7094892b8c161fab0730f8197711a239.ppt
- Количество слайдов: 35
 Le pharmacien d’officine Pharmacovigilance Patient Safety Ph. Dirk BROECKX Secrétaire général de l’APB broeckx. dirk@mail. apb. be © APB 2009
Le pharmacien d’officine Pharmacovigilance Patient Safety Ph. Dirk BROECKX Secrétaire général de l’APB broeckx. dirk@mail. apb. be © APB 2009
 Menu l Electronic prescription & patient file l Pharmacovigilance active l Conterfeit and quality of medicines © APB 2009
Menu l Electronic prescription & patient file l Pharmacovigilance active l Conterfeit and quality of medicines © APB 2009
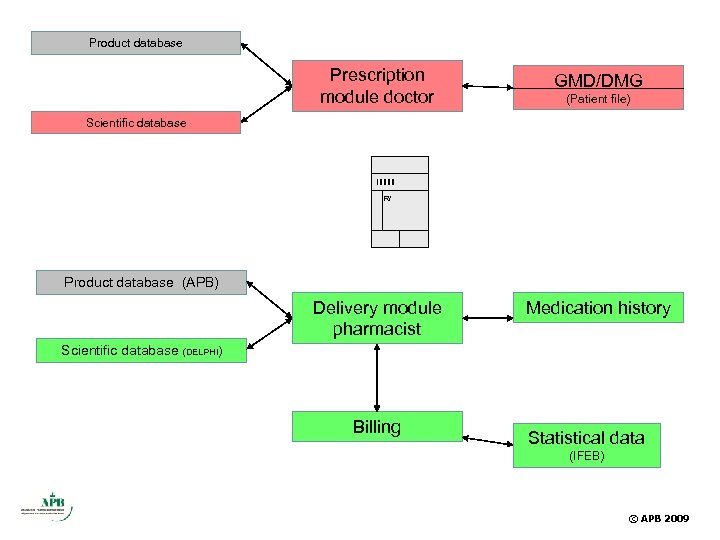 Product database Prescription module doctor GMD/DMG (Patient file) Scientific database R/ Product database (APB) Delivery module pharmacist Medication history Scientific database (DELPHI) Billing Statistical data (IFEB) © APB 2009
Product database Prescription module doctor GMD/DMG (Patient file) Scientific database R/ Product database (APB) Delivery module pharmacist Medication history Scientific database (DELPHI) Billing Statistical data (IFEB) © APB 2009
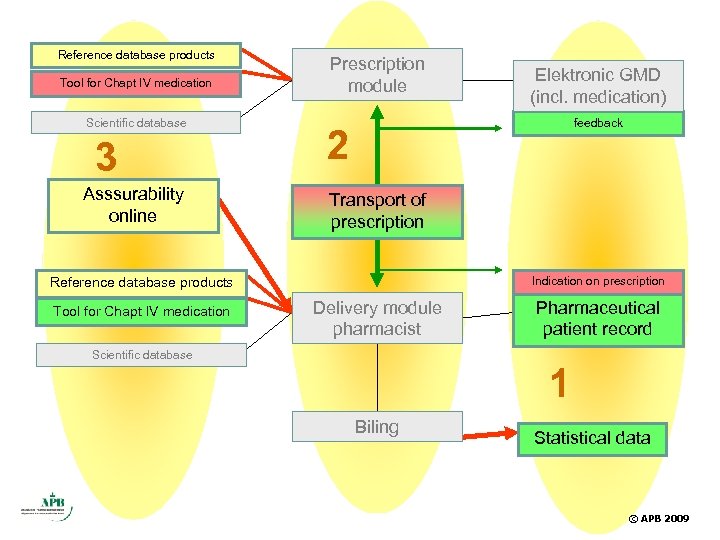 Reference database products Tool for Chapt IV medication Scientific database 3 Asssurability online Prescription module feedback 2 Transport of prescription Indication on prescription Reference database products Tool for Chapt IV medication Elektronic GMD (incl. medication) Delivery module pharmacist Scientific database Pharmaceutical patient record 1 Biling Statistical data © APB 2009
Reference database products Tool for Chapt IV medication Scientific database 3 Asssurability online Prescription module feedback 2 Transport of prescription Indication on prescription Reference database products Tool for Chapt IV medication Elektronic GMD (incl. medication) Delivery module pharmacist Scientific database Pharmaceutical patient record 1 Biling Statistical data © APB 2009
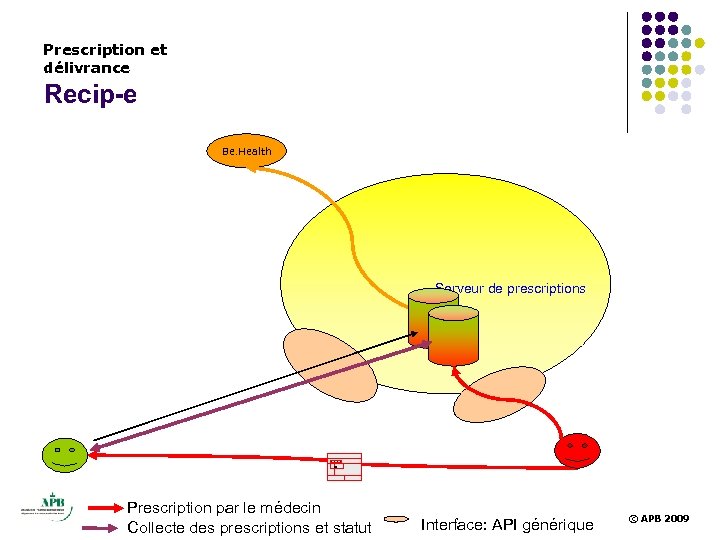 Prescription et délivrance Recip-e Be. Health Serveur de prescriptions 8 R/ Prescription par le médecin Collecte des prescriptions et statut Interface: API générique © APB 2009
Prescription et délivrance Recip-e Be. Health Serveur de prescriptions 8 R/ Prescription par le médecin Collecte des prescriptions et statut Interface: API générique © APB 2009
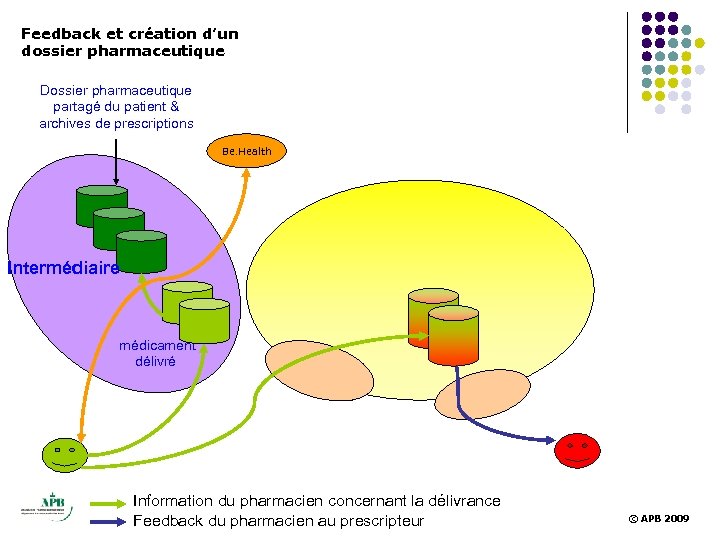 Feedback et création d’un dossier pharmaceutique Dossier pharmaceutique partagé du patient & archives de prescriptions Be. Health Intermédiaire médicament délivré 8 Information du pharmacien concernant la délivrance Feedback du pharmacien au prescripteur © APB 2009
Feedback et création d’un dossier pharmaceutique Dossier pharmaceutique partagé du patient & archives de prescriptions Be. Health Intermédiaire médicament délivré 8 Information du pharmacien concernant la délivrance Feedback du pharmacien au prescripteur © APB 2009
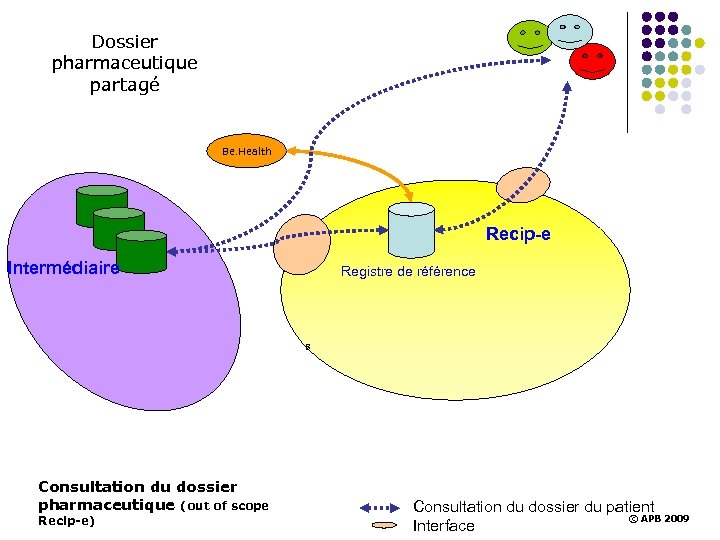 Dossier pharmaceutique partagé Be. Health Recip-e Intermédiaire Registre de référence 8 Consultation du dossier pharmaceutique (out of scope Recip-e) Consultation du dossier du patient © APB 2009 Interface
