bbca677955a8078e7c616707cb6f780f.ppt
- Количество слайдов: 40
 Last Lecture: • Viscosity and relaxation times increase with decreasing temperature: Arrhenius and Vogel. Fulcher equations • First and second-order phase transitions are defined by derivatives of Gibbs’ free energy. • The glass transition occurs at a temperature where tconfig texp and is dependent on thermal history. In a glass, tconfig > texp. • Glass structure is described by a radial distribution function. • Liquid crystals have order between that of liquids and crystals.
Last Lecture: • Viscosity and relaxation times increase with decreasing temperature: Arrhenius and Vogel. Fulcher equations • First and second-order phase transitions are defined by derivatives of Gibbs’ free energy. • The glass transition occurs at a temperature where tconfig texp and is dependent on thermal history. In a glass, tconfig > texp. • Glass structure is described by a radial distribution function. • Liquid crystals have order between that of liquids and crystals.
 3 SMS Phase Separation 13 February, 2007 Lecture 5 See Jones’ Soft Condensed Matter, Chapt. 3 and Appendix A
3 SMS Phase Separation 13 February, 2007 Lecture 5 See Jones’ Soft Condensed Matter, Chapt. 3 and Appendix A
 Today’s Question: When are Two Liquids Miscible? Oil and water When cooled below a critical temperature, miscible liquids will separate into two phases.
Today’s Question: When are Two Liquids Miscible? Oil and water When cooled below a critical temperature, miscible liquids will separate into two phases.
 Basic Guiding Principles • Recall from last week that d. G = Vd. P-Td. S. • Since, S increases or stays the same in an isolated system, at constant P, the condition for thermodynamic equilibrium of a system is that the Gibbs’ free energy, G, goes to a minimum at equilibrium. • Helmholtz free energy: F = U - TS, so that in a phase transition at constant T: DF = DU – TDS (at const. T) • Likewise at constant V, the Helmholtz free energy, F, also goes to a minimum at equilibrium. • We also see that an increase in S or a decrease in U favours a transition. • Whether a transition occurs is thus decided by the balance between DU and DS.
Basic Guiding Principles • Recall from last week that d. G = Vd. P-Td. S. • Since, S increases or stays the same in an isolated system, at constant P, the condition for thermodynamic equilibrium of a system is that the Gibbs’ free energy, G, goes to a minimum at equilibrium. • Helmholtz free energy: F = U - TS, so that in a phase transition at constant T: DF = DU – TDS (at const. T) • Likewise at constant V, the Helmholtz free energy, F, also goes to a minimum at equilibrium. • We also see that an increase in S or a decrease in U favours a transition. • Whether a transition occurs is thus decided by the balance between DU and DS.
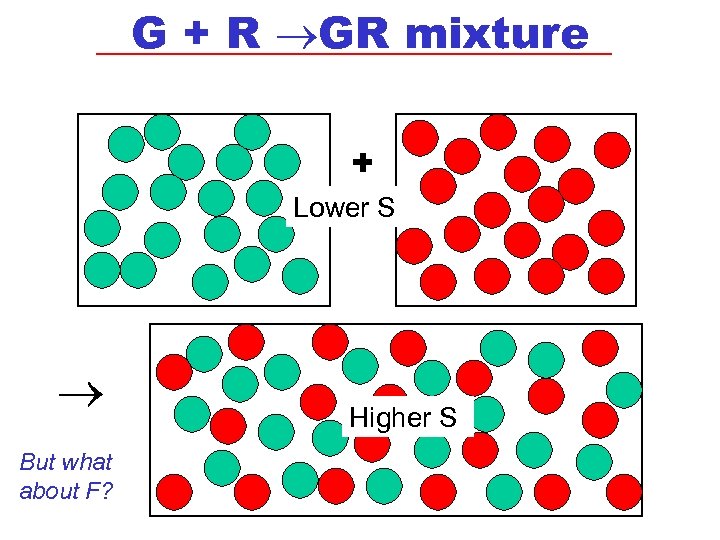 G + R GR mixture + Lower S But what about F? Higher S
G + R GR mixture + Lower S But what about F? Higher S
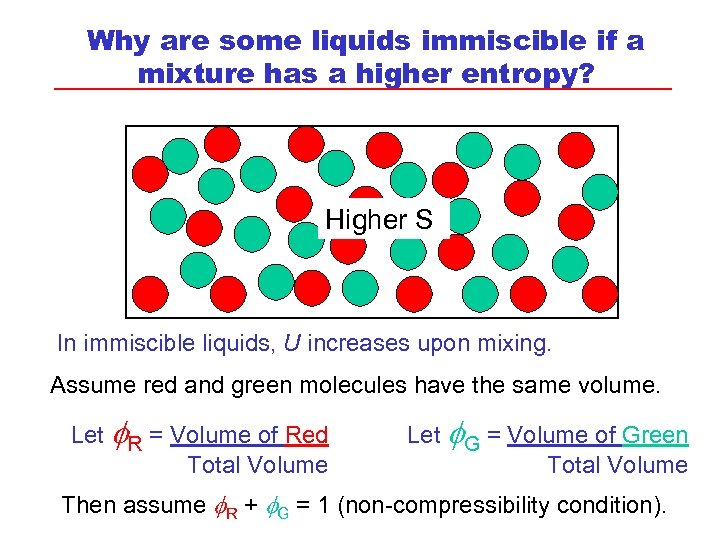 Why are some liquids immiscible if a mixture has a higher entropy? Higher S In immiscible liquids, U increases upon mixing. Assume red and green molecules have the same volume. Let R = Volume of Red Total Volume Let G = Volume of Green Total Volume Then assume R + G = 1 (non-compressibility condition).
Why are some liquids immiscible if a mixture has a higher entropy? Higher S In immiscible liquids, U increases upon mixing. Assume red and green molecules have the same volume. Let R = Volume of Red Total Volume Let G = Volume of Green Total Volume Then assume R + G = 1 (non-compressibility condition).
 Entropy Calculation from Statistical Thermodynamics Boltzmann’s tomb S = k ln The statistical weight, , represents the number of ways of arranging particles (microstate) in a particular energy state (macrostate).
Entropy Calculation from Statistical Thermodynamics Boltzmann’s tomb S = k ln The statistical weight, , represents the number of ways of arranging particles (microstate) in a particular energy state (macrostate).
