Laboratory tests in Rheumatology.ppt
- Количество слайдов: 55

Laboratory tests in Rheumatology Dr Katya Dolnikov D_katya@rambam. health. gov. il 2017
Introduction • In rheumatic disease lab test contribute to diagnosis • Laboratory investigation should be guided by clinical picture • Measurement of biomarkers can be useful to monitor treatment efficacy and safety • Stratification of patients to predict prognosis

Utility of Lab Tests Aims of lab test: 1. Identification of pathological process in the body & evaluation of its severity 2. Support or negation of specific diagnosis 3. Follow up of disease & complications 4. Detection of adverse reactions of drug therapy • Interpretation of lab tests should be done only in relation to certain clinical context. • Without the clinical picture most lab tests are useless.

Diagnostic vs. Evaluative Tests • Need to determine which test is appropriate • Diagnostic tests accurately distinguish a group of patients with a specific disease from a non-disease group • Evaluative tests monitor disease activity over time
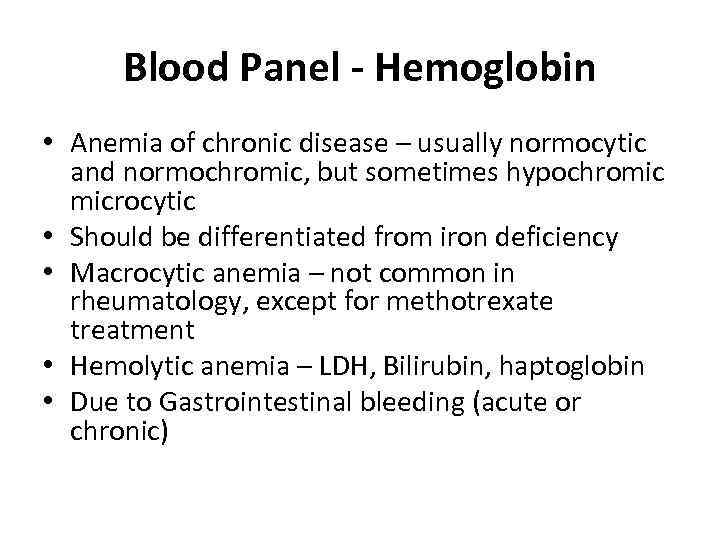
Blood Panel - Hemoglobin • Anemia of chronic disease – usually normocytic and normochromic, but sometimes hypochromic microcytic • Should be differentiated from iron deficiency • Macrocytic anemia – not common in rheumatology, except for methotrexate treatment • Hemolytic anemia – LDH, Bilirubin, haptoglobin • Due to Gastrointestinal bleeding (acute or chronic)
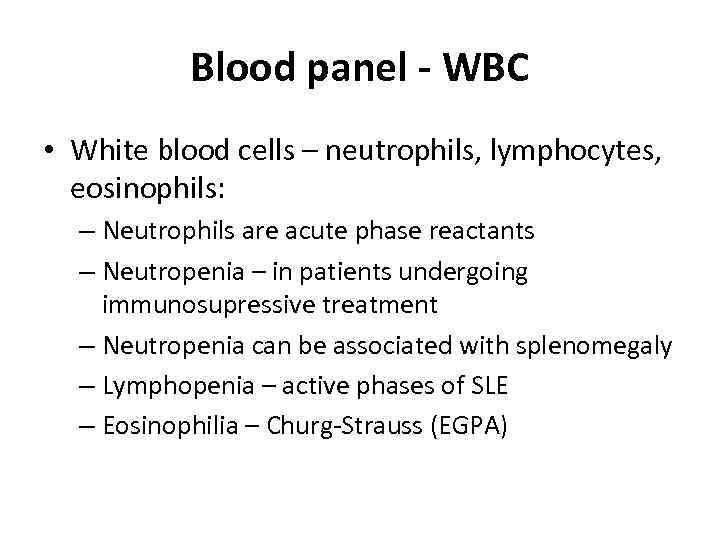
Blood panel - WBC • White blood cells – neutrophils, lymphocytes, eosinophils: – Neutrophils are acute phase reactants – Neutropenia – in patients undergoing immunosupressive treatment – Neutropenia can be associated with splenomegaly – Lymphopenia – active phases of SLE – Eosinophilia – Churg-Strauss (EGPA)
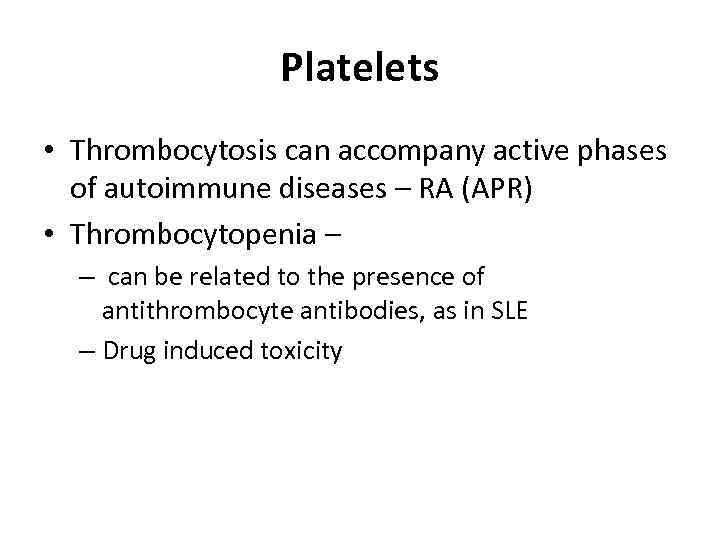
Platelets • Thrombocytosis can accompany active phases of autoimmune diseases – RA (APR) • Thrombocytopenia – – can be related to the presence of antithrombocyte antibodies, as in SLE – Drug induced toxicity
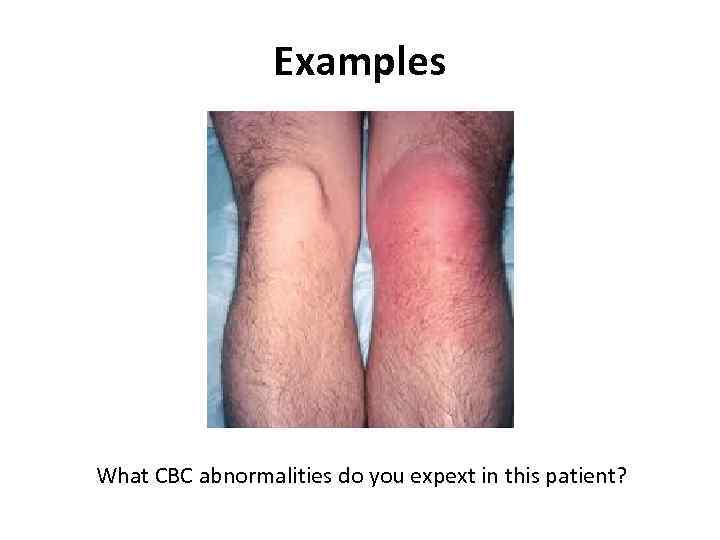
Examples What CBC abnormalities do you expext in this patient?
Biochemical testing- liver • Synthetic activity (albumin, coagulation factors, Glucose, Bil) • Liver enzymes – hepatocellular, cholestatic • Should be ordered before and after initiation of treatment (NSAIDS, DMARDS, including MTX, biological)
Kidney function tests • Connective tissue diseases and systemic vasculitides are frequently associated with kidney involvement – vascular/glomerular/tubular-interstitial • Creatinine/ Creatinine Clearance provide sufficient information • Urinalysis – always part of investigation (hematuria, leukocyturia, proteins) • Monitor for adverse effects of treatment
Uric acid • Commonly included in the workup of patients with arthritis • Elevated in 90% of patients with Gout • Healthy population can also have increased levels of uric acid • Important to monitor urate lowering therapy – goal of <5 -6 mg/d. L
Acute-phase reactants • Are not specific for rheumatic disorders • AP response occurs in a variety of inflammatory conditions – infection, trauma, malignancy. • The most widely used APR – ESR – erythrocyte sedimentation rate – CRP – Ferritin
Acute phase reactants • Produced by hepatocytes upon stimulation by cytokines (IL-1, IL -6, TNF – tumor necrosis factor) • Examples – CRP, fibrinogen, ferritin, haptoglobin, ceruloplasmin, amyloid protein A, complement (C 3, immunoglobilins • ESR and CRP are useful for monitoring the level of inflammation, however sometimes are not sensitive enough and sometimes are “slow” and should not guide the clinical decisions