Dossier pharmaceutique partagé Be. Health Recip-e Intermédiaire Registre de référence 8 Consultation du dossier pharmaceutique (out of scope Recip-e) Consultation du dossier du patient © APB 2009 Interface
 Menu l Electronic prescription & patient file l Pharmacovigilance active l Conterfeit and quality of medicines © APB 2009
Menu l Electronic prescription & patient file l Pharmacovigilance active l Conterfeit and quality of medicines © APB 2009
 “Pharmacovigilance active” l 2004: 25% “ seulement “ des soignants communiquent des informations au CBPH l Nouvelle approche: pharmacovigilance “active” Groupe restreint qui mentionne plus spécifiquement certains effets indésirables de manière systématique. l Phase de test (2008 -2009) l l l Objectif 200 300 professionnels des soins de santé volontaires Généralistes, spécialistes, médecins, pharmaciens hospitaliers et pharmaciens d’officine Pourquoi également les pharmaciens ? l l Dossier pharmaceutique des patients l l Soins pharmaceutiques = dépistage de problèmes liés aux médicaments Contact très fréquent et direct avec les patients (ou leur représentant) Un nouveau système convivial de rapport en ligne l Grand besoin d’un système administratif efficace et simple ! l Opérationnel quand en 2009 ? Entrée des données à l’aide de “fiches types” ? © APB 2009
“Pharmacovigilance active” l 2004: 25% “ seulement “ des soignants communiquent des informations au CBPH l Nouvelle approche: pharmacovigilance “active” Groupe restreint qui mentionne plus spécifiquement certains effets indésirables de manière systématique. l Phase de test (2008 -2009) l l l Objectif 200 300 professionnels des soins de santé volontaires Généralistes, spécialistes, médecins, pharmaciens hospitaliers et pharmaciens d’officine Pourquoi également les pharmaciens ? l l Dossier pharmaceutique des patients l l Soins pharmaceutiques = dépistage de problèmes liés aux médicaments Contact très fréquent et direct avec les patients (ou leur représentant) Un nouveau système convivial de rapport en ligne l Grand besoin d’un système administratif efficace et simple ! l Opérationnel quand en 2009 ? Entrée des données à l’aide de “fiches types” ? © APB 2009
 Quels effets indésirables? l “Au départ”: Mentionner tout effet indésirable grave, inattendu ou suspect. l Vigilance particulière pour: l Des groupes vulnérables de la population. (enfants, femmes enceintes, allaitement, personnes âgés) l La première administration d’un médicament innovant ou générique. l L’administration de vaccins. l L’utilisation erronée de médicaments (off label). © APB 2009
Quels effets indésirables? l “Au départ”: Mentionner tout effet indésirable grave, inattendu ou suspect. l Vigilance particulière pour: l Des groupes vulnérables de la population. (enfants, femmes enceintes, allaitement, personnes âgés) l La première administration d’un médicament innovant ou générique. l L’administration de vaccins. l L’utilisation erronée de médicaments (off label). © APB 2009
 Avantages inhérents à la participation? l Formations en matière de pharmacovigilance. l Soutien téléphonique et par mail du CBPH. l Bulletin d’information électronique mensuel “ VIG-NEWS” reprenant des informations sur le profil de sécurité des médicaments. l (? ) Feedback concernant les rapports - réponse individualisée à des questions portant sur les effets indésirables des médicaments. l (? ) Reconnaissance et “rémunération” de la participation (points d’accréditation / honoraire spécifique pour les soins pharmaceutiques ? ). © APB 2009
