5918755d940e626c8e5bd9db715a9741.ppt
- Количество слайдов: 43
 Lecture 17 Tissue fluorescence (Part II)
Lecture 17 Tissue fluorescence (Part II)
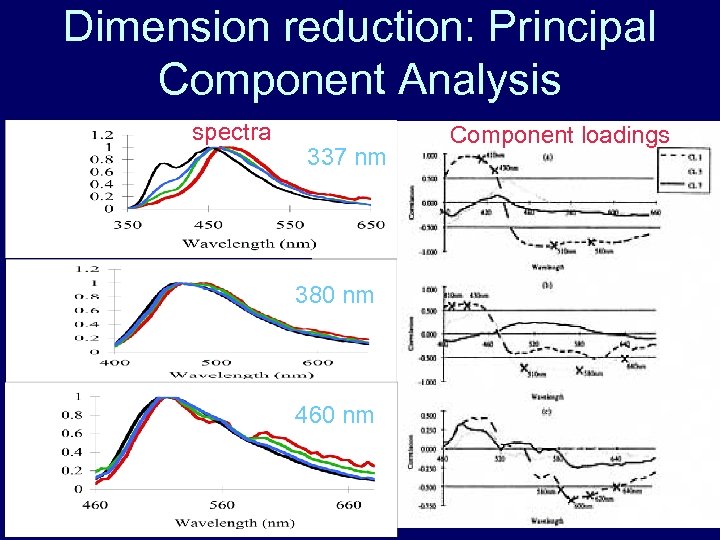 Dimension reduction: Principal Component Analysis spectra 337 nm 380 nm 460 nm Component loadings
Dimension reduction: Principal Component Analysis spectra 337 nm 380 nm 460 nm Component loadings
 Spectroscopic analysis using PCA • Uses full spectrum information to optimize sensitivity and specificity • Relatively easy to implement (automated software) • Provides no intuition with regards to the origin of spectral differences
Spectroscopic analysis using PCA • Uses full spectrum information to optimize sensitivity and specificity • Relatively easy to implement (automated software) • Provides no intuition with regards to the origin of spectral differences
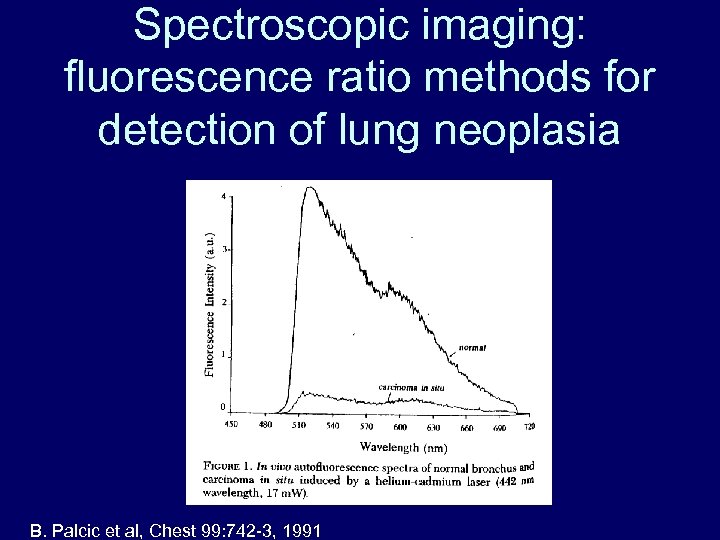 Spectroscopic imaging: fluorescence ratio methods for detection of lung neoplasia B. Palcic et al, Chest 99: 742 -3, 1991
Spectroscopic imaging: fluorescence ratio methods for detection of lung neoplasia B. Palcic et al, Chest 99: 742 -3, 1991
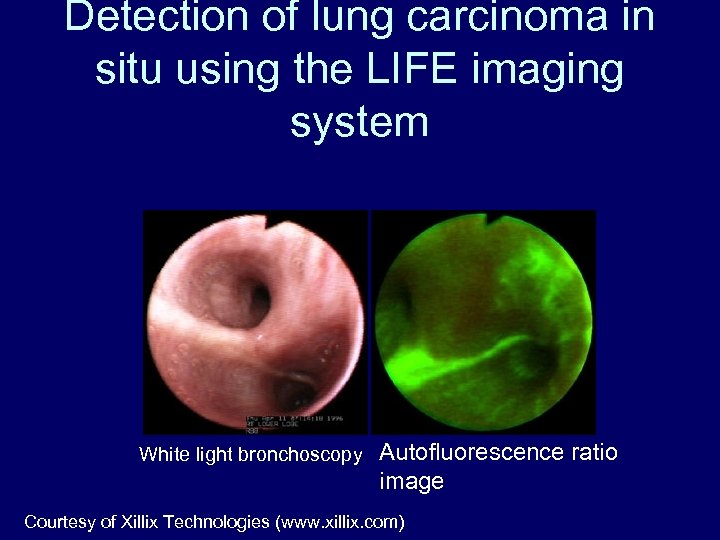 Detection of lung carcinoma in situ using the LIFE imaging system White light bronchoscopy Autofluorescence ratio image Courtesy of Xillix Technologies (www. xillix. com)
Detection of lung carcinoma in situ using the LIFE imaging system White light bronchoscopy Autofluorescence ratio image Courtesy of Xillix Technologies (www. xillix. com)
 Fluorescence imaging based on ratio methods • Wide field of view (probably a huge advantage for most clinical settings) • Eliminates effects of distance and angle of illumination • Easy to implement • Provides no intuition with regards to origins of spectral differences
Fluorescence imaging based on ratio methods • Wide field of view (probably a huge advantage for most clinical settings) • Eliminates effects of distance and angle of illumination • Easy to implement • Provides no intuition with regards to origins of spectral differences
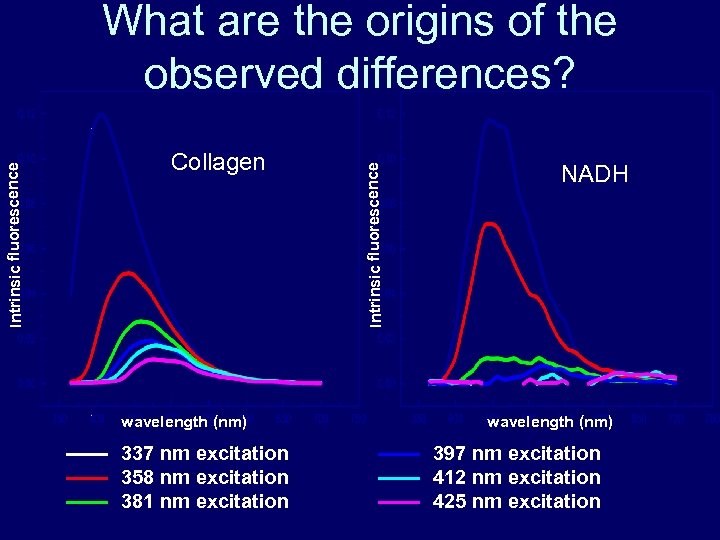 Collagen wavelength (nm) 337 nm excitation 358 nm excitation 381 nm excitation Intrinsic fluorescence What are the origins of the observed differences? NADH wavelength (nm) 397 nm excitation 412 nm excitation 425 nm excitation
Collagen wavelength (nm) 337 nm excitation 358 nm excitation 381 nm excitation Intrinsic fluorescence What are the origins of the observed differences? NADH wavelength (nm) 397 nm excitation 412 nm excitation 425 nm excitation