Example What lab abnormalities do you expect in this patient?
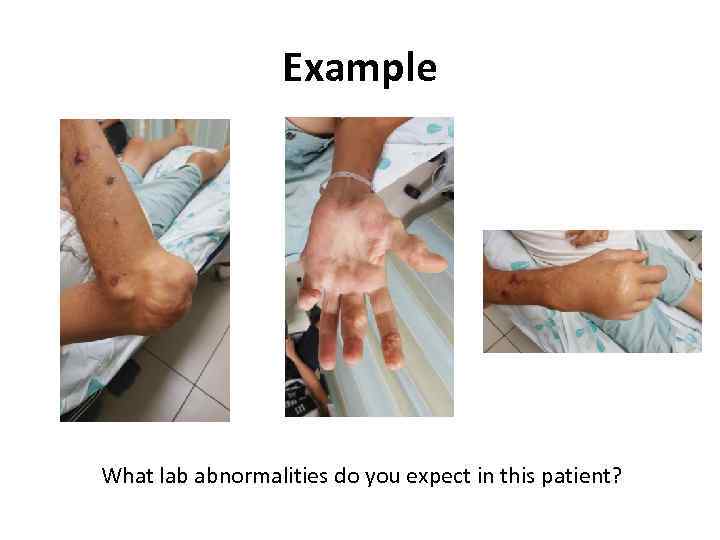
Example What lab abnormalities do you expect in this patient?

