6f23412ce4620aa6c4b8cdb9df8cb088.ppt
- Количество слайдов: 68
 Latest update on Cervical Cancer Screening guidelines and clinical published data Michael D. Randell MD Obstetrician and Gynecologist Atlanta, Georgia
Latest update on Cervical Cancer Screening guidelines and clinical published data Michael D. Randell MD Obstetrician and Gynecologist Atlanta, Georgia
 Objectives of Today’s Presentation 1. 2. 3. 4. 5. 6. Burden of cervical cancer on women’s lives & U. S. prevention efforts Latest guidelines for HPV DNA testing & interval extension HPV test: Predictor of a woman’s risk for developing cervical disease What do patients want to know about cervical cancer screening? HPV vaccines - Will that make cervical screening obsolete? Are the other HPV tests clinically better?
Objectives of Today’s Presentation 1. 2. 3. 4. 5. 6. Burden of cervical cancer on women’s lives & U. S. prevention efforts Latest guidelines for HPV DNA testing & interval extension HPV test: Predictor of a woman’s risk for developing cervical disease What do patients want to know about cervical cancer screening? HPV vaccines - Will that make cervical screening obsolete? Are the other HPV tests clinically better?
 Cervical Cancer in U. S. Estimated Cancer New Cancer and Deaths in 2009 § 11, 270 cases - invasive cervical cancer § 4, 070 deaths from cervical cancer § 1 out of 50 cancer-related deaths Source: American Cancer Society - Facts and Figures 2009
Cervical Cancer in U. S. Estimated Cancer New Cancer and Deaths in 2009 § 11, 270 cases - invasive cervical cancer § 4, 070 deaths from cervical cancer § 1 out of 50 cancer-related deaths Source: American Cancer Society - Facts and Figures 2009
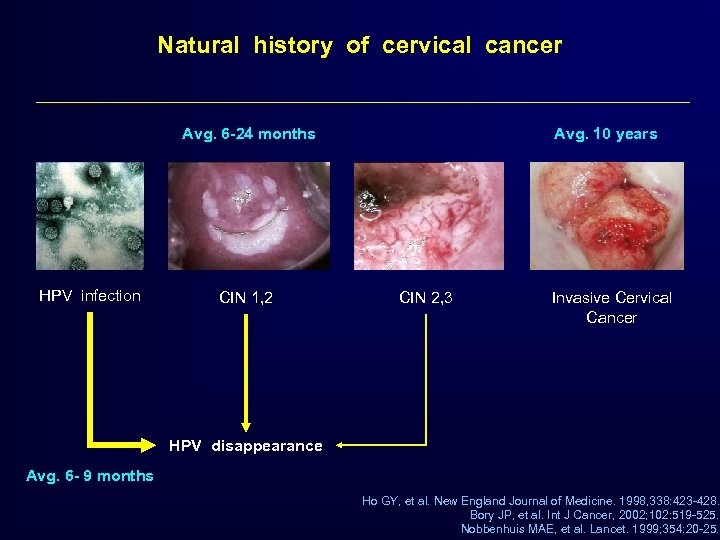 Natural history of cervical cancer Avg. 6 -24 months HPV infection CIN 1, 2 Avg. 10 years CIN 2, 3 Invasive Cervical Cancer HPV disappearance Avg. 6 - 9 months Ho GY, et al. New England Journal of Medicine. 1998, 338: 423 -428. Bory JP, et al. Int J Cancer, 2002; 102: 519 -525. Nobbenhuis MAE, et al. Lancet. 1999; 354: 20 -25.
Natural history of cervical cancer Avg. 6 -24 months HPV infection CIN 1, 2 Avg. 10 years CIN 2, 3 Invasive Cervical Cancer HPV disappearance Avg. 6 - 9 months Ho GY, et al. New England Journal of Medicine. 1998, 338: 423 -428. Bory JP, et al. Int J Cancer, 2002; 102: 519 -525. Nobbenhuis MAE, et al. Lancet. 1999; 354: 20 -25.
 Longitudinal Study of HPV persistence and CIN 2+ Critical role of age and duration of HPV infection • More than 90% of new infections with carcinogenic HPV types clear • Regardless of the woman’s age, newly detected infections were associated with very low absolute risks of persistence, CIN 2, or worse disease • Long duration of infection predicts – Risk of further persistence – Risk associated with CIN 2+ – Cervical Cancer Rodriguez AC et al, J Natl Cancer Inst 2010; 102: 315– 324
Longitudinal Study of HPV persistence and CIN 2+ Critical role of age and duration of HPV infection • More than 90% of new infections with carcinogenic HPV types clear • Regardless of the woman’s age, newly detected infections were associated with very low absolute risks of persistence, CIN 2, or worse disease • Long duration of infection predicts – Risk of further persistence – Risk associated with CIN 2+ – Cervical Cancer Rodriguez AC et al, J Natl Cancer Inst 2010; 102: 315– 324
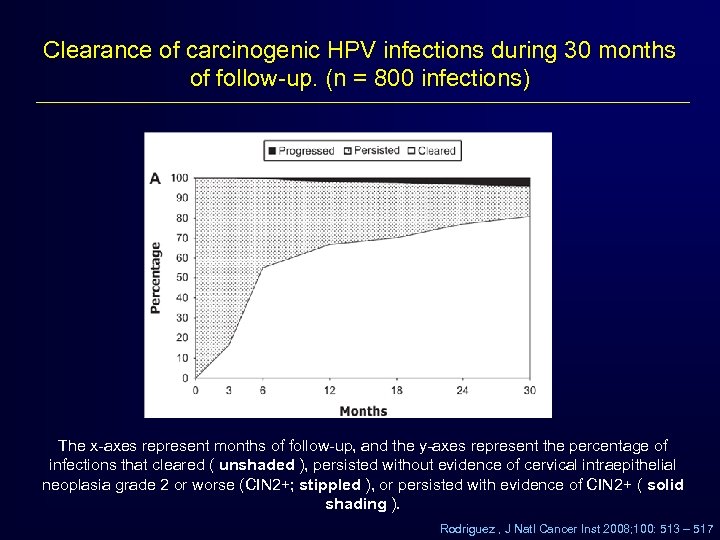 Clearance of carcinogenic HPV infections during 30 months of follow-up. (n = 800 infections) The x-axes represent months of follow-up, and the y-axes represent the percentage of infections that cleared ( unshaded ), persisted without evidence of cervical intraepithelial neoplasia grade 2 or worse (CIN 2+; stippled ), or persisted with evidence of CIN 2+ ( solid shading ). Rodriguez , J Natl Cancer Inst 2008; 100: 513 – 517
Clearance of carcinogenic HPV infections during 30 months of follow-up. (n = 800 infections) The x-axes represent months of follow-up, and the y-axes represent the percentage of infections that cleared ( unshaded ), persisted without evidence of cervical intraepithelial neoplasia grade 2 or worse (CIN 2+; stippled ), or persisted with evidence of CIN 2+ ( solid shading ). Rodriguez , J Natl Cancer Inst 2008; 100: 513 – 517
 HPV Infection Persistence • How persistence is defined: Most studies of HPV persistence have defined persistence as the detection of the same HPV type at 2 and 3 consecutive visits, 2 to 24 months apart. • Risk factors for persistence: – HPV type – Weak immune system – Infection with multiple types – Age Sycuro LA et al. , J Infect Dis. 2008, 198: 971=978
HPV Infection Persistence • How persistence is defined: Most studies of HPV persistence have defined persistence as the detection of the same HPV type at 2 and 3 consecutive visits, 2 to 24 months apart. • Risk factors for persistence: – HPV type – Weak immune system – Infection with multiple types – Age Sycuro LA et al. , J Infect Dis. 2008, 198: 971=978
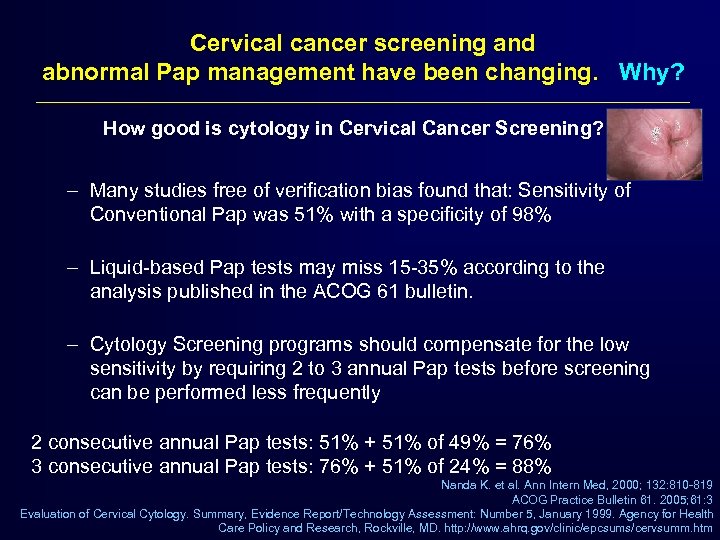 Cervical cancer screening and abnormal Pap management have been changing. Why? How good is cytology in Cervical Cancer Screening? – Many studies free of verification bias found that: Sensitivity of Conventional Pap was 51% with a specificity of 98% – Liquid-based Pap tests may miss 15 -35% according to the analysis published in the ACOG 61 bulletin. – Cytology Screening programs should compensate for the low sensitivity by requiring 2 to 3 annual Pap tests before screening can be performed less frequently 2 consecutive annual Pap tests: 51% + 51% of 49% = 76% 3 consecutive annual Pap tests: 76% + 51% of 24% = 88% Nanda K. et al. Ann Intern Med, 2000; 132: 810 -819 ACOG Practice Bulletin 61. 2005; 61: 3 Evaluation of Cervical Cytology. Summary, Evidence Report/Technology Assessment: Number 5, January 1999. Agency for Health Care Policy and Research, Rockville, MD. http: //www. ahrq. gov/clinic/epcsums/cervsumm. htm
Cervical cancer screening and abnormal Pap management have been changing. Why? How good is cytology in Cervical Cancer Screening? – Many studies free of verification bias found that: Sensitivity of Conventional Pap was 51% with a specificity of 98% – Liquid-based Pap tests may miss 15 -35% according to the analysis published in the ACOG 61 bulletin. – Cytology Screening programs should compensate for the low sensitivity by requiring 2 to 3 annual Pap tests before screening can be performed less frequently 2 consecutive annual Pap tests: 51% + 51% of 49% = 76% 3 consecutive annual Pap tests: 76% + 51% of 24% = 88% Nanda K. et al. Ann Intern Med, 2000; 132: 810 -819 ACOG Practice Bulletin 61. 2005; 61: 3 Evaluation of Cervical Cytology. Summary, Evidence Report/Technology Assessment: Number 5, January 1999. Agency for Health Care Policy and Research, Rockville, MD. http: //www. ahrq. gov/clinic/epcsums/cervsumm. htm
 The following is based on good and consistent scientific evidence “Because HPV DNA testing is more sensitive than cervical cytology in detecting CIN 2, 3, … [ ] …. . women with negative concurrent test results can be reassured that their risk of unidentified CIN 2, 3 or cervical cancer is approximately 1 in 1, 000. ” ACOG Practice Bulletin. April 2005; 61: 9