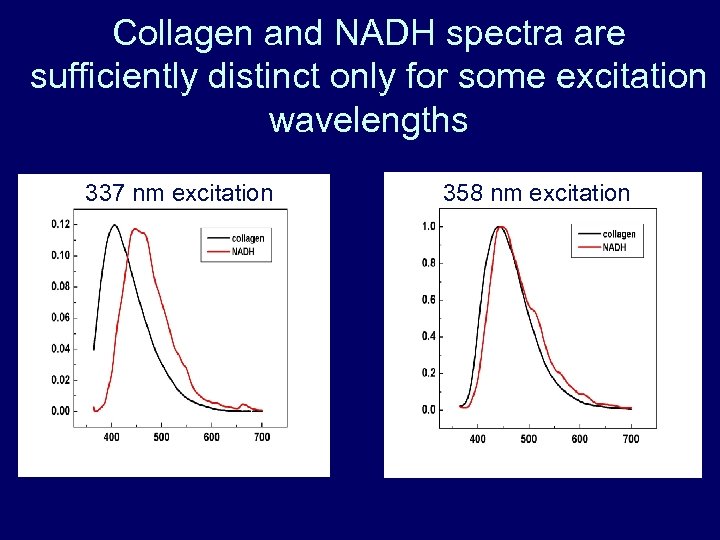 Collagen and NADH spectra are sufficiently distinct only for some excitation wavelengths 337 nm excitation 358 nm excitation
Collagen and NADH spectra are sufficiently distinct only for some excitation wavelengths 337 nm excitation 358 nm excitation
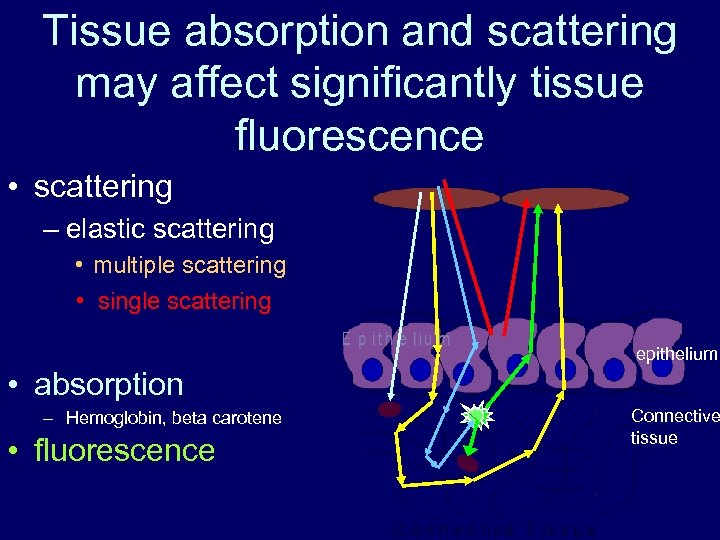 Tissue absorption and scattering may affect significantly tissue fluorescence • scattering – elastic scattering • multiple scattering • single scattering epithelium • absorption – Hemoglobin, beta carotene • fluorescence Connective tissue
Tissue absorption and scattering may affect significantly tissue fluorescence • scattering – elastic scattering • multiple scattering • single scattering epithelium • absorption – Hemoglobin, beta carotene • fluorescence Connective tissue
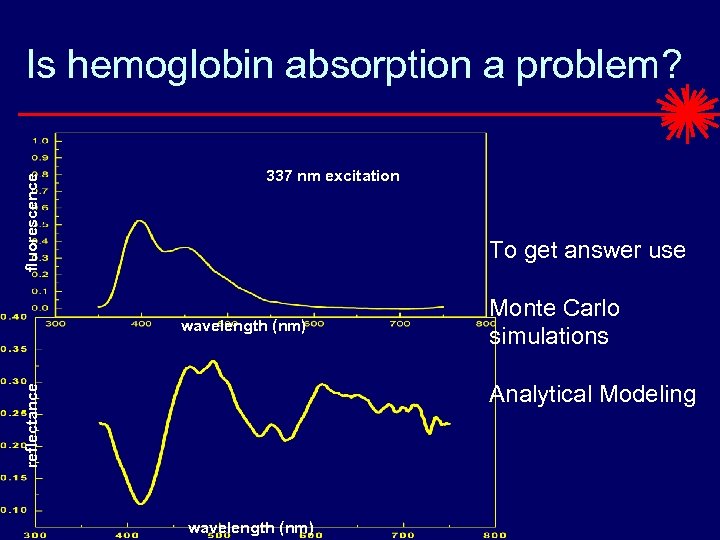 fluorescence Is hemoglobin absorption a problem? 337 nm excitation To get answer use wavelength (nm) Monte Carlo simulations reflectance Analytical Modeling wavelength (nm)
fluorescence Is hemoglobin absorption a problem? 337 nm excitation To get answer use wavelength (nm) Monte Carlo simulations reflectance Analytical Modeling wavelength (nm)
 Monte Carlo simulations • Treat light as individual photons • Assign to each photon a probability of being absorbed or being scattered in a particular direction • Collect photons that make it out of the tissue • Relatively easy to model different light delivery/collection geometries and multiple tissue layers • Require a lot of time for acquiring statistically valid results
Monte Carlo simulations • Treat light as individual photons • Assign to each photon a probability of being absorbed or being scattered in a particular direction • Collect photons that make it out of the tissue • Relatively easy to model different light delivery/collection geometries and multiple tissue layers • Require a lot of time for acquiring statistically valid results
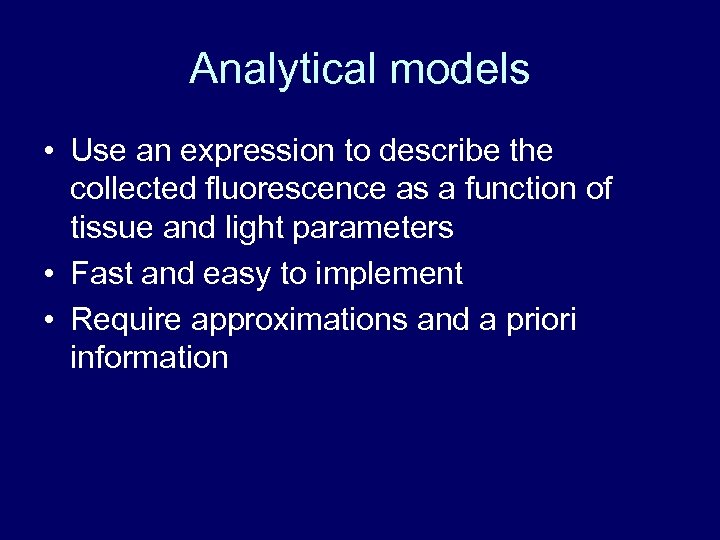 Analytical models • Use an expression to describe the collected fluorescence as a function of tissue and light parameters • Fast and easy to implement • Require approximations and a priori information
Analytical models • Use an expression to describe the collected fluorescence as a function of tissue and light parameters • Fast and easy to implement • Require approximations and a priori information