Avantages inhérents à la participation? l Formations en matière de pharmacovigilance. l Soutien téléphonique et par mail du CBPH. l Bulletin d’information électronique mensuel “ VIG-NEWS” reprenant des informations sur le profil de sécurité des médicaments. l (? ) Feedback concernant les rapports - réponse individualisée à des questions portant sur les effets indésirables des médicaments. l (? ) Reconnaissance et “rémunération” de la participation (points d’accréditation / honoraire spécifique pour les soins pharmaceutiques ? ). © APB 2009
 Menu l Electronic prescription & patient file l Pharmacovigilance active l Conterfeit and quality of medicines © APB 2009
Menu l Electronic prescription & patient file l Pharmacovigilance active l Conterfeit and quality of medicines © APB 2009
 Definition « counterfeit drug » (WHO) l Deliberately and fraudulently mislabeled with respect to identity and/or source l Can apply to both branded and generic products l May include products with : l The correct or the wrong ingredients l Without active ingredients or with insufficient active ingredients l With fake packaging © APB 2009
Definition « counterfeit drug » (WHO) l Deliberately and fraudulently mislabeled with respect to identity and/or source l Can apply to both branded and generic products l May include products with : l The correct or the wrong ingredients l Without active ingredients or with insufficient active ingredients l With fake packaging © APB 2009
 Belgium NOT at stake ? ? ? l Sep 2006 : 100. 000 tabs. counterfeit VIAGRA seized at Brussels National Airport l July 2007: 600. 000 tabs. of counterfeit antibiotic seized at Brussels National Airport (Dubai, Belgian importer, destination unknown) l 2007: two Belgian wholesalers involved in shipping counterfeit Casodex to UK-wholesalers with legal US-market as final destination (source: Rapport Annuel 2007 – Cellule Multidisciplinaire Hormones) l Sep 2008: 2. 134. 000 units of counterfeit Tramal and Fansidar coming from Mumbai (India) seized at Brussels National Airport © APB 2009
Belgium NOT at stake ? ? ? l Sep 2006 : 100. 000 tabs. counterfeit VIAGRA seized at Brussels National Airport l July 2007: 600. 000 tabs. of counterfeit antibiotic seized at Brussels National Airport (Dubai, Belgian importer, destination unknown) l 2007: two Belgian wholesalers involved in shipping counterfeit Casodex to UK-wholesalers with legal US-market as final destination (source: Rapport Annuel 2007 – Cellule Multidisciplinaire Hormones) l Sep 2008: 2. 134. 000 units of counterfeit Tramal and Fansidar coming from Mumbai (India) seized at Brussels National Airport © APB 2009
 The problem goes beyond the economic context l Economic issue l Trade l Financial l Intellectual Property l Social issue l Undermines public confidence in : l Health care systems l Health care professionals l Pharmaceutical manufacturers l Health problem l Treatment failure l Serious intoxication / injury l Death © APB 2009
