81757f8675de0ee7bb29842caf59be72.ppt
- Количество слайдов: 23
 Lec 1 a: Life in Water Properties • All life basically is aquatic – Life on Earth evolved in and is sustained by water – Terrestrial organisms maintain an internal aquatic environment for their organs and tissues • Determines human distribution and population sizes • Water is both a renewable and a non-renewable resource • Available fresh water is relatively scarce 1
Lec 1 a: Life in Water Properties • All life basically is aquatic – Life on Earth evolved in and is sustained by water – Terrestrial organisms maintain an internal aquatic environment for their organs and tissues • Determines human distribution and population sizes • Water is both a renewable and a non-renewable resource • Available fresh water is relatively scarce 1
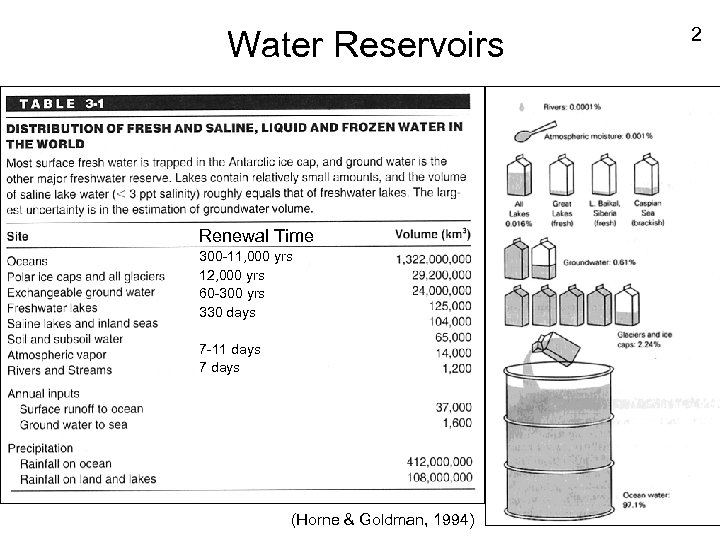 Water Reservoirs Renewal Time 300 -11, 000 yrs 12, 000 yrs 60 -300 yrs 330 days 7 -11 days 7 days (Horne & Goldman, 1994) 2
Water Reservoirs Renewal Time 300 -11, 000 yrs 12, 000 yrs 60 -300 yrs 330 days 7 -11 days 7 days (Horne & Goldman, 1994) 2
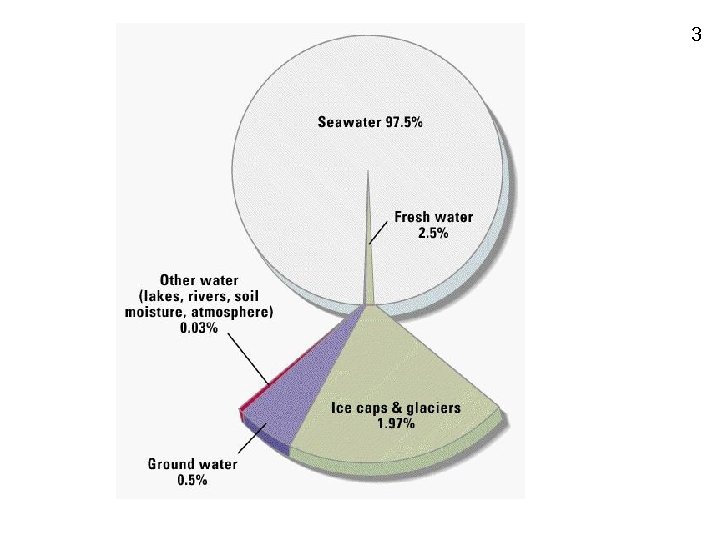 3
3
 Human Water Use Summary – Pressures on a Key Resource • Only 1% of water in lakes, 0. 01% in rivers – (as % of inland liquid water) • Surface water provides majority of water (Fig 1. 4) • U. S. water use ~2000 m 3 / person /year –e. g. versus Israel ~500 m 3 / person / year • Much water use for industry and irrigation, not just home use (Fig 1. 3) 4
