227b2f461920fb51e33a4dd0c5589e59.ppt
- Количество слайдов: 25

Late Breaking Clinical Trials – ACC 2011 PROTECTION AMI Inhibition of d-Protein Kinase C for Reduction of Infarct Size in Acute Myocardial Infarction A. Michael Lincoff, M. D. Director, C 5 Research (Cleveland Clinic Coordinating Center for Clinical Research) Vice Chairman for Clinical Research, Lerner Research Institute Vice Chairman of Cardiovascular Medicine Professor of Medicine Heart and Vascular Institute

Speaker Disclosure – A. Michael Lincoff, MD Relationships with Industry Research Sponsors · · · · Anthera Astra. Zeneca Atherosys Bristol-Myers Squibb (BMS) Centocor Cordis Guidant Heartscape J&J KAI Pharma Lilly Mannkind Medicines Company · · · Medtronic Novartis Novo Nordisk Pfizer Resverlogix Roche / Genentech Sankyo Sanofi-Aventis Schering-Plough Scios Takeda Vaso. Genix Consultant · · · · Schering-Plough Bioline BMS Merck Baxter Aztra Zeneca Roche

d. PKC in Ischemia-Reperfusion Injury d. PKC Activation in Response to IR Injury § activated in a variety of human, rodent and pig cells exposed to ischemic conditions in vitro and in vivo § during reperfusion, activated d. PKC translocates to the mitochondria and mediates necrosis and apoptosis § d. PKC KO mice have reduced free radical production from endothelial cells and decreased damage following cardiac ischemia, and reduced infarct size after stroke.