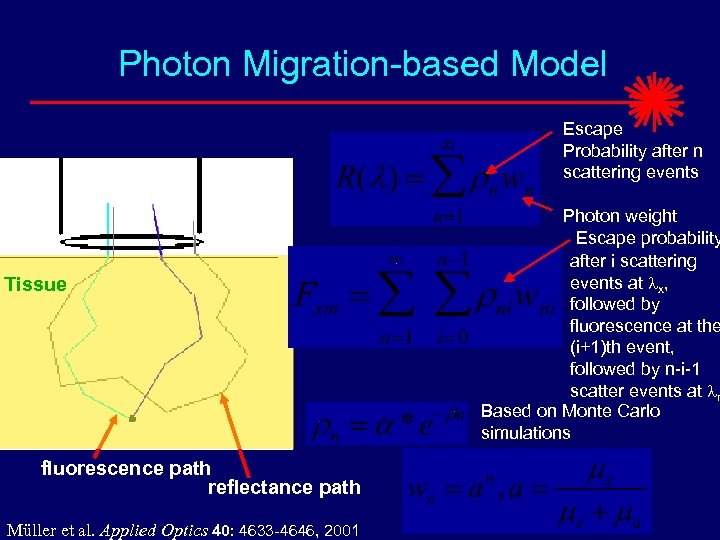 Photon Migration-based Model Escape Probability after n scattering events Tissue fluorescence path reflectance path Müller et al. Applied Optics 40: 4633 -4646, 2001 Photon weight Escape probability after i scattering events at lx, followed by fluorescence at the (i+1)th event, followed by n-i-1 scatter events at lm Based on Monte Carlo simulations
Photon Migration-based Model Escape Probability after n scattering events Tissue fluorescence path reflectance path Müller et al. Applied Optics 40: 4633 -4646, 2001 Photon weight Escape probability after i scattering events at lx, followed by fluorescence at the (i+1)th event, followed by n-i-1 scatter events at lm Based on Monte Carlo simulations
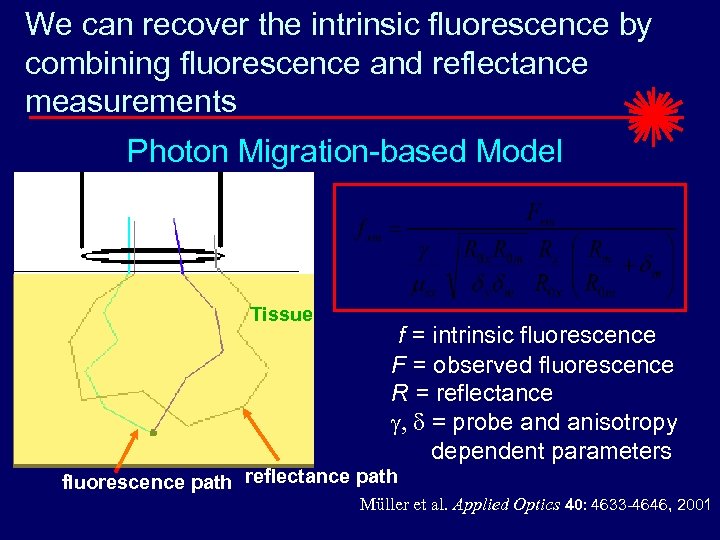 We can recover the intrinsic fluorescence by combining fluorescence and reflectance measurements Photon Migration-based Model Tissue f = intrinsic fluorescence F = observed fluorescence R = reflectance g, d = probe and anisotropy dependent parameters fluorescence path reflectance path Müller et al. Applied Optics 40: 4633 -4646, 2001
We can recover the intrinsic fluorescence by combining fluorescence and reflectance measurements Photon Migration-based Model Tissue f = intrinsic fluorescence F = observed fluorescence R = reflectance g, d = probe and anisotropy dependent parameters fluorescence path reflectance path Müller et al. Applied Optics 40: 4633 -4646, 2001
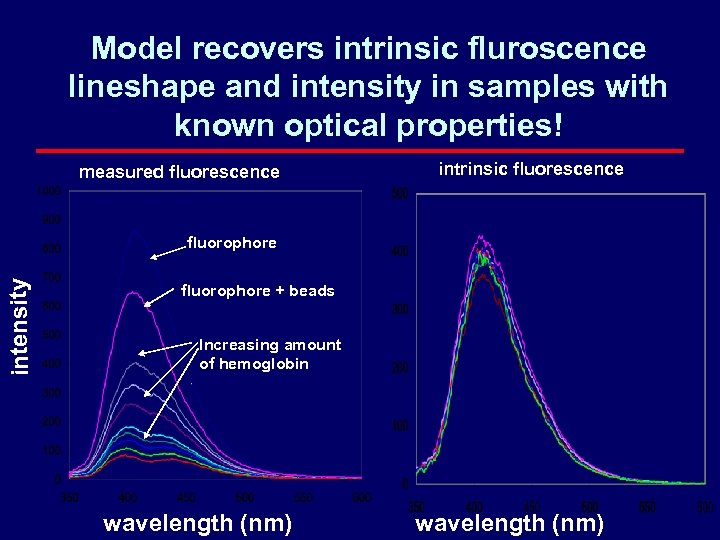 Model recovers intrinsic fluroscence lineshape and intensity in samples with known optical properties! measured fluorescence intrinsic fluorescence intensity fluorophore + beads Increasing amount of hemoglobin wavelength (nm)
Model recovers intrinsic fluroscence lineshape and intensity in samples with known optical properties! measured fluorescence intrinsic fluorescence intensity fluorophore + beads Increasing amount of hemoglobin wavelength (nm)
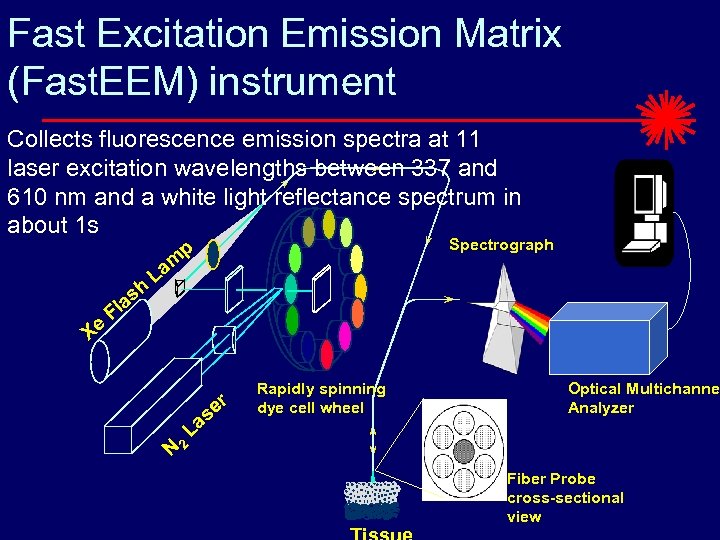 Fast Excitation Emission Matrix (Fast. EEM) instrument Collects fluorescence emission spectra at 11 laser excitation wavelengths between 337 and 610 nm and a white light reflectance spectrum in about 1 s Xe Fl sh a Spectrograph p m La er N 2 as L Rapidly spinning dye cell wheel Optical Multichannel Analyzer Fiber Probe cross-sectional view
Fast Excitation Emission Matrix (Fast. EEM) instrument Collects fluorescence emission spectra at 11 laser excitation wavelengths between 337 and 610 nm and a white light reflectance spectrum in about 1 s Xe Fl sh a Spectrograph p m La er N 2 as L Rapidly spinning dye cell wheel Optical Multichannel Analyzer Fiber Probe cross-sectional view

