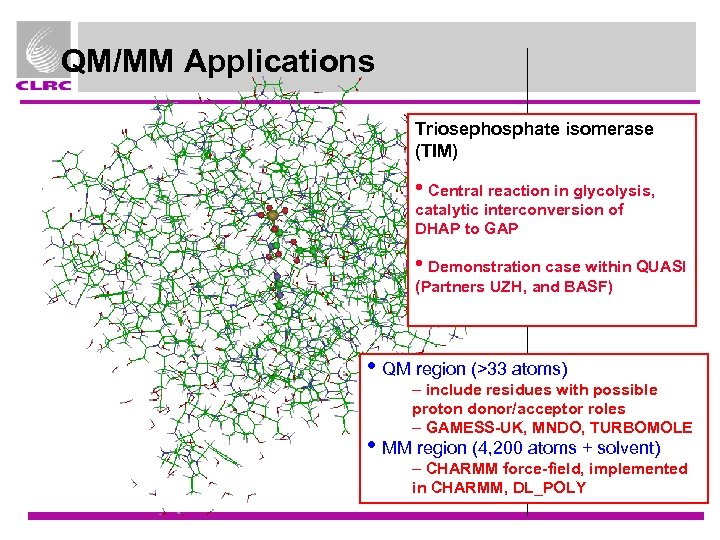 QM/MM Applications Triosephosphate isomerase (TIM) • Central reaction in glycolysis, catalytic interconversion of DHAP to GAP • Demonstration case within QUASI (Partners UZH, and BASF) • QM region (>33 atoms) – include residues with possible proton donor/acceptor roles – GAMESS-UK, MNDO, TURBOMOLE • MM region (4, 200 atoms + solvent) – CHARMM force-field, implemented in CHARMM, DL_POLY
QM/MM Applications Triosephosphate isomerase (TIM) • Central reaction in glycolysis, catalytic interconversion of DHAP to GAP • Demonstration case within QUASI (Partners UZH, and BASF) • QM region (>33 atoms) – include residues with possible proton donor/acceptor roles – GAMESS-UK, MNDO, TURBOMOLE • MM region (4, 200 atoms + solvent) – CHARMM force-field, implemented in CHARMM, DL_POLY
 Enzyme QM/MM Applications - TIM
Enzyme QM/MM Applications - TIM
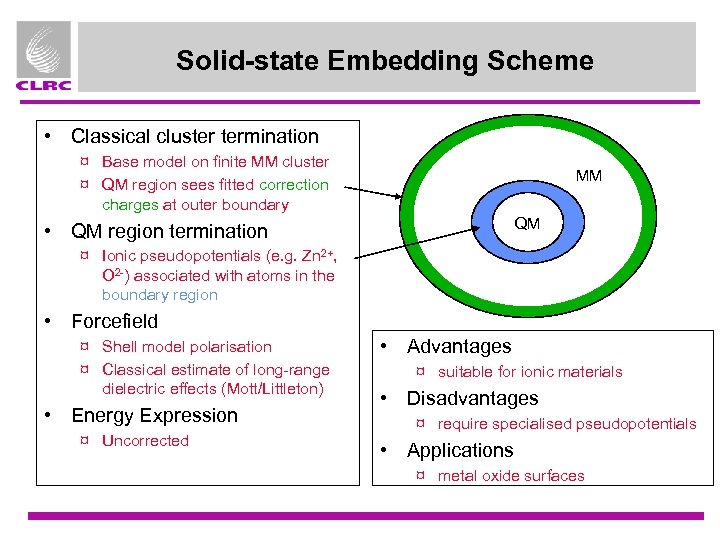 Solid-state Embedding Scheme • Classical cluster termination ¤ Base model on finite MM cluster ¤ QM region sees fitted correction charges at outer boundary MM QM • QM region termination ¤ Ionic pseudopotentials (e. g. Zn 2+, O 2 -) associated with atoms in the boundary region • Forcefield ¤ Shell model polarisation ¤ Classical estimate of long-range dielectric effects (Mott/Littleton) • Energy Expression ¤ Uncorrected • Advantages ¤ suitable for ionic materials • Disadvantages ¤ require specialised pseudopotentials • Applications ¤ metal oxide surfaces
Solid-state Embedding Scheme • Classical cluster termination ¤ Base model on finite MM cluster ¤ QM region sees fitted correction charges at outer boundary MM QM • QM region termination ¤ Ionic pseudopotentials (e. g. Zn 2+, O 2 -) associated with atoms in the boundary region • Forcefield ¤ Shell model polarisation ¤ Classical estimate of long-range dielectric effects (Mott/Littleton) • Energy Expression ¤ Uncorrected • Advantages ¤ suitable for ionic materials • Disadvantages ¤ require specialised pseudopotentials • Applications ¤ metal oxide surfaces
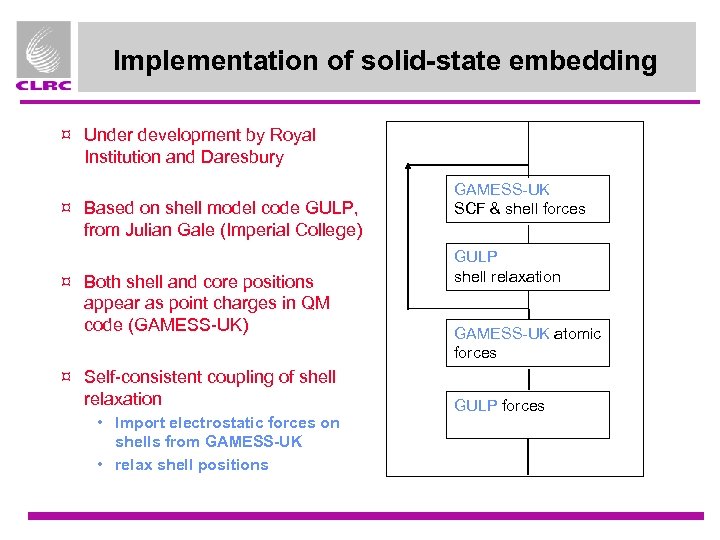 Implementation of solid-state embedding ¤ Under development by Royal Institution and Daresbury ¤ Based on shell model code GULP, from Julian Gale (Imperial College) ¤ Both shell and core positions appear as point charges in QM code (GAMESS-UK) ¤ Self-consistent coupling of shell relaxation • Import electrostatic forces on shells from GAMESS-UK • relax shell positions GAMESS-UK SCF & shell forces GULP shell relaxation GAMESS-UK atomic forces GULP forces