Delcasertib Peptide Inhibitor of PKC Localization - Selective Inhibitor • disrupts binding of activated d. PKC with its RACK (Receptor for Activated C-Kinase) • reaches steady state within 5 - 30 minutes after the start of infusion, terminal T 1/2 of 2 to 5 minutes • well-tolerated across range of doses Animal Models: • reduced infarct size, myocyte and endothelial cellular damage • enhanced recovery of myocardial metabolic activity and regional LV function • improved infarct zone microvascular flow Ikeno F et al. Cardiovasc Res 2007; 73: 699 -709
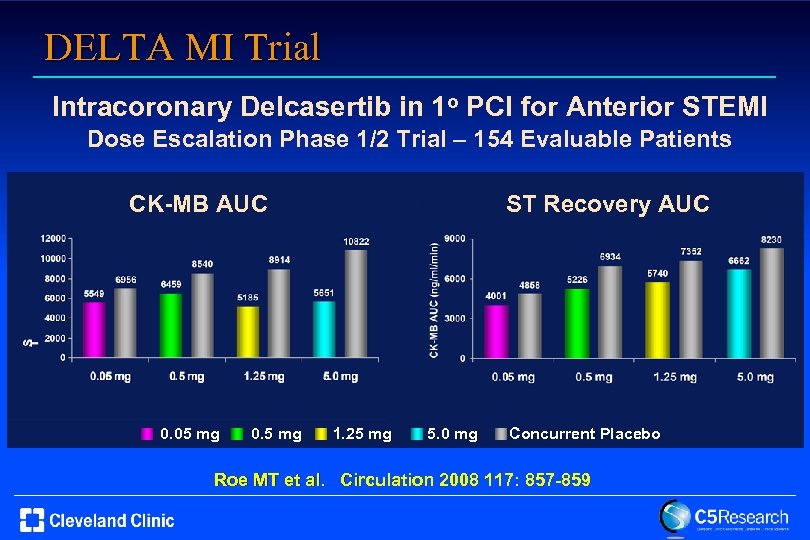
DELTA MI Trial Intracoronary Delcasertib in 1 o PCI for Anterior STEMI Dose Escalation Phase 1/2 Trial – 154 Evaluable Patients CK-MB AUC 0. 05 mg 0. 5 mg ST Recovery AUC 1. 25 mg 5. 0 mg Concurrent Placebo Roe MT et al. Circulation 2008 117: 857 -859
Delcasertib in STEMI Inhibition of δ-PROTEin kinase C for the reduc. TION of infarct size in Acute Myocardial Infarction (PROTECTION AMI) Study Hypothesis: intravenous administration of delcasertib will reduce infarct size in subjects with anterior ST elevation myocardial infarction (STEMI) undergoing primary PCI. § international, multi-center Phase 2 b trial § randomized, placebo-controlled, double-blind, parallel group § acute STEMI subjects undergoing primary PCI