Human Water Use Summary – Pressures on a Key Resource • Only 1% of water in lakes, 0. 01% in rivers – (as % of inland liquid water) • Surface water provides majority of water (Fig 1. 4) • U. S. water use ~2000 m 3 / person /year –e. g. versus Israel ~500 m 3 / person / year • Much water use for industry and irrigation, not just home use (Fig 1. 3) 4
 Factors Affecting Human Need for Fresh Water • Population pressure and growth – Now approximately 6. 6 billion humans – Human population doubling every 50 years • Pollution –Reduces amount of fresh water available for use • Development and Technology –New technologies in manufacturing and agriculture often result in increased per capita need for fresh water –Development often interferes with the water cycle (e. g. , reduction of vegetation, paving, etc. ) 5
Factors Affecting Human Need for Fresh Water • Population pressure and growth – Now approximately 6. 6 billion humans – Human population doubling every 50 years • Pollution –Reduces amount of fresh water available for use • Development and Technology –New technologies in manufacturing and agriculture often result in increased per capita need for fresh water –Development often interferes with the water cycle (e. g. , reduction of vegetation, paving, etc. ) 5
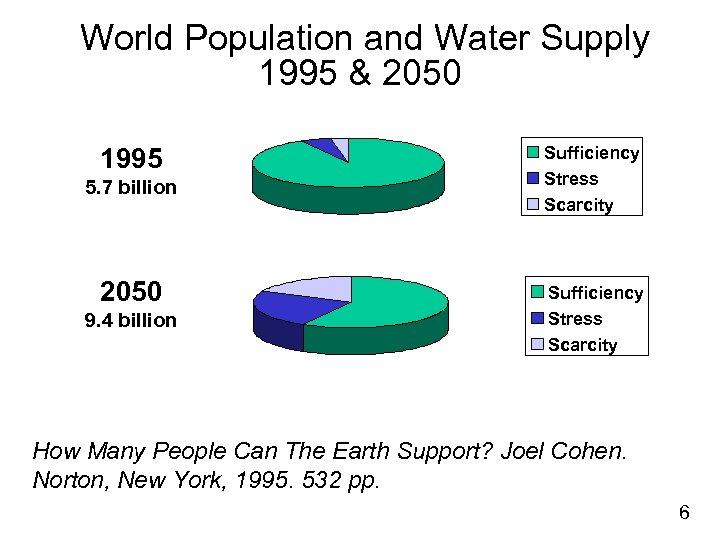 World Population and Water Supply 1995 & 2050 5% 3% 1995 Sufficiency Stress Scarcity 5. 7 billion 92% 18% 2050 9. 4 billion 24% 58% Sufficiency Stress Scarcity How Many People Can The Earth Support? Joel Cohen. Norton, New York, 1995. 532 pp. 6
World Population and Water Supply 1995 & 2050 5% 3% 1995 Sufficiency Stress Scarcity 5. 7 billion 92% 18% 2050 9. 4 billion 24% 58% Sufficiency Stress Scarcity How Many People Can The Earth Support? Joel Cohen. Norton, New York, 1995. 532 pp. 6