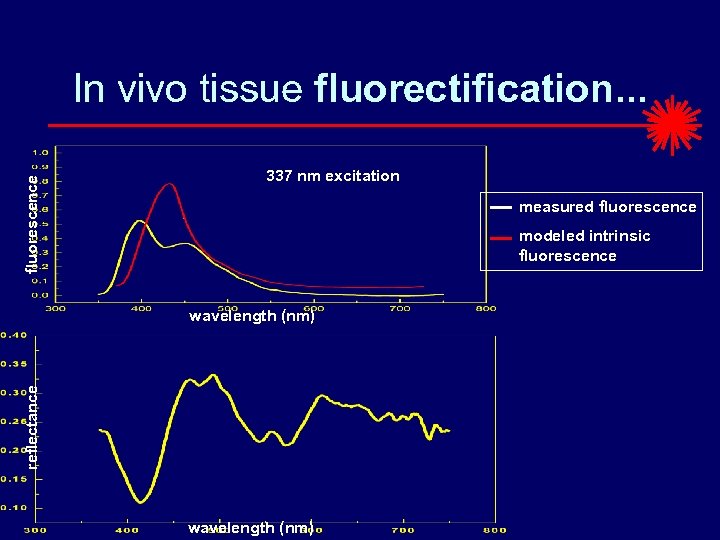 fluorescence In vivo tissue fluorectification. . . 337 nm excitation measured fluorescence modeled intrinsic fluorescence reflectance wavelength (nm)
fluorescence In vivo tissue fluorectification. . . 337 nm excitation measured fluorescence modeled intrinsic fluorescence reflectance wavelength (nm)
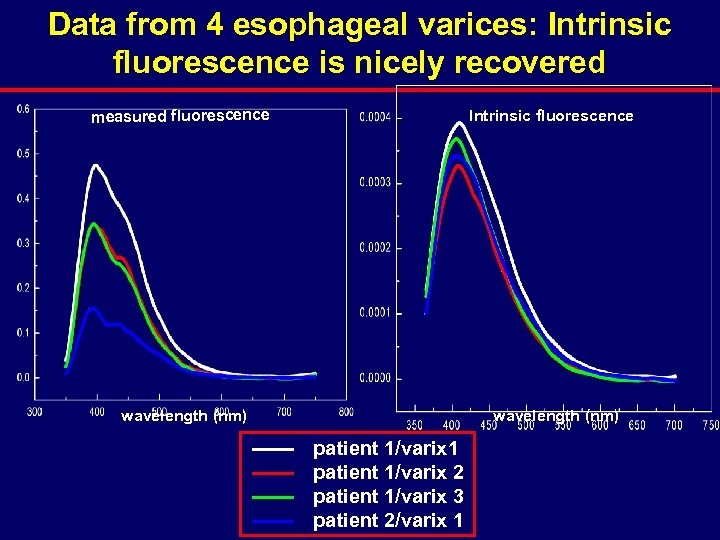 Data from 4 esophageal varices: Intrinsic fluorescence is nicely recovered measured fluorescence Intrinsic fluorescence wavelength (nm) patient 1/varix 1 patient 1/varix 2 patient 1/varix 3 patient 2/varix 1
Data from 4 esophageal varices: Intrinsic fluorescence is nicely recovered measured fluorescence Intrinsic fluorescence wavelength (nm) patient 1/varix 1 patient 1/varix 2 patient 1/varix 3 patient 2/varix 1
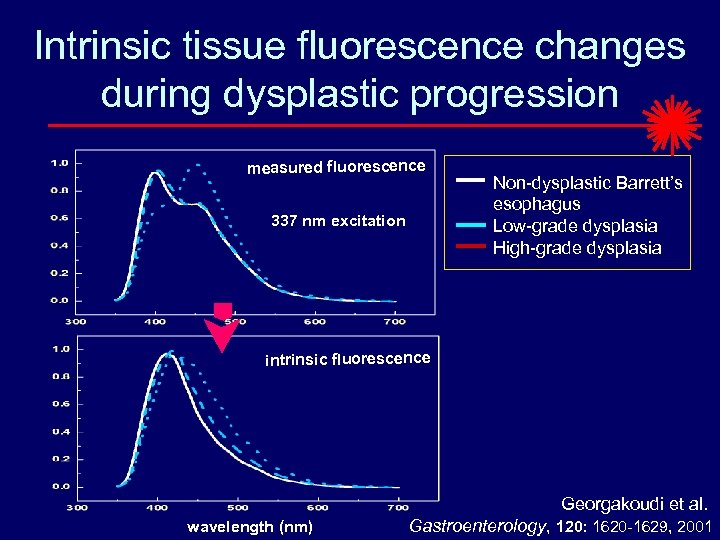 Intrinsic tissue fluorescence changes during dysplastic progression measured fluorescence 337 nm excitation Non-dysplastic Barrett’s esophagus Low-grade dysplasia High-grade dysplasia intrinsic fluorescence Georgakoudi et al. wavelength (nm) Gastroenterology, 120: 1620 -1629, 2001
Intrinsic tissue fluorescence changes during dysplastic progression measured fluorescence 337 nm excitation Non-dysplastic Barrett’s esophagus Low-grade dysplasia High-grade dysplasia intrinsic fluorescence Georgakoudi et al. wavelength (nm) Gastroenterology, 120: 1620 -1629, 2001
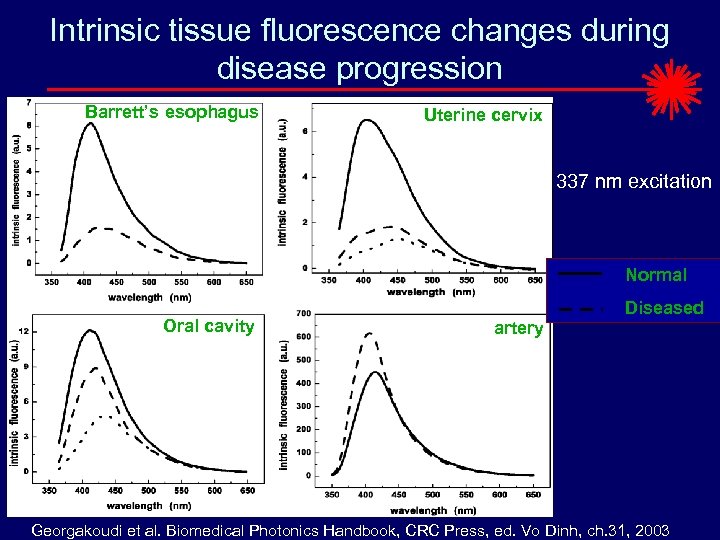 Intrinsic tissue fluorescence changes during disease progression Barrett’s esophagus Uterine cervix 337 nm excitation Normal Oral cavity Diseased artery Georgakoudi et al. Biomedical Photonics Handbook, CRC Press, ed. Vo Dinh, ch. 31, 2003
Intrinsic tissue fluorescence changes during disease progression Barrett’s esophagus Uterine cervix 337 nm excitation Normal Oral cavity Diseased artery Georgakoudi et al. Biomedical Photonics Handbook, CRC Press, ed. Vo Dinh, ch. 31, 2003
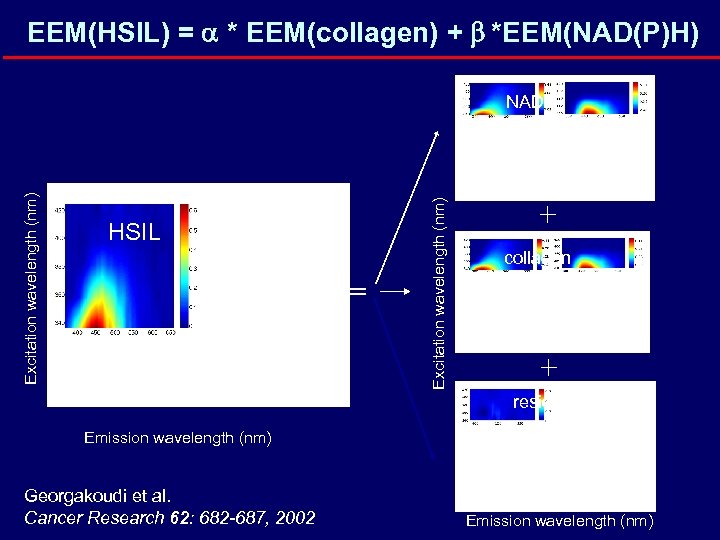 EEM(HSIL) = a * EEM(collagen) + b *EEM(NAD(P)H) HSIL = = Excitation wavelength (nm) NADH + collagen + + residual Emission wavelength (nm) Georgakoudi et al. Cancer Research 62: 682 -687, 2002 + Emission wavelength (nm)
EEM(HSIL) = a * EEM(collagen) + b *EEM(NAD(P)H) HSIL = = Excitation wavelength (nm) NADH + collagen + + residual Emission wavelength (nm) Georgakoudi et al. Cancer Research 62: 682 -687, 2002 + Emission wavelength (nm)