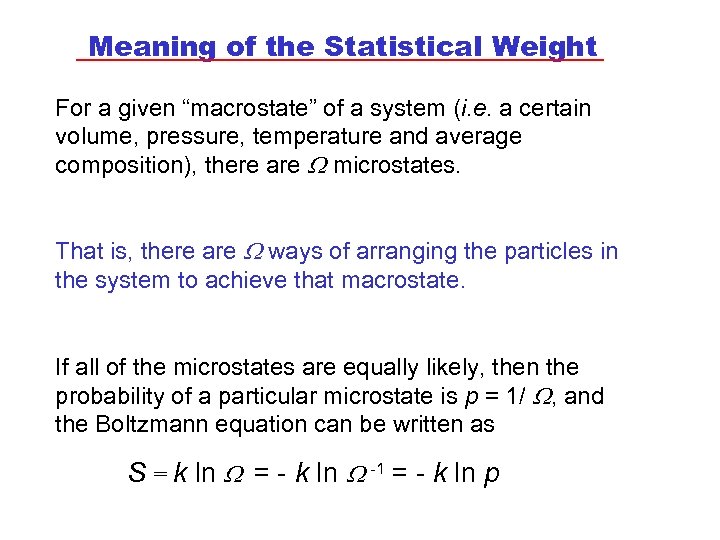 Meaning of the Statistical Weight For a given “macrostate” of a system (i. e. a certain volume, pressure, temperature and average composition), there are microstates. That is, there are ways of arranging the particles in the system to achieve that macrostate. If all of the microstates are equally likely, then the probability of a particular microstate is p = 1/ , and the Boltzmann equation can be written as S = k ln = - k ln -1 = - k ln p
Meaning of the Statistical Weight For a given “macrostate” of a system (i. e. a certain volume, pressure, temperature and average composition), there are microstates. That is, there are ways of arranging the particles in the system to achieve that macrostate. If all of the microstates are equally likely, then the probability of a particular microstate is p = 1/ , and the Boltzmann equation can be written as S = k ln = - k ln -1 = - k ln p
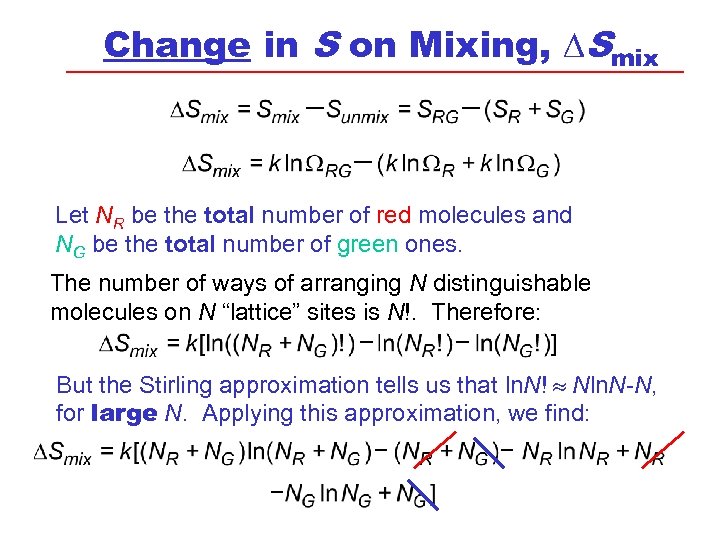 Change in S on Mixing, DSmix Let NR be the total number of red molecules and NG be the total number of green ones. The number of ways of arranging N distinguishable molecules on N “lattice” sites is N!. Therefore: But the Stirling approximation tells us that ln. N! Nln. N-N, for large N. Applying this approximation, we find:
Change in S on Mixing, DSmix Let NR be the total number of red molecules and NG be the total number of green ones. The number of ways of arranging N distinguishable molecules on N “lattice” sites is N!. Therefore: But the Stirling approximation tells us that ln. N! Nln. N-N, for large N. Applying this approximation, we find:
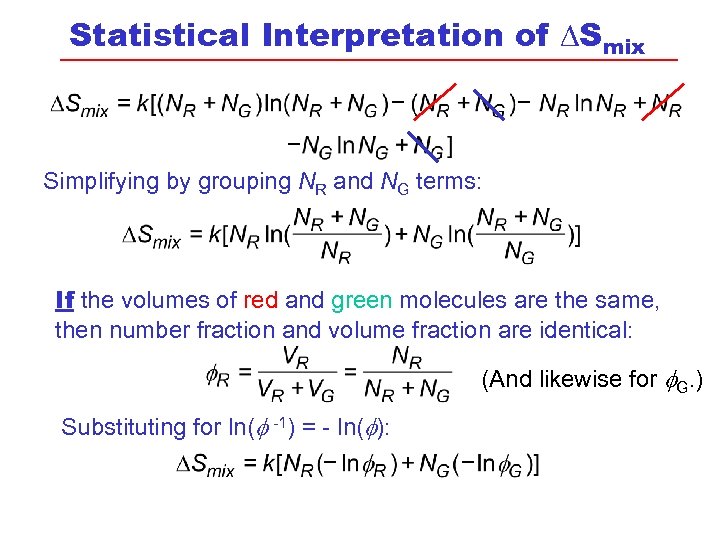 Statistical Interpretation of DSmix Simplifying by grouping NR and NG terms: If the volumes of red and green molecules are the same, then number fraction and volume fraction are identical: (And likewise for G. ) Substituting for ln( -1) = - ln( ):
Statistical Interpretation of DSmix Simplifying by grouping NR and NG terms: If the volumes of red and green molecules are the same, then number fraction and volume fraction are identical: (And likewise for G. ) Substituting for ln( -1) = - ln( ):
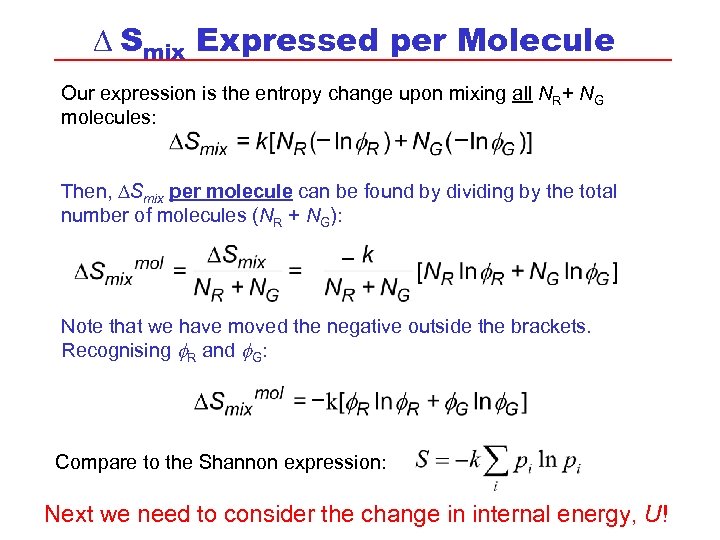 D Smix Expressed per Molecule Our expression is the entropy change upon mixing all NR+ NG molecules: Then, DSmix per molecule can be found by dividing by the total number of molecules (NR + NG): Note that we have moved the negative outside the brackets. Recognising R and G: Compare to the Shannon expression: Next we need to consider the change in internal energy, U!
D Smix Expressed per Molecule Our expression is the entropy change upon mixing all NR+ NG molecules: Then, DSmix per molecule can be found by dividing by the total number of molecules (NR + NG): Note that we have moved the negative outside the brackets. Recognising R and G: Compare to the Shannon expression: Next we need to consider the change in internal energy, U!
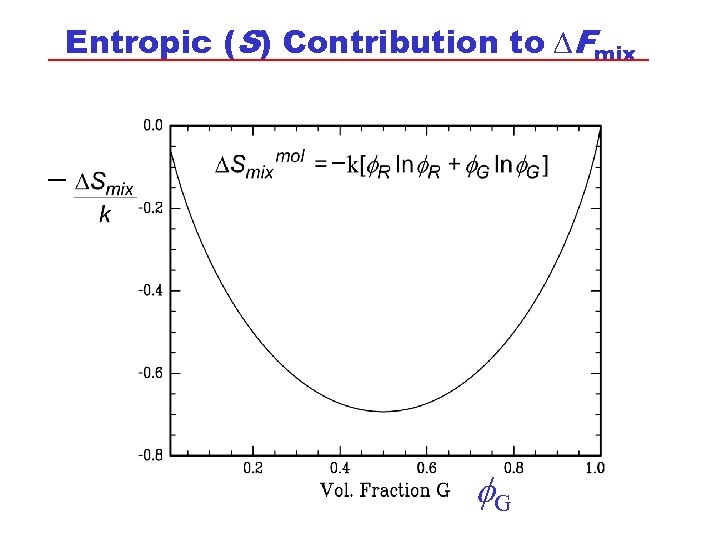 Entropic (S) Contribution to DFmix G
Entropic (S) Contribution to DFmix G
 Change in U on Mixing, DUmix • Previously, we considered the energy of interaction between pairs of molecules, w(r), for a variety of different interactions, e. g. van der Waals, Coulombic, polar, etc. • We assumed the interaction energies (w) are additive! • When unmixed, there are interaction energies between like molecules only: w. RR and w. GG. • When mixed, there is then a new interaction energy between unlike molecules: w. RG. • At a constant T, the kinetic energy does not change with mixing; only the potential energies change. • So, DUmix = WR+G - (WRR + WGG), which is the difference between the mixed and the unmixed states.