The following is based on good and consistent scientific evidence “Because HPV DNA testing is more sensitive than cervical cytology in detecting CIN 2, 3, … [ ] …. . women with negative concurrent test results can be reassured that their risk of unidentified CIN 2, 3 or cervical cancer is approximately 1 in 1, 000. ” ACOG Practice Bulletin. April 2005; 61: 9
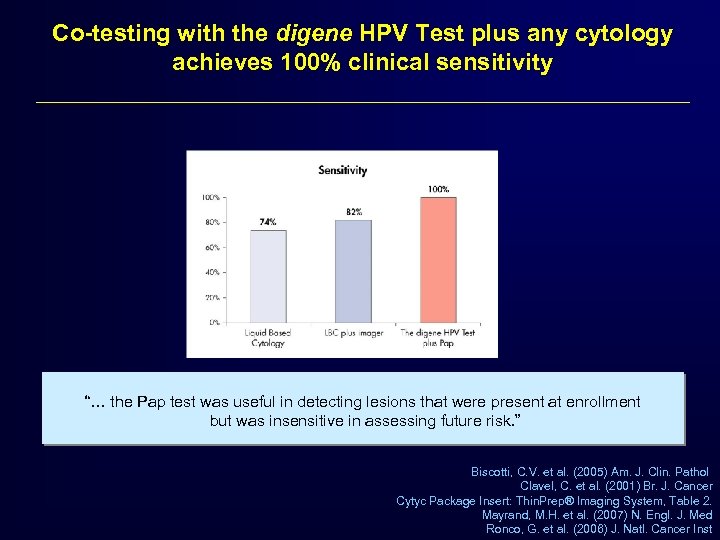 Co-testing with the digene HPV Test plus any cytology achieves 100% clinical sensitivity “… the Pap test was useful in detecting lesions that were present at enrollment but was insensitive in assessing future risk. ” Biscotti, C. V. et al. (2005) Am. J. Clin. Pathol Clavel, C. et al. (2001) Br. J. Cancer Cytyc Package Insert: Thin. Prep® Imaging System, Table 2. Mayrand, M. H. et al. (2007) N. Engl. J. Med Ronco, G. et al. (2006) J. Natl. Cancer Inst
Co-testing with the digene HPV Test plus any cytology achieves 100% clinical sensitivity “… the Pap test was useful in detecting lesions that were present at enrollment but was insensitive in assessing future risk. ” Biscotti, C. V. et al. (2005) Am. J. Clin. Pathol Clavel, C. et al. (2001) Br. J. Cancer Cytyc Package Insert: Thin. Prep® Imaging System, Table 2. Mayrand, M. H. et al. (2007) N. Engl. J. Med Ronco, G. et al. (2006) J. Natl. Cancer Inst
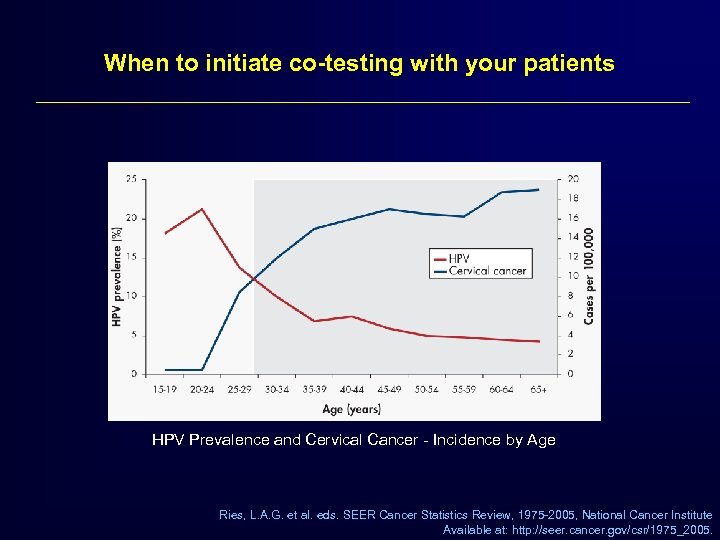 When to initiate co-testing with your patients HPV Prevalence and Cervical Cancer - Incidence by Age Ries, L. A. G. et al. eds. SEER Cancer Statistics Review, 1975 -2005, National Cancer Institute Available at: http: //seer. cancer. gov/csr/1975_2005.
When to initiate co-testing with your patients HPV Prevalence and Cervical Cancer - Incidence by Age Ries, L. A. G. et al. eds. SEER Cancer Statistics Review, 1975 -2005, National Cancer Institute Available at: http: //seer. cancer. gov/csr/1975_2005.
 Why use HPV testing in cervical cancer screening? • • More sensitive than cytology Detects disease earlier therefore enables longer screening intervals Can be automated, centralized and standardized among labs Evidence that screening with HPV reduces cervical cancer incidence and mortality • Improves detection of glandular lesions & adenocarcinomas
Why use HPV testing in cervical cancer screening? • • More sensitive than cytology Detects disease earlier therefore enables longer screening intervals Can be automated, centralized and standardized among labs Evidence that screening with HPV reduces cervical cancer incidence and mortality • Improves detection of glandular lesions & adenocarcinomas
 Examples of randomized clinical trials that use HC 2 HPV testing in screening • • HART Trial: United Kingdom POBASCAM Study: The Nederlands Indian Trial: Osmanabad district ARTISTIC Trial: United Kingdom NTCC Study: Italy SWEDESCAN: Sweden CCCa. ST Study: Canada
Examples of randomized clinical trials that use HC 2 HPV testing in screening • • HART Trial: United Kingdom POBASCAM Study: The Nederlands Indian Trial: Osmanabad district ARTISTIC Trial: United Kingdom NTCC Study: Italy SWEDESCAN: Sweden CCCa. ST Study: Canada

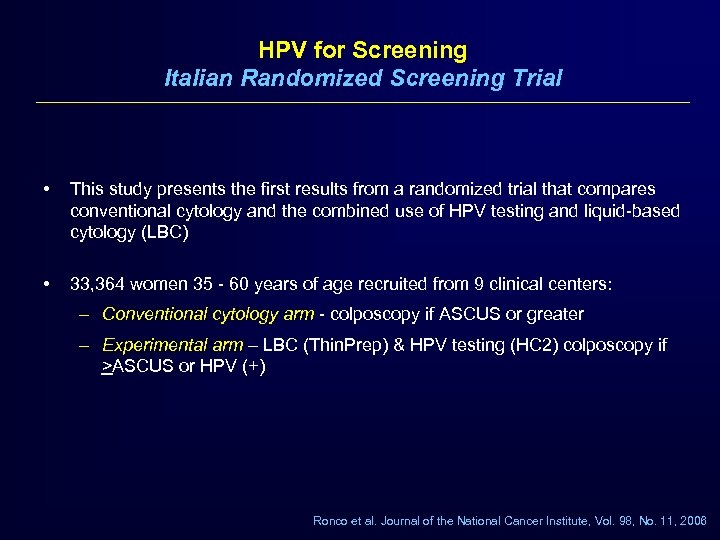 HPV for Screening Italian Randomized Screening Trial • This study presents the first results from a randomized trial that compares conventional cytology and the combined use of HPV testing and liquid-based cytology (LBC) • 33, 364 women 35 - 60 years of age recruited from 9 clinical centers: – Conventional cytology arm - colposcopy if ASCUS or greater – Experimental arm – LBC (Thin. Prep) & HPV testing (HC 2) colposcopy if >ASCUS or HPV (+) Ronco et al. Journal of the National Cancer Institute, Vol. 98, No. 11, 2006
HPV for Screening Italian Randomized Screening Trial • This study presents the first results from a randomized trial that compares conventional cytology and the combined use of HPV testing and liquid-based cytology (LBC) • 33, 364 women 35 - 60 years of age recruited from 9 clinical centers: – Conventional cytology arm - colposcopy if ASCUS or greater – Experimental arm – LBC (Thin. Prep) & HPV testing (HC 2) colposcopy if >ASCUS or HPV (+) Ronco et al. Journal of the National Cancer Institute, Vol. 98, No. 11, 2006
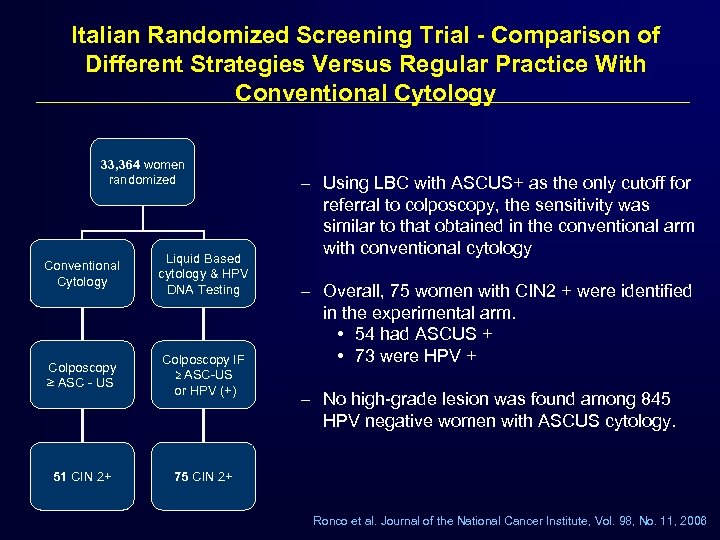 Italian Randomized Screening Trial - Comparison of Different Strategies Versus Regular Practice With Conventional Cytology 33, 364 women randomized Conventional Cytology Liquid Based cytology & HPV DNA Testing Colposcopy ≥ ASC - US Colposcopy IF ≥ ASC-US or HPV (+) 51 CIN 2+ – Using LBC with ASCUS+ as the only cutoff for referral to colposcopy, the sensitivity was similar to that obtained in the conventional arm with conventional cytology 75 CIN 2+ – Overall, 75 women with CIN 2 + were identified in the experimental arm. • 54 had ASCUS + • 73 were HPV + – No high-grade lesion was found among 845 HPV negative women with ASCUS cytology. Ronco et al. Journal of the National Cancer Institute, Vol. 98, No. 11, 2006
Italian Randomized Screening Trial - Comparison of Different Strategies Versus Regular Practice With Conventional Cytology 33, 364 women randomized Conventional Cytology Liquid Based cytology & HPV DNA Testing Colposcopy ≥ ASC - US Colposcopy IF ≥ ASC-US or HPV (+) 51 CIN 2+ – Using LBC with ASCUS+ as the only cutoff for referral to colposcopy, the sensitivity was similar to that obtained in the conventional arm with conventional cytology 75 CIN 2+ – Overall, 75 women with CIN 2 + were identified in the experimental arm. • 54 had ASCUS + • 73 were HPV + – No high-grade lesion was found among 845 HPV negative women with ASCUS cytology. Ronco et al. Journal of the National Cancer Institute, Vol. 98, No. 11, 2006
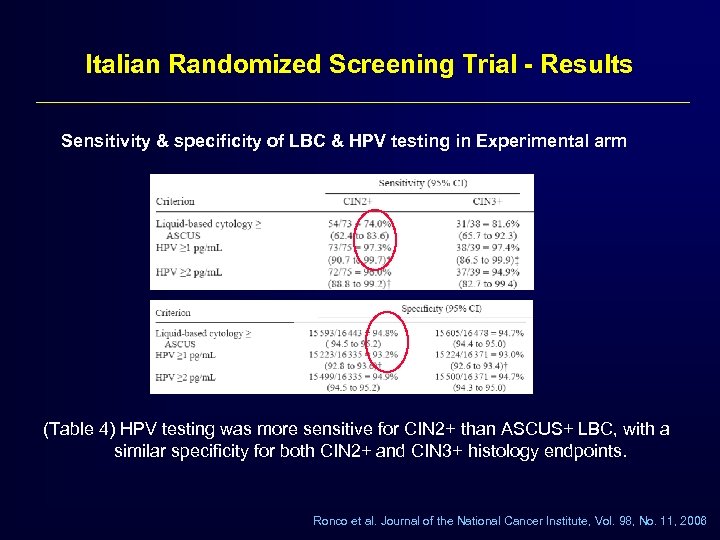 Italian Randomized Screening Trial - Results Sensitivity & specificity of LBC & HPV testing in Experimental arm (Table 4) HPV testing was more sensitive for CIN 2+ than ASCUS+ LBC, with a similar specificity for both CIN 2+ and CIN 3+ histology endpoints. Ronco et al. Journal of the National Cancer Institute, Vol. 98, No. 11, 2006
Italian Randomized Screening Trial - Results Sensitivity & specificity of LBC & HPV testing in Experimental arm (Table 4) HPV testing was more sensitive for CIN 2+ than ASCUS+ LBC, with a similar specificity for both CIN 2+ and CIN 3+ histology endpoints. Ronco et al. Journal of the National Cancer Institute, Vol. 98, No. 11, 2006
 Italian Randomized Screening Trial Conclusions • HPV testing alone was more sensitive than conventional cytology among women 35 – 60 years old. • HPV testing supplemented by LBC led to a substantial (47%) increase in sensitivity for CIN 2+ Ronco et al. Journal of the National Cancer Institute, Vol. 98, No. 11, 2006
Italian Randomized Screening Trial Conclusions • HPV testing alone was more sensitive than conventional cytology among women 35 – 60 years old. • HPV testing supplemented by LBC led to a substantial (47%) increase in sensitivity for CIN 2+ Ronco et al. Journal of the National Cancer Institute, Vol. 98, No. 11, 2006
 HPV used in screening randomized trial Canadian Cervical Cancer Screening Trial (CCCa. ST) • 10, 456 women 30 -69 yrs of age seeking screening in Montreal or St. Johns (Canada) • Women Had BOTH HC 2 & conventional cytology. Women positive on either test had colposcopy • First large randomized trial where HPV testing has been compared directly as a stand alone test with Pap in North American population with access to quality care N Engl. J Med. 2007; 357(16): 1579 -88.