The problem goes beyond the economic context l Economic issue l Trade l Financial l Intellectual Property l Social issue l Undermines public confidence in : l Health care systems l Health care professionals l Pharmaceutical manufacturers l Health problem l Treatment failure l Serious intoxication / injury l Death © APB 2009
 Why is counterfeiting medicines on the rise ? l Globalization l Parallel trade (relabelling and repackaging) l Supply chain complexity l Not readily detectable l Low public awareness l Lack of political awareness l Poor legal framework l Inadequate enforcement capacity l Weak penal sanctions l Internet sales l Organised crime moving into medicines © APB 2009
Why is counterfeiting medicines on the rise ? l Globalization l Parallel trade (relabelling and repackaging) l Supply chain complexity l Not readily detectable l Low public awareness l Lack of political awareness l Poor legal framework l Inadequate enforcement capacity l Weak penal sanctions l Internet sales l Organised crime moving into medicines © APB 2009
 Counterfeiting T-shirts or medicines. . ? U. S. Federal Criminal Code Trafficking in Counterfeit Goods or Services, 18 U. S. C. § 2320 Ø 1 st offence : 10 -year prison $2 mio max. fine Ø 2 nd offence : 20 -year prison $5 mio max. fine Federal Food Drug and Cosmetic Act Counterfeit Drugs, 21 U. S. C. § 331 (i) Ø 1 st offence Ø 2 nd offence 1 -year misdemeanor 3 -year prison & « significant fines » © APB 2009
Counterfeiting T-shirts or medicines. . ? U. S. Federal Criminal Code Trafficking in Counterfeit Goods or Services, 18 U. S. C. § 2320 Ø 1 st offence : 10 -year prison $2 mio max. fine Ø 2 nd offence : 20 -year prison $5 mio max. fine Federal Food Drug and Cosmetic Act Counterfeit Drugs, 21 U. S. C. § 331 (i) Ø 1 st offence Ø 2 nd offence 1 -year misdemeanor 3 -year prison & « significant fines » © APB 2009
 EFPIA proposal l EFPIA proposes to verify the authenticity of each product at the point of dispensing l To employ a common European product-coding standard in order to capture the cross-border trade l Unique coding standard : 2 D Data Matrix ECC-200 l Central piece of IT-infrastructure is PILL (Pharmaceutical Interchange Logistics Link) © APB 2009
EFPIA proposal l EFPIA proposes to verify the authenticity of each product at the point of dispensing l To employ a common European product-coding standard in order to capture the cross-border trade l Unique coding standard : 2 D Data Matrix ECC-200 l Central piece of IT-infrastructure is PILL (Pharmaceutical Interchange Logistics Link) © APB 2009
 Belgium : a unique country 1885 § The Pharmaceutical Practice Law introduces the principle of « NO FAULT responsibility » § The pharmacist has the final responsibility for every product (s)he delivers § « Modernised » Royal Decree (21/1/2009) confirms the full legal responsibility of every pharmacist for every product he delivers © APB 2009
Belgium : a unique country 1885 § The Pharmaceutical Practice Law introduces the principle of « NO FAULT responsibility » § The pharmacist has the final responsibility for every product (s)he delivers § « Modernised » Royal Decree (21/1/2009) confirms the full legal responsibility of every pharmacist for every product he delivers © APB 2009