Change in U on Mixing, DUmix • Previously, we considered the energy of interaction between pairs of molecules, w(r), for a variety of different interactions, e. g. van der Waals, Coulombic, polar, etc. • We assumed the interaction energies (w) are additive! • When unmixed, there are interaction energies between like molecules only: w. RR and w. GG. • When mixed, there is then a new interaction energy between unlike molecules: w. RG. • At a constant T, the kinetic energy does not change with mixing; only the potential energies change. • So, DUmix = WR+G - (WRR + WGG), which is the difference between the mixed and the unmixed states.
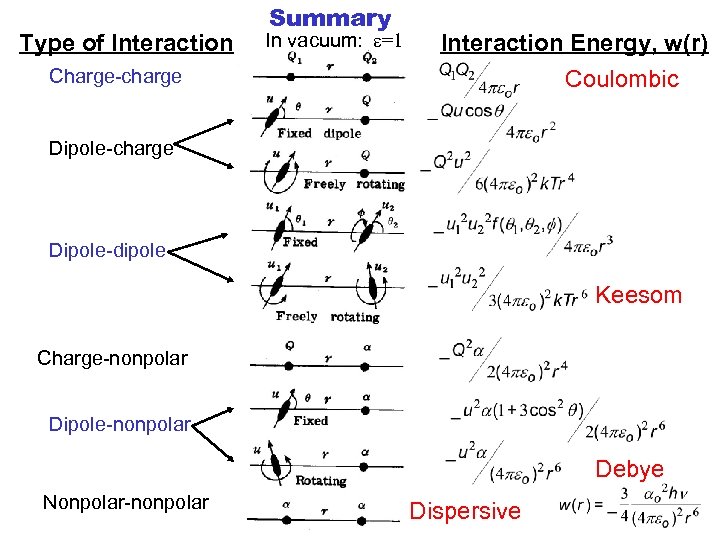 Type of Interaction Charge-charge Summary In vacuum: e=1 Interaction Energy, w(r) Coulombic Dipole-charge Dipole-dipole Keesom Charge-nonpolar Dipole-nonpolar Debye Nonpolar-nonpolar Dispersive
Type of Interaction Charge-charge Summary In vacuum: e=1 Interaction Energy, w(r) Coulombic Dipole-charge Dipole-dipole Keesom Charge-nonpolar Dipole-nonpolar Debye Nonpolar-nonpolar Dispersive
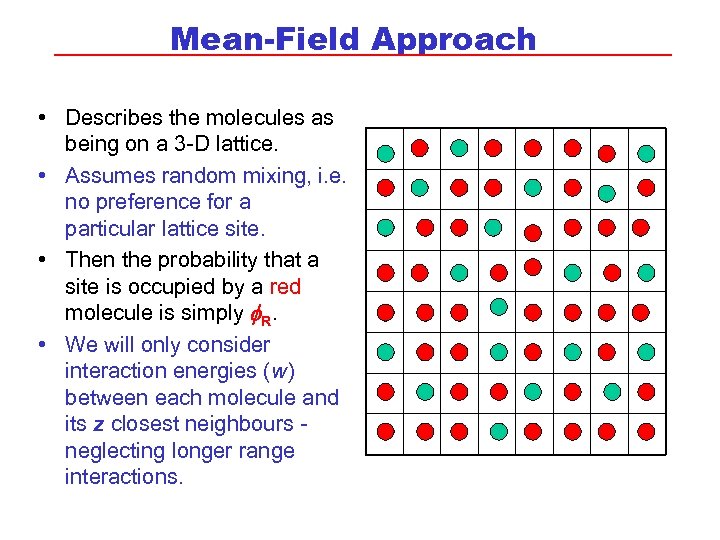 Mean-Field Approach • Describes the molecules as being on a 3 -D lattice. • Assumes random mixing, i. e. no preference for a particular lattice site. • Then the probability that a site is occupied by a red molecule is simply f. R. • We will only consider interaction energies (w) between each molecule and its z closest neighbours neglecting longer range interactions.
Mean-Field Approach • Describes the molecules as being on a 3 -D lattice. • Assumes random mixing, i. e. no preference for a particular lattice site. • Then the probability that a site is occupied by a red molecule is simply f. R. • We will only consider interaction energies (w) between each molecule and its z closest neighbours neglecting longer range interactions.
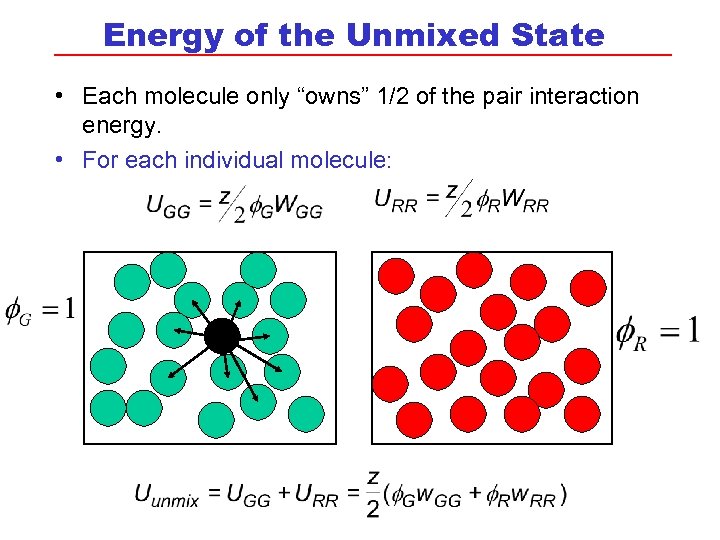 Energy of the Unmixed State • Each molecule only “owns” 1/2 of the pair interaction energy. • For each individual molecule:
Energy of the Unmixed State • Each molecule only “owns” 1/2 of the pair interaction energy. • For each individual molecule:
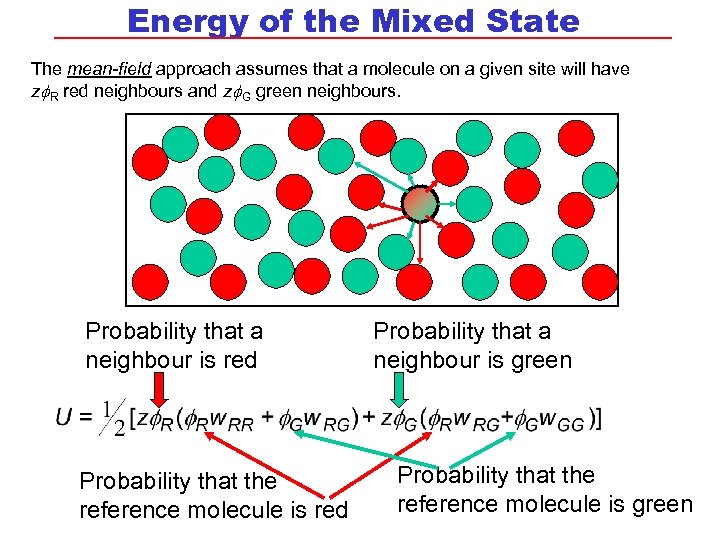 Energy of the Mixed State The mean-field approach assumes that a molecule on a given site will have z R red neighbours and z G green neighbours. Probability that a neighbour is red Probability that the reference molecule is red Probability that a neighbour is green Probability that the reference molecule is green