Implementation of solid-state embedding ¤ Under development by Royal Institution and Daresbury ¤ Based on shell model code GULP, from Julian Gale (Imperial College) ¤ Both shell and core positions appear as point charges in QM code (GAMESS-UK) ¤ Self-consistent coupling of shell relaxation • Import electrostatic forces on shells from GAMESS-UK • relax shell positions GAMESS-UK SCF & shell forces GULP shell relaxation GAMESS-UK atomic forces GULP forces
 QUASI - Surface catalysis applications Methanol synthesis from synthesis gas (CO, CO 2 and H 2) using the ternary catalyst system Cu/Zn. O/Al 2 O 3 e. g. CO + 2 H 2 -> CH 3(OH) Demonstration phase ¤ Geometry and electronic structure of bulk and surface QM clusters as a function of cluster size. ¤ Adsorption of Cu(I) on the Zn. O surface ¤ Absorption energies, IR spectra and PES for CO on Cu and Zn sites Lead Partner: ICI Target Applications ¤ Stability of Cu clusters of different sizes and ox. states ¤ Structure and energetics of absorption formate, methoxy and carbonate on the surface, 13 C chemical shifts ¤ Transition states for proton and hydride transfer steps ¤ Understanding promoter action
QUASI - Surface catalysis applications Methanol synthesis from synthesis gas (CO, CO 2 and H 2) using the ternary catalyst system Cu/Zn. O/Al 2 O 3 e. g. CO + 2 H 2 -> CH 3(OH) Demonstration phase ¤ Geometry and electronic structure of bulk and surface QM clusters as a function of cluster size. ¤ Adsorption of Cu(I) on the Zn. O surface ¤ Absorption energies, IR spectra and PES for CO on Cu and Zn sites Lead Partner: ICI Target Applications ¤ Stability of Cu clusters of different sizes and ox. states ¤ Structure and energetics of absorption formate, methoxy and carbonate on the surface, 13 C chemical shifts ¤ Transition states for proton and hydride transfer steps ¤ Understanding promoter action
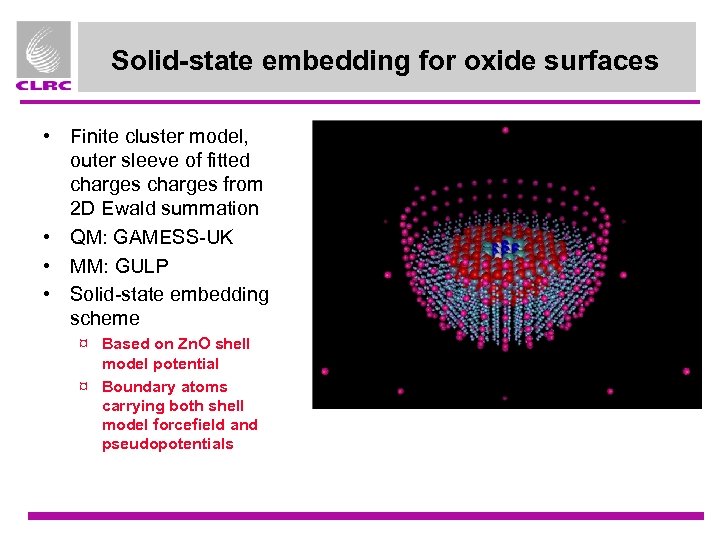 Solid-state embedding for oxide surfaces • Finite cluster model, outer sleeve of fitted charges from 2 D Ewald summation • QM: GAMESS-UK • MM: GULP • Solid-state embedding scheme ¤ Based on Zn. O shell model potential ¤ Boundary atoms carrying both shell model forcefield and pseudopotentials
Solid-state embedding for oxide surfaces • Finite cluster model, outer sleeve of fitted charges from 2 D Ewald summation • QM: GAMESS-UK • MM: GULP • Solid-state embedding scheme ¤ Based on Zn. O shell model potential ¤ Boundary atoms carrying both shell model forcefield and pseudopotentials
