The Haber process.pptx
- Количество слайдов: 15

Learning outcomes You will be able to describe the industrial manufacture of ammonia and be able to understand it as an equilibrium process. You will be able to describe and explain the environmental impact of oxides of nitrogen in the atmosphere and nitrates in soils and water supplies You will be able to evaluate ways of minimising these environmental impacts

The Haber process Ammonia is used to make fertilisers, explosives, dyes, household cleaners and nylon. It is also the most important raw material in the manufacture of nitric acid. Ammonia is manufactured by combining nitrogen and hydrogen in an important industrial process called the Haber process.

The Haber process The raw materials for this process are hydrogen and nitrogen. Hydrogen is obtained by reacting natural gas - methane - with steam, or through the cracking of oil. Nitrogen is obtained by burning hydrogen in air. Air is 80 per cent nitrogen; nearly all the rest is oxygen. When hydrogen is burned in air, the oxygen combines with the hydrogen, leaving nitrogen behind. Nitrogen and hydrogen will react together under these conditions: • a high temperature - about 450ºC • a high pressure - about 200 atmospheres (200 times normal pressure) • an iron catalyst
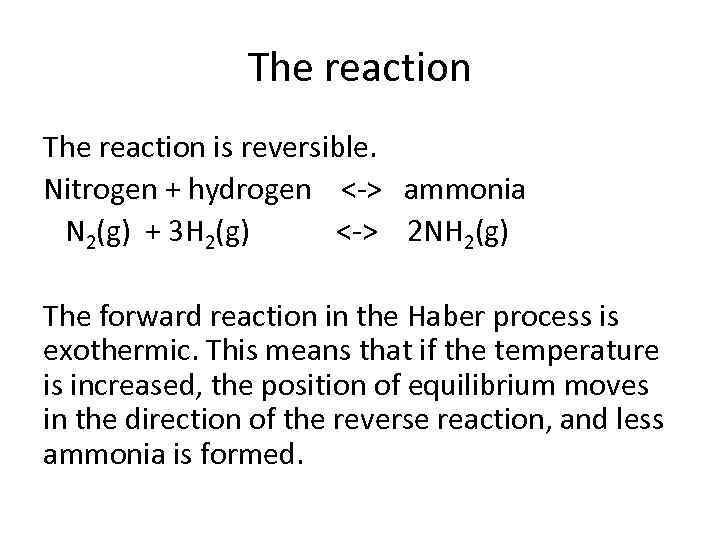
The reaction is reversible. Nitrogen + hydrogen <-> ammonia N 2(g) + 3 H 2(g) <-> 2 NH 2(g) The forward reaction in the Haber process is exothermic. This means that if the temperature is increased, the position of equilibrium moves in the direction of the reverse reaction, and less ammonia is formed.
Factors affecting equilibrium If the Haber process was carried out at low temperatures the yield would increase as the forward reaction is exothermic. However the reaction rate would be so slow it is commercially unviable for this to occur and a compromise temperature is chosen: low enough to get a good yield of ammonia but high enough to obtain a reasonable rate of reaction.