 Belgium : a unique country § 1885 legal « NO FAULT responsibility » § 1952 Start-up by APB of the Medicines Control Laboratory (MCL – DGO – SCM) to combat postwar fraud of penicillin-containing pharmaceuticals. § 1974 Official legal recognition and compulsory contribution by all pharmacists to guanatee the quality of all medicines delivered in Belgian pharmacies © APB 2009
Belgium : a unique country § 1885 legal « NO FAULT responsibility » § 1952 Start-up by APB of the Medicines Control Laboratory (MCL – DGO – SCM) to combat postwar fraud of penicillin-containing pharmaceuticals. § 1974 Official legal recognition and compulsory contribution by all pharmacists to guanatee the quality of all medicines delivered in Belgian pharmacies © APB 2009
 Medicines Control Lab l l l Financed by all Belgian pharmacies (0, 013€ per pack) Staff : +/-60 (pharmacists & lab technicians) Co-management with FAGG/AFMPS DGO-SCM operates centralised recall procedure 173 batches of 94 products recalled in 2006 © APB 2009
Medicines Control Lab l l l Financed by all Belgian pharmacies (0, 013€ per pack) Staff : +/-60 (pharmacists & lab technicians) Co-management with FAGG/AFMPS DGO-SCM operates centralised recall procedure 173 batches of 94 products recalled in 2006 © APB 2009
 Belgium : a unique country § 1885 legal « NO FAULT responsibility » § 1952 APB starts the Medicines Control Laboratory § 1974 Official legal recognition and compulsory contribution by all pharmacists § 2004 Healthcare Authorities introduce Unique Barcode per package: mass serialisation of reimbursed pharmaceuticals to combat healthcare insurance fraud © APB 2009
Belgium : a unique country § 1885 legal « NO FAULT responsibility » § 1952 APB starts the Medicines Control Laboratory § 1974 Official legal recognition and compulsory contribution by all pharmacists § 2004 Healthcare Authorities introduce Unique Barcode per package: mass serialisation of reimbursed pharmaceuticals to combat healthcare insurance fraud © APB 2009
 Belgium : a unique country § 1885 legal « NO FAULT responsibility » § 1952 APB starts the Medicines Control Laboratory § 1974 Official legal recognition and compulsory contribution by all pharmacists § 2004 Unique Barcode per package to combat healthcare insurance fraud § 2006 APB and Aegate start collaboration, using existing mass serialisation to combat counterfeiting of medicines first launch worldwide of an authentication system © APB 2009
Belgium : a unique country § 1885 legal « NO FAULT responsibility » § 1952 APB starts the Medicines Control Laboratory § 1974 Official legal recognition and compulsory contribution by all pharmacists § 2004 Unique Barcode per package to combat healthcare insurance fraud § 2006 APB and Aegate start collaboration, using existing mass serialisation to combat counterfeiting of medicines first launch worldwide of an authentication system © APB 2009