Energy of the Mixed State The mean-field approach assumes that a molecule on a given site will have z R red neighbours and z G green neighbours. Probability that a neighbour is red Probability that the reference molecule is red Probability that a neighbour is green Probability that the reference molecule is green
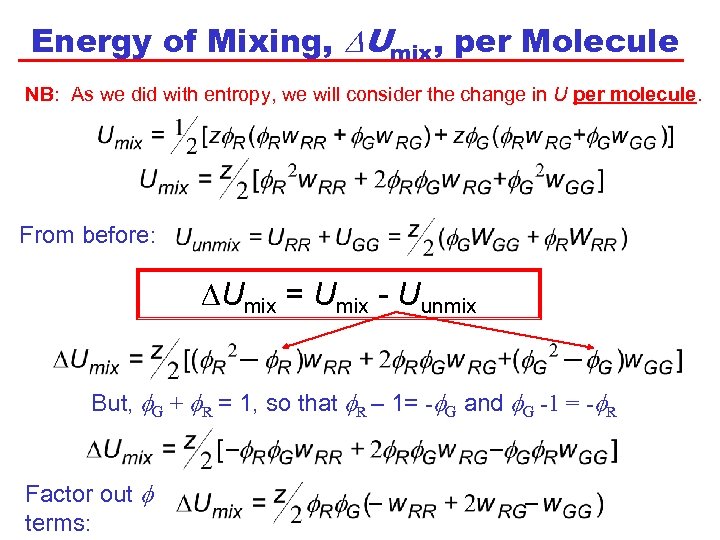 Energy of Mixing, DUmix, per Molecule NB: As we did with entropy, we will consider the change in U per molecule. From before: DUmix = Umix - Uunmix But, G + R = 1, so that R – 1= - G and G -1 = - R Factor out terms:
Energy of Mixing, DUmix, per Molecule NB: As we did with entropy, we will consider the change in U per molecule. From before: DUmix = Umix - Uunmix But, G + R = 1, so that R – 1= - G and G -1 = - R Factor out terms:
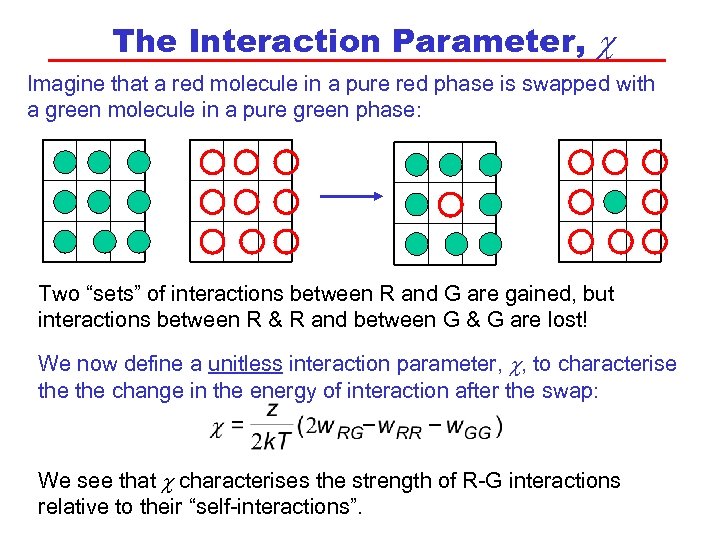 The Interaction Parameter, c Imagine that a red molecule in a pure red phase is swapped with a green molecule in a pure green phase: Two “sets” of interactions between R and G are gained, but interactions between R & R and between G & G are lost! We now define a unitless interaction parameter, c, to characterise the change in the energy of interaction after the swap: We see that c characterises the strength of R-G interactions relative to their “self-interactions”.
The Interaction Parameter, c Imagine that a red molecule in a pure red phase is swapped with a green molecule in a pure green phase: Two “sets” of interactions between R and G are gained, but interactions between R & R and between G & G are lost! We now define a unitless interaction parameter, c, to characterise the change in the energy of interaction after the swap: We see that c characterises the strength of R-G interactions relative to their “self-interactions”.
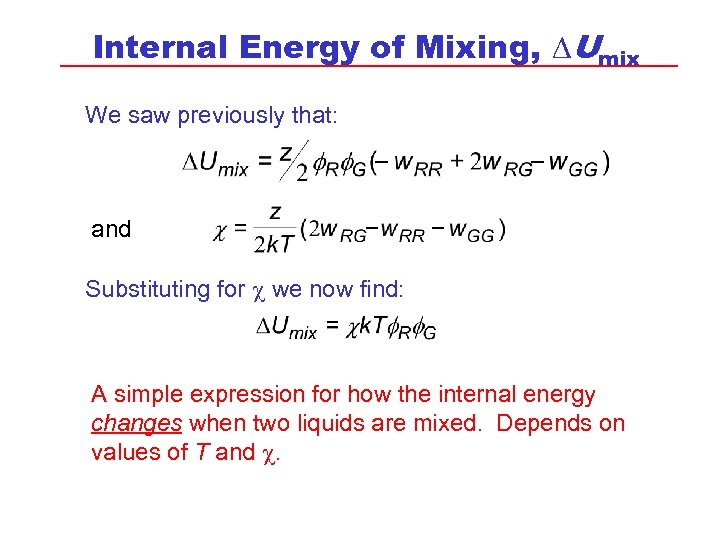 Internal Energy of Mixing, DUmix We saw previously that: and Substituting for c we now find: A simple expression for how the internal energy changes when two liquids are mixed. Depends on values of T and c.
Internal Energy of Mixing, DUmix We saw previously that: and Substituting for c we now find: A simple expression for how the internal energy changes when two liquids are mixed. Depends on values of T and c.