HPV used in screening randomized trial Canadian Cervical Cancer Screening Trial (CCCa. ST) • 10, 456 women 30 -69 yrs of age seeking screening in Montreal or St. Johns (Canada) • Women Had BOTH HC 2 & conventional cytology. Women positive on either test had colposcopy • First large randomized trial where HPV testing has been compared directly as a stand alone test with Pap in North American population with access to quality care N Engl. J Med. 2007; 357(16): 1579 -88.
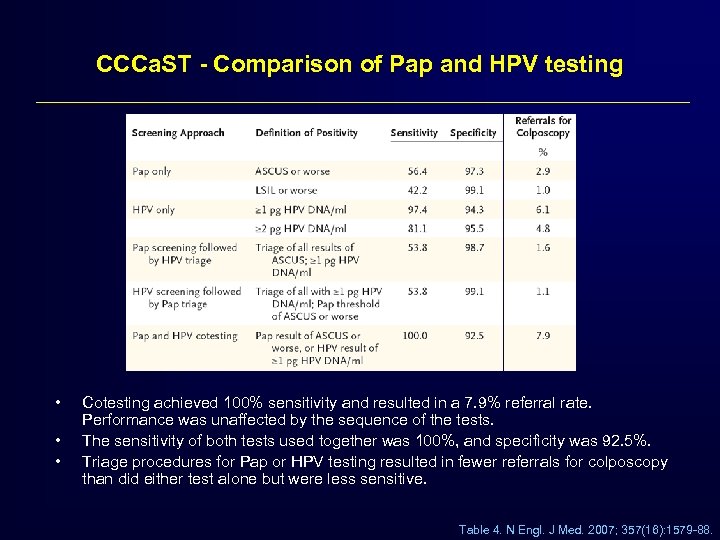 CCCa. ST - Comparison of Pap and HPV testing • • • Cotesting achieved 100% sensitivity and resulted in a 7. 9% referral rate. Performance was unaffected by the sequence of the tests. The sensitivity of both tests used together was 100%, and specificity was 92. 5%. Triage procedures for Pap or HPV testing resulted in fewer referrals for colposcopy than did either test alone but were less sensitive. Table 4. N Engl. J Med. 2007; 357(16): 1579 -88.
CCCa. ST - Comparison of Pap and HPV testing • • • Cotesting achieved 100% sensitivity and resulted in a 7. 9% referral rate. Performance was unaffected by the sequence of the tests. The sensitivity of both tests used together was 100%, and specificity was 92. 5%. Triage procedures for Pap or HPV testing resulted in fewer referrals for colposcopy than did either test alone but were less sensitive. Table 4. N Engl. J Med. 2007; 357(16): 1579 -88.
 CCCa. ST – Key Conclusions • Pap + DNA is the most sensitive method for cancer detection. Regardless of what type of Pap test you perform, you can only achieve 100% sensitivity by coupling it with the HPV DNA test. • The small decrease in specificity (2. 7%) compared to Pap is off-set by the dramatic increase in cancer detection. • This study attracted international attention and domestic TV and newspaper coverage because it proposed “a shift from cellular to viral testing” (i. e. “HPV followed by Pap”) as a possible future strategy for improved cancer detection. “As compared with Pap testing, HPV testing has greater sensitivity for the detection of cervical intraepithelial neoplasia. ” N Engl. J Med. 2007; 357(16): 1579 -88.
CCCa. ST – Key Conclusions • Pap + DNA is the most sensitive method for cancer detection. Regardless of what type of Pap test you perform, you can only achieve 100% sensitivity by coupling it with the HPV DNA test. • The small decrease in specificity (2. 7%) compared to Pap is off-set by the dramatic increase in cancer detection. • This study attracted international attention and domestic TV and newspaper coverage because it proposed “a shift from cellular to viral testing” (i. e. “HPV followed by Pap”) as a possible future strategy for improved cancer detection. “As compared with Pap testing, HPV testing has greater sensitivity for the detection of cervical intraepithelial neoplasia. ” N Engl. J Med. 2007; 357(16): 1579 -88.
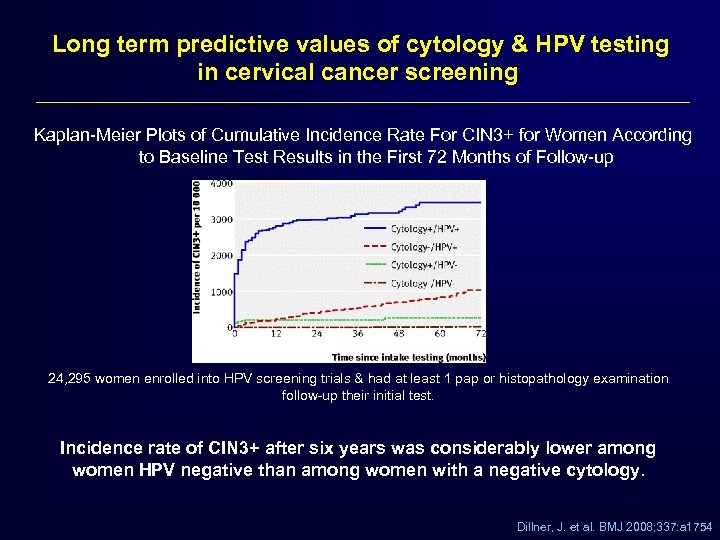 Long term predictive values of cytology & HPV testing in cervical cancer screening Kaplan-Meier Plots of Cumulative Incidence Rate For CIN 3+ for Women According to Baseline Test Results in the First 72 Months of Follow-up 24, 295 women enrolled into HPV screening trials & had at least 1 pap or histopathology examination follow-up their initial test. Incidence rate of CIN 3+ after six years was considerably lower among women HPV negative than among women with a negative cytology. Dillner, J. et al. BMJ 2008; 337: a 1754
Long term predictive values of cytology & HPV testing in cervical cancer screening Kaplan-Meier Plots of Cumulative Incidence Rate For CIN 3+ for Women According to Baseline Test Results in the First 72 Months of Follow-up 24, 295 women enrolled into HPV screening trials & had at least 1 pap or histopathology examination follow-up their initial test. Incidence rate of CIN 3+ after six years was considerably lower among women HPV negative than among women with a negative cytology. Dillner, J. et al. BMJ 2008; 337: a 1754
 2009 NEJM - Mortality Study (2000 -2007) – 131, 746 women aged 30 -59 years • Married at time of trial or previously married – Randomly assigned to four groups • Screening by the digene HPV Test (34, 126) • Pap (32, 058) • visual inspection with acetic acid - VIA (34, 074) • Control – only counseling (31, 488) – Women who had positive results on screening underwent colposcopy and biopsy – Women with precancerous lesions or cancer received treatment Sankaranarayanan R, et al. N Engl J Med 2009; 360: 1385 -94.
2009 NEJM - Mortality Study (2000 -2007) – 131, 746 women aged 30 -59 years • Married at time of trial or previously married – Randomly assigned to four groups • Screening by the digene HPV Test (34, 126) • Pap (32, 058) • visual inspection with acetic acid - VIA (34, 074) • Control – only counseling (31, 488) – Women who had positive results on screening underwent colposcopy and biopsy – Women with precancerous lesions or cancer received treatment Sankaranarayanan R, et al. N Engl J Med 2009; 360: 1385 -94.
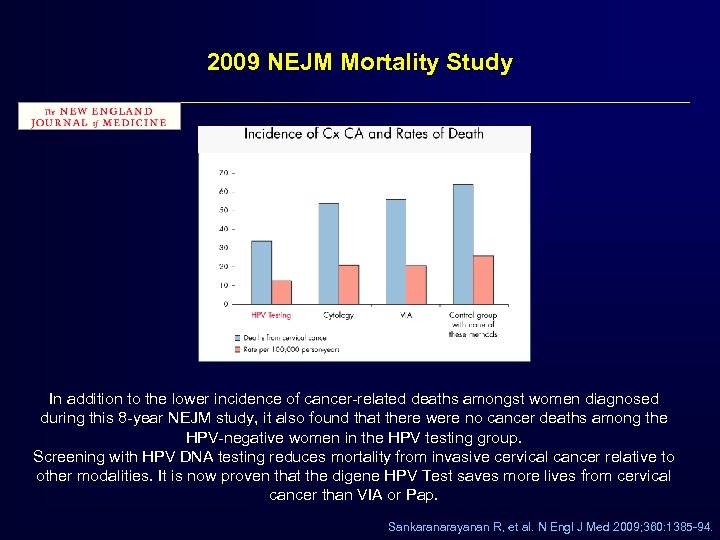 2009 NEJM Mortality Study In addition to the lower incidence of cancer-related deaths amongst women diagnosed during this 8 -year NEJM study, it also found that there were no cancer deaths among the HPV-negative women in the HPV testing group. Screening with HPV DNA testing reduces mortality from invasive cervical cancer relative to other modalities. It is now proven that the digene HPV Test saves more lives from cervical cancer than VIA or Pap. Sankaranarayanan R, et al. N Engl J Med 2009; 360: 1385 -94.
2009 NEJM Mortality Study In addition to the lower incidence of cancer-related deaths amongst women diagnosed during this 8 -year NEJM study, it also found that there were no cancer deaths among the HPV-negative women in the HPV testing group. Screening with HPV DNA testing reduces mortality from invasive cervical cancer relative to other modalities. It is now proven that the digene HPV Test saves more lives from cervical cancer than VIA or Pap. Sankaranarayanan R, et al. N Engl J Med 2009; 360: 1385 -94.
 New England Journal of Medicine India Screening Study – “Our study found that a single round of HPV testing was associated with a significant decline in the rate of advanced cervical cancer and associated deaths. . . ” – “We found that HPV testing was the most objective and reproducible of all cervical screening tests and was less demanding in terms of training and quality assurance. ” Sankaranarayanan R, et al. N Engl J Med 2009; 360: 1385 -94.
New England Journal of Medicine India Screening Study – “Our study found that a single round of HPV testing was associated with a significant decline in the rate of advanced cervical cancer and associated deaths. . . ” – “We found that HPV testing was the most objective and reproducible of all cervical screening tests and was less demanding in terms of training and quality assurance. ” Sankaranarayanan R, et al. N Engl J Med 2009; 360: 1385 -94.