 APB’s rationale for collaboration l Logical extension of APB’s efforts relating to quality assurance of pharmaceuticals and to patient safety l Pro-activity is better than reactivity (when safety of patients is at stake) l Opportunity to steer the evolution of the system (content, functionalities, ease of use etc) to the benefit of the pharmacist l Have impact on and control of dispensing data l Have impact on and control of a powerfull communication tool at the most important communication moment © APB 2009
APB’s rationale for collaboration l Logical extension of APB’s efforts relating to quality assurance of pharmaceuticals and to patient safety l Pro-activity is better than reactivity (when safety of patients is at stake) l Opportunity to steer the evolution of the system (content, functionalities, ease of use etc) to the benefit of the pharmacist l Have impact on and control of dispensing data l Have impact on and control of a powerfull communication tool at the most important communication moment © APB 2009
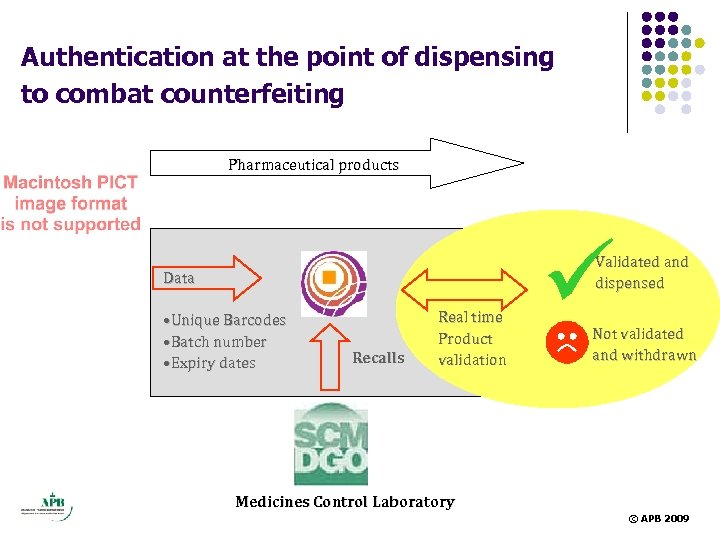 Authentication at the point of dispensing to combat counterfeiting Pharmaceutical products ü Validated and dispensed Data • Unique Barcodes • Batch number • Expiry dates Recalls Real time Product validation Not validated and withdrawn Medicines Control Laboratory © APB 2009
Authentication at the point of dispensing to combat counterfeiting Pharmaceutical products ü Validated and dispensed Data • Unique Barcodes • Batch number • Expiry dates Recalls Real time Product validation Not validated and withdrawn Medicines Control Laboratory © APB 2009
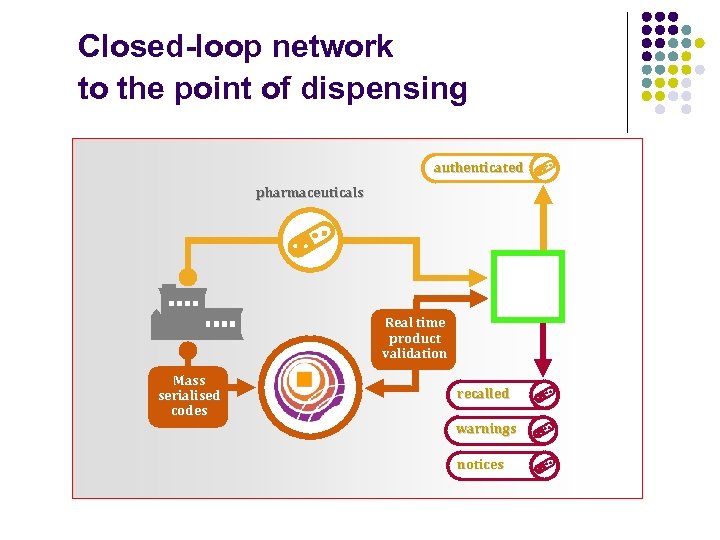 Closed-loop network to the point of dispensing authenticated pharmaceuticals Real time product validation Mass serialised codes recalled warnings notices
Closed-loop network to the point of dispensing authenticated pharmaceuticals Real time product validation Mass serialised codes recalled warnings notices
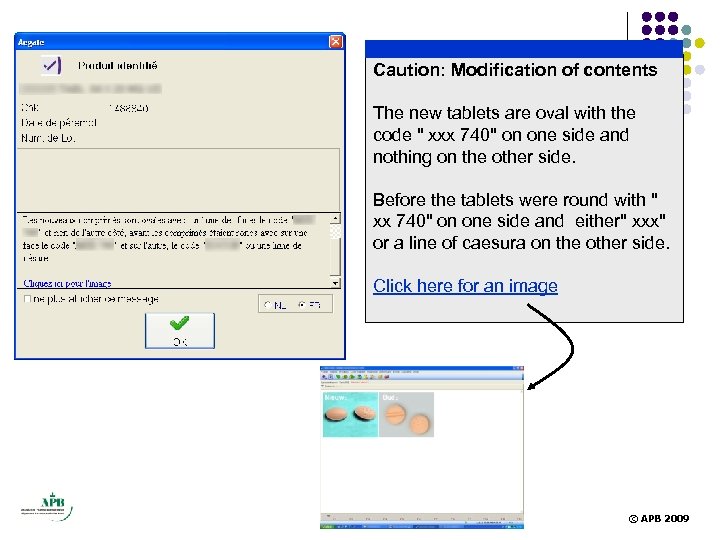 Caution: Modification of contents The new tablets are oval with the code " xxx 740" on one side and nothing on the other side. Before the tablets were round with " xx 740" on one side and either" xxx" or a line of caesura on the other side. Click here for an image © APB 2009
Caution: Modification of contents The new tablets are oval with the code " xxx 740" on one side and nothing on the other side. Before the tablets were round with " xx 740" on one side and either" xxx" or a line of caesura on the other side. Click here for an image © APB 2009
 Pharmacovigilance August 2008 : EMEA and Belgian Federal Agency for Medicines and Health Products recommend restricting the use of oral moxifloxacin-containing medicines APB programs message in Aegate system for all Moxifloxacincontaining oral forms available on Belgian market (within same day). Informatie van APB Moxifloxacine: nieuwe aanbevelingen van het Europees Geneesmiddelenbureau (EMEA) Klik hier voor het artikel – 04 08 2008 Hyperlink to BFAMH website opening in new screen Message live for 2 -3 weeks (depending on turnover) © APB 2009
Pharmacovigilance August 2008 : EMEA and Belgian Federal Agency for Medicines and Health Products recommend restricting the use of oral moxifloxacin-containing medicines APB programs message in Aegate system for all Moxifloxacincontaining oral forms available on Belgian market (within same day). Informatie van APB Moxifloxacine: nieuwe aanbevelingen van het Europees Geneesmiddelenbureau (EMEA) Klik hier voor het artikel – 04 08 2008 Hyperlink to BFAMH website opening in new screen Message live for 2 -3 weeks (depending on turnover) © APB 2009