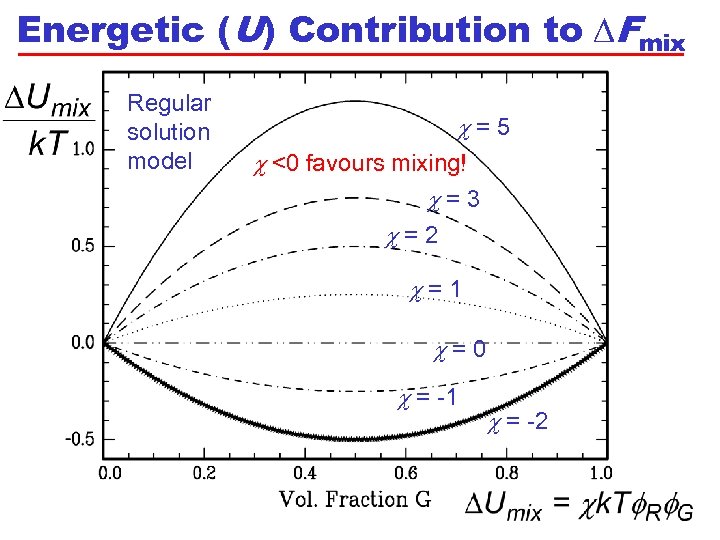 Energetic (U) Contribution to DFmix Regular solution model c=5 c <0 favours mixing! c=3 c=2 c=1 c=0 c = -1 c = -2
Energetic (U) Contribution to DFmix Regular solution model c=5 c <0 favours mixing! c=3 c=2 c=1 c=0 c = -1 c = -2
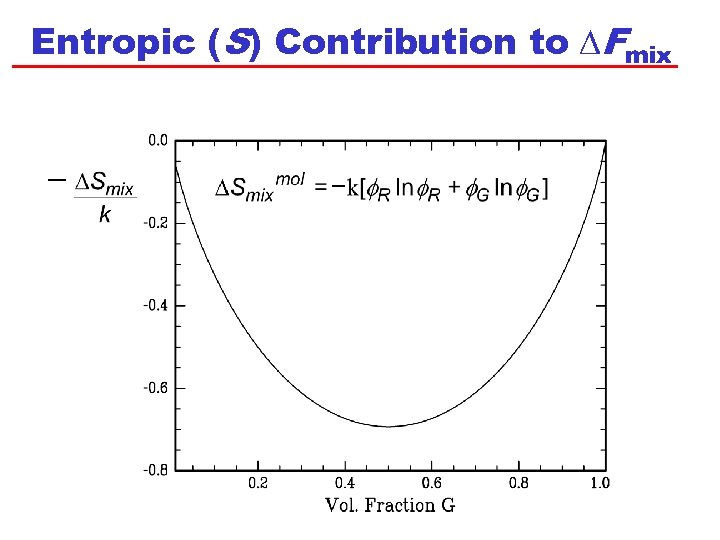 Entropic (S) Contribution to DFmix
Entropic (S) Contribution to DFmix
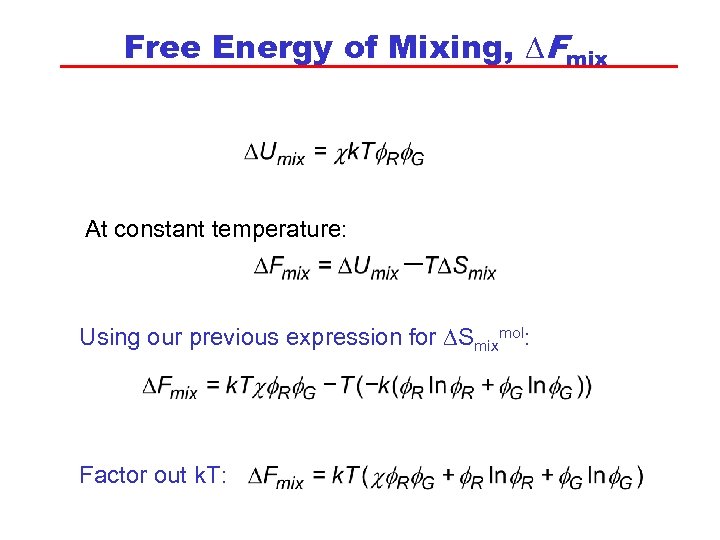 Free Energy of Mixing, DFmix At constant temperature: Using our previous expression for DSmixmol: Factor out k. T:
Free Energy of Mixing, DFmix At constant temperature: Using our previous expression for DSmixmol: Factor out k. T:
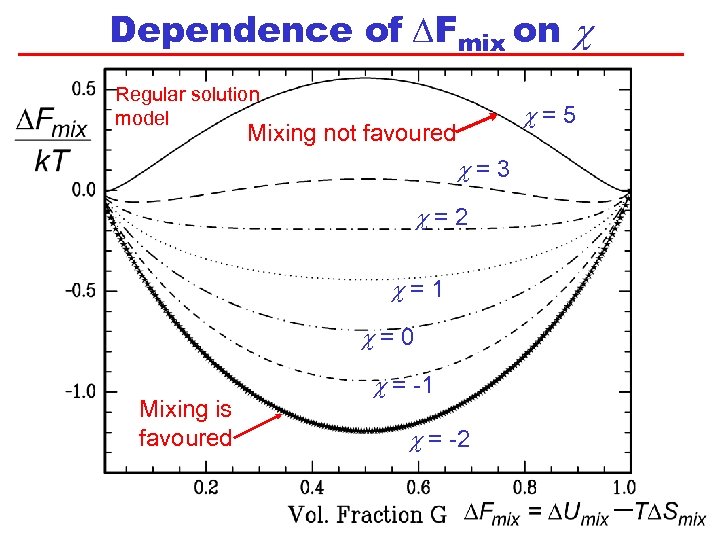 Dependence of DFmix on c Regular solution model c=5 Mixing not favoured c=3 c=2 c=1 c=0 Mixing is favoured c = -1 c = -2
Dependence of DFmix on c Regular solution model c=5 Mixing not favoured c=3 c=2 c=1 c=0 Mixing is favoured c = -1 c = -2
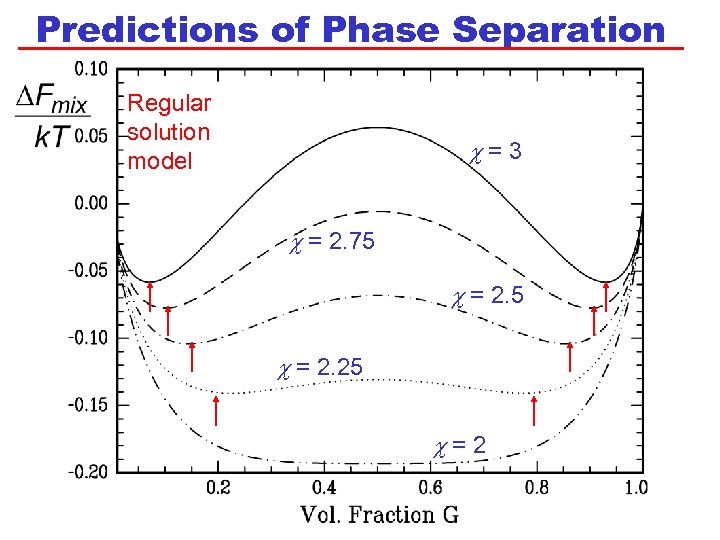 Predictions of Phase Separation Regular solution model c=3 c = 2. 75 c = 2. 25 c=2
Predictions of Phase Separation Regular solution model c=3 c = 2. 75 c = 2. 25 c=2
 Summary of Observations We have assumed non-compressibility, that molecules are on a lattice, and that volume fraction equals number fraction. When c < 2, there is a single minimum at R = 0. 5 When c 2, there are two minima in DFmix and a maximum at R = G = 0. 5. As c increases, the two compositions at the DFmix minima become more different. How does this dependence of DFmix on determine the composition of phases in a mixture of liquids?
Summary of Observations We have assumed non-compressibility, that molecules are on a lattice, and that volume fraction equals number fraction. When c < 2, there is a single minimum at R = 0. 5 When c 2, there are two minima in DFmix and a maximum at R = G = 0. 5. As c increases, the two compositions at the DFmix minima become more different. How does this dependence of DFmix on determine the composition of phases in a mixture of liquids?