 New screening guidelines: incorporates knowledge of the role of HPV
New screening guidelines: incorporates knowledge of the role of HPV
 HPV Testing in cervical cancer screening Age to initiate co-testing: Women 30 years and older FDA approval ACOG Practice Bulletins ASCCP Guidelines SGO Recommendations on Genotyping
HPV Testing in cervical cancer screening Age to initiate co-testing: Women 30 years and older FDA approval ACOG Practice Bulletins ASCCP Guidelines SGO Recommendations on Genotyping
 HPV DNA Testing for Screening Current state of science - 2010 • Large number of cross-sectional studies demonstrating superiority of HPV DNA testing compared to cytology • Randomized screening trials are now being completed Data is overwhelmingly in favor of any Pap plus HPV Test offering women the best protection against cervical disease
HPV DNA Testing for Screening Current state of science - 2010 • Large number of cross-sectional studies demonstrating superiority of HPV DNA testing compared to cytology • Randomized screening trials are now being completed Data is overwhelmingly in favor of any Pap plus HPV Test offering women the best protection against cervical disease
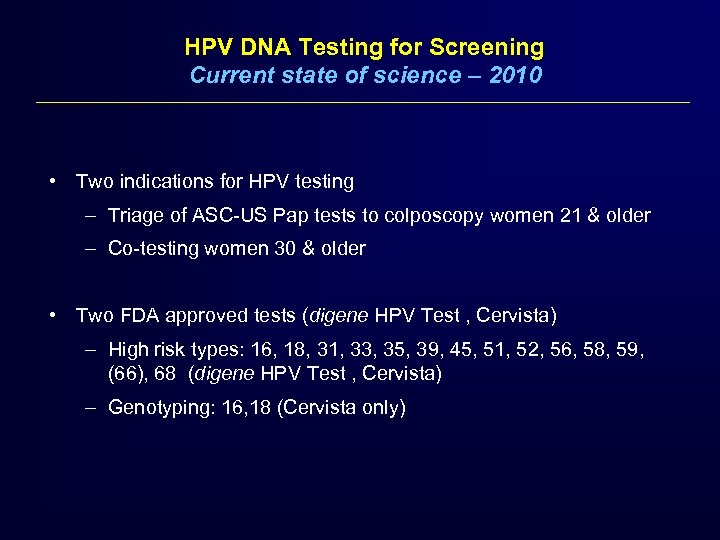 HPV DNA Testing for Screening Current state of science – 2010 • Two indications for HPV testing – Triage of ASC-US Pap tests to colposcopy women 21 & older – Co-testing women 30 & older • Two FDA approved tests (digene HPV Test , Cervista) – High risk types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, (66), 68 (digene HPV Test , Cervista) – Genotyping: 16, 18 (Cervista only)
HPV DNA Testing for Screening Current state of science – 2010 • Two indications for HPV testing – Triage of ASC-US Pap tests to colposcopy women 21 & older – Co-testing women 30 & older • Two FDA approved tests (digene HPV Test , Cervista) – High risk types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, (66), 68 (digene HPV Test , Cervista) – Genotyping: 16, 18 (Cervista only)
 ACOG Guidelines Recommend HPV co-testing since 2003 2009 2005 2008
ACOG Guidelines Recommend HPV co-testing since 2003 2009 2005 2008
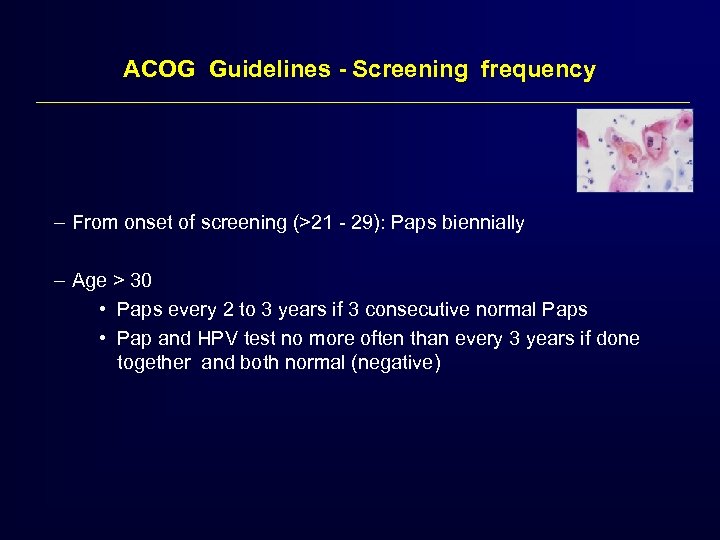 ACOG Guidelines - Screening frequency – From onset of screening (>21 - 29): Paps biennially – Age > 30 • Paps every 2 to 3 years if 3 consecutive normal Paps • Pap and HPV test no more often than every 3 years if done together and both normal (negative)
ACOG Guidelines - Screening frequency – From onset of screening (>21 - 29): Paps biennially – Age > 30 • Paps every 2 to 3 years if 3 consecutive normal Paps • Pap and HPV test no more often than every 3 years if done together and both normal (negative)
 ACOG Practice Bulletin 109 upgraded recommendations for HPV co-testing n Women 30+ years with both (-) Pap test and (-) HR HPV test results are at extremely low risk of developing CIN 2+ during the next 4– 6 years. n HPV co-testing for women 30 and older has now been upgraded to a Level A Recommendation n Seven studies were analyzed to compare HPV and Pap co-testing versus Pap alone in a total of 24, 295 women. q The risk of CIN 3+ after 6 years of follow-up was 0. 28% in women who tested (-) on co-testing at baseline. This rate for co-testing was considerably lower than for women with (-) Pap results at 0. 97%. ACOG Practice Bulletin, Number 109. Obstet Gynecol. 2009; 114: 1409 -1420.
ACOG Practice Bulletin 109 upgraded recommendations for HPV co-testing n Women 30+ years with both (-) Pap test and (-) HR HPV test results are at extremely low risk of developing CIN 2+ during the next 4– 6 years. n HPV co-testing for women 30 and older has now been upgraded to a Level A Recommendation n Seven studies were analyzed to compare HPV and Pap co-testing versus Pap alone in a total of 24, 295 women. q The risk of CIN 3+ after 6 years of follow-up was 0. 28% in women who tested (-) on co-testing at baseline. This rate for co-testing was considerably lower than for women with (-) Pap results at 0. 97%. ACOG Practice Bulletin, Number 109. Obstet Gynecol. 2009; 114: 1409 -1420.
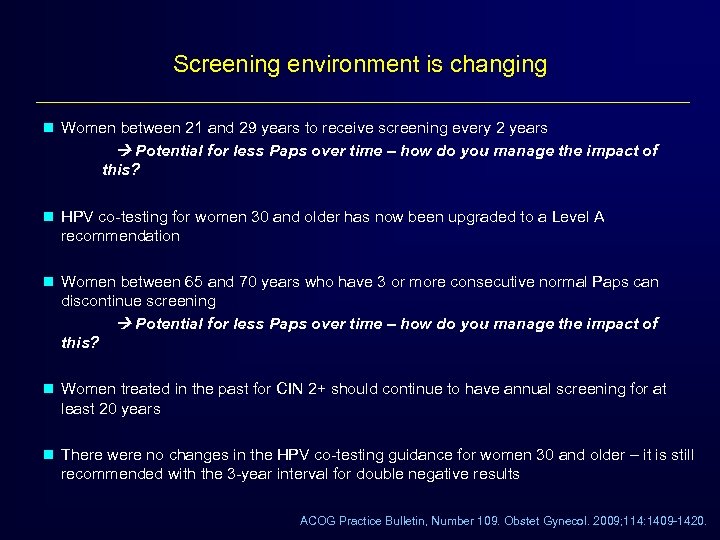 Screening environment is changing n Women between 21 and 29 years to receive screening every 2 years Potential for less Paps over time – how do you manage the impact of this? n HPV co-testing for women 30 and older has now been upgraded to a Level A recommendation n Women between 65 and 70 years who have 3 or more consecutive normal Paps can discontinue screening Potential for less Paps over time – how do you manage the impact of this? n Women treated in the past for CIN 2+ should continue to have annual screening for at least 20 years n There were no changes in the HPV co-testing guidance for women 30 and older – it is still recommended with the 3 -year interval for double negative results ACOG Practice Bulletin, Number 109. Obstet Gynecol. 2009; 114: 1409 -1420.
Screening environment is changing n Women between 21 and 29 years to receive screening every 2 years Potential for less Paps over time – how do you manage the impact of this? n HPV co-testing for women 30 and older has now been upgraded to a Level A recommendation n Women between 65 and 70 years who have 3 or more consecutive normal Paps can discontinue screening Potential for less Paps over time – how do you manage the impact of this? n Women treated in the past for CIN 2+ should continue to have annual screening for at least 20 years n There were no changes in the HPV co-testing guidance for women 30 and older – it is still recommended with the 3 -year interval for double negative results ACOG Practice Bulletin, Number 109. Obstet Gynecol. 2009; 114: 1409 -1420.
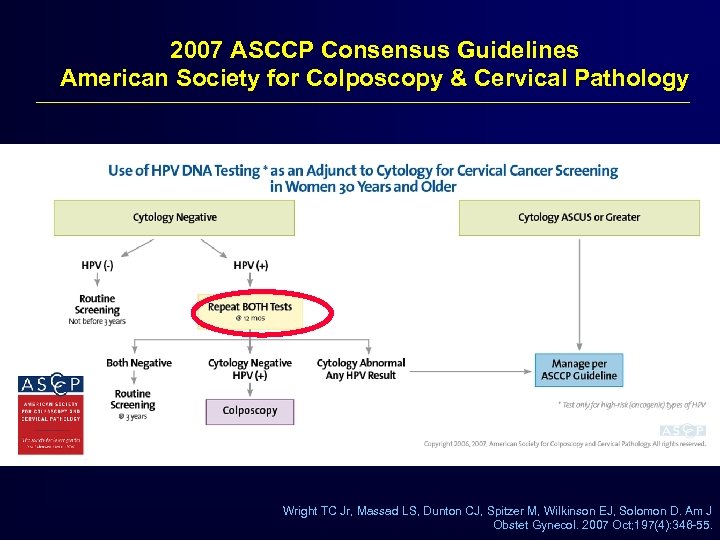 2007 ASCCP Consensus Guidelines American Society for Colposcopy & Cervical Pathology Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. Am J Obstet Gynecol. 2007 Oct; 197(4): 346 -55.
2007 ASCCP Consensus Guidelines American Society for Colposcopy & Cervical Pathology Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. Am J Obstet Gynecol. 2007 Oct; 197(4): 346 -55.
