c9a196c9a5e65fe887856fe24237af50.ppt
- Количество слайдов: 38
 Lecture 13. 0 Chemical Mechanical Polishing
Lecture 13. 0 Chemical Mechanical Polishing
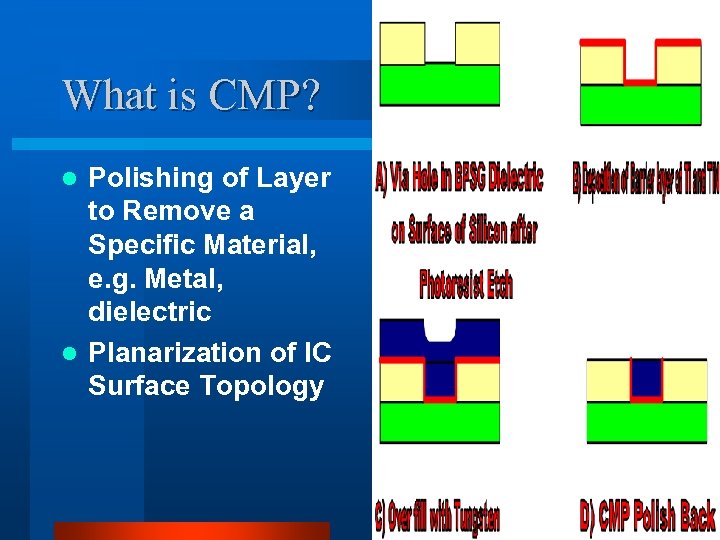 What is CMP? Polishing of Layer to Remove a Specific Material, e. g. Metal, dielectric l Planarization of IC Surface Topology l
What is CMP? Polishing of Layer to Remove a Specific Material, e. g. Metal, dielectric l Planarization of IC Surface Topology l
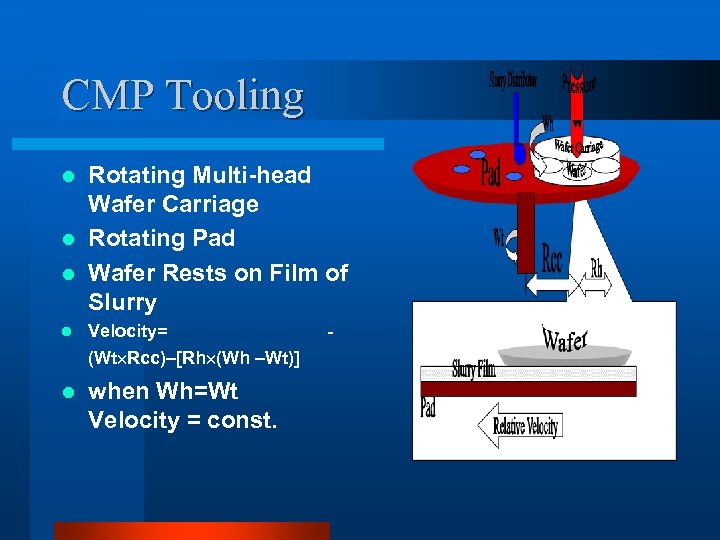 CMP Tooling Rotating Multi-head Wafer Carriage l Rotating Pad l Wafer Rests on Film of Slurry l l Velocity= (Wt Rcc)–[Rh (Wh –Wt)] l when Wh=Wt Velocity = const. -
CMP Tooling Rotating Multi-head Wafer Carriage l Rotating Pad l Wafer Rests on Film of Slurry l l Velocity= (Wt Rcc)–[Rh (Wh –Wt)] l when Wh=Wt Velocity = const. -
 Slurry l Aqueous Chemical Mixture – Material to be removed is soluble in liquid – Material to be removed reacts to form an oxide layer which is abraded by abrasive l Abrasive – 5 -20% wgt of ~200± 50 nm particles • Narrow PSD, high purity(<100 ppm) • Fumed particle = fractal aggregates of spherical primary particles (15 -30 nm)
Slurry l Aqueous Chemical Mixture – Material to be removed is soluble in liquid – Material to be removed reacts to form an oxide layer which is abraded by abrasive l Abrasive – 5 -20% wgt of ~200± 50 nm particles • Narrow PSD, high purity(<100 ppm) • Fumed particle = fractal aggregates of spherical primary particles (15 -30 nm)
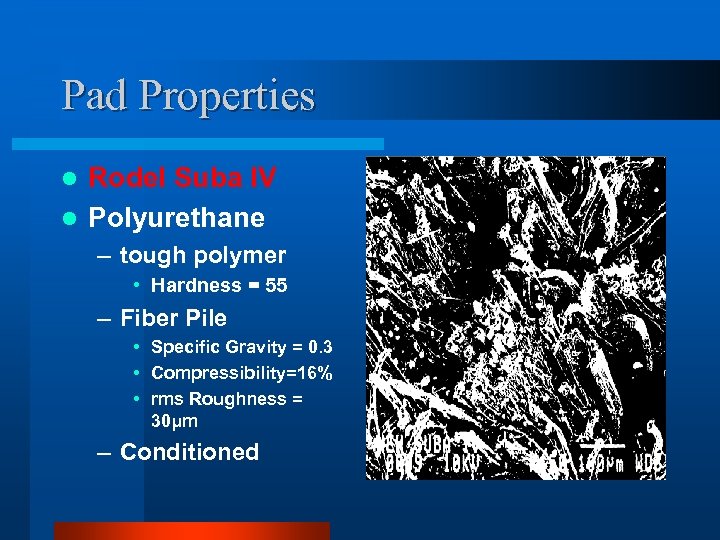 Pad Properties Rodel Suba IV l Polyurethane l – tough polymer • Hardness = 55 – Fiber Pile • Specific Gravity = 0. 3 • Compressibility=16% • rms Roughness = 30μm – Conditioned
Pad Properties Rodel Suba IV l Polyurethane l – tough polymer • Hardness = 55 – Fiber Pile • Specific Gravity = 0. 3 • Compressibility=16% • rms Roughness = 30μm – Conditioned
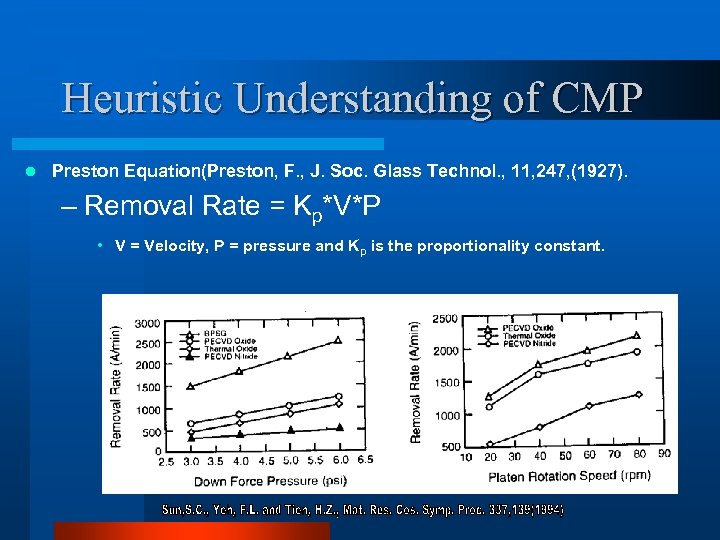 Heuristic Understanding of CMP l Preston Equation(Preston, F. , J. Soc. Glass Technol. , 11, 247, (1927). – Removal Rate = Kp*V*P • V = Velocity, P = pressure and Kp is the proportionality constant.