Serologic testing • Testing for autoantibodies is frequently used in the diagnoses of rheumatic conditions and sometimes for monitoring of disease activity. • An adjunct to diagnosis and management rather than precise clinical guide.
Rheumatoid Factor • Autoantibodies directed against Fc– chains of Ig. G molecules • Laboratories test only for Ig. M RF • The main immunoglobilin classes of RF that can be easily detected are Ig. A and Ig. M RF • Ig. M RF is produced in many chronic inflammatory conditions – endocarditis, hepatitis B/C, tuberculosis, Idiopathic pulmonary fibrosis, mixed connective tissue disease, SLE, cryoglobulinemia • Can be present in 5% of normal elderly population
Rheumatoid Factor • Not specific for Rheumatoid Arthritis • The main indication for RF testing – suspicion for RA and Sjogren syndrome • The specificity of RF increases with higher titers • Higher titers are associated with more aggressive and erosive disease
Antibodies to citrullinated protein and peptide antigens -ACPA • Citrullination of proteins (arginine – citrullin) occurs as the result of synovial inflammation and inflammation induced apoptosis • RA patients react to such modified proteins by creating ACPAs • ACPAs are especially prevalent in RF patients but can be found in 25% of RF negative patients • ACPAs predict later development of erosive RA • Helpful in discriminating between RA and psoriasis with erosive arthritis
Anti-nuclear Antibodies (ANA) • Immunoglobulins directed against structures within the cell ( i. e. DNA, ribonuclear proteins, histones, and centromere) • Screening tool • Titer/ pattern • Found in a variety of autoimmune diseases such as SLE, MCTD, JRA, scleroderma, Sjogren’s syndrome in high titres (>1: 320) • Almost always present in SLE (95 -98%) • High titer increases the likelihood that the presence of ANA is related to autoimmune disease
ANA • ANAs do not correlate with disease activity • Consider using as a screening test in only symptomatic patients (arthritis, rash, serositis, proteinuria) • Must measure ANAs in patients with JIA (esp. oligoarticular) to assess risk of uveitis

ANA • Low titres (<= 1: 160) found in: – Infections (EBV, CMV, Hepatitis B, bacterial endocarditis, HIV) – Drugs (hydralazine, INH, dilantin, tegretol, ETX, PCN, and sulfas) – Neoplasias (lymphoma) • It is sensitive but not specific • ~ 10% of the population have a positive low titer ANA and can be asymptomatic • As one ages, ANA titers increase
ANA detection and measurement • IIF - the indirect immunofluorescence test is the most widely used assay for the detection of ANA and remains the reference method of choice for the detection of these antibodies • Nuclear staining patterns include: homogeneous, speckled, centromere, and nucleolar
ANA patterns • In the homogeneous staining pattern, the entire nucleus is diffusely stained. EX: Antibodies to histone proteins, DNA, and DNA-histone complexes. • In the speckled staining pattern, fine or coarse speckles are seen throughout the nucleus. Ex: Antibodies against U 1 RNP, Sm, and La antigens. • The centromere pattern - anti centromere • The nucleolar pattern refers to homogeneous or speckled staining of the nucleolus; Ex: fibrillarin, RNA polymerase I and III, Th, PM-Scl, and RNA helicase.
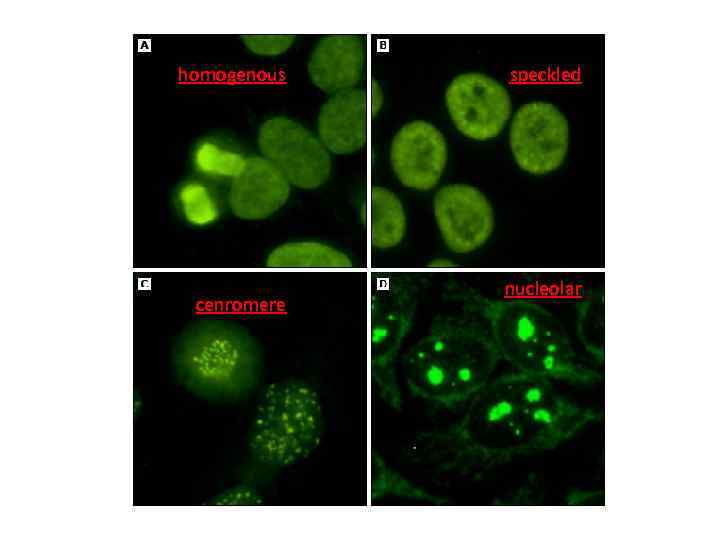
homogenous cenromere speckled nucleolar
ELISA method • Solid phase assays - enzyme-linked immunoabsorbant assays (ELISA) • A panel of purified native or recombinant autoantigens is prepared and each antigen is immobilized on a solid surface • The panel of antigens used in solid phase assays may include all or some of the following: Ro, La, Sm, U 1 RNP, Scl -70, PM-Scl, Jo-1, centromere, histone, ribosomal P, and DNA. • Diluted human serum is incubated with the immobilized antigen and, as with the indirect immunofluorescence assay, a secondary antibody is used to detect bound autoantibodies.