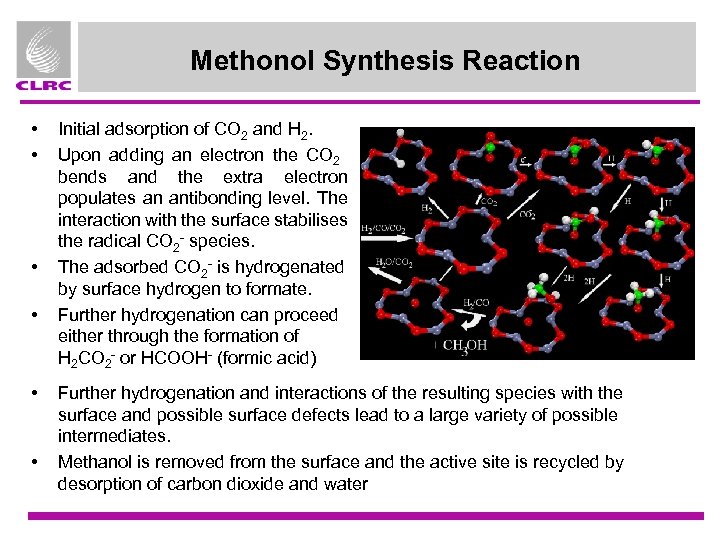 Methonol Synthesis Reaction • • • Initial adsorption of CO 2 and H 2. Upon adding an electron the CO 2 bends and the extra electron populates an antibonding level. The interaction with the surface stabilises the radical CO 2 - species. The adsorbed CO 2 - is hydrogenated by surface hydrogen to formate. Further hydrogenation can proceed either through the formation of H 2 CO 2 - or HCOOH- (formic acid) Further hydrogenation and interactions of the resulting species with the surface and possible surface defects lead to a large variety of possible intermediates. Methanol is removed from the surface and the active site is recycled by desorption of carbon dioxide and water
Methonol Synthesis Reaction • • • Initial adsorption of CO 2 and H 2. Upon adding an electron the CO 2 bends and the extra electron populates an antibonding level. The interaction with the surface stabilises the radical CO 2 - species. The adsorbed CO 2 - is hydrogenated by surface hydrogen to formate. Further hydrogenation can proceed either through the formation of H 2 CO 2 - or HCOOH- (formic acid) Further hydrogenation and interactions of the resulting species with the surface and possible surface defects lead to a large variety of possible intermediates. Methanol is removed from the surface and the active site is recycled by desorption of carbon dioxide and water
 Adsorption of copper clusters
Adsorption of copper clusters
 Acknowledgements • QUASI software developments ¤ Geometry optimisation, CHARMM interfacing, G 98 interface • Walter Thiel, Frank Terstegen, Salomon Billeter, Alex Turner ¤ TURBOMOLE interface • Ansgar Schäfer, Christian Lennartz ¤ Solid-state embedding • Alexei Sokol, Sam French, Richard Catlow • Other Collaborators ¤ CHARMM/GAMESS-UK • Bernie Brooks, Eric Billings ¤ Chem. Shell developments, models for zeolites • Alex de Vries, Simon Collins, Ian Hillier, Steve Greatbanks • CEC, Shell SIOP Amsterdam
Acknowledgements • QUASI software developments ¤ Geometry optimisation, CHARMM interfacing, G 98 interface • Walter Thiel, Frank Terstegen, Salomon Billeter, Alex Turner ¤ TURBOMOLE interface • Ansgar Schäfer, Christian Lennartz ¤ Solid-state embedding • Alexei Sokol, Sam French, Richard Catlow • Other Collaborators ¤ CHARMM/GAMESS-UK • Bernie Brooks, Eric Billings ¤ Chem. Shell developments, models for zeolites • Alex de Vries, Simon Collins, Ian Hillier, Steve Greatbanks • CEC, Shell SIOP Amsterdam