Heuristic Understanding of CMP l Preston Equation(Preston, F. , J. Soc. Glass Technol. , 11, 247, (1927). – Removal Rate = Kp*V*P • V = Velocity, P = pressure and Kp is the proportionality constant.
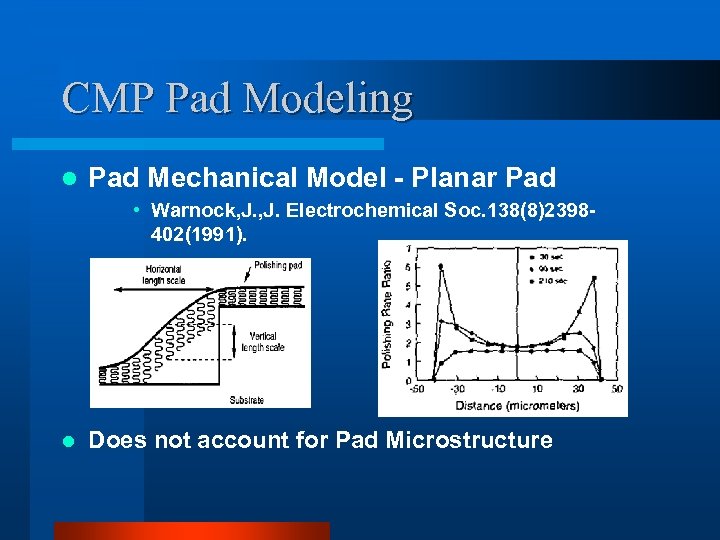 CMP Pad Modeling l Pad Mechanical Model - Planar Pad • Warnock, J. Electrochemical Soc. 138(8)2398402(1991). l Does not account for Pad Microstructure
CMP Pad Modeling l Pad Mechanical Model - Planar Pad • Warnock, J. Electrochemical Soc. 138(8)2398402(1991). l Does not account for Pad Microstructure
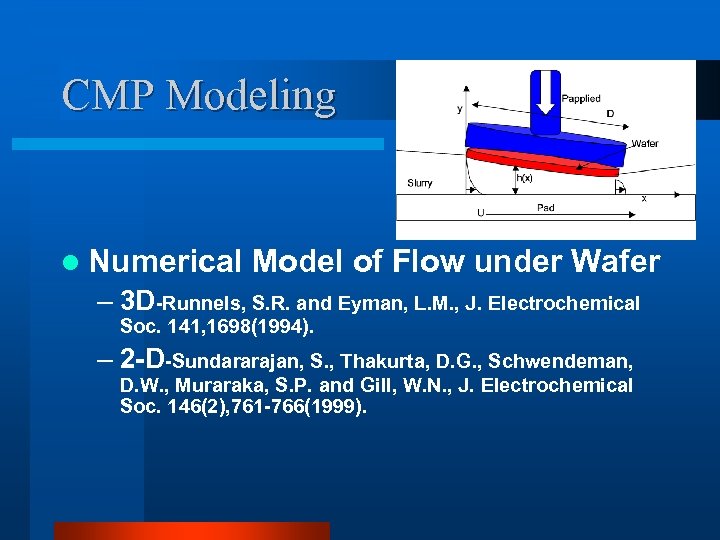 CMP Modeling l Numerical Model of Flow under Wafer – 3 D-Runnels, S. R. and Eyman, L. M. , J. Electrochemical Soc. 141, 1698(1994). – 2 -D-Sundararajan, S. , Thakurta, D. G. , Schwendeman, D. W. , Muraraka, S. P. and Gill, W. N. , J. Electrochemical Soc. 146(2), 761 -766(1999).
CMP Modeling l Numerical Model of Flow under Wafer – 3 D-Runnels, S. R. and Eyman, L. M. , J. Electrochemical Soc. 141, 1698(1994). – 2 -D-Sundararajan, S. , Thakurta, D. G. , Schwendeman, D. W. , Muraraka, S. P. and Gill, W. N. , J. Electrochemical Soc. 146(2), 761 -766(1999).
 Abrasive in 2 D Flow Model l In the 2 -D approach the effect of the slurry and specifically the particles in the slurry is reduced to that of an unknown constant, , determined by experimental measurements l where w is the shear stress at the wafer surface and CA is the concentration of abrasive. Sundararajan, Thakurta, Schwendeman, Mararka and Gill, J. Electro Chemical Soc. 146(2), 761 -766(1999).
Abrasive in 2 D Flow Model l In the 2 -D approach the effect of the slurry and specifically the particles in the slurry is reduced to that of an unknown constant, , determined by experimental measurements l where w is the shear stress at the wafer surface and CA is the concentration of abrasive. Sundararajan, Thakurta, Schwendeman, Mararka and Gill, J. Electro Chemical Soc. 146(2), 761 -766(1999).