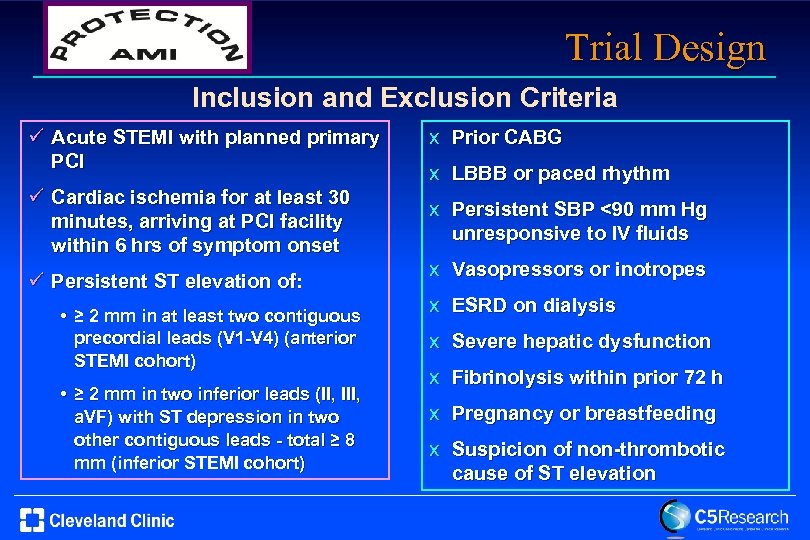
Trial Design Inclusion and Exclusion Criteria ü Acute STEMI with planned primary PCI ü Cardiac ischemia for at least 30 minutes, arriving at PCI facility within 6 hrs of symptom onset ü Persistent ST elevation of: • ≥ 2 mm in at least two contiguous precordial leads (V 1 -V 4) (anterior STEMI cohort) • ≥ 2 mm in two inferior leads (II, III, a. VF) with ST depression in two other contiguous leads - total ≥ 8 mm (inferior STEMI cohort) x Prior CABG x LBBB or paced rhythm x Persistent SBP <90 mm Hg unresponsive to IV fluids x Vasopressors or inotropes x ESRD on dialysis x Severe hepatic dysfunction x Fibrinolysis within prior 72 h x Pregnancy or breastfeeding x Suspicion of non-thrombotic cause of ST elevation
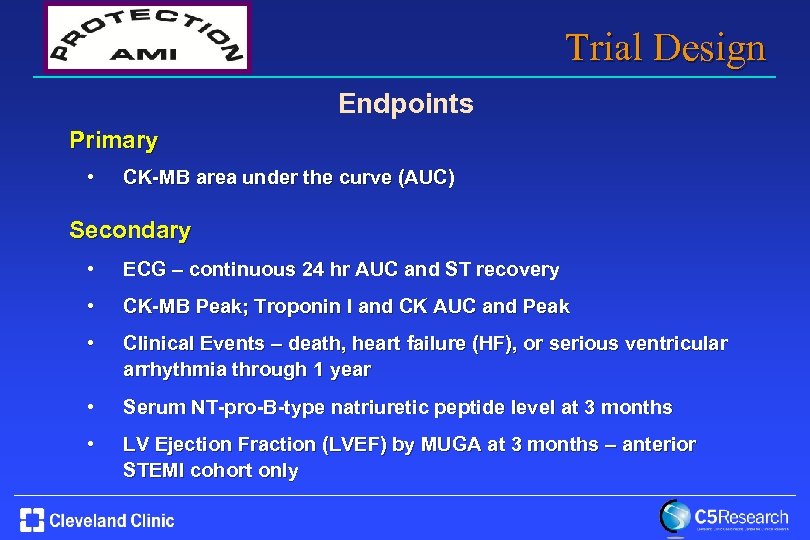
Trial Design Endpoints Primary • CK-MB area under the curve (AUC) Secondary • ECG – continuous 24 hr AUC and ST recovery • CK-MB Peak; Troponin I and CK AUC and Peak • Clinical Events – death, heart failure (HF), or serious ventricular arrhythmia through 1 year • Serum NT-pro-B-type natriuretic peptide level at 3 months • LV Ejection Fraction (LVEF) by MUGA at 3 months – anterior STEMI cohort only
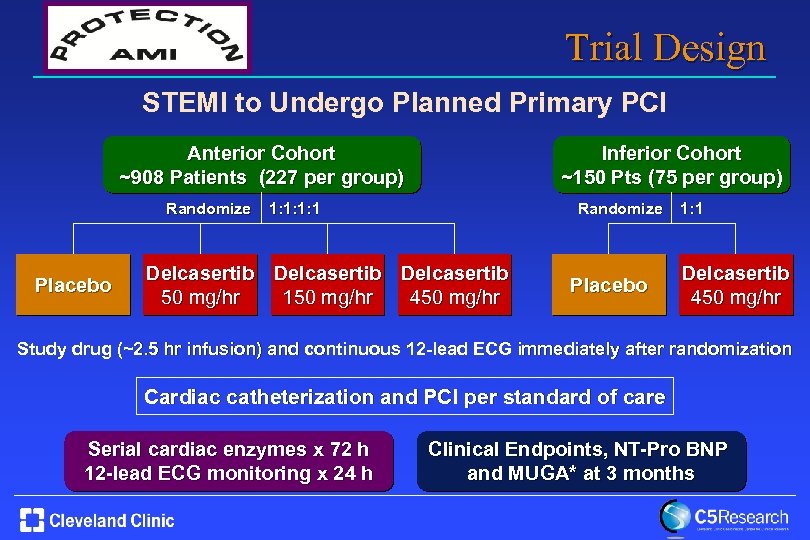