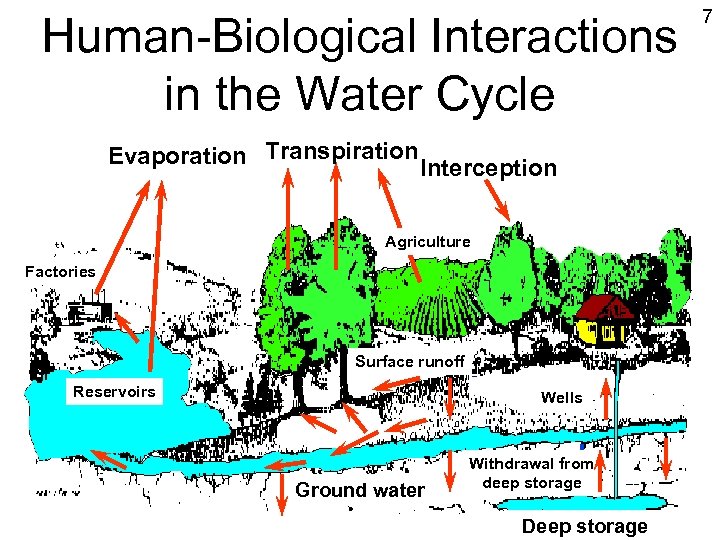 Human-Biological Interactions in the Water Cycle Evaporation Transpiration Interception Agriculture Factories Surface runoff Reservoirs Wells Ground water Withdrawal from deep storage Deep storage 7
Human-Biological Interactions in the Water Cycle Evaporation Transpiration Interception Agriculture Factories Surface runoff Reservoirs Wells Ground water Withdrawal from deep storage Deep storage 7
 Water Properties (See Table 2. 1 for a summary) Depiction of the three phases of water: steam, liquid water, and ice ©Time, Inc. All rights reserved. 8
Water Properties (See Table 2. 1 for a summary) Depiction of the three phases of water: steam, liquid water, and ice ©Time, Inc. All rights reserved. 8
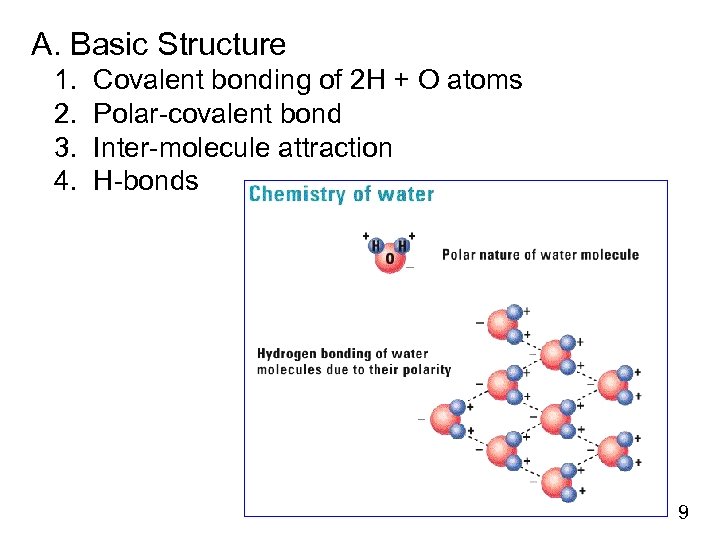 A. Basic Structure 1. 2. 3. 4. Covalent bonding of 2 H + O atoms Polar-covalent bond Inter-molecule attraction H-bonds 9
A. Basic Structure 1. 2. 3. 4. Covalent bonding of 2 H + O atoms Polar-covalent bond Inter-molecule attraction H-bonds 9
 B. Cohesion 1. Wave formation and other water movements 2. Distribution of heat, gases, nutrients, plankton, etc. 10
B. Cohesion 1. Wave formation and other water movements 2. Distribution of heat, gases, nutrients, plankton, etc. 10
 C. Surface Tension 1. Pressure needed to break surface 2. Only Hg is higher 3. Implications for organisms? -Related to what characteristics? 11
C. Surface Tension 1. Pressure needed to break surface 2. Only Hg is higher 3. Implications for organisms? -Related to what characteristics? 11