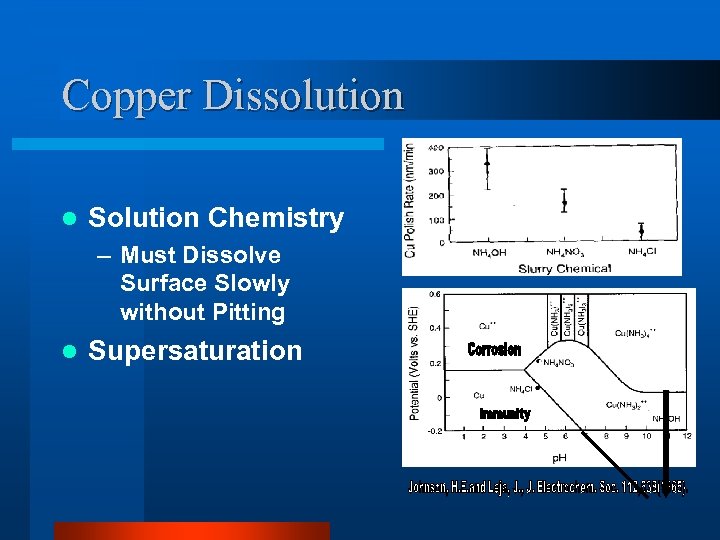 Copper Dissolution l Solution Chemistry – Must Dissolve Surface Slowly without Pitting l Supersaturation
Copper Dissolution l Solution Chemistry – Must Dissolve Surface Slowly without Pitting l Supersaturation
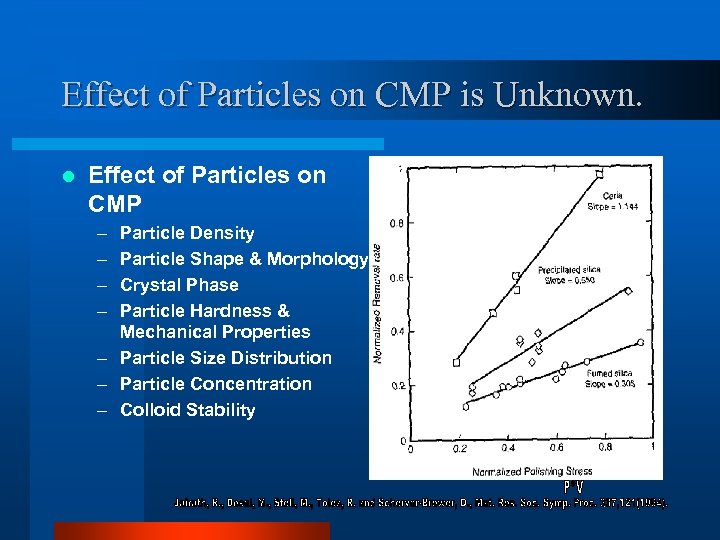 Effect of Particles on CMP is Unknown. l Effect of Particles on CMP – – Particle Density Particle Shape & Morphology Crystal Phase Particle Hardness & Mechanical Properties – Particle Size Distribution – Particle Concentration – Colloid Stability
Effect of Particles on CMP is Unknown. l Effect of Particles on CMP – – Particle Density Particle Shape & Morphology Crystal Phase Particle Hardness & Mechanical Properties – Particle Size Distribution – Particle Concentration – Colloid Stability
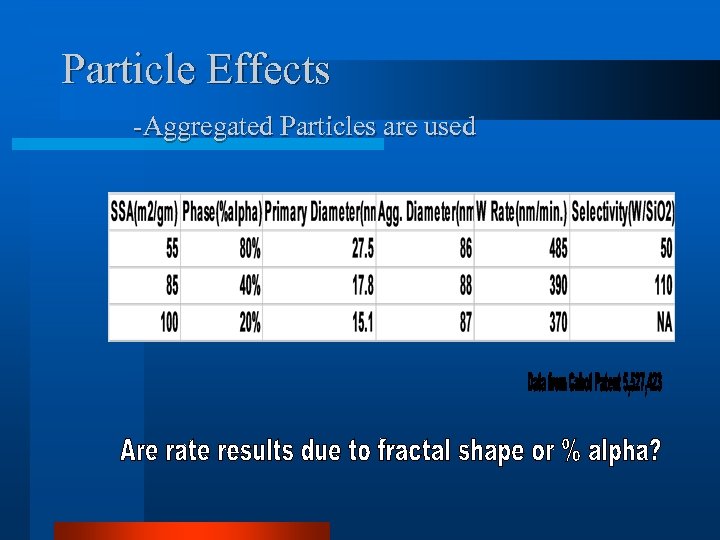 Particle Effects -Aggregated Particles are used
Particle Effects -Aggregated Particles are used
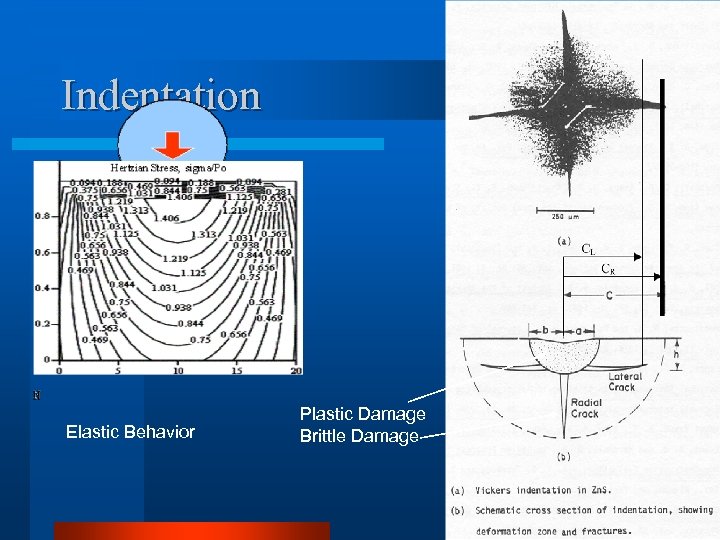 Indentation Elastic Behavior Plastic Damage Brittle Damage
Indentation Elastic Behavior Plastic Damage Brittle Damage
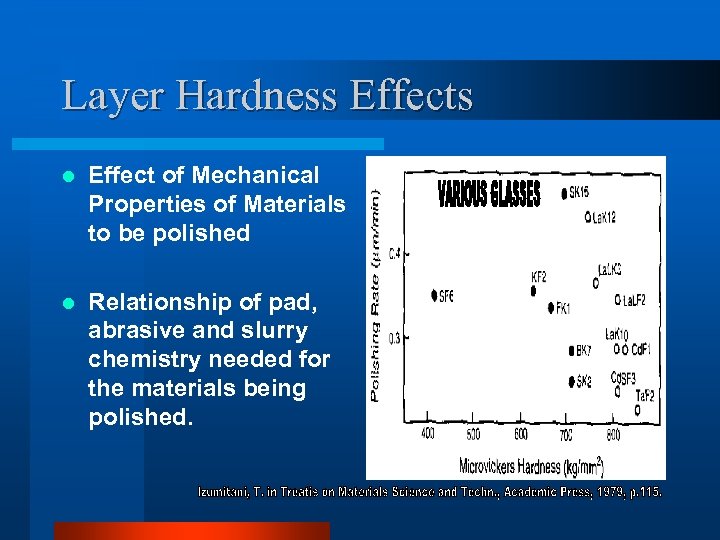 Layer Hardness Effects l Effect of Mechanical Properties of Materials to be polished l Relationship of pad, abrasive and slurry chemistry needed for the materials being polished.
Layer Hardness Effects l Effect of Mechanical Properties of Materials to be polished l Relationship of pad, abrasive and slurry chemistry needed for the materials being polished.