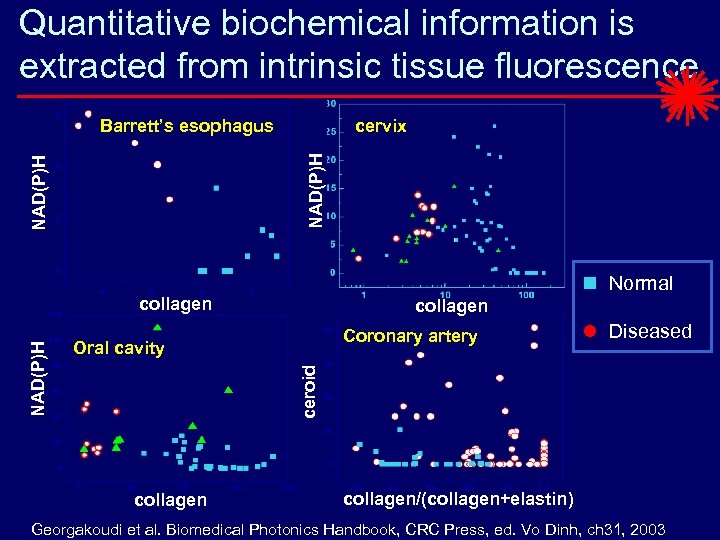 Quantitative biochemical information is extracted from intrinsic tissue fluorescence cervix NAD(P)H Barrett’s esophagus Normal collagen Coronary artery Oral cavity Diseased ceroid NAD(P)H collagen/(collagen+elastin) Georgakoudi et al. Biomedical Photonics Handbook, CRC Press, ed. Vo Dinh, ch 31, 2003
Quantitative biochemical information is extracted from intrinsic tissue fluorescence cervix NAD(P)H Barrett’s esophagus Normal collagen Coronary artery Oral cavity Diseased ceroid NAD(P)H collagen/(collagen+elastin) Georgakoudi et al. Biomedical Photonics Handbook, CRC Press, ed. Vo Dinh, ch 31, 2003
 Model based fluorescence spectral analysis • Utilizes full spectrum information • Model development may require assumptions/approximations • Results are quantitative and provide insights into the origins of spectral difference • Instrument optimization • Disease understanding
Model based fluorescence spectral analysis • Utilizes full spectrum information • Model development may require assumptions/approximations • Results are quantitative and provide insights into the origins of spectral difference • Instrument optimization • Disease understanding
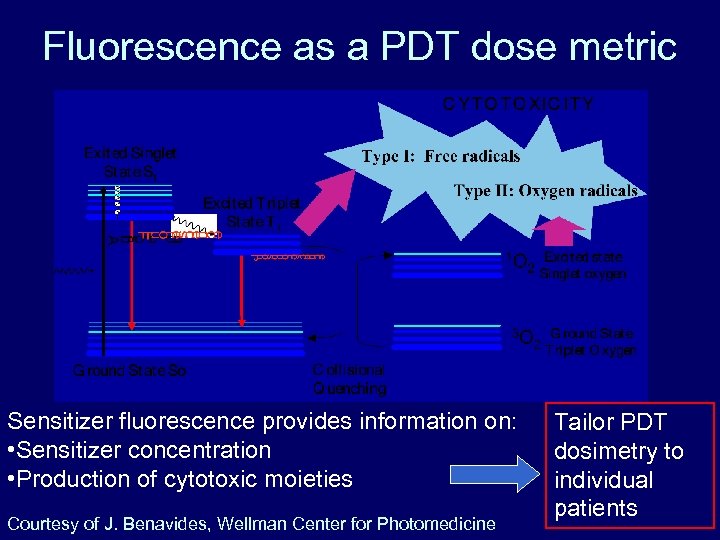 Fluorescence as a PDT dose metric Sensitizer fluorescence provides information on: • Sensitizer concentration • Production of cytotoxic moieties Courtesy of J. Benavides, Wellman Center for Photomedicine Tailor PDT dosimetry to individual patients
Fluorescence as a PDT dose metric Sensitizer fluorescence provides information on: • Sensitizer concentration • Production of cytotoxic moieties Courtesy of J. Benavides, Wellman Center for Photomedicine Tailor PDT dosimetry to individual patients
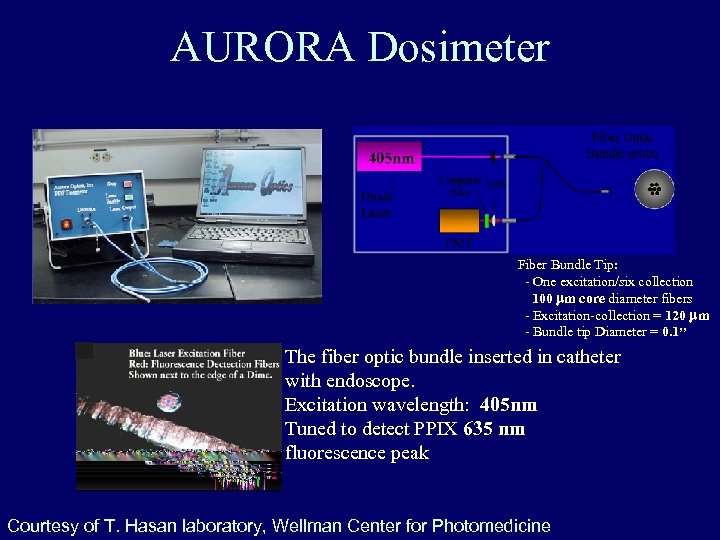 AURORA Dosimeter Fiber Bundle Tip: - One excitation/six collection 100 mm core diameter fibers - Excitation-collection = 120 mm - Bundle tip Diameter = 0. 1’’ The fiber optic bundle inserted in catheter with endoscope. Excitation wavelength: 405 nm Tuned to detect PPIX 635 nm fluorescence peak Courtesy of T. Hasan laboratory, Wellman Center for Photomedicine
AURORA Dosimeter Fiber Bundle Tip: - One excitation/six collection 100 mm core diameter fibers - Excitation-collection = 120 mm - Bundle tip Diameter = 0. 1’’ The fiber optic bundle inserted in catheter with endoscope. Excitation wavelength: 405 nm Tuned to detect PPIX 635 nm fluorescence peak Courtesy of T. Hasan laboratory, Wellman Center for Photomedicine