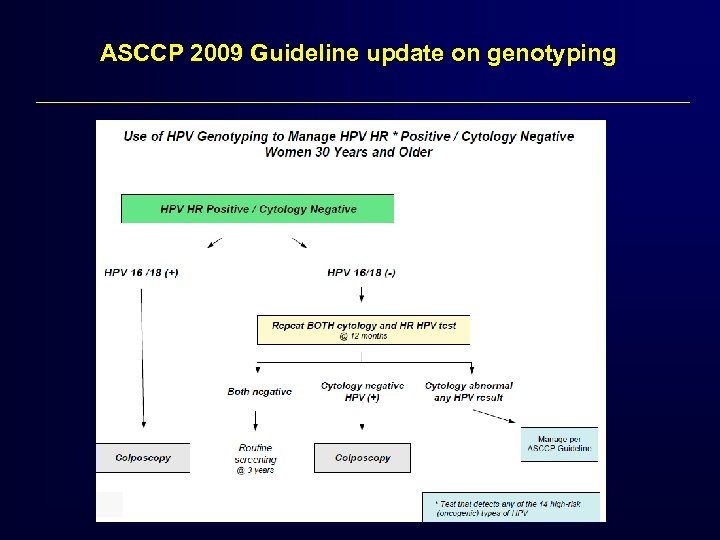 ASCCP 2009 Guideline update on genotyping
ASCCP 2009 Guideline update on genotyping
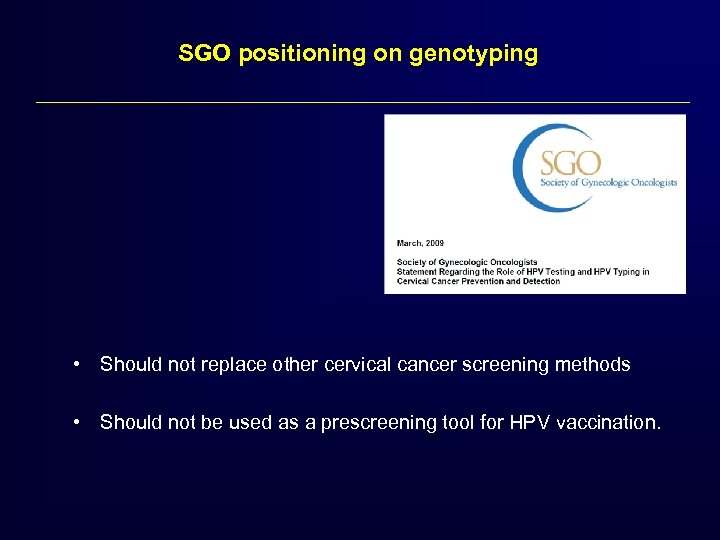 SGO positioning on genotyping • Should not replace other cervical cancer screening methods • Should not be used as a prescreening tool for HPV vaccination.
SGO positioning on genotyping • Should not replace other cervical cancer screening methods • Should not be used as a prescreening tool for HPV vaccination.
 HPV testing as predictor of a woman’s risk for developing cervical disease & cost to society Screening is all about risk. So what is the risk?
HPV testing as predictor of a woman’s risk for developing cervical disease & cost to society Screening is all about risk. So what is the risk?
 HPV Testing for Screening A risk stratifier § A normal Pap and a negative HPV test give a 99 -100% assurance that cervical cancer is not present and will not likely occur in the next few years. Providing increased safety with less frequent screening § A positive HPV test and a normal Pap reflects increased risk for either missed disease or for the subsequent development of CIN 2/3 and cancer. Requiring increased surveillance
HPV Testing for Screening A risk stratifier § A normal Pap and a negative HPV test give a 99 -100% assurance that cervical cancer is not present and will not likely occur in the next few years. Providing increased safety with less frequent screening § A positive HPV test and a normal Pap reflects increased risk for either missed disease or for the subsequent development of CIN 2/3 and cancer. Requiring increased surveillance
 Considering co-testing your patients? Common questions § What is the rationale? § Will I be overloaded with counseling HPV (+) women? § HPV positive - Pap negative. How many are there? § HPV positive - Pap negative. Probability of HPV infection regressing § What does HPV positive / Pap negative mean?
Considering co-testing your patients? Common questions § What is the rationale? § Will I be overloaded with counseling HPV (+) women? § HPV positive - Pap negative. How many are there? § HPV positive - Pap negative. Probability of HPV infection regressing § What does HPV positive / Pap negative mean?
 What is the rationale for combined screening? § “. . persistent, high-risk HPV infection is necessary for the development of cervical cancer” § “. . an obvious corollary is that the absence of HPV means that the risk of cervical cancer is negligible. ” Wright TC, Schiffman MH NEJM 2003; 348: 489 -90
What is the rationale for combined screening? § “. . persistent, high-risk HPV infection is necessary for the development of cervical cancer” § “. . an obvious corollary is that the absence of HPV means that the risk of cervical cancer is negligible. ” Wright TC, Schiffman MH NEJM 2003; 348: 489 -90
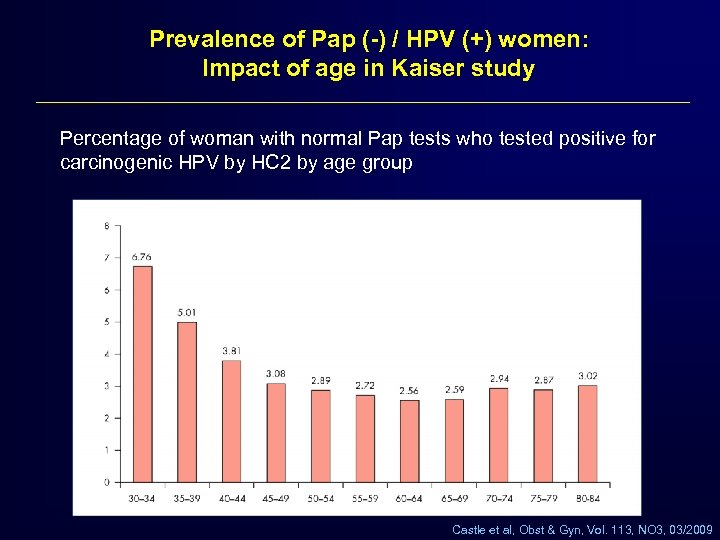 Prevalence of Pap (-) / HPV (+) women: Impact of age in Kaiser study Percentage of woman with normal Pap tests who tested positive for carcinogenic HPV by HC 2 by age group Castle et al, Obst & Gyn, Vol. 113, NO 3, 03/2009
Prevalence of Pap (-) / HPV (+) women: Impact of age in Kaiser study Percentage of woman with normal Pap tests who tested positive for carcinogenic HPV by HC 2 by age group Castle et al, Obst & Gyn, Vol. 113, NO 3, 03/2009
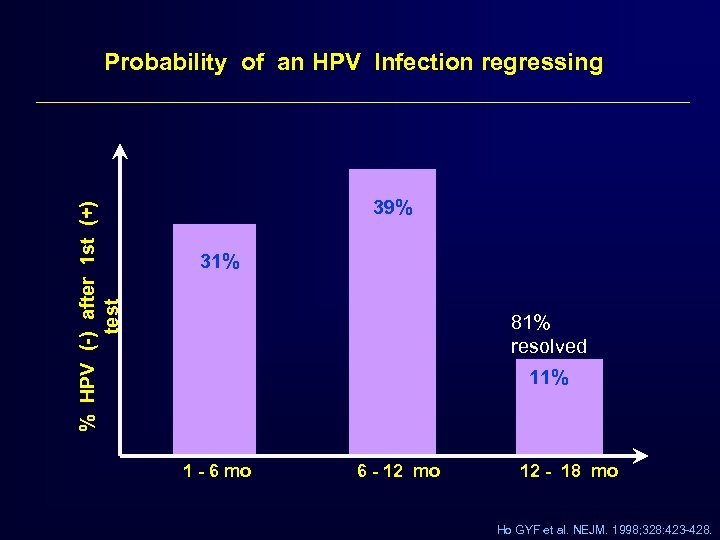 % HPV (-) after 1 st (+) test Probability of an HPV Infection regressing 39% 31% 81% resolved 11% 1 - 6 mo 6 - 12 mo 12 - 18 mo Ho GYF et al. NEJM. 1998; 328: 423 -428.
% HPV (-) after 1 st (+) test Probability of an HPV Infection regressing 39% 31% 81% resolved 11% 1 - 6 mo 6 - 12 mo 12 - 18 mo Ho GYF et al. NEJM. 1998; 328: 423 -428.
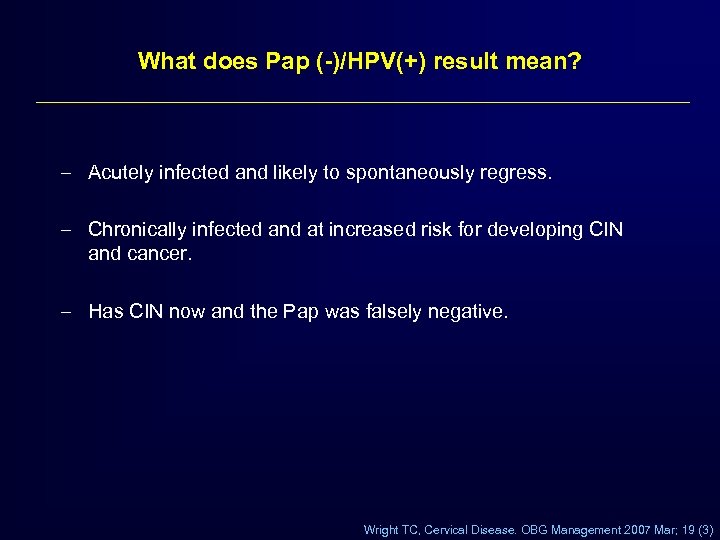 What does Pap (-)/HPV(+) result mean? – Acutely infected and likely to spontaneously regress. – Chronically infected and at increased risk for developing CIN and cancer. – Has CIN now and the Pap was falsely negative. Wright TC, Cervical Disease. OBG Management 2007 Mar; 19 (3)
What does Pap (-)/HPV(+) result mean? – Acutely infected and likely to spontaneously regress. – Chronically infected and at increased risk for developing CIN and cancer. – Has CIN now and the Pap was falsely negative. Wright TC, Cervical Disease. OBG Management 2007 Mar; 19 (3)
 What do patients and providers want to know about cervical cancer screening reimbursement?
What do patients and providers want to know about cervical cancer screening reimbursement?
 Provider concerns with co-testing Extended screening intervals • Disconnect “the Pap” from the “annual exam” • The Pap is just one test done at intervals now more tailored to the individual’s needs • Women still need annual preventive health care › Contraception › Breast and pelvic exam › Screening for STDs as needed › Cardiovascular, diabetes and other risk assessment › Bone density, peri- & postmenopausal concerns
Provider concerns with co-testing Extended screening intervals • Disconnect “the Pap” from the “annual exam” • The Pap is just one test done at intervals now more tailored to the individual’s needs • Women still need annual preventive health care › Contraception › Breast and pelvic exam › Screening for STDs as needed › Cardiovascular, diabetes and other risk assessment › Bone density, peri- & postmenopausal concerns
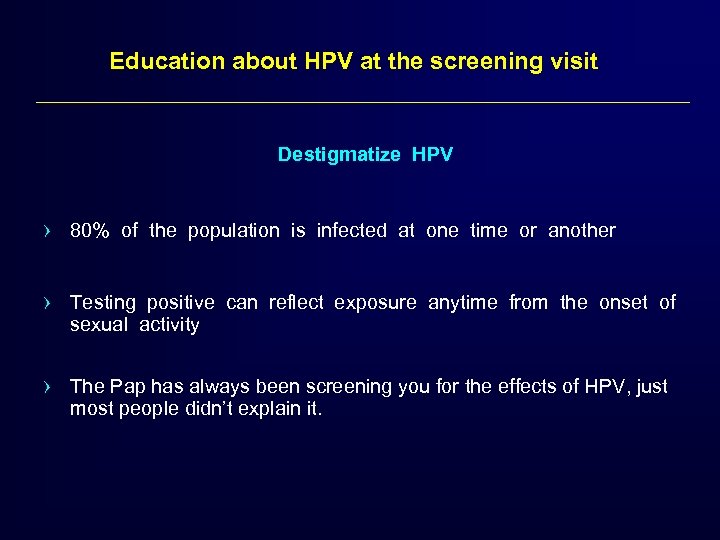 Education about HPV at the screening visit Destigmatize HPV › 80% of the population is infected at one time or another › Testing positive can reflect exposure anytime from the onset of sexual activity › The Pap has always been screening you for the effects of HPV, just most people didn’t explain it.
Education about HPV at the screening visit Destigmatize HPV › 80% of the population is infected at one time or another › Testing positive can reflect exposure anytime from the onset of sexual activity › The Pap has always been screening you for the effects of HPV, just most people didn’t explain it.