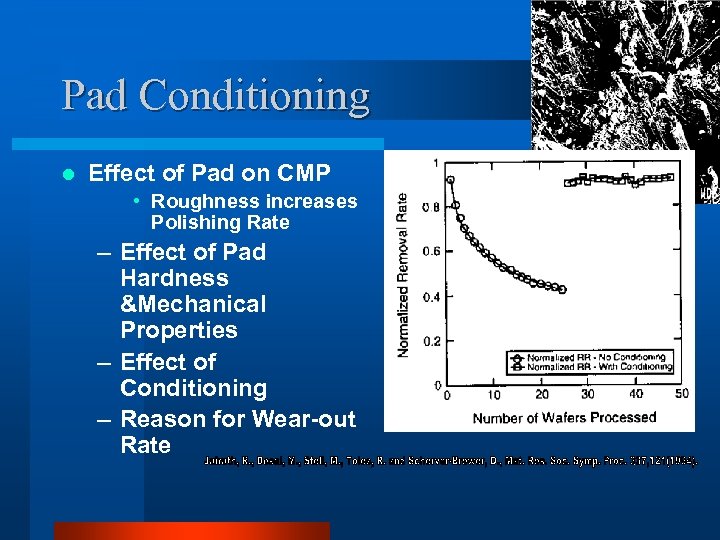 Pad Conditioning l Effect of Pad on CMP • Roughness increases Polishing Rate – Effect of Pad Hardness &Mechanical Properties – Effect of Conditioning – Reason for Wear-out Rate
Pad Conditioning l Effect of Pad on CMP • Roughness increases Polishing Rate – Effect of Pad Hardness &Mechanical Properties – Effect of Conditioning – Reason for Wear-out Rate
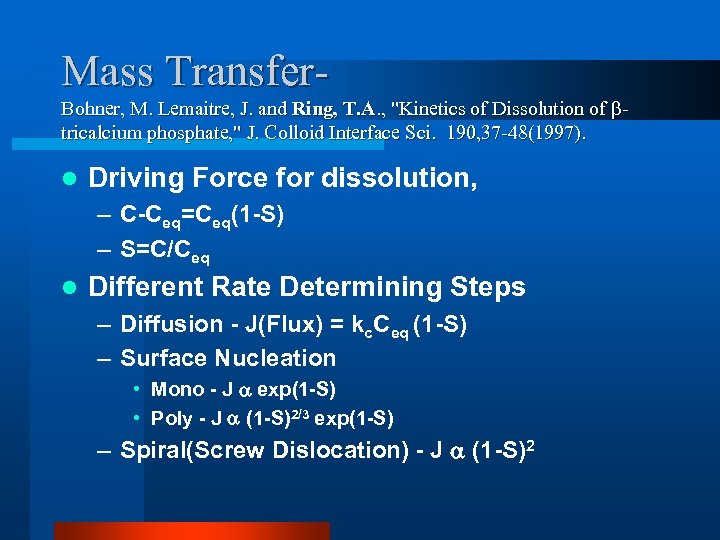 Mass Transfer- Bohner, M. Lemaitre, J. and Ring, T. A. , "Kinetics of Dissolution of tricalcium phosphate, " J. Colloid Interface Sci. 190, 37 -48(1997). l Driving Force for dissolution, – C-Ceq=Ceq(1 -S) – S=C/Ceq l Different Rate Determining Steps – Diffusion - J(Flux) = kc. Ceq (1 -S) – Surface Nucleation • Mono - J exp(1 -S) • Poly - J (1 -S)2/3 exp(1 -S) – Spiral(Screw Dislocation) - J (1 -S)2
Mass Transfer- Bohner, M. Lemaitre, J. and Ring, T. A. , "Kinetics of Dissolution of tricalcium phosphate, " J. Colloid Interface Sci. 190, 37 -48(1997). l Driving Force for dissolution, – C-Ceq=Ceq(1 -S) – S=C/Ceq l Different Rate Determining Steps – Diffusion - J(Flux) = kc. Ceq (1 -S) – Surface Nucleation • Mono - J exp(1 -S) • Poly - J (1 -S)2/3 exp(1 -S) – Spiral(Screw Dislocation) - J (1 -S)2
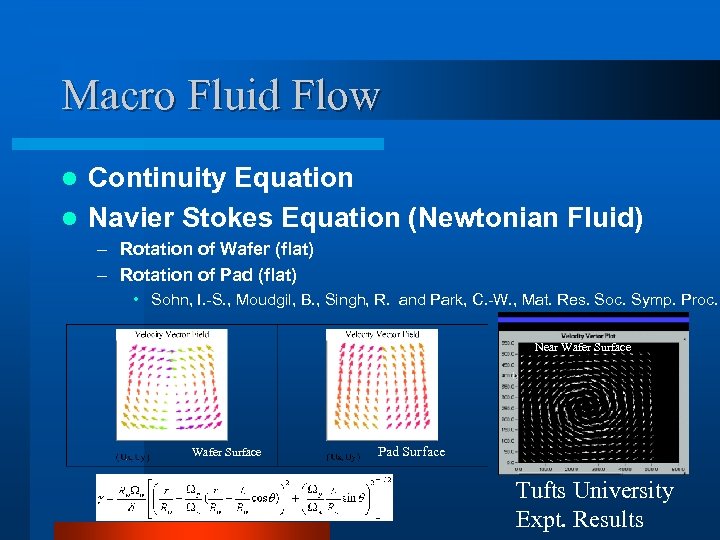 Macro Fluid Flow Continuity Equation l Navier Stokes Equation (Newtonian Fluid) l – Rotation of Wafer (flat) – Rotation of Pad (flat) • Sohn, I. -S. , Moudgil, B. , Singh, R. and Park, C. -W. , Mat. Res. Soc. Symp. Proc. Near Wafer Surface Pad Surface Tufts University Expt. Results
Macro Fluid Flow Continuity Equation l Navier Stokes Equation (Newtonian Fluid) l – Rotation of Wafer (flat) – Rotation of Pad (flat) • Sohn, I. -S. , Moudgil, B. , Singh, R. and Park, C. -W. , Mat. Res. Soc. Symp. Proc. Near Wafer Surface Pad Surface Tufts University Expt. Results
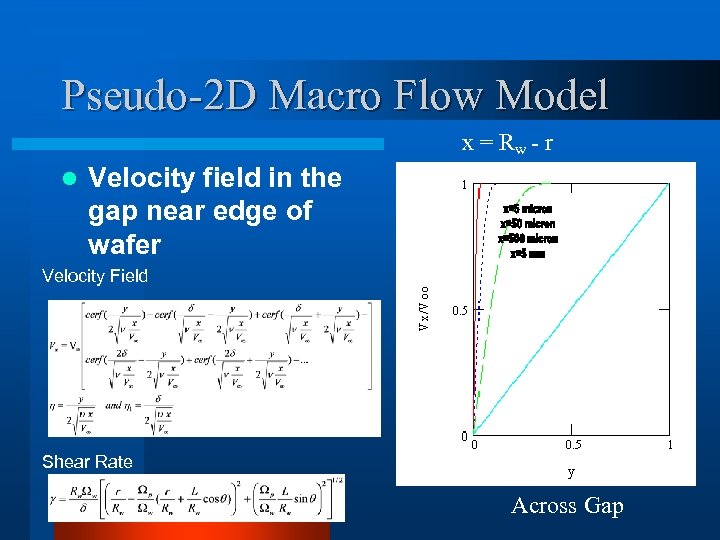 Pseudo-2 D Macro Flow Model x = Rw - r l Velocity field in the gap near edge of wafer Velocity Field Shear Rate Across Gap