Factors affecting equilibrium The presence of a catalyst does not affect the position of the equilibrium, but it does increase the rate of the reaction. This means the ammonia is produced in a shorter time, reducing the cost of the process. Iron is a cheap catalyst.
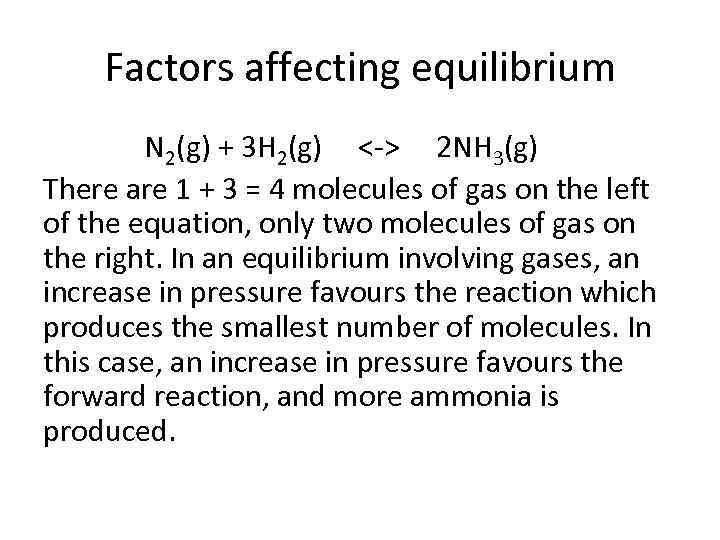
Factors affecting equilibrium N 2(g) + 3 H 2(g) <-> 2 NH 3(g) There are 1 + 3 = 4 molecules of gas on the left of the equation, only two molecules of gas on the right. In an equilibrium involving gases, an increase in pressure favours the reaction which produces the smallest number of molecules. In this case, an increase in pressure favours the forward reaction, and more ammonia is produced.
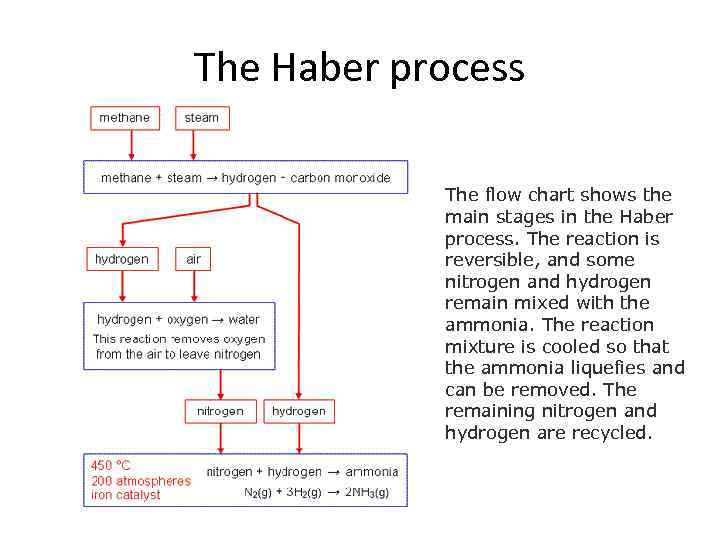
The Haber process The flow chart shows the main stages in the Haber process. The reaction is reversible, and some nitrogen and hydrogen remain mixed with the ammonia. The reaction mixture is cooled so that the ammonia liquefies and can be removed. The remaining nitrogen and hydrogen are recycled.
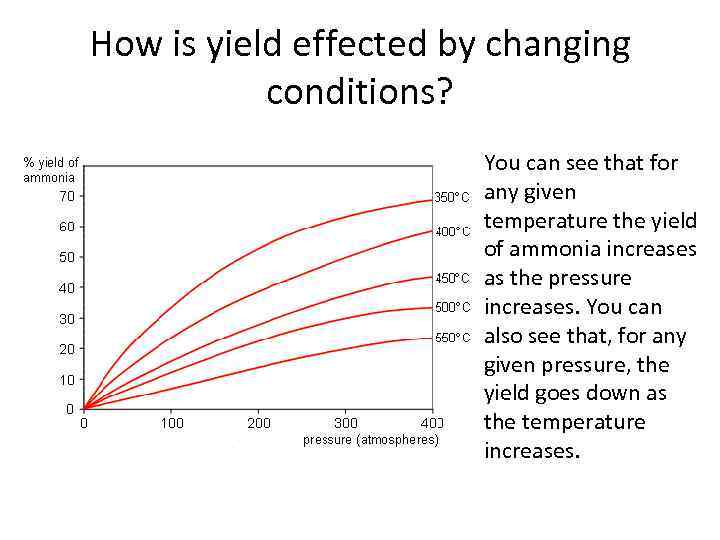
How is yield effected by changing conditions? You can see that for any given temperature the yield of ammonia increases as the pressure increases. You can also see that, for any given pressure, the yield goes down as the temperature increases.
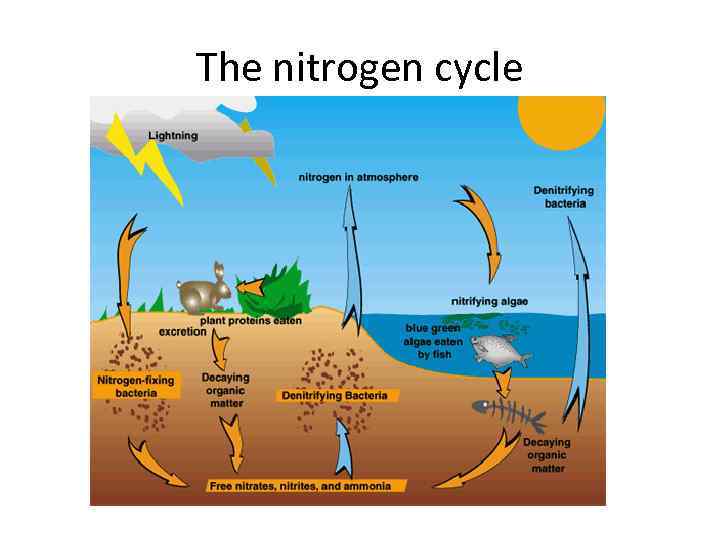
The nitrogen cycle
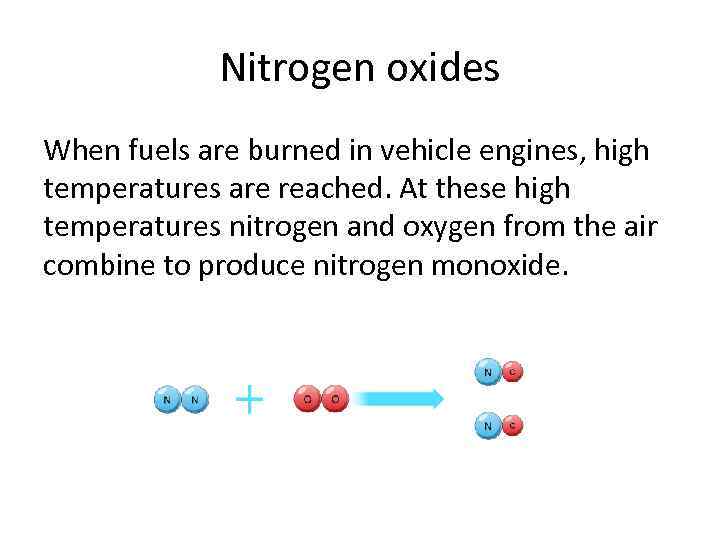
Nitrogen oxides When fuels are burned in vehicle engines, high temperatures are reached. At these high temperatures nitrogen and oxygen from the air combine to produce nitrogen monoxide.
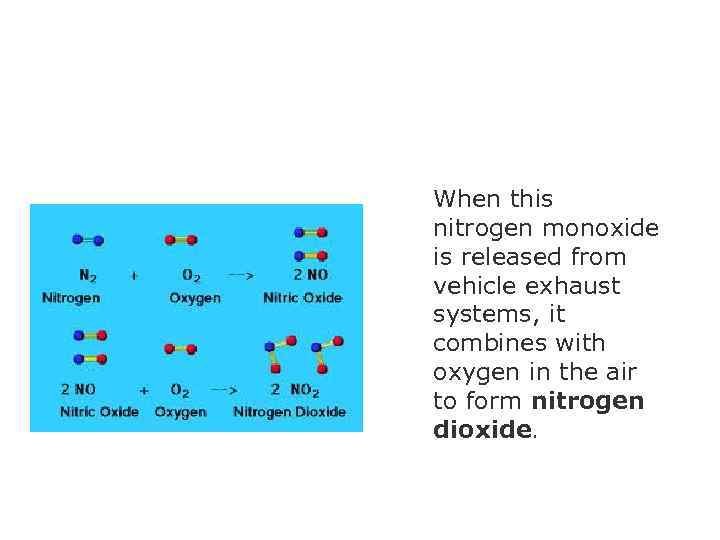
When this nitrogen monoxide is released from vehicle exhaust systems, it combines with oxygen in the air to form nitrogen dioxide.