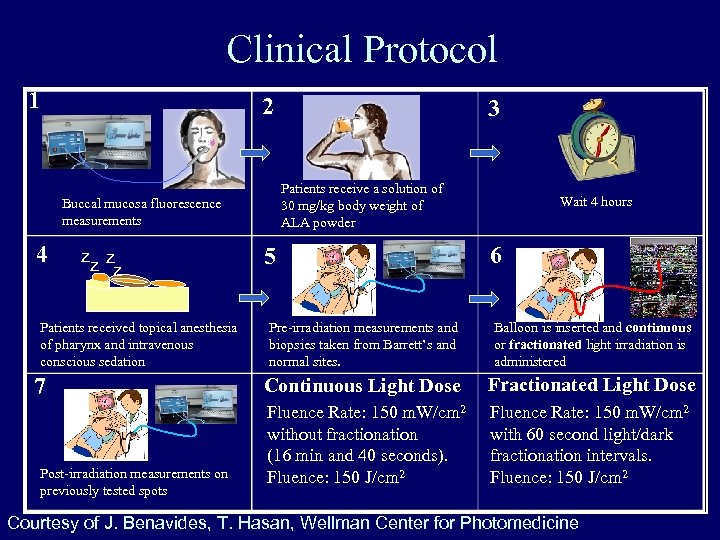 Clinical Protocol 1 2 Patients receive a solution of 30 mg/kg body weight of ALA powder Buccal mucosa fluorescence measurements 4 zz z z Patients received topical anesthesia of pharynx and intravenous conscious sedation 7 Post-irradiation measurements on previously tested spots 3 5 Wait 4 hours 6 Pre-irradiation measurements and biopsies taken from Barrett’s and normal sites. Balloon is inserted and continuous or fractionated light irradiation is administered Continuous Light Dose Fractionated Light Dose Fluence Rate: 150 m. W/cm 2 without fractionation (16 min and 40 seconds). Fluence: 150 J/cm 2 Fluence Rate: 150 m. W/cm 2 with 60 second light/dark fractionation intervals. Fluence: 150 J/cm 2 Courtesy of J. Benavides, T. Hasan, Wellman Center for Photomedicine
Clinical Protocol 1 2 Patients receive a solution of 30 mg/kg body weight of ALA powder Buccal mucosa fluorescence measurements 4 zz z z Patients received topical anesthesia of pharynx and intravenous conscious sedation 7 Post-irradiation measurements on previously tested spots 3 5 Wait 4 hours 6 Pre-irradiation measurements and biopsies taken from Barrett’s and normal sites. Balloon is inserted and continuous or fractionated light irradiation is administered Continuous Light Dose Fractionated Light Dose Fluence Rate: 150 m. W/cm 2 without fractionation (16 min and 40 seconds). Fluence: 150 J/cm 2 Fluence Rate: 150 m. W/cm 2 with 60 second light/dark fractionation intervals. Fluence: 150 J/cm 2 Courtesy of J. Benavides, T. Hasan, Wellman Center for Photomedicine
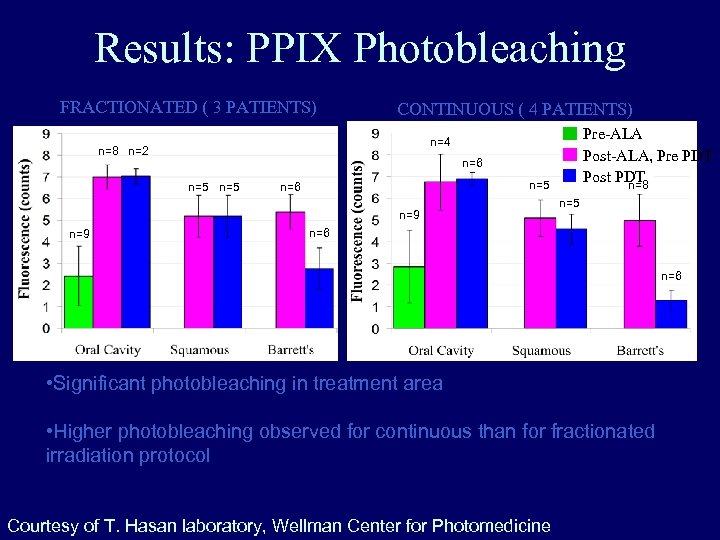 Results: PPIX Photobleaching FRACTIONATED ( 3 PATIENTS) n=8 n=2 CONTINUOUS ( 4 PATIENTS) n=6 n=5 n=5 n=6 n=9 Pre-ALA Post-ALA, Pre PDT Post PDT n=8 n=4 n = number of sites n=5 n=6 • Significant photobleaching in treatment area • Higher photobleaching observed for continuous than for fractionated irradiation protocol Courtesy of T. Hasan laboratory, Wellman Center for Photomedicine
Results: PPIX Photobleaching FRACTIONATED ( 3 PATIENTS) n=8 n=2 CONTINUOUS ( 4 PATIENTS) n=6 n=5 n=5 n=6 n=9 Pre-ALA Post-ALA, Pre PDT Post PDT n=8 n=4 n = number of sites n=5 n=6 • Significant photobleaching in treatment area • Higher photobleaching observed for continuous than for fractionated irradiation protocol Courtesy of T. Hasan laboratory, Wellman Center for Photomedicine
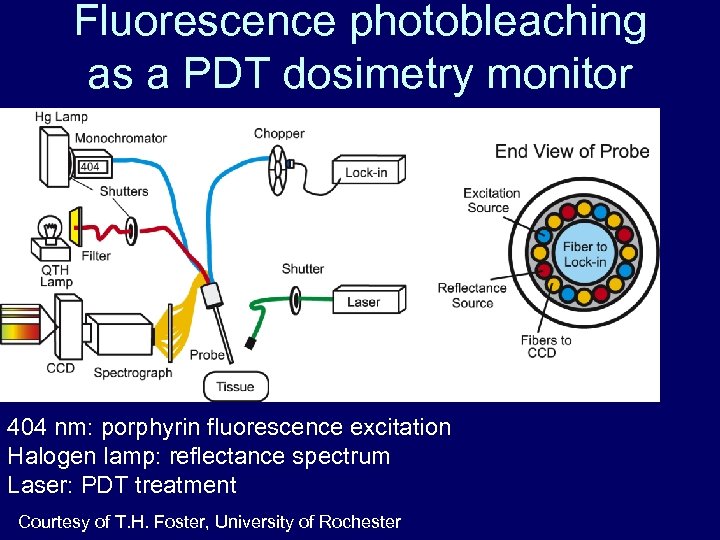 Fluorescence photobleaching as a PDT dosimetry monitor 404 nm: porphyrin fluorescence excitation Halogen lamp: reflectance spectrum Laser: PDT treatment Courtesy of T. H. Foster, University of Rochester