Pseudo-2 D Macro Flow Model x = Rw - r l Velocity field in the gap near edge of wafer Velocity Field Shear Rate Across Gap
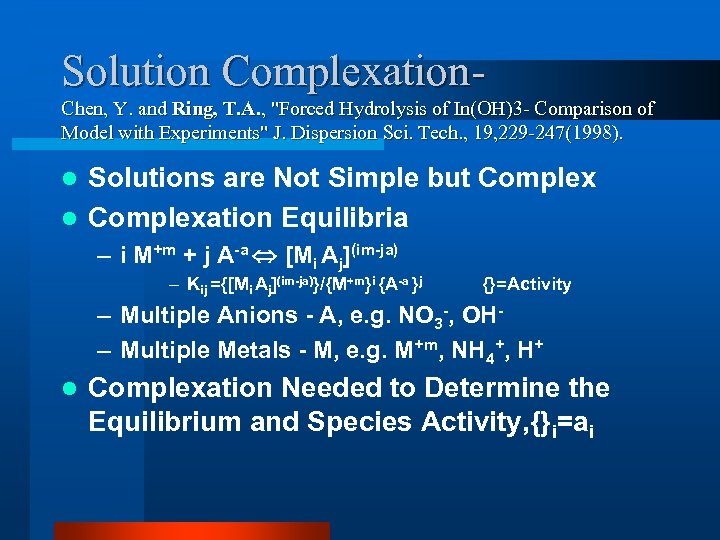 Solution Complexation. Chen, Y. and Ring, T. A. , "Forced Hydrolysis of In(OH)3 - Comparison of Model with Experiments" J. Dispersion Sci. Tech. , 19, 229 -247(1998). Solutions are Not Simple but Complex l Complexation Equilibria l – i M+m + j A-a [Mi Aj](im-ja) – Kij ={[Mi Aj](im-ja)}/{M+m}i {A-a }j {}=Activity – Multiple Anions - A, e. g. NO 3 -, OH– Multiple Metals - M, e. g. M+m, NH 4+, H+ l Complexation Needed to Determine the Equilibrium and Species Activity, {}i=ai
Solution Complexation. Chen, Y. and Ring, T. A. , "Forced Hydrolysis of In(OH)3 - Comparison of Model with Experiments" J. Dispersion Sci. Tech. , 19, 229 -247(1998). Solutions are Not Simple but Complex l Complexation Equilibria l – i M+m + j A-a [Mi Aj](im-ja) – Kij ={[Mi Aj](im-ja)}/{M+m}i {A-a }j {}=Activity – Multiple Anions - A, e. g. NO 3 -, OH– Multiple Metals - M, e. g. M+m, NH 4+, H+ l Complexation Needed to Determine the Equilibrium and Species Activity, {}i=ai
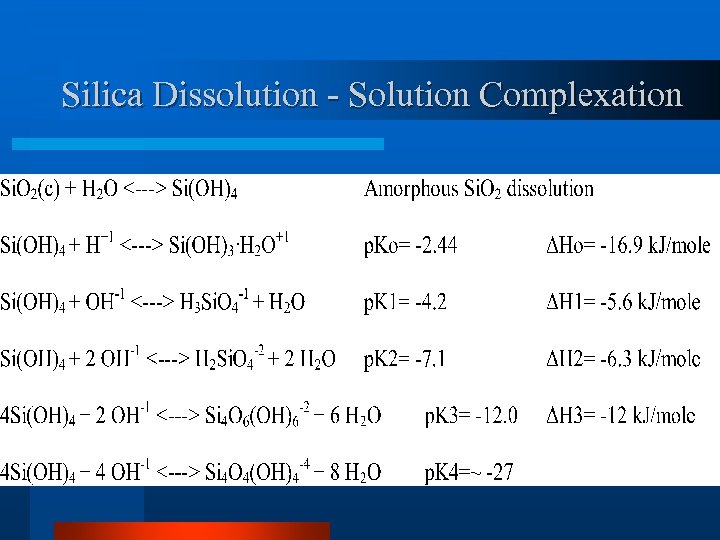 Silica Dissolution - Solution Complexation
Silica Dissolution - Solution Complexation
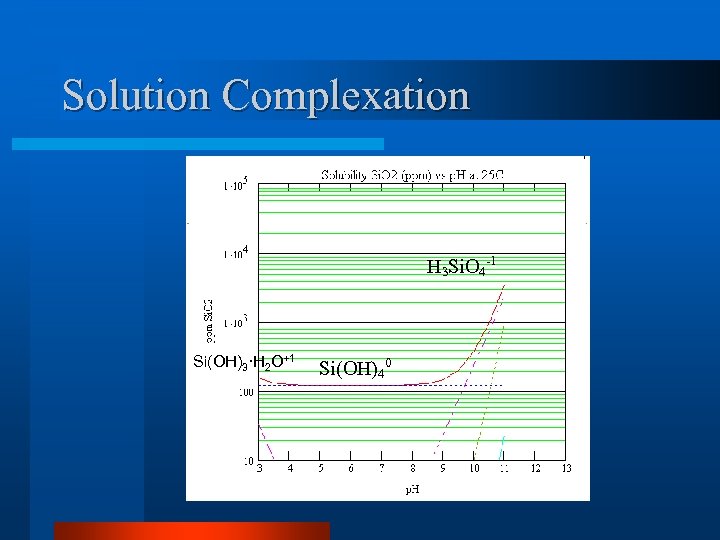 Solution Complexation H 3 Si. O 4 -1 Si(OH)3·H 2 O+1 Si(OH)40
Solution Complexation H 3 Si. O 4 -1 Si(OH)3·H 2 O+1 Si(OH)40
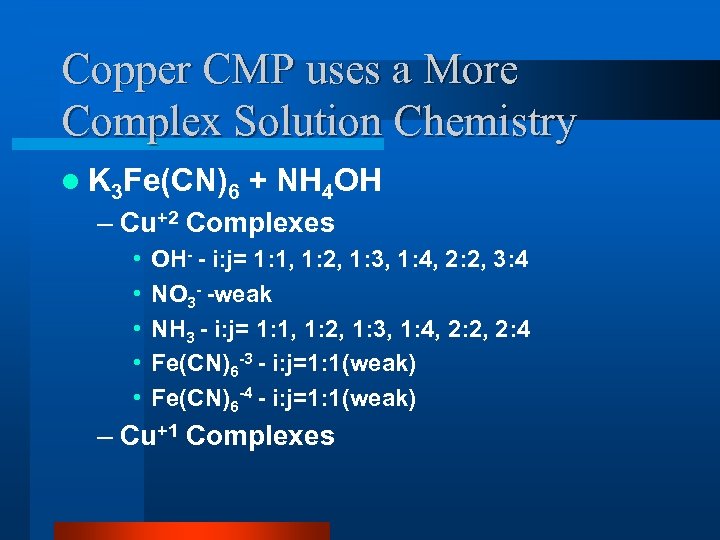 Copper CMP uses a More Complex Solution Chemistry l K 3 Fe(CN)6 + NH 4 OH – Cu+2 Complexes • • • OH- - i: j= 1: 1, 1: 2, 1: 3, 1: 4, 2: 2, 3: 4 NO 3 - -weak NH 3 - i: j= 1: 1, 1: 2, 1: 3, 1: 4, 2: 2, 2: 4 Fe(CN)6 -3 - i: j=1: 1(weak) Fe(CN)6 -4 - i: j=1: 1(weak) – Cu+1 Complexes
Copper CMP uses a More Complex Solution Chemistry l K 3 Fe(CN)6 + NH 4 OH – Cu+2 Complexes • • • OH- - i: j= 1: 1, 1: 2, 1: 3, 1: 4, 2: 2, 3: 4 NO 3 - -weak NH 3 - i: j= 1: 1, 1: 2, 1: 3, 1: 4, 2: 2, 2: 4 Fe(CN)6 -3 - i: j=1: 1(weak) Fe(CN)6 -4 - i: j=1: 1(weak) – Cu+1 Complexes