Trial Design STEMI to Undergo Planned Primary PCI Anterior Cohort ~908 Patients (227 per group) Randomize Placebo Inferior Cohort ~150 Pts (75 per group) Randomize 1: 1: 1: 1 Delcasertib 50 mg/hr 150 mg/hr 450 mg/hr Placebo 1: 1 Delcasertib 450 mg/hr Study drug (~2. 5 hr infusion) and continuous 12 -lead ECG immediately after randomization Cardiac catheterization and PCI per standard of care Serial cardiac enzymes x 72 h 12 -lead ECG monitoring x 24 h Clinical Endpoints, NT-Pro BNP and MUGA* at 3 months
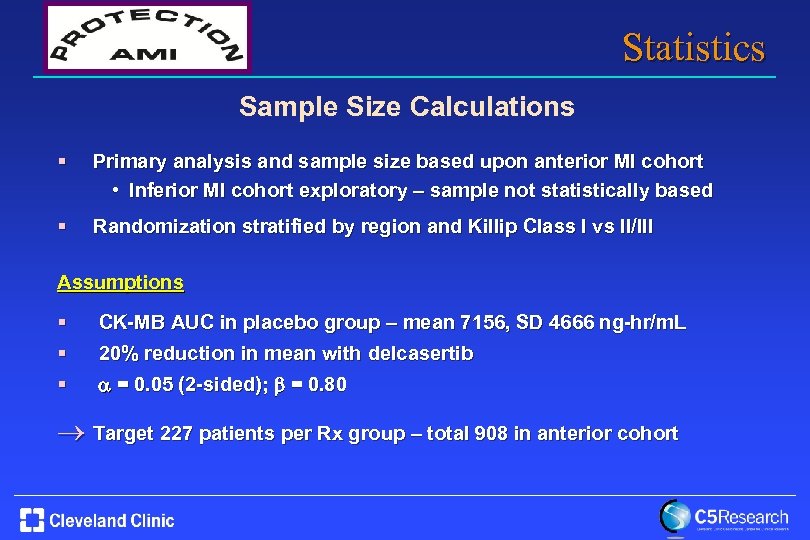
Statistics Sample Size Calculations § Primary analysis and sample size based upon anterior MI cohort • Inferior MI cohort exploratory – sample not statistically based § Randomization stratified by region and Killip Class I vs II/III Assumptions § § CK-MB AUC in placebo group – mean 7156, SD 4666 ng-hr/m. L § a = 0. 05 (2 -sided); b = 0. 80 20% reduction in mean with delcasertib ® Target 227 patients per Rx group – total 908 in anterior cohort

Academic Leadership Steering Committee / National Coordinators A. Michael Lincoff (PI) Mitchell Krucoff (Co-PI) Operations Matthew Roe, USA Committee John Galla, USA Phil Aylward, Australia Andrezej Rynkiewcz, Poland Victor Guetta, Israel Micheal Zelizko, Czech Republic Neal Kleiman, USA Tamàs Forster, Hungary Antonio Fernández-Ortiz, Spain Harry Suryapranata, Netherlands David Erlinge, Sweden