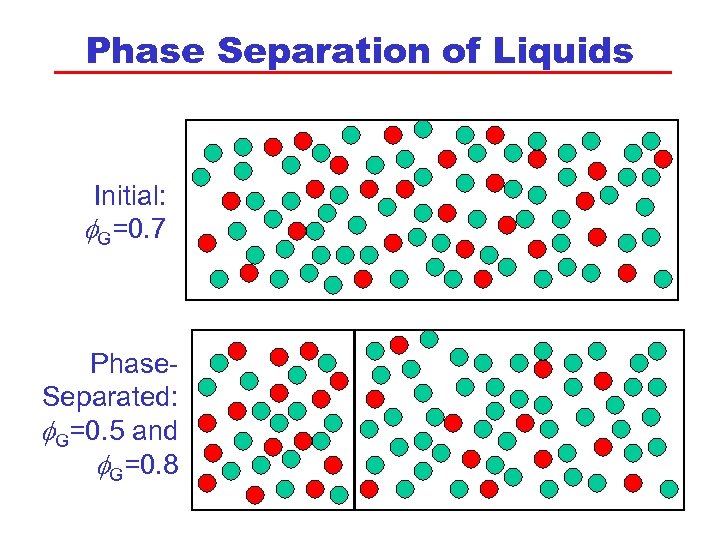 Phase Separation of Liquids Initial: G=0. 7 Phase. Separated: G=0. 5 and G=0. 8
Phase Separation of Liquids Initial: G=0. 7 Phase. Separated: G=0. 5 and G=0. 8
 Free Energy of a System of Two Liquids • A system of two mixed liquids (G and R) will have a certain initial volume fraction of liquid G of o. • At a certain temperature, this mixture separates into two phases with volume fractions of G of 1 and 2. • The total volume of the system is conserved when there is phase separation. • The free energy of the phase-separated system can be shown to be: Fsep can be easily interpreted graphically!
Free Energy of a System of Two Liquids • A system of two mixed liquids (G and R) will have a certain initial volume fraction of liquid G of o. • At a certain temperature, this mixture separates into two phases with volume fractions of G of 1 and 2. • The total volume of the system is conserved when there is phase separation. • The free energy of the phase-separated system can be shown to be: Fsep can be easily interpreted graphically!
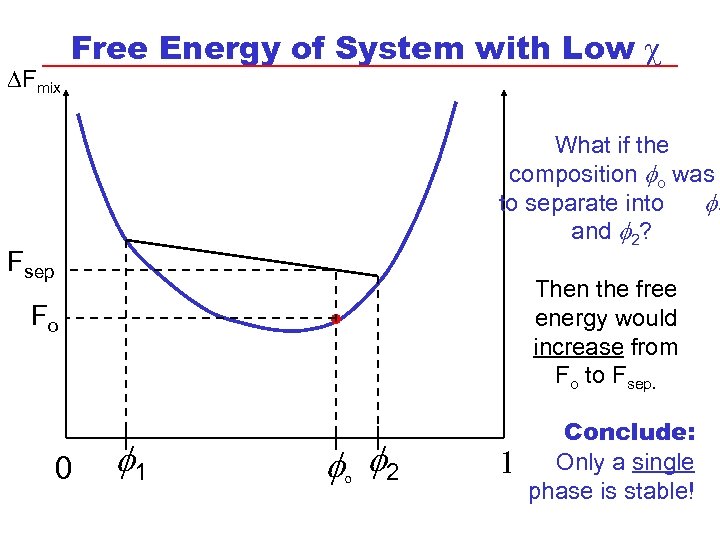 DFmix Free Energy of System with Low c . Fsep Fo 0 1 o 2 What if the composition o was to separate into 1 and 2? Then the free energy would increase from Fo to Fsep. 1 Conclude: Only a single phase is stable!
DFmix Free Energy of System with Low c . Fsep Fo 0 1 o 2 What if the composition o was to separate into 1 and 2? Then the free energy would increase from Fo to Fsep. 1 Conclude: Only a single phase is stable!
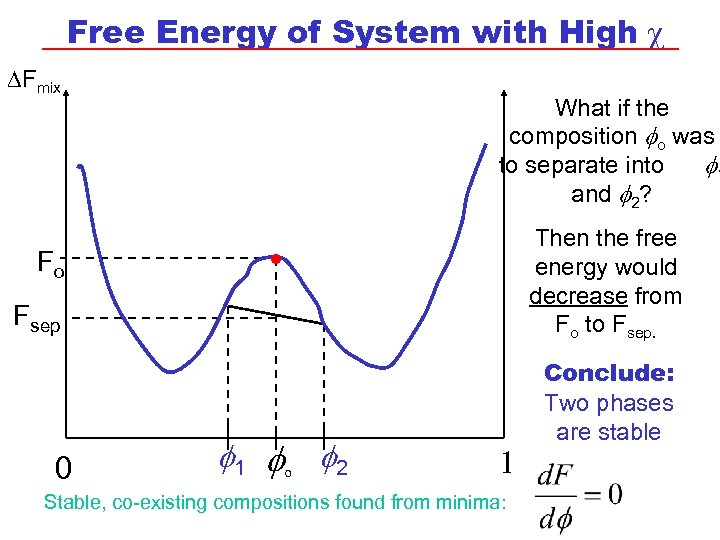 Free Energy of System with High c DFmix Fo . What if the composition o was to separate into 1 and 2? Then the free energy would decrease from Fo to Fsep 0 1 o 2 1 Stable, co-existing compositions found from minima: Conclude: Two phases are stable
Free Energy of System with High c DFmix Fo . What if the composition o was to separate into 1 and 2? Then the free energy would decrease from Fo to Fsep 0 1 o 2 1 Stable, co-existing compositions found from minima: Conclude: Two phases are stable
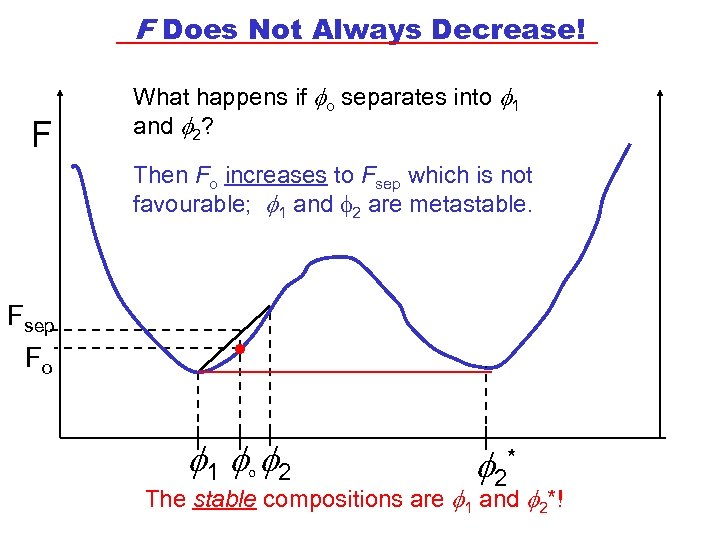 F Does Not Always Decrease! F What happens if o separates into 1 and 2? Then Fo increases to Fsep which is not favourable; 1 and f 2 are metastable. Fsep Fo . 1 o 2 2* The stable compositions are 1 and 2*!
F Does Not Always Decrease! F What happens if o separates into 1 and 2? Then Fo increases to Fsep which is not favourable; 1 and f 2 are metastable. Fsep Fo . 1 o 2 2* The stable compositions are 1 and 2*!