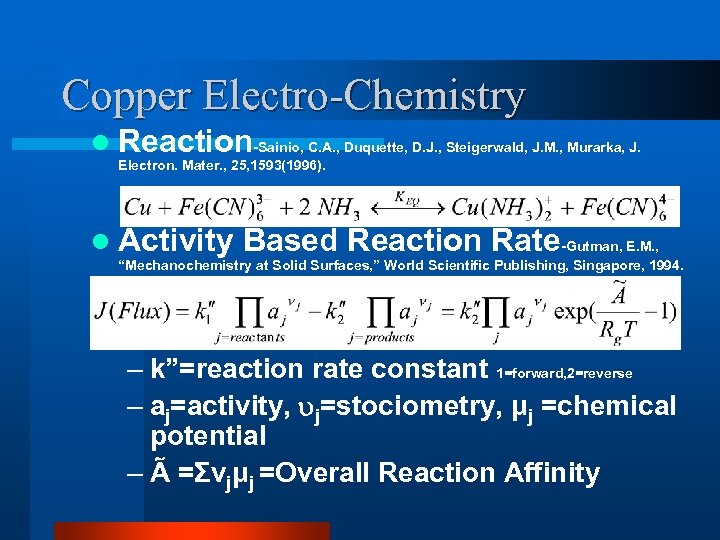 Copper Electro-Chemistry l Reaction-Sainio, C. A. , Duquette, D. J. , Steigerwald, J. M. , Murarka, J. Electron. Mater. , 25, 1593(1996). l Activity Based Reaction Rate-Gutman, E. M. , “Mechanochemistry at Solid Surfaces, ” World Scientific Publishing, Singapore, 1994. – k”=reaction rate constant 1=forward, 2=reverse – aj=activity, j=stociometry, μj =chemical potential – Ã =Σνjμj =Overall Reaction Affinity
Copper Electro-Chemistry l Reaction-Sainio, C. A. , Duquette, D. J. , Steigerwald, J. M. , Murarka, J. Electron. Mater. , 25, 1593(1996). l Activity Based Reaction Rate-Gutman, E. M. , “Mechanochemistry at Solid Surfaces, ” World Scientific Publishing, Singapore, 1994. – k”=reaction rate constant 1=forward, 2=reverse – aj=activity, j=stociometry, μj =chemical potential – Ã =Σνjμj =Overall Reaction Affinity
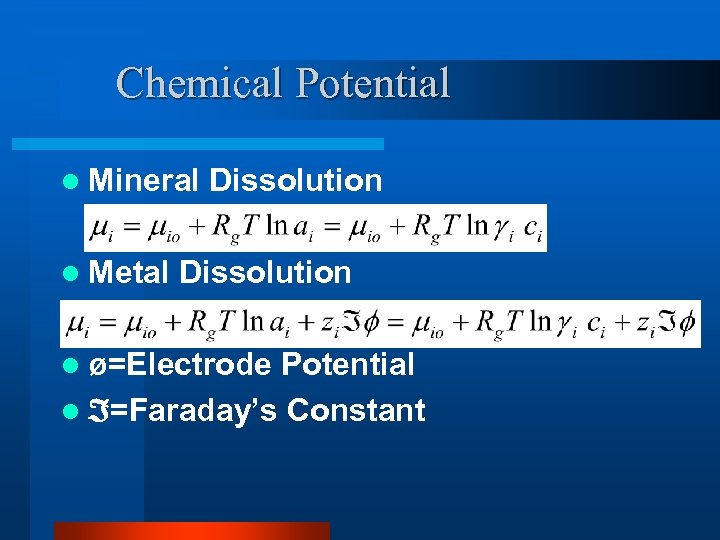 Chemical Potential l Mineral l Metal Dissolution l ø=Electrode Potential l =Faraday’s Constant
Chemical Potential l Mineral l Metal Dissolution l ø=Electrode Potential l =Faraday’s Constant
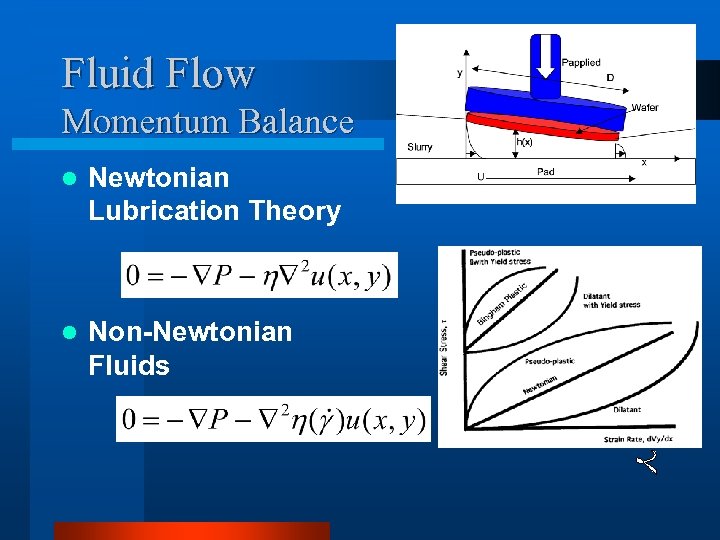 Fluid Flow Momentum Balance l Newtonian Lubrication Theory l Non-Newtonian Fluids
Fluid Flow Momentum Balance l Newtonian Lubrication Theory l Non-Newtonian Fluids
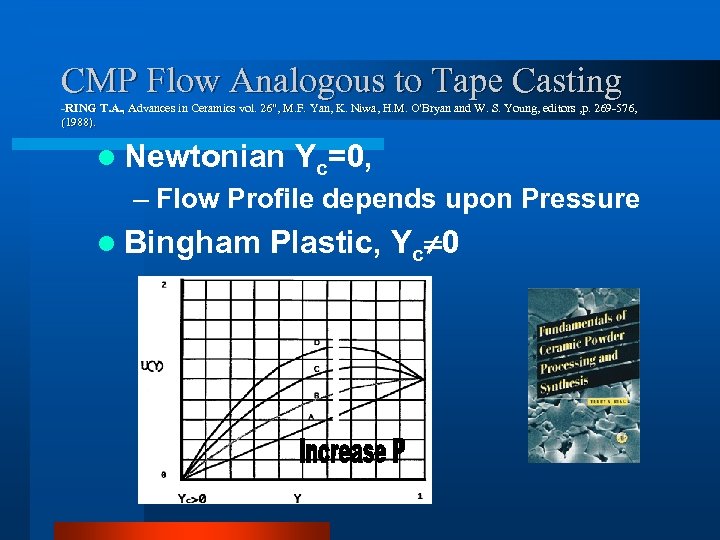 CMP Flow Analogous to Tape Casting -RING T. A. , Advances in Ceramics vol. 26", M. F. Yan, K. Niwa, H. M. O'Bryan and W. S. Young, editors , p. 269 -576, Yan, Niwa, (1988). l Newtonian Yc=0, – Flow Profile depends upon Pressure l Bingham Plastic, Yc 0
CMP Flow Analogous to Tape Casting -RING T. A. , Advances in Ceramics vol. 26", M. F. Yan, K. Niwa, H. M. O'Bryan and W. S. Young, editors , p. 269 -576, Yan, Niwa, (1988). l Newtonian Yc=0, – Flow Profile depends upon Pressure l Bingham Plastic, Yc 0
 Wall Shear Rate, w l Product of – Viscosity at wall shear stress – Velocity Gradient at wall
Wall Shear Rate, w l Product of – Viscosity at wall shear stress – Velocity Gradient at wall