Svend Eggert Jensen, Denmark Harvey White, New Zealand Danny Schoors, Belgium Peter Radke, Germany Shamir Mehta, Canada Shaun Goodman, Canada Dan Atar, Norway Michael Laine, Finland Jorge Manuel dos Santos Ferreira, Portugal Guido Belli, Italy

Trial Organization Cleveland Clinic Coordinating Center for Clinical Research Ellen Mc. Erlean, RN – Project Mnger Trial Sponsors Gregory Bell, MD - KAI Fred Fiedorek, MD - BMS Data Safety Monitoring Committee CRO Medpace, Inc Cincinnati, OH Michel Bertrand, MD - Chair Christopher Cannon, MD David Holmes, MD Kerry Lee, Ph. D

Enrollment Dec 4, 2008 June 21, 2010 18 Countries 114 Hospitals 1176 Patients Randomize d Country National Coordinator Patients Poland Prof. Andrezej Rynkiewcz 226 Israel Prof. Victor Guetta 222 Czech Republic Dr. Micheal Zelizko 133 Australia Prof. Phil Aylward 85 United States Dr. Neal Kleiman 77 Netherlands Dr. Harry Suryapranata 53 Hungary Prof. Tamàs Forster 52 Spain Dr. Antonio Fernández-Ortiz 50 Sweden Prof. David Erlinge 49 Denmark Dr. Svend Eggert Jensen 45 New Zealand Dr. Harvey White 45 Belgium Dr. Danny Schoors 39 Germany Dr. Peter Radke 28 Canada Drs. Shamir Mehta/Shaun Goodman 23 Norway Dr. Dan Atar 22 Finland Dr. Michael Laine 19 Portugal Dr. Jorge Manuel dos Santos Ferreira 12 Italy Dr. Guido Belli 1
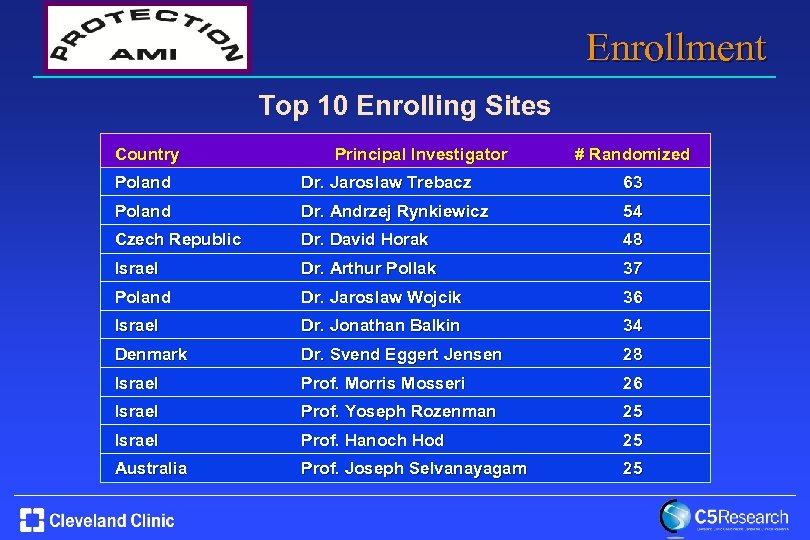