 Info on new product launches Every 1 st of the month for ± 5 products - New active principles, new routes of administration, new therapeutic indication - Short, basic pharmacotherapeutic information (before complete information is available in pharmacy software) Ex. Vaniqa 11, 5 % Nieuwe specialiteit - Informatie van APB Eflornithine hydrochloride monohydraat Behandeling van hirsutisme in het aangezicht bij de vrouw. 2 maal daags een dunne laag crème aanbrengen met minimum 8 uur interval tussen 2 applicatie Message live from 31/10/2008 to 31/12/2008 © APB 2009
Info on new product launches Every 1 st of the month for ± 5 products - New active principles, new routes of administration, new therapeutic indication - Short, basic pharmacotherapeutic information (before complete information is available in pharmacy software) Ex. Vaniqa 11, 5 % Nieuwe specialiteit - Informatie van APB Eflornithine hydrochloride monohydraat Behandeling van hirsutisme in het aangezicht bij de vrouw. 2 maal daags een dunne laag crème aanbrengen met minimum 8 uur interval tussen 2 applicatie Message live from 31/10/2008 to 31/12/2008 © APB 2009
 Independent Audit confirms Drug Authentication protects Patients in « real world » Setting System was independently audited by Katholieke Universiteit Leuven (June - Aug 08) : l Tested in 116 pharmacies l 656 authentic products l 327 recalled products l 220 expired products l 106 suspicious products l The actual response for each test code corresponded with the correct response l Reliability of [ 99. 8%-100%] (95% confidence interval) l The Aegate system protects patients from harmful products at point of dispense © APB 2009
Independent Audit confirms Drug Authentication protects Patients in « real world » Setting System was independently audited by Katholieke Universiteit Leuven (June - Aug 08) : l Tested in 116 pharmacies l 656 authentic products l 327 recalled products l 220 expired products l 106 suspicious products l The actual response for each test code corresponded with the correct response l Reliability of [ 99. 8%-100%] (95% confidence interval) l The Aegate system protects patients from harmful products at point of dispense © APB 2009
 October 2008 report 695 78. 2 million Products that can be authenticated UBC in database 35 Recalled products currently in database 308 Product messages added 1283 776. 635 0 493 3784 6207 0. 3041 sec Pharmacies scanning Items scanned Notifications suspicious Notifications expired Notifications soon to expire Notifications has been recalled Response time © APB 2009
October 2008 report 695 78. 2 million Products that can be authenticated UBC in database 35 Recalled products currently in database 308 Product messages added 1283 776. 635 0 493 3784 6207 0. 3041 sec Pharmacies scanning Items scanned Notifications suspicious Notifications expired Notifications soon to expire Notifications has been recalled Response time © APB 2009