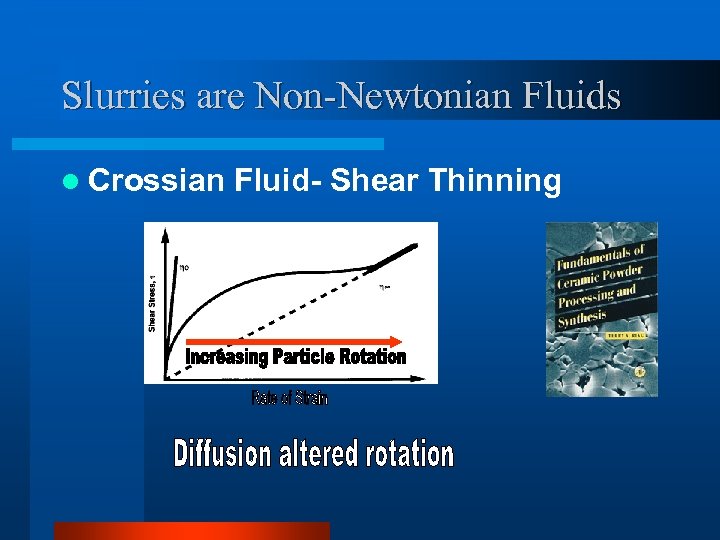 Slurries are Non-Newtonian Fluids l Crossian Fluid- Shear Thinning
Slurries are Non-Newtonian Fluids l Crossian Fluid- Shear Thinning
 Mass Transfer into Slurries l No Known Theories! l 2 -D CMP Model gives this Heuristic Shear Stress, w and Abrasive Concentration, CA are Important! l Wall
Mass Transfer into Slurries l No Known Theories! l 2 -D CMP Model gives this Heuristic Shear Stress, w and Abrasive Concentration, CA are Important! l Wall
 Mechanical Properties l Elastic Deformation l Plastic Damage l Plastic Deformation – Scratching
Mechanical Properties l Elastic Deformation l Plastic Damage l Plastic Deformation – Scratching
 Abrasive Particles Cause Surface Stress A. Evans “Mechanical Abrasion” l Collisions with Wafer Surface Cause Hertzian Stress l Collision Rate ? l Stress l Due To Collision P[ =(H tan 2 )1/3 Uk 2/3] is the peak load (N) due to the incident kinetic energy of the particles, Uk, The load is spread over the contact area
Abrasive Particles Cause Surface Stress A. Evans “Mechanical Abrasion” l Collisions with Wafer Surface Cause Hertzian Stress l Collision Rate ? l Stress l Due To Collision P[ =(H tan 2 )1/3 Uk 2/3] is the peak load (N) due to the incident kinetic energy of the particles, Uk, The load is spread over the contact area
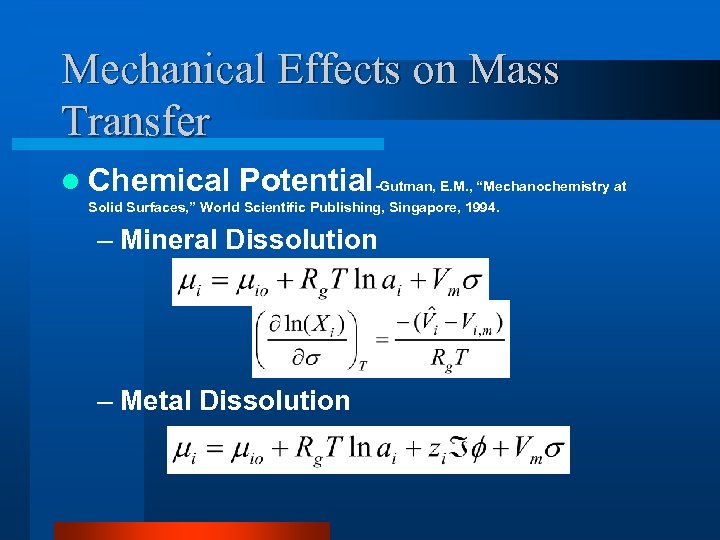 Mechanical Effects on Mass Transfer l Chemical Potential-Gutman, E. M. , “Mechanochemistry at Solid Surfaces, ” World Scientific Publishing, Singapore, 1994. – Mineral Dissolution – Metal Dissolution
Mechanical Effects on Mass Transfer l Chemical Potential-Gutman, E. M. , “Mechanochemistry at Solid Surfaces, ” World Scientific Publishing, Singapore, 1994. – Mineral Dissolution – Metal Dissolution
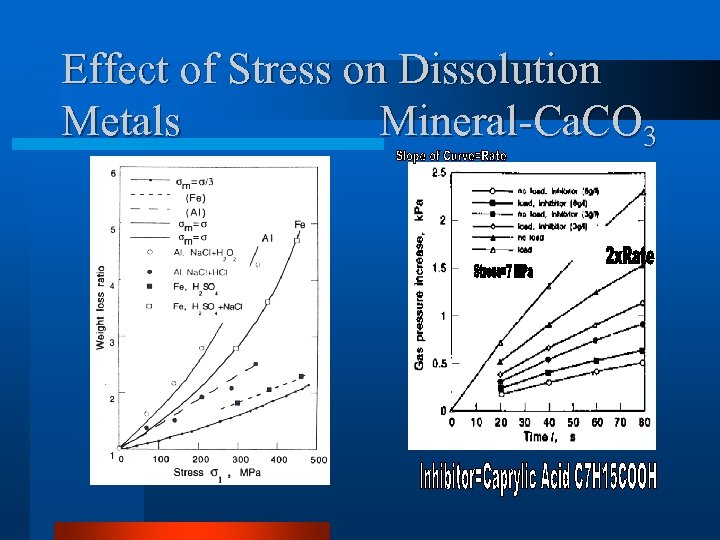 Effect of Stress on Dissolution Metals Mineral-Ca. CO 3
Effect of Stress on Dissolution Metals Mineral-Ca. CO 3
 Mechano-Chemical Effect – Effect on Chemical Potential of solid – Effect of Activity of Solid l As a result, Dissolution Rate of Metal and Mineral are Enhanced by Stress.