 D. Liquid at ambient temperatures E. Low density solid (ice floats!) -Critical for life on earth F. High heat capacity -Specific Heat - 1. 0 (also called Heat Capacity) calories required to raise 1 g H 2 O 1 OC (e. g. from 14. 5 to 15. 5 OC) -Exceeded only by Liquid NH 3 1. 23 Liquid H 2 3. 40 -Heat transfer by water is very important 12
D. Liquid at ambient temperatures E. Low density solid (ice floats!) -Critical for life on earth F. High heat capacity -Specific Heat - 1. 0 (also called Heat Capacity) calories required to raise 1 g H 2 O 1 OC (e. g. from 14. 5 to 15. 5 OC) -Exceeded only by Liquid NH 3 1. 23 Liquid H 2 3. 40 -Heat transfer by water is very important 12
 G. Good Solvent (for some things) Difference on land vs. water % of air as oxygen Concentration =21% =210 ml / L What about in water? (at 15 deg. C and 1 atm) Solubility of oxygen in water =34 ml / L Solubility of carbon dioxide in water =1019 ml / L …. Why the disparity? So, volume of oxygen at equil. with air: = 34. 1 ml/L * 21% = 7. 16 ml / L (30 x less than air!) What is the effect of temperature on gas solubility? (in eq. with Atm) Water Temperature (deg C) ml / L 0 10. 3 10 8. 0 15 7. 2 20 6. 6 30 5. 6 (This is why hot water supposedly freezes more quickly than cold water) 13
G. Good Solvent (for some things) Difference on land vs. water % of air as oxygen Concentration =21% =210 ml / L What about in water? (at 15 deg. C and 1 atm) Solubility of oxygen in water =34 ml / L Solubility of carbon dioxide in water =1019 ml / L …. Why the disparity? So, volume of oxygen at equil. with air: = 34. 1 ml/L * 21% = 7. 16 ml / L (30 x less than air!) What is the effect of temperature on gas solubility? (in eq. with Atm) Water Temperature (deg C) ml / L 0 10. 3 10 8. 0 15 7. 2 20 6. 6 30 5. 6 (This is why hot water supposedly freezes more quickly than cold water) 13