Problems caused by NOx gases Helps form acid rain It contributes to global warming It hampers the growth of plants Small levels of NOx can cause nausea, irritated eyes and/or nose, fluid forming in lungs and shortness of breath NOx can cause visual impairment.
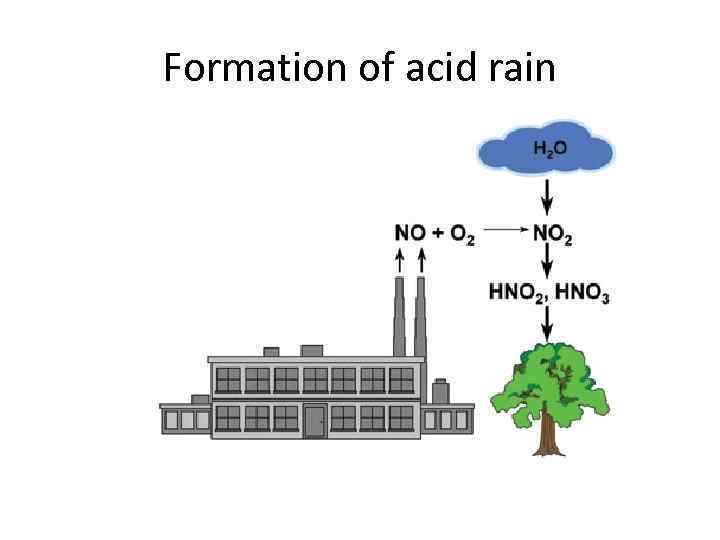
Formation of acid rain

Task You will research the uses of nitrogenous fertilisers. Group 1 will prepare a presentation showing the benefits of their use. Group 2 will prepare a presentation showing the problems caused by their use.
The Haber process.pptx