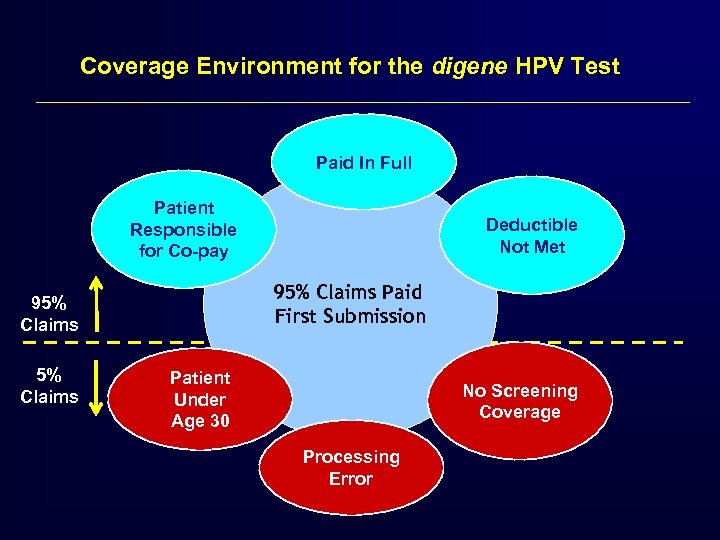 Coverage Environment for the digene HPV Test Paid In Full Patient Responsible for Co-pay 95% Claims Paid First Submission 95% Claims Deductible Not Met Patient Under Age 30 No Screening Coverage Processing Error
Coverage Environment for the digene HPV Test Paid In Full Patient Responsible for Co-pay 95% Claims Paid First Submission 95% Claims Deductible Not Met Patient Under Age 30 No Screening Coverage Processing Error
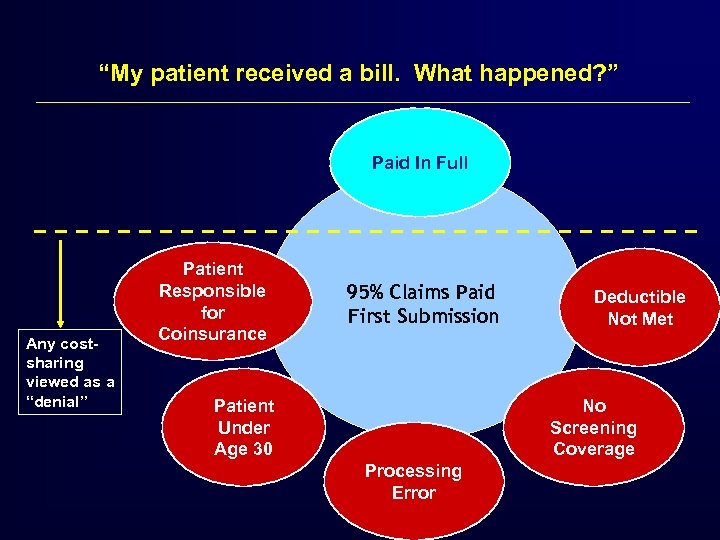 “My patient received a bill. What happened? ” Paid In Full Any costsharing viewed as a “denial” Patient Responsible for Coinsurance 95% Claims Paid First Submission Patient Under Age 30 Deductible Not Met No Screening Coverage Processing Error
“My patient received a bill. What happened? ” Paid In Full Any costsharing viewed as a “denial” Patient Responsible for Coinsurance 95% Claims Paid First Submission Patient Under Age 30 Deductible Not Met No Screening Coverage Processing Error
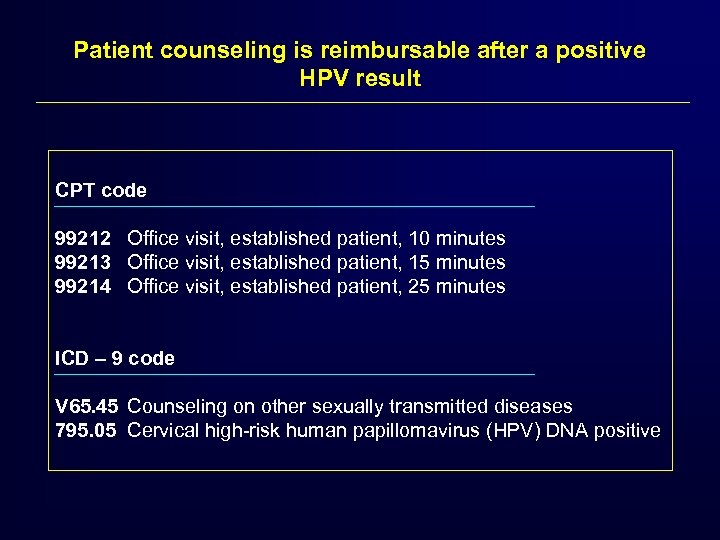 Patient counseling is reimbursable after a positive HPV result CPT code 99212 Office visit, established patient, 10 minutes 99213 Office visit, established patient, 15 minutes 99214 Office visit, established patient, 25 minutes ICD – 9 code V 65. 45 Counseling on other sexually transmitted diseases 795. 05 Cervical high-risk human papillomavirus (HPV) DNA positive
Patient counseling is reimbursable after a positive HPV result CPT code 99212 Office visit, established patient, 10 minutes 99213 Office visit, established patient, 15 minutes 99214 Office visit, established patient, 25 minutes ICD – 9 code V 65. 45 Counseling on other sexually transmitted diseases 795. 05 Cervical high-risk human papillomavirus (HPV) DNA positive
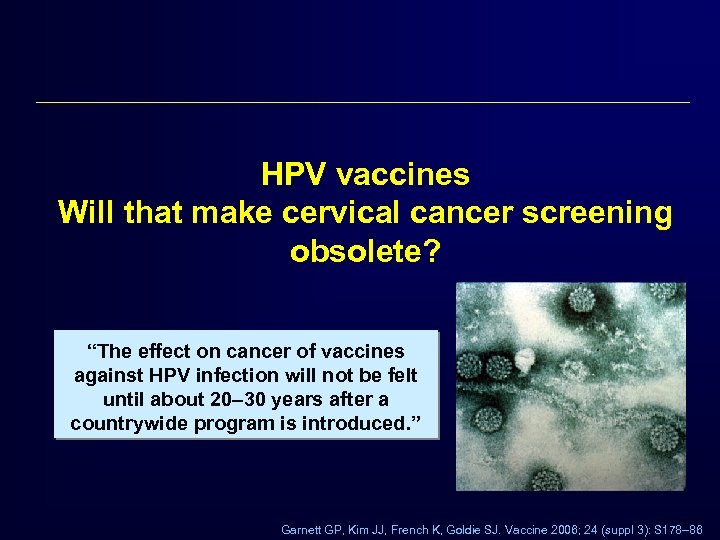 HPV vaccines Will that make cervical cancer screening obsolete? “The effect on cancer of vaccines against HPV infection will not be felt until about 20– 30 years after a countrywide program is introduced. ” Garnett GP, Kim JJ, French K, Goldie SJ. Vaccine 2006; 24 (suppl 3): S 178– 86
HPV vaccines Will that make cervical cancer screening obsolete? “The effect on cancer of vaccines against HPV infection will not be felt until about 20– 30 years after a countrywide program is introduced. ” Garnett GP, Kim JJ, French K, Goldie SJ. Vaccine 2006; 24 (suppl 3): S 178– 86
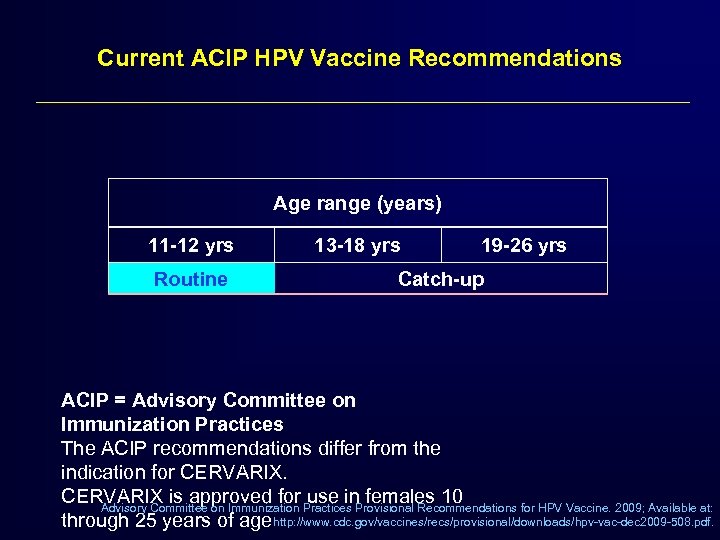 Current ACIP HPV Vaccine Recommendations Age range (years) 11 -12 yrs Routine 13 -18 yrs 19 -26 yrs Catch-up ACIP = Advisory Committee on Immunization Practices The ACIP recommendations differ from the indication for CERVARIX is approved for Practices Provisional Recommendations for HPV Vaccine. 2009; Available at: use in females 10 Advisory Committee on Immunization through 25 years of age http: //www. cdc. gov/vaccines/recs/provisional/downloads/hpv-vac-dec 2009 -508. pdf.
Current ACIP HPV Vaccine Recommendations Age range (years) 11 -12 yrs Routine 13 -18 yrs 19 -26 yrs Catch-up ACIP = Advisory Committee on Immunization Practices The ACIP recommendations differ from the indication for CERVARIX is approved for Practices Provisional Recommendations for HPV Vaccine. 2009; Available at: use in females 10 Advisory Committee on Immunization through 25 years of age http: //www. cdc. gov/vaccines/recs/provisional/downloads/hpv-vac-dec 2009 -508. pdf.