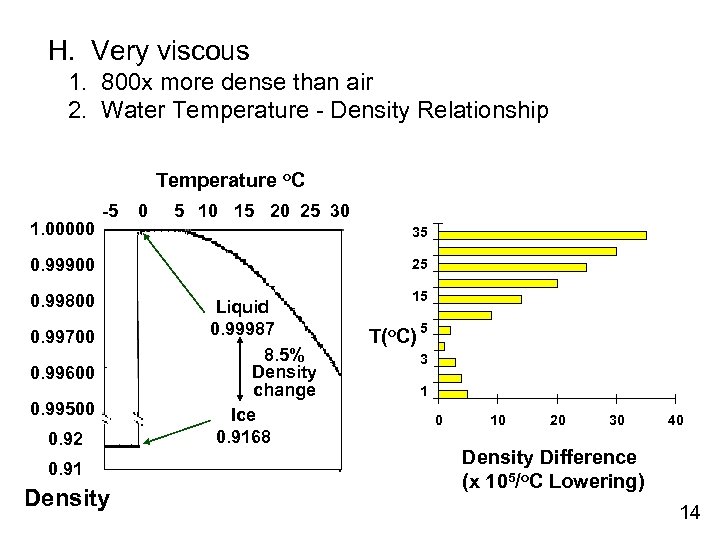 H. Very viscous 1. 800 x more dense than air 2. Water Temperature - Density Relationship Temperature o. C 1. 00000 -5 0 5 10 15 20 25 30 35 0. 99900 0. 99800 0. 99700 0. 99600 0. 99500 0. 92 0. 91 Density 25 Liquid 0. 99987 8. 5% Density change Ice 0. 9168 15 T(o. C) 5 3 1 0 10 20 30 40 Density Difference (x 105/o. C Lowering) 14
H. Very viscous 1. 800 x more dense than air 2. Water Temperature - Density Relationship Temperature o. C 1. 00000 -5 0 5 10 15 20 25 30 35 0. 99900 0. 99800 0. 99700 0. 99600 0. 99500 0. 92 0. 91 Density 25 Liquid 0. 99987 8. 5% Density change Ice 0. 9168 15 T(o. C) 5 3 1 0 10 20 30 40 Density Difference (x 105/o. C Lowering) 14
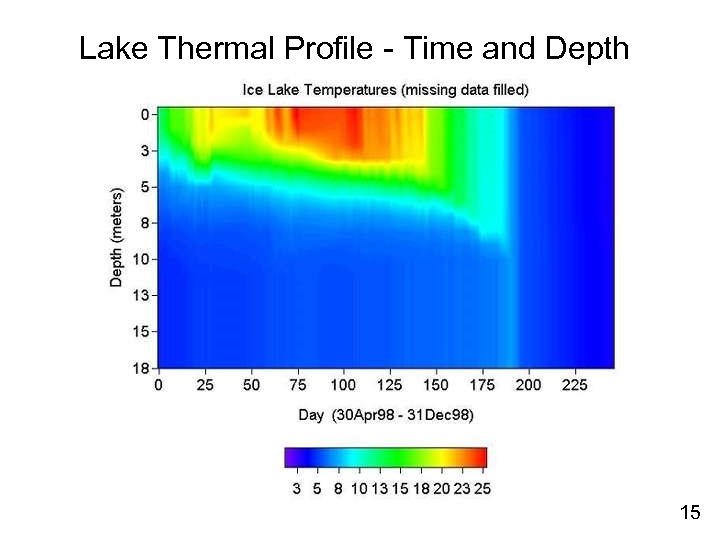 Lake Thermal Profile - Time and Depth 15
Lake Thermal Profile - Time and Depth 15
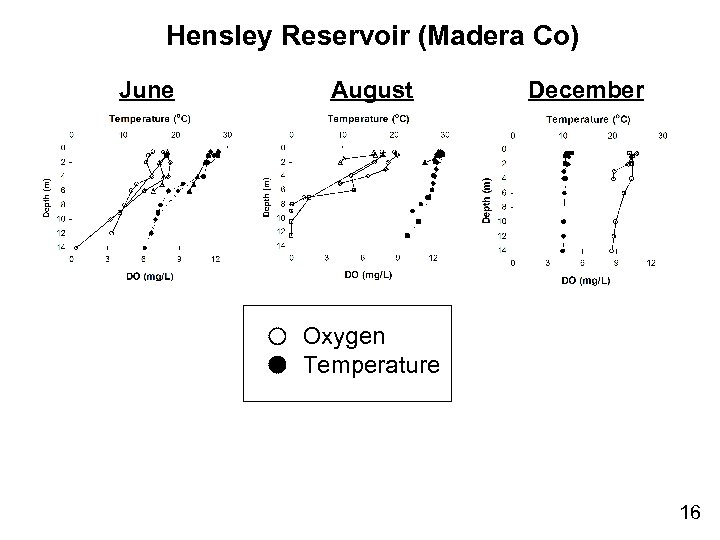 Hensley Reservoir (Madera Co) June August December Oxygen Temperature 16
Hensley Reservoir (Madera Co) June August December Oxygen Temperature 16
 Relationships among Water Viscosity, Inertia, and Physical Parameters • Hydrogen bonding becomes more important at smaller scales, altering both viscosity and inertia • Viscosity is the resistance to change in form (internal friction) • Inertia is the resistance of a body to a change in its state of motion • Reynolds number incorporates both 17