Mechano-Chemical Effect – Effect on Chemical Potential of solid – Effect of Activity of Solid l As a result, Dissolution Rate of Metal and Mineral are Enhanced by Stress.
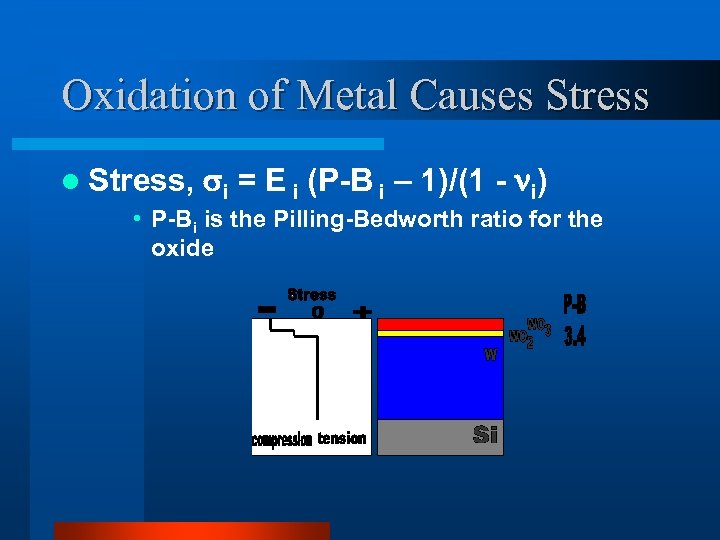 Oxidation of Metal Causes Stress l Stress, i = E i (P-B i – 1)/(1 - i) • P-Bi is the Pilling-Bedworth ratio for the oxide
Oxidation of Metal Causes Stress l Stress, i = E i (P-B i – 1)/(1 - i) • P-Bi is the Pilling-Bedworth ratio for the oxide
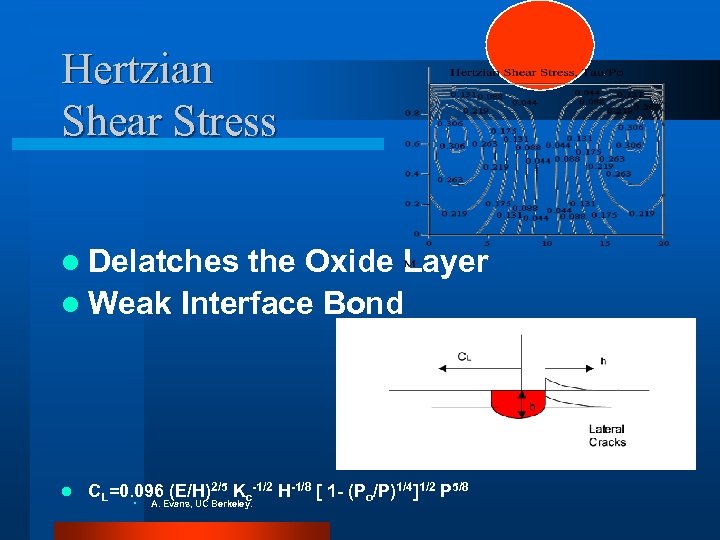 Hertzian Shear Stress l Delatches the Oxide Layer l Weak Interface Bond l CL=0. 096 (E/H)2/5 Kc-1/2 H-1/8 [ 1 - (Po/P)1/4]1/2 P 5/8 • A. Evans, UC Berkeley.
Hertzian Shear Stress l Delatches the Oxide Layer l Weak Interface Bond l CL=0. 096 (E/H)2/5 Kc-1/2 H-1/8 [ 1 - (Po/P)1/4]1/2 P 5/8 • A. Evans, UC Berkeley.
 CMP Problems l Defectivity – WIWNU – Dishing and Erosion – Line Erosion – Scratching
CMP Problems l Defectivity – WIWNU – Dishing and Erosion – Line Erosion – Scratching
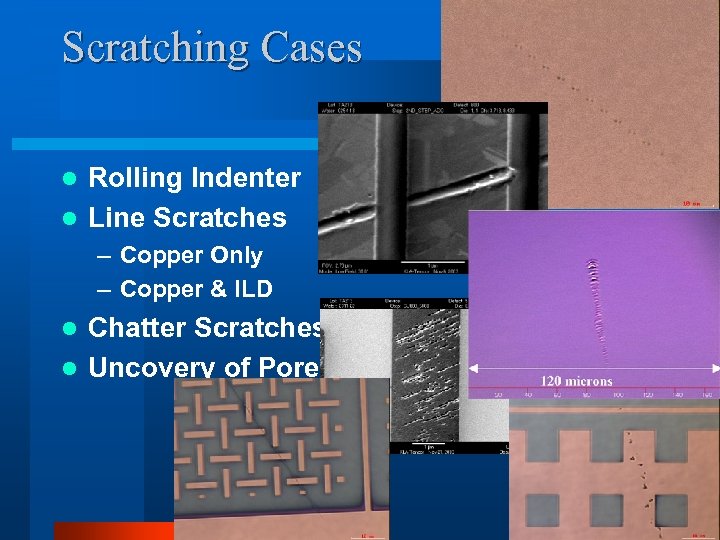 Scratching Cases Rolling Indenter l Line Scratches l – Copper Only – Copper & ILD Chatter Scratches l Uncovery of Pores l
Scratching Cases Rolling Indenter l Line Scratches l – Copper Only – Copper & ILD Chatter Scratches l Uncovery of Pores l