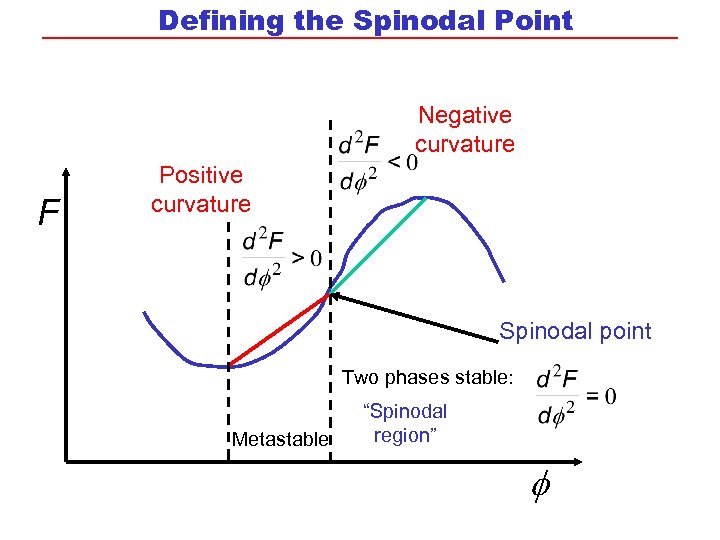 Defining the Spinodal Point Negative curvature F Positive curvature Spinodal point Two phases stable: Metastable “Spinodal region”
Defining the Spinodal Point Negative curvature F Positive curvature Spinodal point Two phases stable: Metastable “Spinodal region”
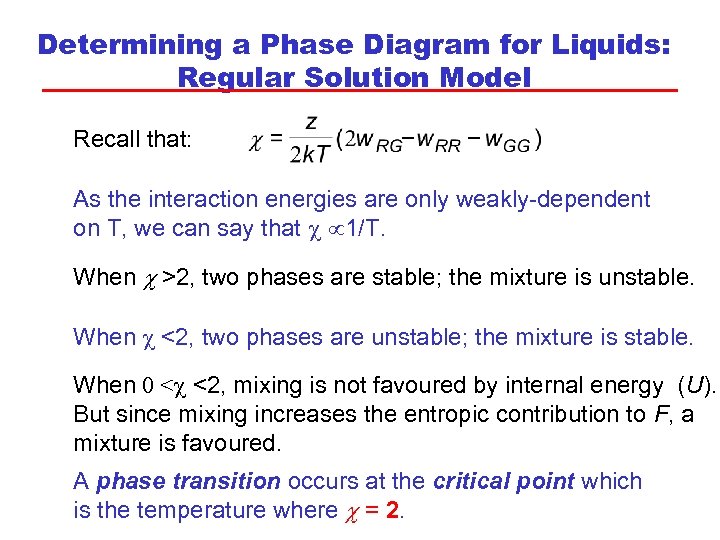 Determining a Phase Diagram for Liquids: Regular Solution Model Recall that: As the interaction energies are only weakly-dependent on T, we can say that c 1/T. When c >2, two phases are stable; the mixture is unstable. When c <2, two phases are unstable; the mixture is stable. When 0
Determining a Phase Diagram for Liquids: Regular Solution Model Recall that: As the interaction energies are only weakly-dependent on T, we can say that c 1/T. When c >2, two phases are stable; the mixture is unstable. When c <2, two phases are unstable; the mixture is stable. When 0
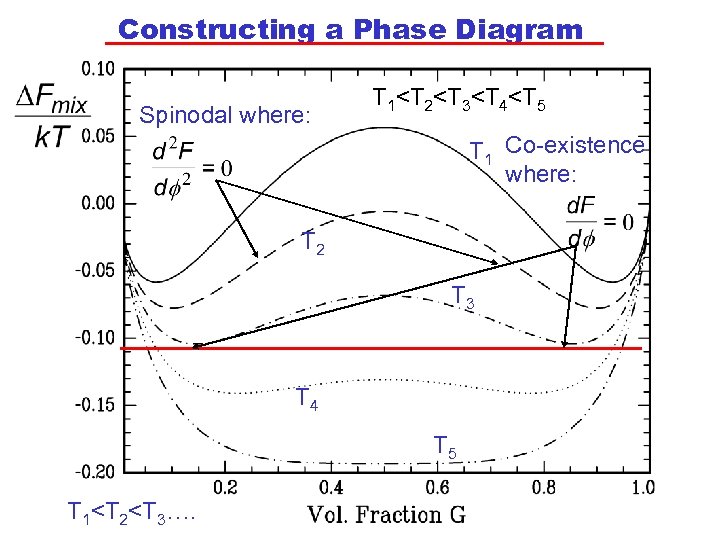 Constructing a Phase Diagram Spinodal where: T 1
Constructing a Phase Diagram Spinodal where: T 1
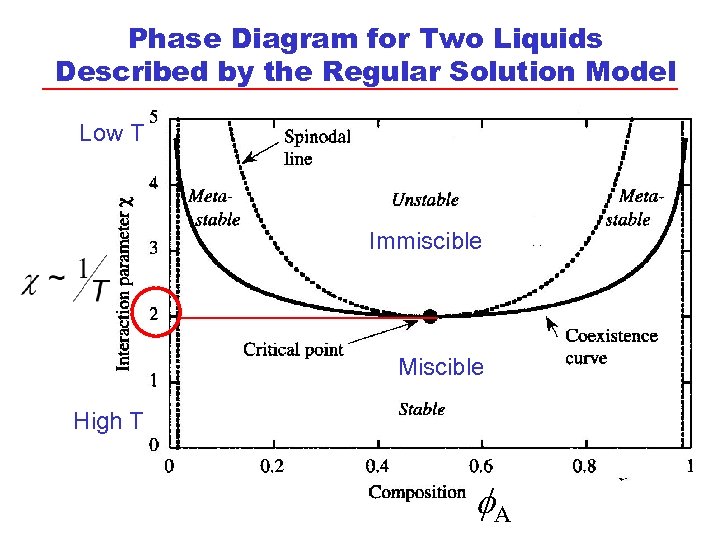 Phase Diagram for Two Liquids Described by the Regular Solution Model Low T Immiscible Miscible High T A
Phase Diagram for Two Liquids Described by the Regular Solution Model Low T Immiscible Miscible High T A
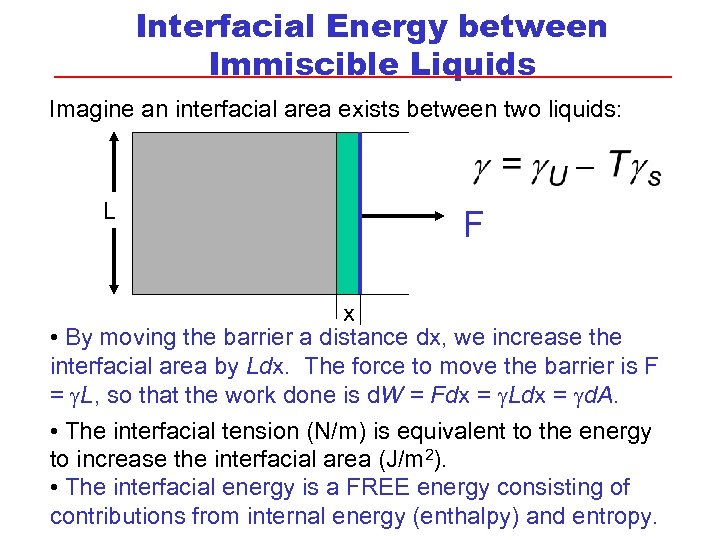 Interfacial Energy between Immiscible Liquids Imagine an interfacial area exists between two liquids: L F x • By moving the barrier a distance dx, we increase the interfacial area by Ldx. The force to move the barrier is F = g. L, so that the work done is d. W = Fdx = g. Ldx = gd. A. • The interfacial tension (N/m) is equivalent to the energy to increase the interfacial area (J/m 2). • The interfacial energy is a FREE energy consisting of contributions from internal energy (enthalpy) and entropy.