Relationships among Water Viscosity, Inertia, and Physical Parameters • Hydrogen bonding becomes more important at smaller scales, altering both viscosity and inertia • Viscosity is the resistance to change in form (internal friction) • Inertia is the resistance of a body to a change in its state of motion • Reynolds number incorporates both 17
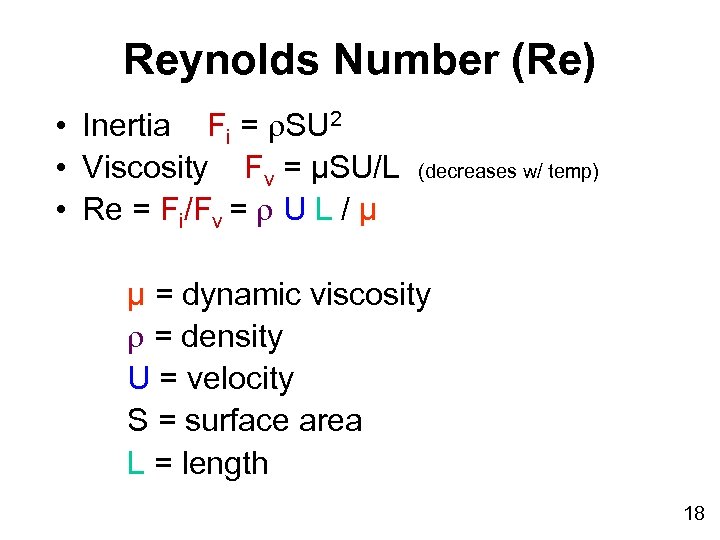 Reynolds Number (Re) • Inertia Fi = SU 2 • Viscosity Fv = µSU/L • Re = Fi/Fv = U L / µ (decreases w/ temp) µ = dynamic viscosity = density U = velocity S = surface area L = length 18
Reynolds Number (Re) • Inertia Fi = SU 2 • Viscosity Fv = µSU/L • Re = Fi/Fv = U L / µ (decreases w/ temp) µ = dynamic viscosity = density U = velocity S = surface area L = length 18
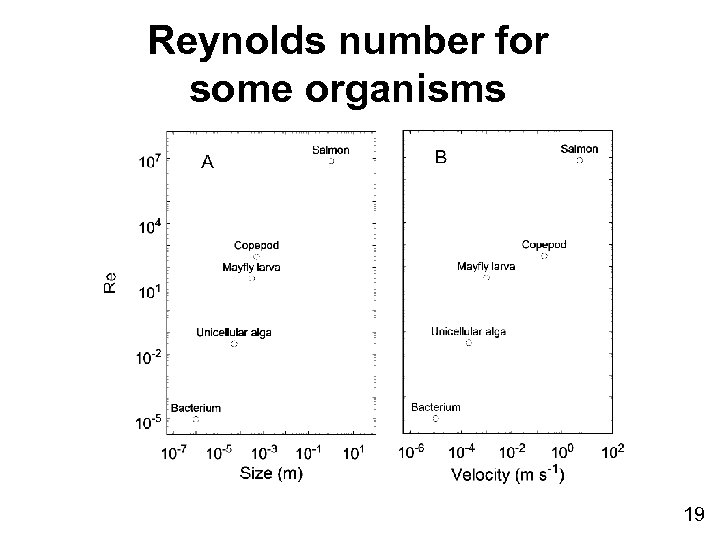 Reynolds number for some organisms 19
Reynolds number for some organisms 19
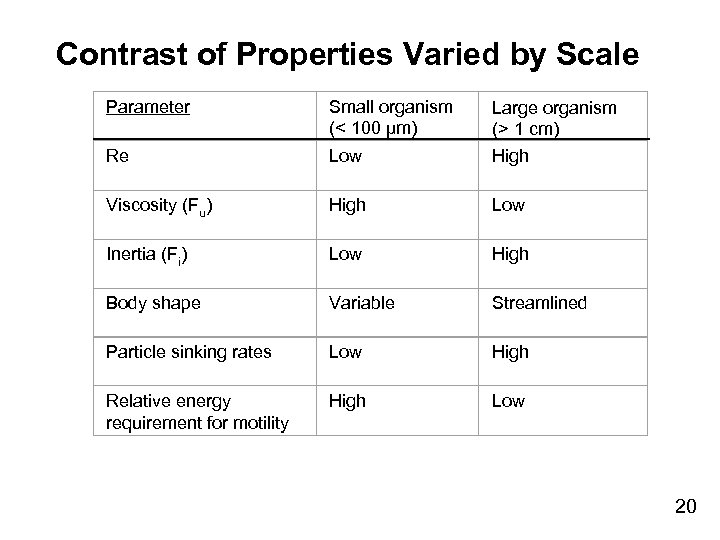 Contrast of Properties Varied by Scale Parameter Small organism (< 100 µm) Re Low Large organism (> 1 cm) High Viscosity (Fu) High Low Inertia (Fi) Low High Body shape Variable Streamlined Particle sinking rates Low High Relative energy requirement for motility High Low 20
Contrast of Properties Varied by Scale Parameter Small organism (< 100 µm) Re Low Large organism (> 1 cm) High Viscosity (Fu) High Low Inertia (Fi) Low High Body shape Variable Streamlined Particle sinking rates Low High Relative energy requirement for motility High Low 20
 Stokes Law • Sinking rate of small spheres is a function of size and density of the sphere and viscosity and density of water Stoke’s Law : where: g = gravitational acceleration (m / s 2) = coefficient of viscosity of the medium (kg/m/s) densp = density of particle densm = density of fluid r = radius of the particle • Cells alter shape to change sinking rate (Melosira example) 21