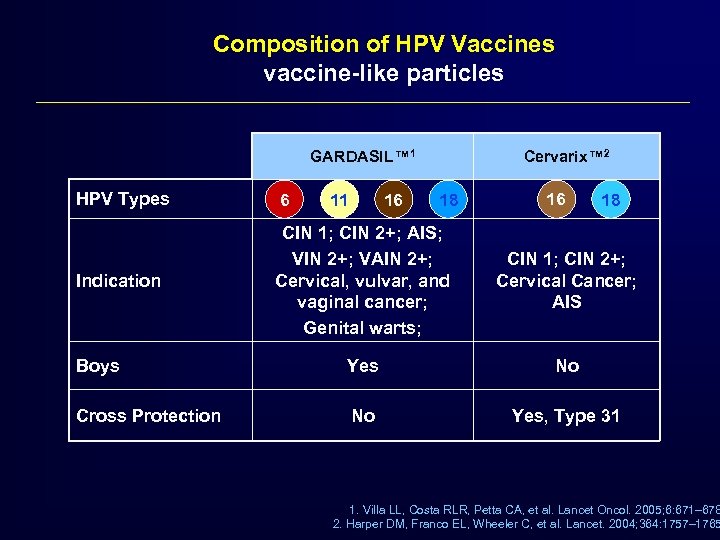 Composition of HPV Vaccines vaccine-like particles GARDASIL™ 1 Cervarix™ 2 HPV Types 6 Indication CIN 1; CIN 2+; AIS; VIN 2+; VAIN 2+; Cervical, vulvar, and vaginal cancer; Genital warts; CIN 1; CIN 2+; Cervical Cancer; AIS Boys Yes No Cross Protection No Yes, Type 31 11 16 18 1. Villa LL, Costa RLR, Petta CA, et al. Lancet Oncol. 2005; 6: 671– 678 2. Harper DM, Franco EL, Wheeler C, et al. Lancet. 2004; 364: 1757– 1765
Composition of HPV Vaccines vaccine-like particles GARDASIL™ 1 Cervarix™ 2 HPV Types 6 Indication CIN 1; CIN 2+; AIS; VIN 2+; VAIN 2+; Cervical, vulvar, and vaginal cancer; Genital warts; CIN 1; CIN 2+; Cervical Cancer; AIS Boys Yes No Cross Protection No Yes, Type 31 11 16 18 1. Villa LL, Costa RLR, Petta CA, et al. Lancet Oncol. 2005; 6: 671– 678 2. Harper DM, Franco EL, Wheeler C, et al. Lancet. 2004; 364: 1757– 1765
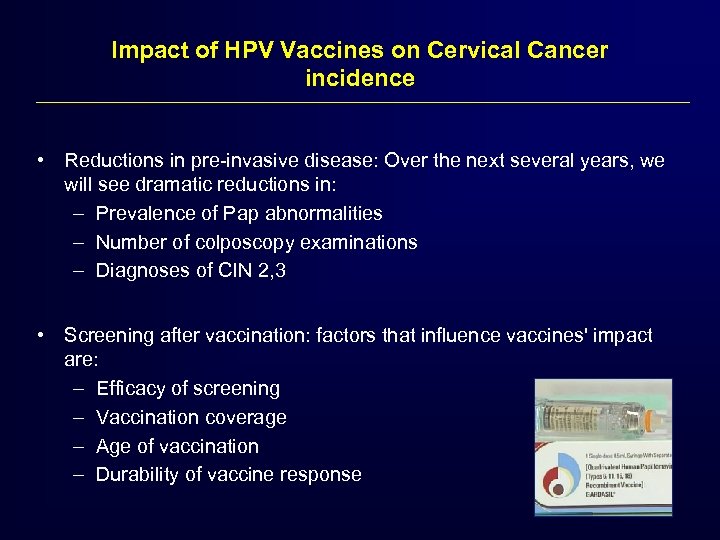 Impact of HPV Vaccines on Cervical Cancer incidence • Reductions in pre-invasive disease: Over the next several years, we will see dramatic reductions in: – Prevalence of Pap abnormalities – Number of colposcopy examinations – Diagnoses of CIN 2, 3 • Screening after vaccination: factors that influence vaccines' impact are: – Efficacy of screening – Vaccination coverage – Age of vaccination – Durability of vaccine response
Impact of HPV Vaccines on Cervical Cancer incidence • Reductions in pre-invasive disease: Over the next several years, we will see dramatic reductions in: – Prevalence of Pap abnormalities – Number of colposcopy examinations – Diagnoses of CIN 2, 3 • Screening after vaccination: factors that influence vaccines' impact are: – Efficacy of screening – Vaccination coverage – Age of vaccination – Durability of vaccine response
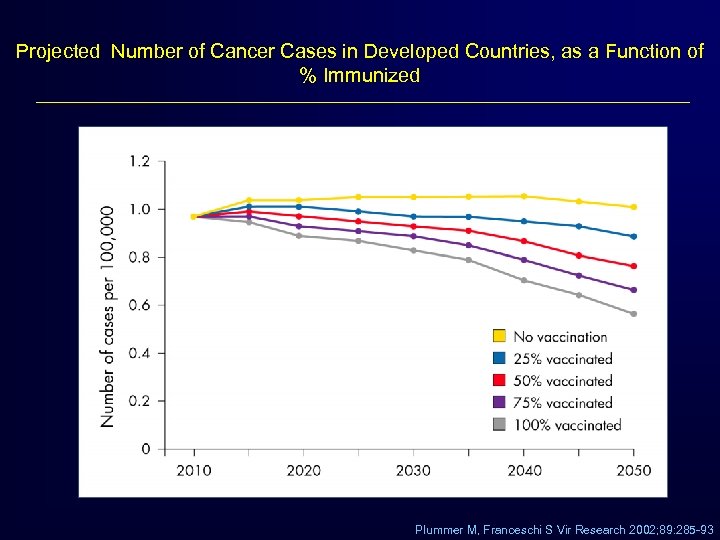 Projected Number of Cancer Cases in Developed Countries, as a Function of % Immunized Plummer M, Franceschi S Vir Research 2002; 89: 285 -93
Projected Number of Cancer Cases in Developed Countries, as a Function of % Immunized Plummer M, Franceschi S Vir Research 2002; 89: 285 -93
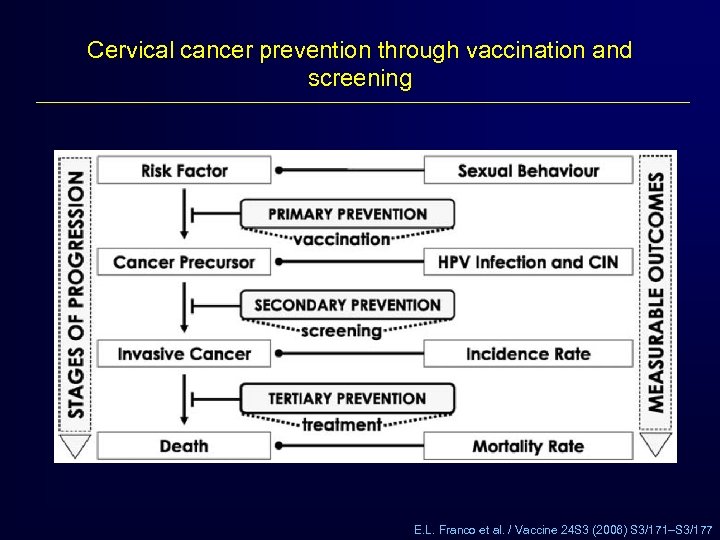 Cervical cancer prevention through vaccination and screening E. L. Franco et al. / Vaccine 24 S 3 (2006) S 3/171–S 3/177
Cervical cancer prevention through vaccination and screening E. L. Franco et al. / Vaccine 24 S 3 (2006) S 3/171–S 3/177
 Are other HPV tests clinically better?
Are other HPV tests clinically better?
 CIN 2 is the clinical threshold for patient treatment co-testing allows to detect more women at risk. “In a well screened population, the risk of CIN 2+ in HPV positive, cytology negative women ranges from 2. 4 to 5. 1%. ” 1 ACOG Practice Bulletin 99 (December 2008) “High-grade (grade 2 or higher) cervical intraepithelial neoplasia is the accepted end point for cervical screening and an actionable finding for clinical management of the disease. ” 2 Mayrand, M-H. , NEJM (2007) 1 - ACOG Practice Bulletin, Number 99. Obstet Gynecol 2 - Mayrand, M-H. , Duarte-Franco, E. , NEJM (2007)
CIN 2 is the clinical threshold for patient treatment co-testing allows to detect more women at risk. “In a well screened population, the risk of CIN 2+ in HPV positive, cytology negative women ranges from 2. 4 to 5. 1%. ” 1 ACOG Practice Bulletin 99 (December 2008) “High-grade (grade 2 or higher) cervical intraepithelial neoplasia is the accepted end point for cervical screening and an actionable finding for clinical management of the disease. ” 2 Mayrand, M-H. , NEJM (2007) 1 - ACOG Practice Bulletin, Number 99. Obstet Gynecol 2 - Mayrand, M-H. , Duarte-Franco, E. , NEJM (2007)
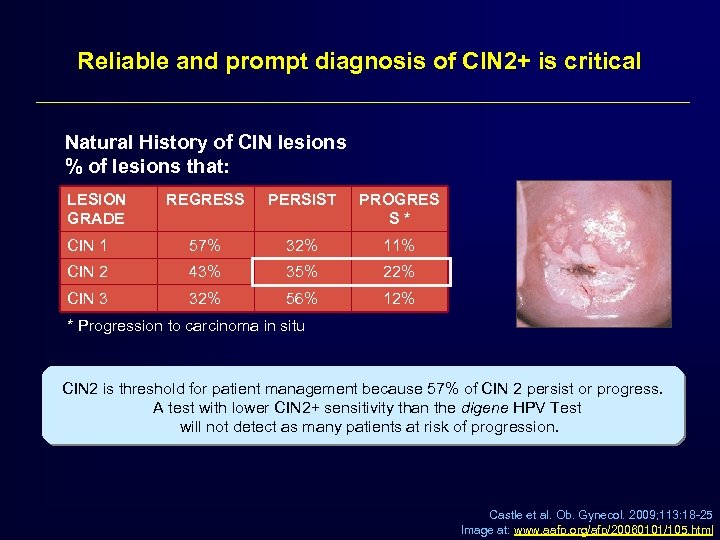 Reliable and prompt diagnosis of CIN 2+ is critical Natural History of CIN lesions % of lesions that: LESION GRADE REGRESS PERSIST PROGRES S* CIN 1 57% 32% 11% CIN 2 43% 35% 22% CIN 3 32% 56% 12% * Progression to carcinoma in situ CIN 2 is threshold for patient management because 57% of CIN 2 persist or progress. A test with lower CIN 2+ sensitivity than the digene HPV Test will not detect as many patients at risk of progression. Castle et al. Ob. Gynecol. 2009; 113: 18 -25 Image at: www. aafp. org/afp/20060101/105. html
Reliable and prompt diagnosis of CIN 2+ is critical Natural History of CIN lesions % of lesions that: LESION GRADE REGRESS PERSIST PROGRES S* CIN 1 57% 32% 11% CIN 2 43% 35% 22% CIN 3 32% 56% 12% * Progression to carcinoma in situ CIN 2 is threshold for patient management because 57% of CIN 2 persist or progress. A test with lower CIN 2+ sensitivity than the digene HPV Test will not detect as many patients at risk of progression. Castle et al. Ob. Gynecol. 2009; 113: 18 -25 Image at: www. aafp. org/afp/20060101/105. html
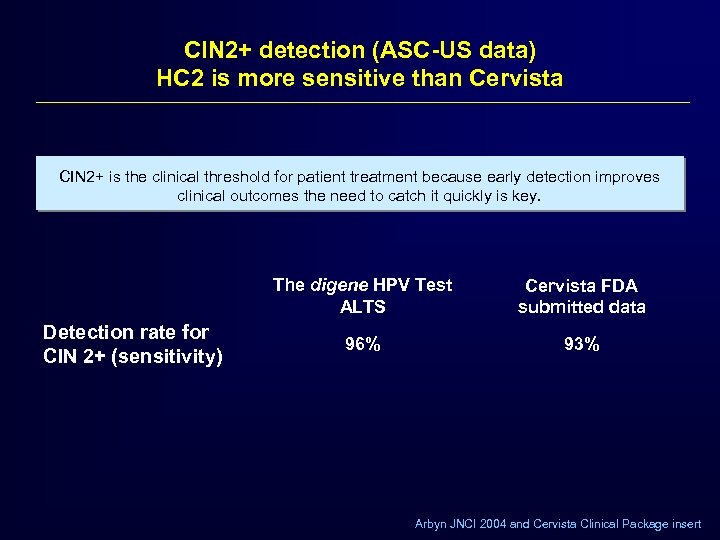 CIN 2+ detection (ASC-US data) HC 2 is more sensitive than Cervista CIN 2+ is the clinical threshold for patient treatment because early detection improves clinical outcomes the need to catch it quickly is key. The digene HPV Test ALTS Detection rate for CIN 2+ (sensitivity) Cervista FDA submitted data 96% 93% Arbyn JNCI 2004 and Cervista Clinical Package insert
CIN 2+ detection (ASC-US data) HC 2 is more sensitive than Cervista CIN 2+ is the clinical threshold for patient treatment because early detection improves clinical outcomes the need to catch it quickly is key. The digene HPV Test ALTS Detection rate for CIN 2+ (sensitivity) Cervista FDA submitted data 96% 93% Arbyn JNCI 2004 and Cervista Clinical Package insert
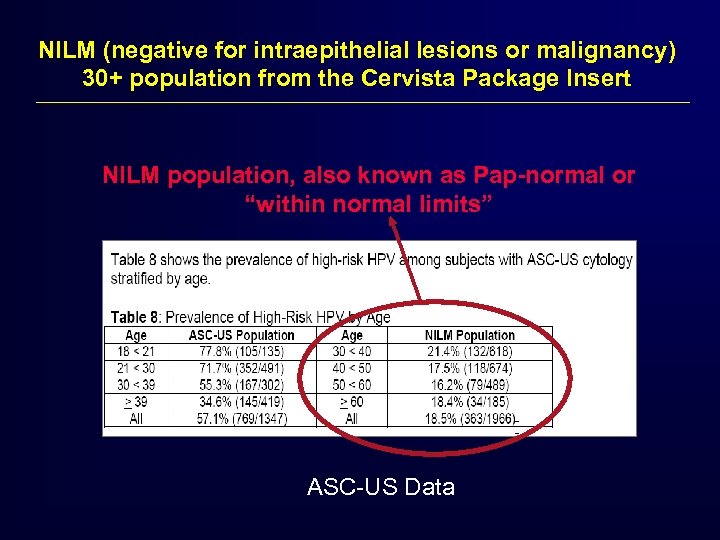 NILM (negative for intraepithelial lesions or malignancy) 30+ population from the Cervista Package Insert NILM population, also known as Pap-normal or “within normal limits” ASC-US Data
NILM (negative for intraepithelial lesions or malignancy) 30+ population from the Cervista Package Insert NILM population, also known as Pap-normal or “within normal limits” ASC-US Data
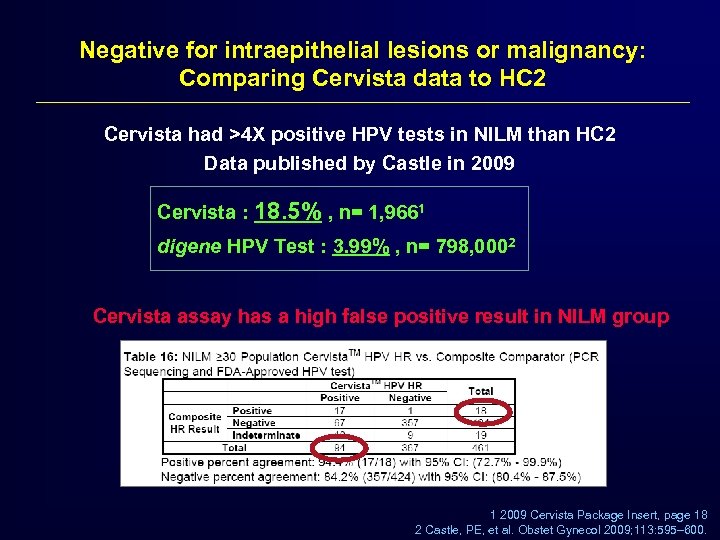 Negative for intraepithelial lesions or malignancy: Comparing Cervista data to HC 2 Cervista had >4 X positive HPV tests in NILM than HC 2 Data published by Castle in 2009 Cervista : 18. 5% , n= 1, 9661 digene HPV Test : 3. 99% , n= 798, 0002 Cervista assay has a high false positive result in NILM group 1 2009 Cervista Package Insert, page 18 2 Castle, PE, et al. Obstet Gynecol 2009; 113: 595– 600.