Interfacial Energy between Immiscible Liquids Imagine an interfacial area exists between two liquids: L F x • By moving the barrier a distance dx, we increase the interfacial area by Ldx. The force to move the barrier is F = g. L, so that the work done is d. W = Fdx = g. Ldx = gd. A. • The interfacial tension (N/m) is equivalent to the energy to increase the interfacial area (J/m 2). • The interfacial energy is a FREE energy consisting of contributions from internal energy (enthalpy) and entropy.
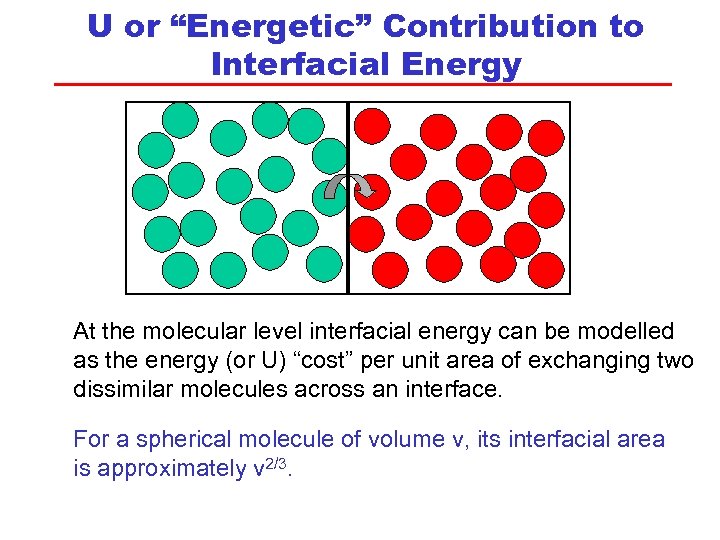 U or “Energetic” Contribution to Interfacial Energy At the molecular level interfacial energy can be modelled as the energy (or U) “cost” per unit area of exchanging two dissimilar molecules across an interface. For a spherical molecule of volume v, its interfacial area is approximately v 2/3.
U or “Energetic” Contribution to Interfacial Energy At the molecular level interfacial energy can be modelled as the energy (or U) “cost” per unit area of exchanging two dissimilar molecules across an interface. For a spherical molecule of volume v, its interfacial area is approximately v 2/3.
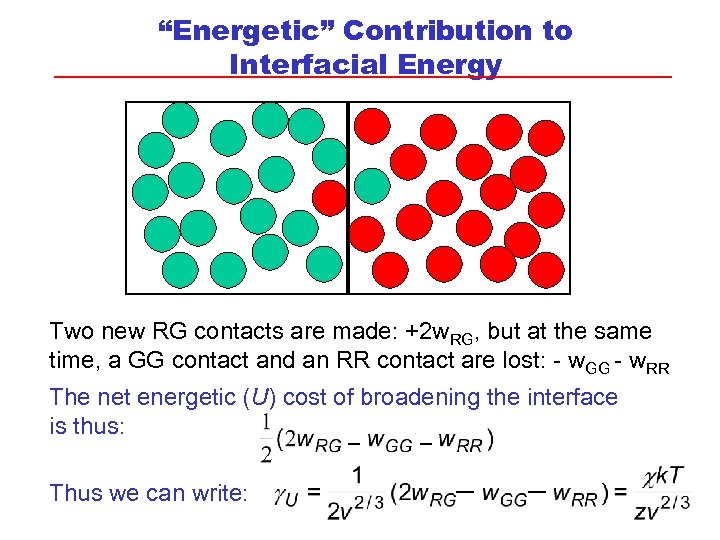 “Energetic” Contribution to Interfacial Energy Two new RG contacts are made: +2 w. RG, but at the same time, a GG contact and an RR contact are lost: - w. GG - w. RR The net energetic (U) cost of broadening the interface is thus: Thus we can write:
“Energetic” Contribution to Interfacial Energy Two new RG contacts are made: +2 w. RG, but at the same time, a GG contact and an RR contact are lost: - w. GG - w. RR The net energetic (U) cost of broadening the interface is thus: Thus we can write:
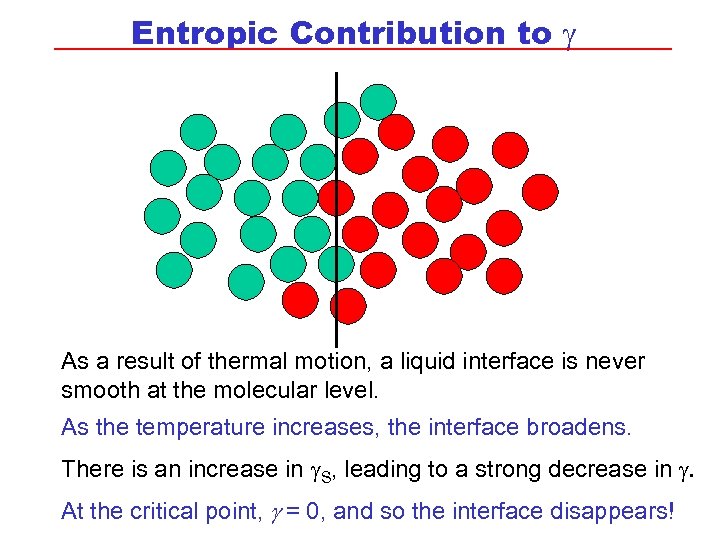 Entropic Contribution to g As a result of thermal motion, a liquid interface is never smooth at the molecular level. As the temperature increases, the interface broadens. There is an increase in g. S, leading to a strong decrease in g. At the critical point, g = 0, and so the interface disappears!
Entropic Contribution to g As a result of thermal motion, a liquid interface is never smooth at the molecular level. As the temperature increases, the interface broadens. There is an increase in g. S, leading to a strong decrease in g. At the critical point, g = 0, and so the interface disappears!
 Problem Set 3 1. The phase behaviour of a liquid mixture can be described by the regular solution model. The interaction parameter depends on temperature as c = 600/T, with T in degrees Kelvin. (a) Calculate the temperature of the critical point. (b) At a temperature of 273 K, what is the composition (volume fractions) of the co-existing phases? (c) At the same temperature, what are the volume fractions of the phases on the spinodal line? 2. Octane and water are immiscible at room temperature, and their interfacial energy is measured to be about 30 m. Jm-2. The molecular volume of octane and water can be approximated as 2. 4 x 10 -29 m 3. (a) Estimate the c parameter for octane and water. (b) What can you conclude about the difference between the interaction energy of octane and water and the “self-interaction” energy of the two liquids?
Problem Set 3 1. The phase behaviour of a liquid mixture can be described by the regular solution model. The interaction parameter depends on temperature as c = 600/T, with T in degrees Kelvin. (a) Calculate the temperature of the critical point. (b) At a temperature of 273 K, what is the composition (volume fractions) of the co-existing phases? (c) At the same temperature, what are the volume fractions of the phases on the spinodal line? 2. Octane and water are immiscible at room temperature, and their interfacial energy is measured to be about 30 m. Jm-2. The molecular volume of octane and water can be approximated as 2. 4 x 10 -29 m 3. (a) Estimate the c parameter for octane and water. (b) What can you conclude about the difference between the interaction energy of octane and water and the “self-interaction” energy of the two liquids?