Stokes Law • Sinking rate of small spheres is a function of size and density of the sphere and viscosity and density of water Stoke’s Law : where: g = gravitational acceleration (m / s 2) = coefficient of viscosity of the medium (kg/m/s) densp = density of particle densm = density of fluid r = radius of the particle • Cells alter shape to change sinking rate (Melosira example) 21
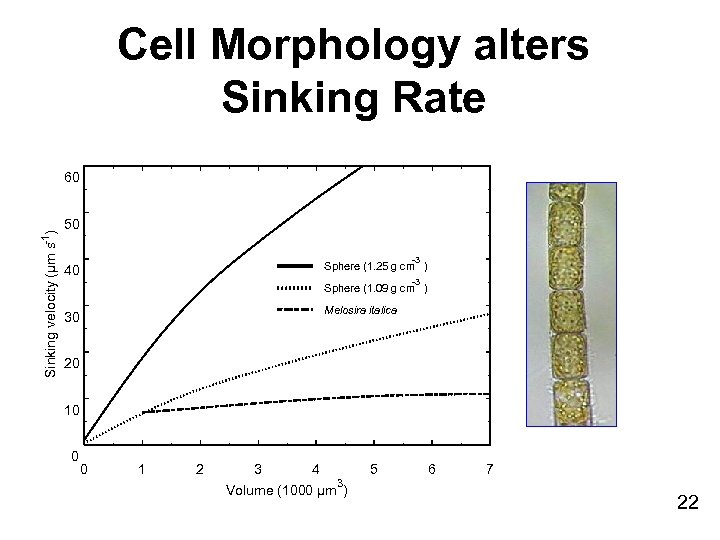 Cell Morphology alters Sinking Rate Sinking velocity (µm s-1) 60 50 -3 Sphere (1. 25 g cm ) 40 -3 Sphere (1. 09 g cm ) Melosira italica 30 20 10 0 0 1 2 3 4 3 Volume (1000 µm ) 5 6 7 22
Cell Morphology alters Sinking Rate Sinking velocity (µm s-1) 60 50 -3 Sphere (1. 25 g cm ) 40 -3 Sphere (1. 09 g cm ) Melosira italica 30 20 10 0 0 1 2 3 4 3 Volume (1000 µm ) 5 6 7 22
 Water Chemical and Physical Properties Summary • Hydrogen bonding • High density, surface tension, heat of vaporization, heat capacity, liquid at earth’s surface, excellent solvent (important for weathering) • Ions more soluble in warmer water, gasses less • Unusual relationship between temperature and density • Influence of water physical properties on organisms 23
Water Chemical and Physical Properties Summary • Hydrogen bonding • High density, surface tension, heat of vaporization, heat capacity, liquid at earth’s surface, excellent solvent (important for weathering) • Ions more soluble in warmer water, gasses less • Unusual relationship between temperature and density • Influence of water physical properties on organisms 23