Enrollment Top 10 Enrolling Sites Country Principal Investigator # Randomized Poland Dr. Jaroslaw Trebacz 63 Poland Dr. Andrzej Rynkiewicz 54 Czech Republic Dr. David Horak 48 Israel Dr. Arthur Pollak 37 Poland Dr. Jaroslaw Wojcik 36 Israel Dr. Jonathan Balkin 34 Denmark Dr. Svend Eggert Jensen 28 Israel Prof. Morris Mosseri 26 Israel Prof. Yoseph Rozenman 25 Israel Prof. Hanoch Hod 25 Australia Prof. Joseph Selvanayagam 25
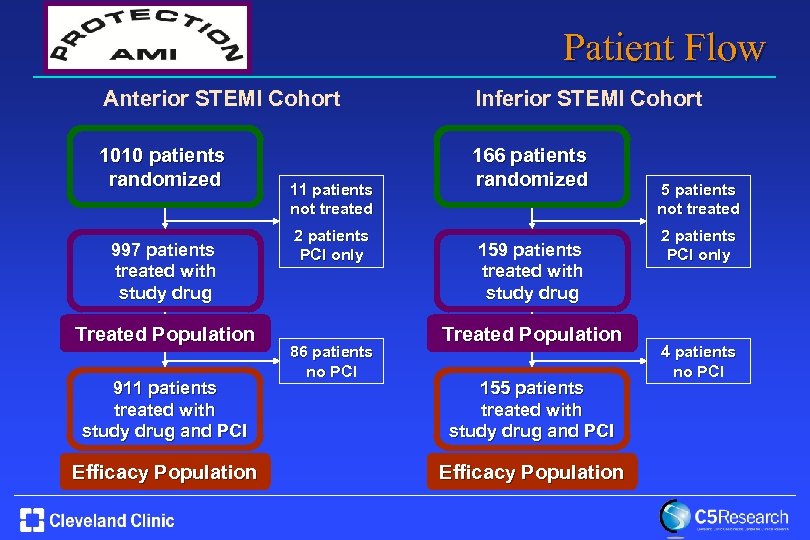
Patient Flow Anterior STEMI Cohort Inferior STEMI Cohort 1010 patients randomized 166 patients randomized 997 patients treated with study drug Treated Population 911 patients treated with study drug and PCI Efficacy Population 11 patients not treated 2 patients PCI only 86 patients no PCI 159 patients treated with study drug Treated Population 155 patients treated with study drug and PCI Efficacy Population 5 patients not treated 2 patients PCI only 4 patients no PCI
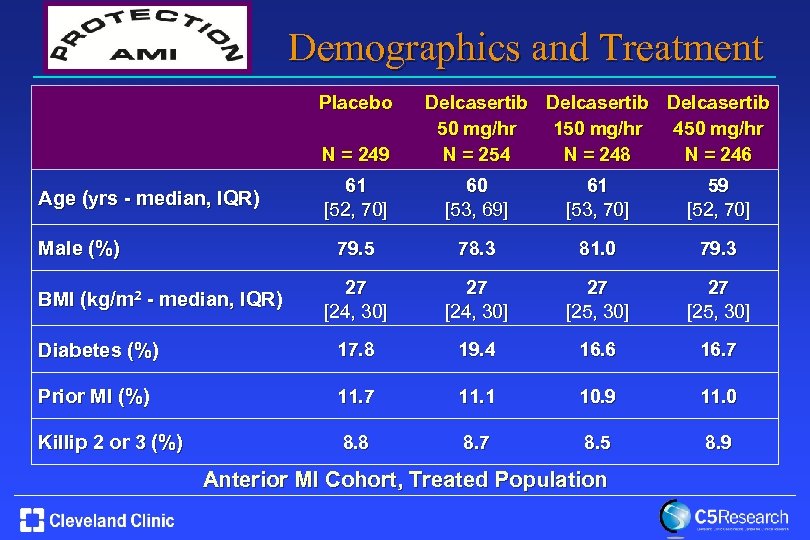
Demographics and Treatment Placebo N = 249 Delcasertib 50 mg/hr 150 mg/hr 450 mg/hr N = 254 N = 248 N = 246 61 [52, 70] 60 [53, 69] 61 [53, 70] 59 [52, 70] 79. 5 78. 3 81. 0 79. 3 27 [24, 30] 27 [25, 30] Diabetes (%) 17. 8 19. 4 16. 6 16. 7 Prior MI (%) 11. 7 11. 1 10. 9 11. 0 Killip 2 or 3 (%) 8. 8 8. 7 8. 5 8. 9 Age (yrs - median, IQR) Male (%) BMI (kg/m 2 - median, IQR) Anterior MI Cohort, Treated Population