Negative for intraepithelial lesions or malignancy: Comparing Cervista data to HC 2 Cervista had >4 X positive HPV tests in NILM than HC 2 Data published by Castle in 2009 Cervista : 18. 5% , n= 1, 9661 digene HPV Test : 3. 99% , n= 798, 0002 Cervista assay has a high false positive result in NILM group 1 2009 Cervista Package Insert, page 18 2 Castle, PE, et al. Obstet Gynecol 2009; 113: 595– 600.
 Impact of increased Pap (-) / HPV (+) Cervista Assay Significant increase in Pap (-) / HPV (+) rate will increase burden of patient counseling § NILM is most relevant for the Ob-Gyn or FP who currently co-tests § In addition to high false positives, the Cervista assay also has higher false negatives in the CIN 2+, ASC-US population § No co-testing data on 30+ yet available for Cervista
Impact of increased Pap (-) / HPV (+) Cervista Assay Significant increase in Pap (-) / HPV (+) rate will increase burden of patient counseling § NILM is most relevant for the Ob-Gyn or FP who currently co-tests § In addition to high false positives, the Cervista assay also has higher false negatives in the CIN 2+, ASC-US population § No co-testing data on 30+ yet available for Cervista
 Non FDA approved tests using PCR technology What are the benefits of PCR tests and limitations?
Non FDA approved tests using PCR technology What are the benefits of PCR tests and limitations?
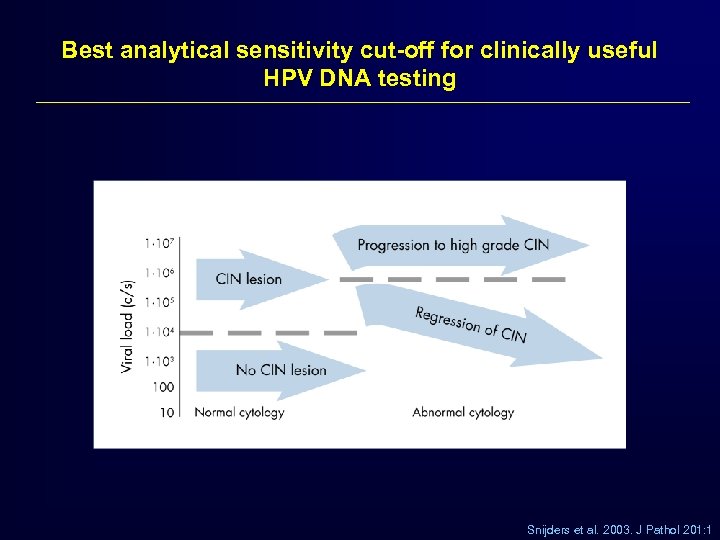 Best analytical sensitivity cut-off for clinically useful HPV DNA testing Snijders et al. 2003. J Pathol 201: 1
Best analytical sensitivity cut-off for clinically useful HPV DNA testing Snijders et al. 2003. J Pathol 201: 1
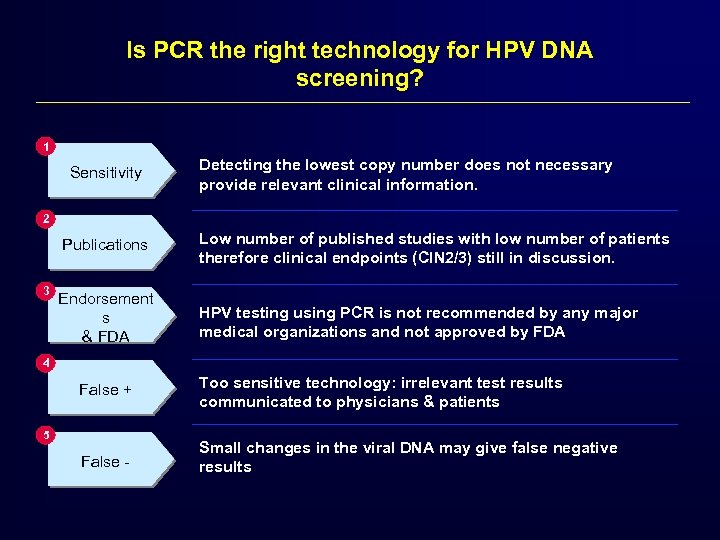 Is PCR the right technology for HPV DNA screening? 1 Sensitivity Detecting the lowest copy number does not necessary provide relevant clinical information. 2 Publications 3 Low number of published studies with low number of patients therefore clinical endpoints (CIN 2/3) still in discussion. Endorsement s & FDA HPV testing using PCR is not recommended by any major medical organizations and not approved by FDA 4 False + Too sensitive technology: irrelevant test results communicated to physicians & patients False - Small changes in the viral DNA may give false negative results 5
Is PCR the right technology for HPV DNA screening? 1 Sensitivity Detecting the lowest copy number does not necessary provide relevant clinical information. 2 Publications 3 Low number of published studies with low number of patients therefore clinical endpoints (CIN 2/3) still in discussion. Endorsement s & FDA HPV testing using PCR is not recommended by any major medical organizations and not approved by FDA 4 False + Too sensitive technology: irrelevant test results communicated to physicians & patients False - Small changes in the viral DNA may give false negative results 5
 Definition of an analytically and clinically validated HPV test for clinical implementation – The test should be capable of detecting at least the 13 high risk HPV types and should not include non-carcinogenic HPV types. – To meet currently achievable standards, the test should have a clinical sensitivity to detect at least 92% ± 3% of CIN 3+ such that the negative predictive value of the test is extremely high.
Definition of an analytically and clinically validated HPV test for clinical implementation – The test should be capable of detecting at least the 13 high risk HPV types and should not include non-carcinogenic HPV types. – To meet currently achievable standards, the test should have a clinical sensitivity to detect at least 92% ± 3% of CIN 3+ such that the negative predictive value of the test is extremely high.
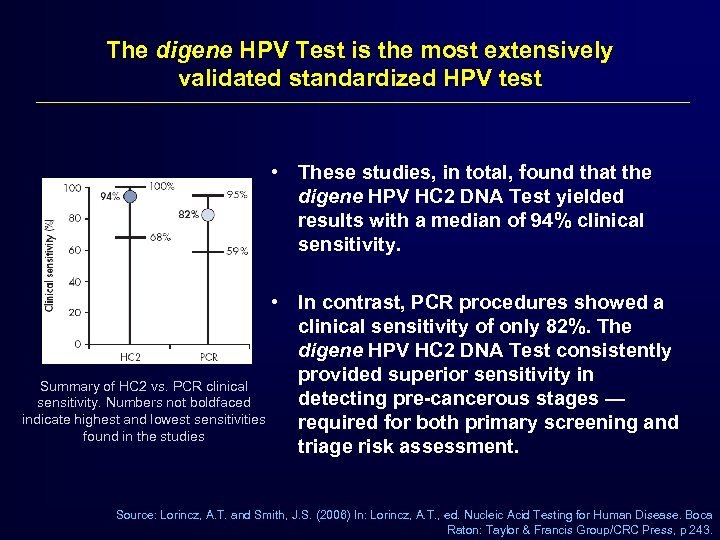 The digene HPV Test is the most extensively validated standardized HPV test • These studies, in total, found that the digene HPV HC 2 DNA Test yielded results with a median of 94% clinical sensitivity. • In contrast, PCR procedures showed a clinical sensitivity of only 82%. The digene HPV HC 2 DNA Test consistently provided superior sensitivity in Summary of HC 2 vs. PCR clinical detecting pre-cancerous stages — sensitivity. Numbers not boldfaced indicate highest and lowest sensitivities required for both primary screening and found in the studies triage risk assessment. Source: Lorincz, A. T. and Smith, J. S. (2006) In: Lorincz, A. T. , ed. Nucleic Acid Testing for Human Disease. Boca Raton: Taylor & Francis Group/CRC Press, p 243.
The digene HPV Test is the most extensively validated standardized HPV test • These studies, in total, found that the digene HPV HC 2 DNA Test yielded results with a median of 94% clinical sensitivity. • In contrast, PCR procedures showed a clinical sensitivity of only 82%. The digene HPV HC 2 DNA Test consistently provided superior sensitivity in Summary of HC 2 vs. PCR clinical detecting pre-cancerous stages — sensitivity. Numbers not boldfaced indicate highest and lowest sensitivities required for both primary screening and found in the studies triage risk assessment. Source: Lorincz, A. T. and Smith, J. S. (2006) In: Lorincz, A. T. , ed. Nucleic Acid Testing for Human Disease. Boca Raton: Taylor & Francis Group/CRC Press, p 243.
 Thank you
Thank you