Fluorescence photobleaching as a PDT dosimetry monitor 404 nm: porphyrin fluorescence excitation Halogen lamp: reflectance spectrum Laser: PDT treatment Courtesy of T. H. Foster, University of Rochester
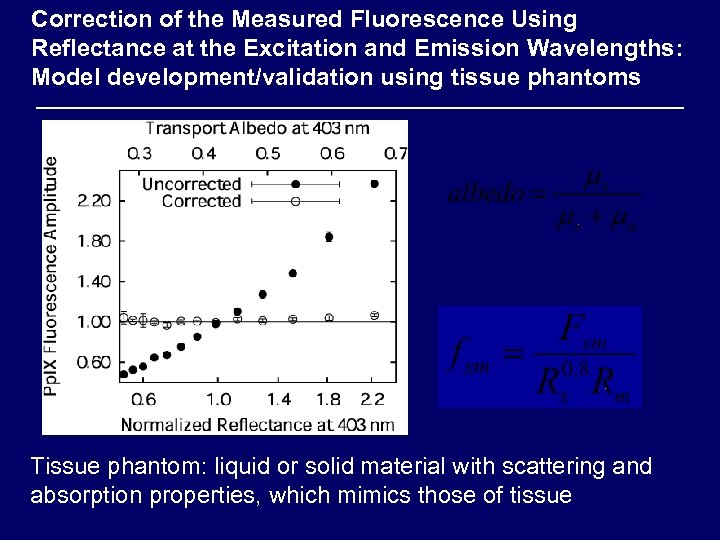 Correction of the Measured Fluorescence Using Reflectance at the Excitation and Emission Wavelengths: Model development/validation using tissue phantoms Tissue phantom: liquid or solid material with scattering and absorption properties, which mimics those of tissue
Correction of the Measured Fluorescence Using Reflectance at the Excitation and Emission Wavelengths: Model development/validation using tissue phantoms Tissue phantom: liquid or solid material with scattering and absorption properties, which mimics those of tissue
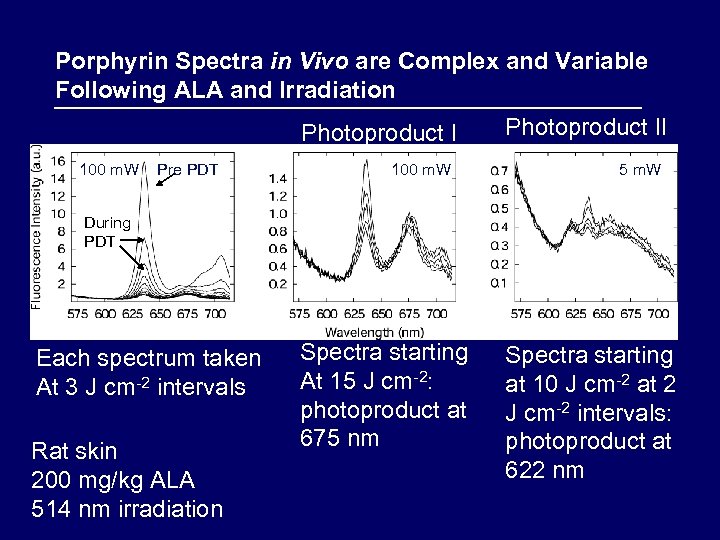 Porphyrin Spectra in Vivo are Complex and Variable Following ALA and Irradiation Photoproduct I 100 m. W Pre PDT 100 m. W Photoproduct II 5 m. W During PDT Each spectrum taken At 3 J cm-2 intervals Rat skin 200 mg/kg ALA 514 nm irradiation Spectra starting At 15 J cm-2: photoproduct at 675 nm Spectra starting at 10 J cm-2 at 2 J cm-2 intervals: photoproduct at 622 nm
Porphyrin Spectra in Vivo are Complex and Variable Following ALA and Irradiation Photoproduct I 100 m. W Pre PDT 100 m. W Photoproduct II 5 m. W During PDT Each spectrum taken At 3 J cm-2 intervals Rat skin 200 mg/kg ALA 514 nm irradiation Spectra starting At 15 J cm-2: photoproduct at 675 nm Spectra starting at 10 J cm-2 at 2 J cm-2 intervals: photoproduct at 622 nm
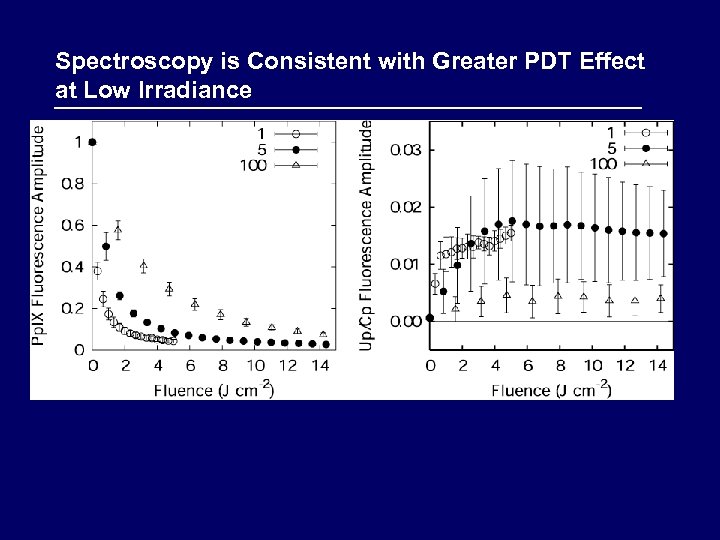 Spectroscopy is Consistent with Greater PDT Effect at Low Irradiance
Spectroscopy is Consistent with Greater PDT Effect at Low Irradiance
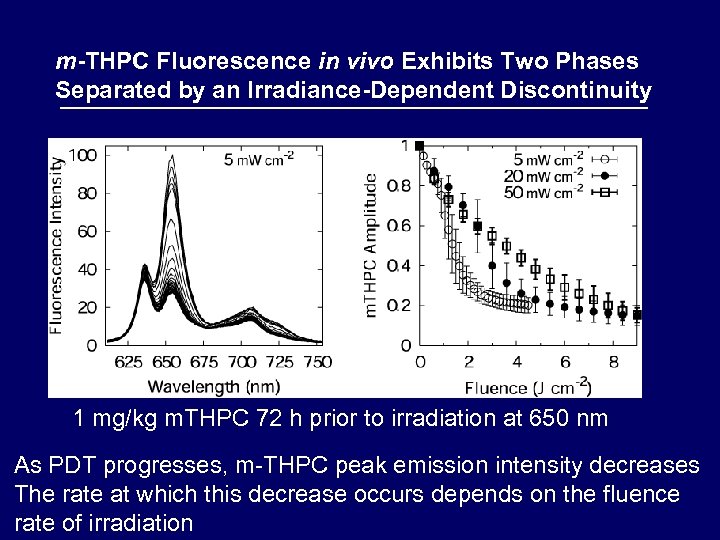 m-THPC Fluorescence in vivo Exhibits Two Phases Separated by an Irradiance-Dependent Discontinuity 1 mg/kg m. THPC 72 h prior to irradiation at 650 nm As PDT progresses, m-THPC peak emission intensity decreases The rate at which this decrease occurs depends on the fluence rate of irradiation
m-THPC Fluorescence in vivo Exhibits Two Phases Separated by an Irradiance-Dependent Discontinuity 1 mg/kg m. THPC 72 h prior to irradiation at 650 nm As PDT progresses, m-THPC peak emission intensity decreases The rate at which this decrease occurs depends on the fluence rate of irradiation
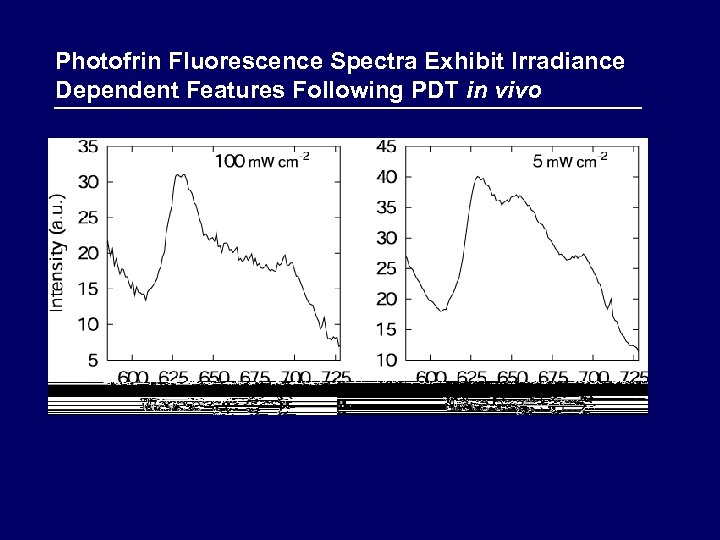 Photofrin Fluorescence Spectra Exhibit Irradiance Dependent Features Following PDT in vivo
Photofrin Fluorescence Spectra Exhibit Irradiance Dependent Features Following PDT in vivo