![Treatment Times Time in minutes Median and [IQR] Placebo N = 249 Delcasertib 50 Treatment Times Time in minutes Median and [IQR] Placebo N = 249 Delcasertib 50](https://present5.com/presentation/227b2f461920fb51e33a4dd0c5589e59/image-17.jpg)
Treatment Times Time in minutes Median and [IQR] Placebo N = 249 Delcasertib 50 mg/hr 150 mg/hr 450 mg/hr N = 254 N = 248 N = 246 Symptoms to hospital presentation 120 [77, 188] 120 [63, 175] 115 [73, 185] 120 [77, 181] Symptoms to study drug initiation 173 [122, 255] 175 [130, 244] 171 [122, 245] 171 [122, 230] Symptoms to PCI 193 [148, 272] 191 [143, 255] 190 [140, 275] 186 [143, 251] 17 [10, 25] 16 [9, 27] 17 [10, 29] 14 [9, 24] Study drug initiation to PCI Anterior MI Cohort, Treated Population
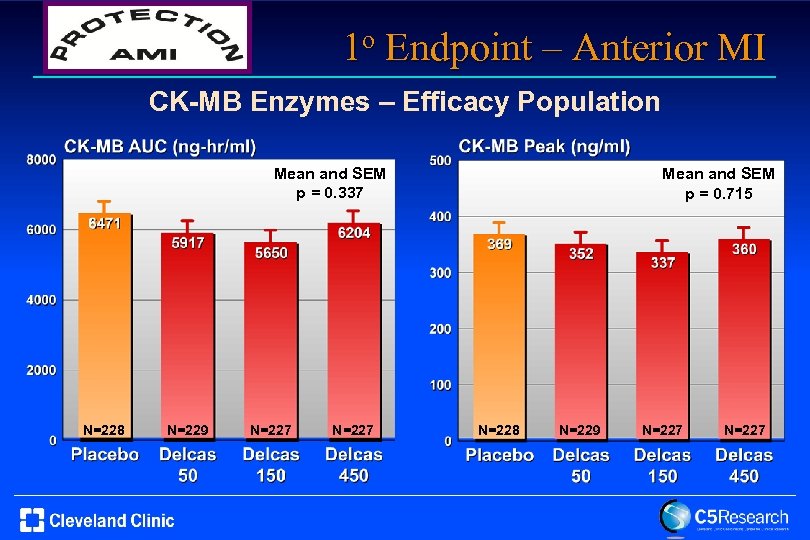
1 o Endpoint – Anterior MI CK-MB Enzymes – Efficacy Population Mean and SEM p = 0. 337 N=228 N=229 N=227 Mean and SEM p = 0. 715 N=228 N=229 N=227
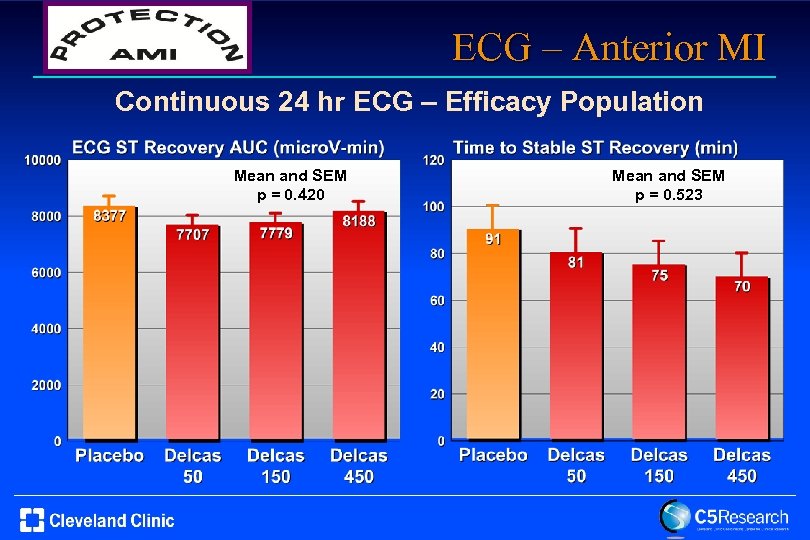
ECG – Anterior MI Continuous 24 hr ECG – Efficacy Population Mean and SEM p = 0. 420 Mean and SEM p = 0. 523
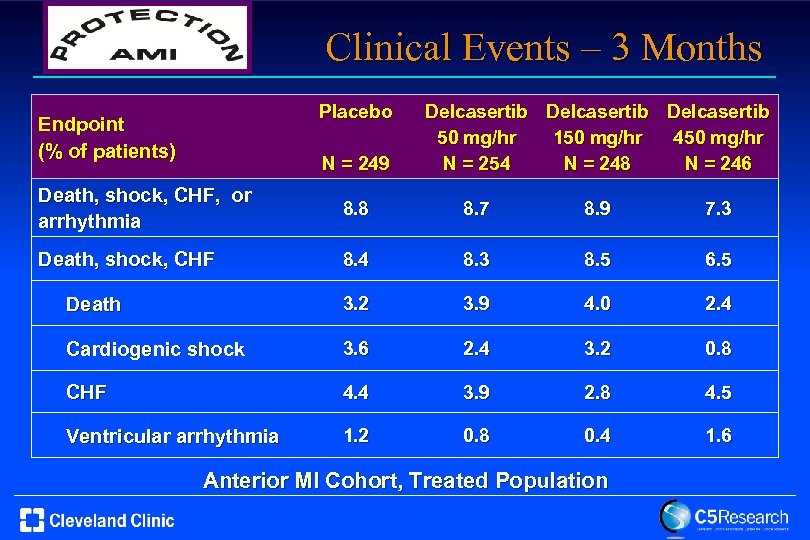