 Practical implications l Allthough imperfect and incomplete, an anti-counterfeiting system is up and running l All the necessary building blocs are in place l Uploading of expiry dates at reception of goods through simple scanning l Real time on-line alerts on recalls are designed and controled by Medicines Control Laboratory (strengthen, speed-up and control) l Ensure proper use of powerfull communication moment (pharmacist has patient in front) l Switch-off function ensured l Strict protection of pharmacists’ proprietary rights of data generated within pharmacies © APB 2009
Practical implications l Allthough imperfect and incomplete, an anti-counterfeiting system is up and running l All the necessary building blocs are in place l Uploading of expiry dates at reception of goods through simple scanning l Real time on-line alerts on recalls are designed and controled by Medicines Control Laboratory (strengthen, speed-up and control) l Ensure proper use of powerfull communication moment (pharmacist has patient in front) l Switch-off function ensured l Strict protection of pharmacists’ proprietary rights of data generated within pharmacies © APB 2009
 (Potential) Issues l Buy-in and co-operation from software providers l Is Big Brother watching again ? l Absolute need for strict and explicit pharmacy data protection l Unique Barcode – based system (presently) limits authentication check to reimbursed products only l « Wait and see » approach (manufacturers and pharmacists) © APB 2009
(Potential) Issues l Buy-in and co-operation from software providers l Is Big Brother watching again ? l Absolute need for strict and explicit pharmacy data protection l Unique Barcode – based system (presently) limits authentication check to reimbursed products only l « Wait and see » approach (manufacturers and pharmacists) © APB 2009
 Added value for patient safety ? Electronic prescription & patient file l Medication errors l Working with the same prescription & patient data / file l Collaboration & communication between healthcare providers Pharmacovigilance active l Post Marketing Surveillance l Safety profile of medicines l Sharing data with authorities and pharmaceutical companies Conterfeit and quality of medicines l Detecting non-quality of medication; counterfeit & quality l Communication at the moment of delivery l Real time, online checks and messaging between HCP & Ph. Com © APB 2009
Added value for patient safety ? Electronic prescription & patient file l Medication errors l Working with the same prescription & patient data / file l Collaboration & communication between healthcare providers Pharmacovigilance active l Post Marketing Surveillance l Safety profile of medicines l Sharing data with authorities and pharmaceutical companies Conterfeit and quality of medicines l Detecting non-quality of medication; counterfeit & quality l Communication at the moment of delivery l Real time, online checks and messaging between HCP & Ph. Com © APB 2009
 The healthcare professionals magazine 6 x/year - FR/NL 8. 000 readers ————— Hot topics seminars driven by reknown opinion leaders
The healthcare professionals magazine 6 x/year - FR/NL 8. 000 readers ————— Hot topics seminars driven by reknown opinion leaders