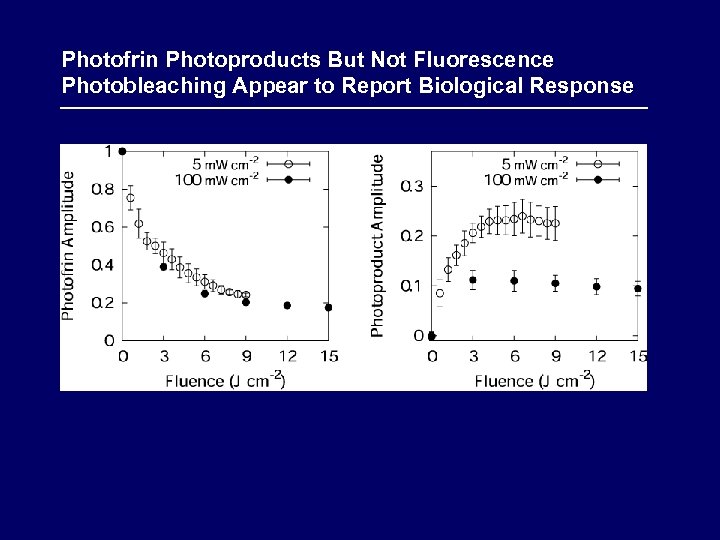 Photofrin Photoproducts But Not Fluorescence Photobleaching Appear to Report Biological Response
Photofrin Photoproducts But Not Fluorescence Photobleaching Appear to Report Biological Response
 Fluorescence spectroscopy in PDT • Enhance accuracy of sensitizer dose • Indirect reporter of deposited cytotoxic dose • Tailor dosimetry to individual patient physiology/biochemistry
Fluorescence spectroscopy in PDT • Enhance accuracy of sensitizer dose • Indirect reporter of deposited cytotoxic dose • Tailor dosimetry to individual patient physiology/biochemistry
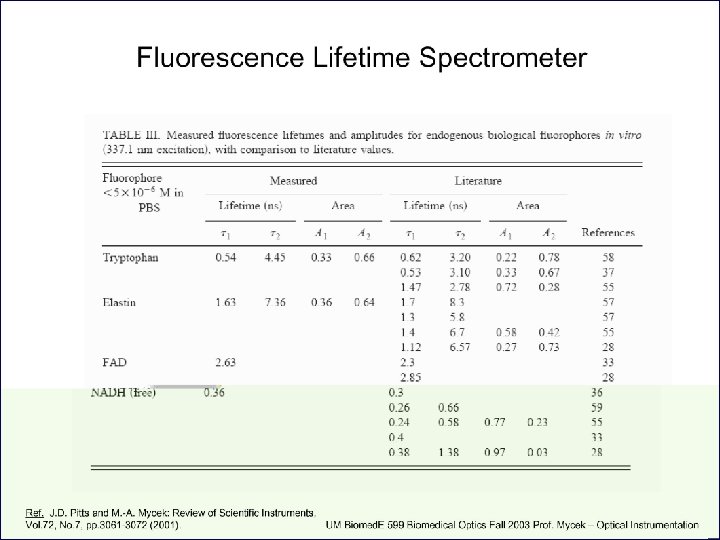
 Fluorescence life-time methods • Provide an additional dimension of information missing in time-integrated steady-state spectral measurements • Sensitive to biochemical microenvironment, including local p. H, oxygenation and binding • Lifetimes unaffected by variations in excitation intensity, concentration or sources of optical loss • Compatible with clinical measurements in vivo Courtesy of M. -A. Mycek, U Michigan
Fluorescence life-time methods • Provide an additional dimension of information missing in time-integrated steady-state spectral measurements • Sensitive to biochemical microenvironment, including local p. H, oxygenation and binding • Lifetimes unaffected by variations in excitation intensity, concentration or sources of optical loss • Compatible with clinical measurements in vivo Courtesy of M. -A. Mycek, U Michigan
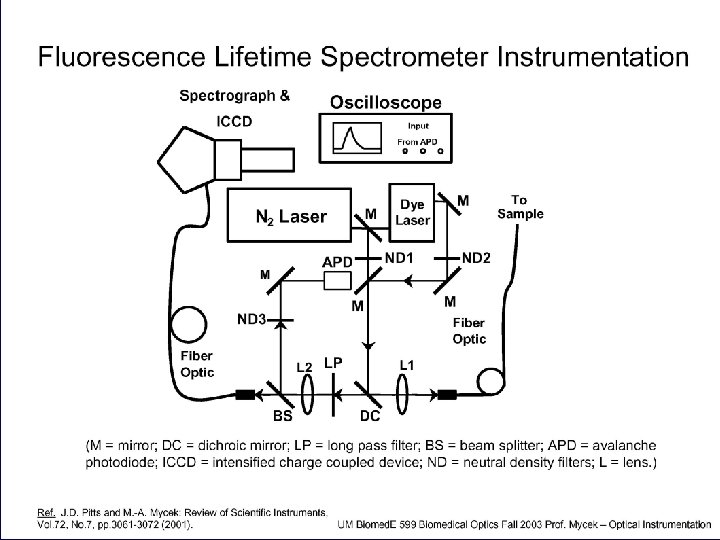
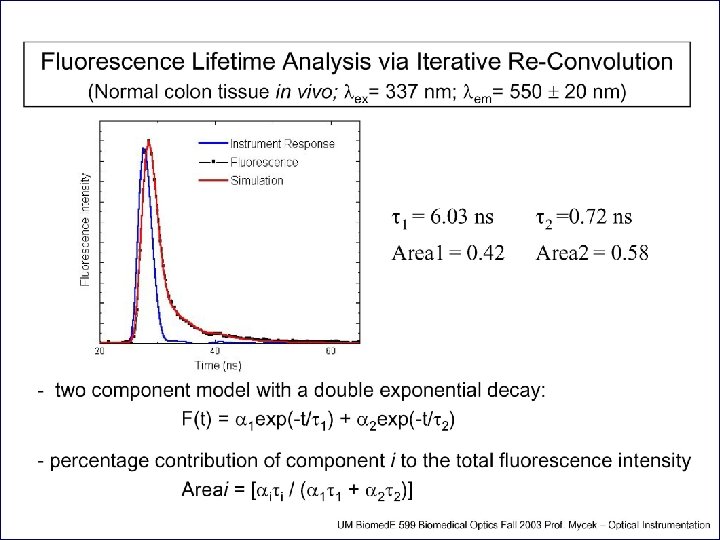
 Fluorescence lifetime measurements
Fluorescence lifetime measurements
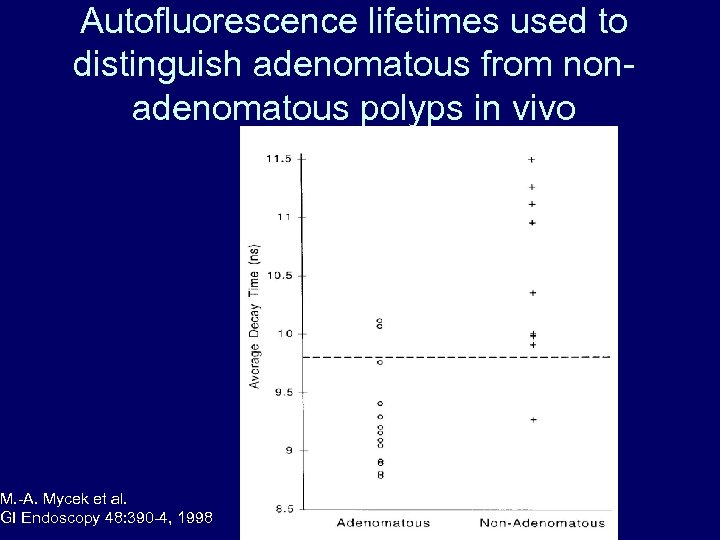 Autofluorescence lifetimes used to distinguish adenomatous from nonadenomatous polyps in vivo M. -A. Mycek et al. GI Endoscopy 48: 390 -4, 1998
Autofluorescence lifetimes used to distinguish adenomatous from nonadenomatous polyps in vivo M. -A. Mycek et al. GI Endoscopy 48: 390 -4, 1998
 Conclusion • Fluorescence spectra provide a rich source of information on tissue state, which may be used to improve current methods of disease detection and treatment • Fluorescence-based instrumentation is relatively simple, compact and compatible with clinical measurements
Conclusion • Fluorescence spectra provide a rich source of information on tissue state, which may be used to improve current methods of disease detection and treatment • Fluorescence-based instrumentation is relatively simple, compact and compatible with clinical measurements