Clinical Events – 3 Months Placebo Endpoint (% of patients) N = 249 Delcasertib 50 mg/hr 150 mg/hr 450 mg/hr N = 254 N = 248 N = 246 Death, shock, CHF, or arrhythmia 8. 8 8. 7 8. 9 7. 3 Death, shock, CHF 8. 4 8. 3 8. 5 6. 5 Death 3. 2 3. 9 4. 0 2. 4 Cardiogenic shock 3. 6 2. 4 3. 2 0. 8 CHF 4. 4 3. 9 2. 8 4. 5 Ventricular arrhythmia 1. 2 0. 8 0. 4 1. 6 Anterior MI Cohort, Treated Population
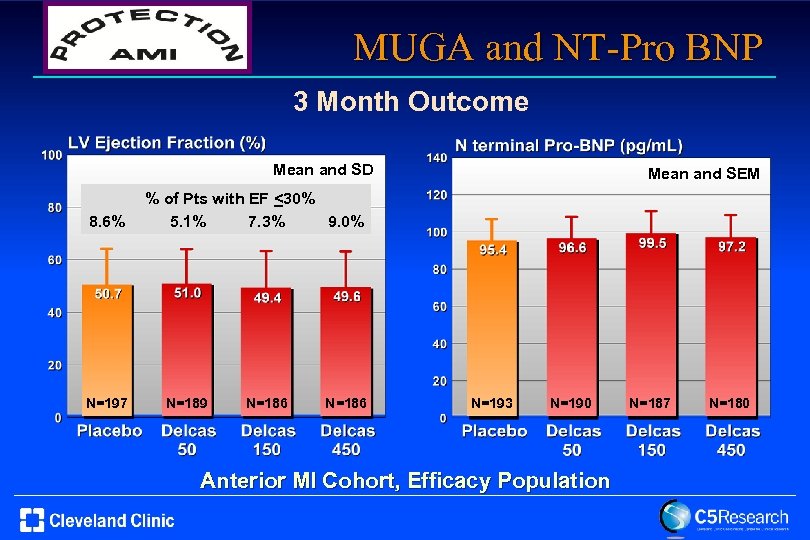
MUGA and NT-Pro BNP 3 Month Outcome Mean and SD 8. 6% N=197 Mean and SEM % of Pts with EF <30% 5. 1% 7. 3% 9. 0% N=189 N=186 N=193 N=190 Anterior MI Cohort, Efficacy Population N=187 N=180
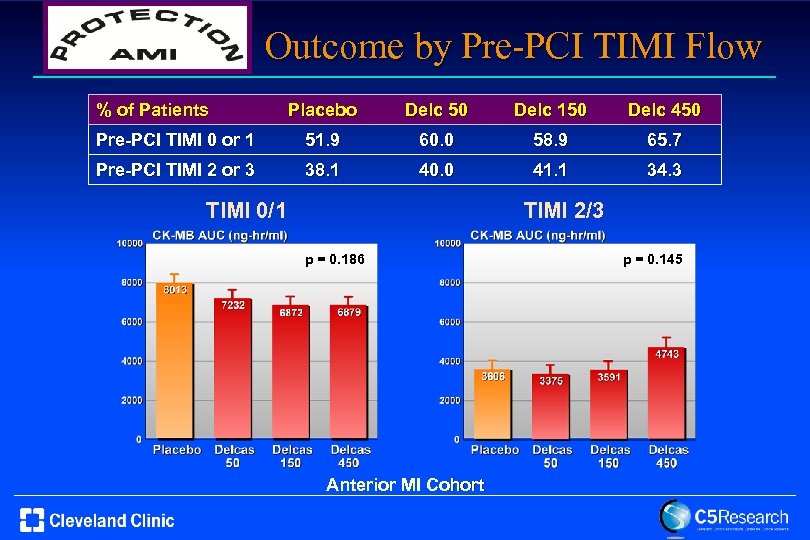
Outcome by Pre-PCI TIMI Flow % of Patients Placebo Delc 50 Delc 150 Delc 450 Pre-PCI TIMI 0 or 1 51. 9 60. 0 58. 9 65. 7 Pre-PCI TIMI 2 or 3 38. 1 40. 0 41. 1 34. 3 TIMI 2/3 TIMI 0/1 p = 0. 186 Anterior MI Cohort p = 0. 145
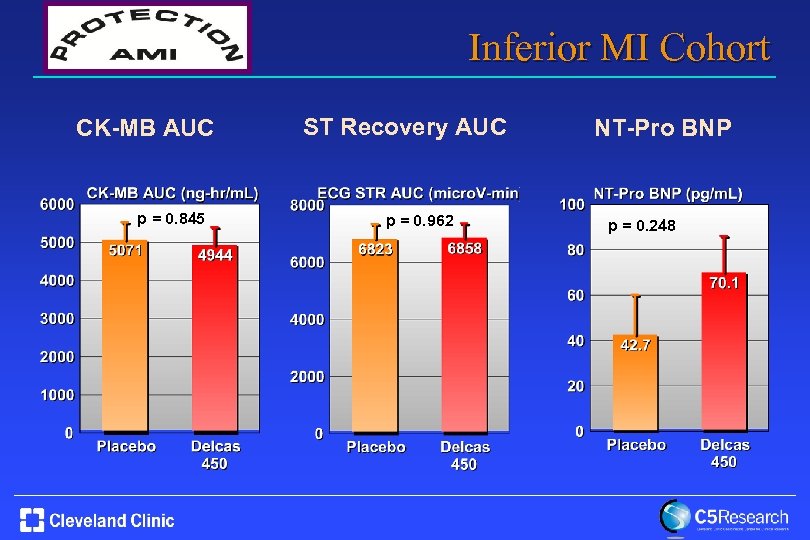
Inferior MI Cohort CK-MB AUC p = 0. 845 ST Recovery AUC p = 0. 962 NT-Pro BNP p = 0. 248
Delcasertib in STEMI PROTECTION AMI - Conclusions Delcasertib, administered IV prior to and during primary PCI for acute anterior STEMI, did not reduce myocardial infarct size or improve clinical outcome. No differences in biomarkers of: § enzymatic infarct size § ECG markers of reperfusion § LV function by 3 months § NT-Pro BNP Other potential applications to IR injury yet to be investigated

Heart and Vascular Institute
227b2f461920fb51e33a4dd0c5589e59.ppt