Advantages and Disadvantages • The major advantage of indirect immunofluorescence is the large number of autoantibodies that can be detected. • Some autoantigens may not be present in the HEp-2 cell substrate – The Ro 60 antigen, (SLE, Sjogren’s) – Anti-ribosomal P antibodies (SLE)
Advantages and Disadvantages • The number of autoantigens that are included in solid phase (ELIZA) assays is limited compared with the number that are present in the HEp-2 cell substrate. As an example, most solid phase assays do not contain antigens found in the nucleolus; patients with autoantibodies directed against these structures will have a falsely negative solid phase ANA result
Anti-ds. DNA antibodies • Antibodies that target DNA • Produce homogenous pattern in ANA IIF • Positive result for anti-ds. DNA screening should be confirmed by additional assays • Anti-ds. DNA antibody testing is very specific (95%), but less sensitive (70%) for SLE • Are associated with disease activity in lupus nephritis

Anti-histone antibodies • Found in 95% of patients with drug-induced lupus syndrome • Seen with: – Procainamide – Quinidine – Hydralazine – Phenytoin or other anti-epileptics

Anti-Sm and anti-RNP antibodies “extractable” (ENA) • Produce coarse speckled pattern in ANA IIF • The nucleoli are spared • Anti-Sm antibodies are almost exclusive for SLE patients, not sensitive (10 -40%) • RNP antibodies are part of diagnosis of Mixed Connective Tissue Disease (a syndrome of arthritis, myositis, Raynauds’ and sclerodactly) • RNP antibodies are not specific for MCTD
Anti-Sm and anti-RNP antibodies “extractable” (ENA) • Anti-Sm antibodies generally remain positive, even when a patient has entered remission. The titer of anti-ds. DNA antibodies may fall into the normal range when a patient’s disease is quiescent • Anti-U 1 RNP antibodies may be found in 3 to 69 percent of patients with SLE. High levels of anti-U 1 RNP antibodies are always present in patients with mixed connective tissue disease (MCTD)
Anti-Ro (SS-A) and anti-La (SS-B) antibodies (ENAs) • Two sets of names assigned by two different groups; first seen in Sjogren’s patients and then seen in SLE patients • Anti Ro/SS-A antibodies seen in: – 5 -15% of normals – 50% of Sjogren’s patients – 30% of SLE patients (many have negative ANA or subacute cutaneous lupus) – Correlates with active nephritis and cytopenias
Anti-Ro (SS-A) and anti-La (SS-B) antibodies • Produce fine speckled pattern in ANA IIF with staining of the nucleoli as well • Are part of the classification criteria for Sjogren syndrome, but are also frequent in SLE patients • Are associated with cutaneous lupus and photosensitivity • Associated with neonatal lupus and congenital heart block
Anticentomere and anti-SCL-70 • Anticentromere antibodies (ACA) produce a typical pattern in ANA IIF by staining the centromere region of the chromosomes – this pattern is pathognomonic • The presence of anti-Scl-70 antibodies should be confirmed using ELIZA • These two antibodies are associated with distinct clinical pictures and are mutually exclusive • Anti-Scl-70 antibodies (also known as antitopoisomerase I) are associated with increased risk of pulmonary fibrosis in both limited and diffuse cutaneous systemic sclerosis
Antineutrophil cytoplasmic antibodies - ANCA • Subgroup of neutrophil specific antibodies • Commonly directed to myeloperoxidase (MPO) - and proteinase 3 (PR 3) • P-ANCA – perinuclear staining (MPO) • C-ANCA – cytoplasmic staining (PR 3) • Positive result on IIF should be confirmed using ELIZA • ANCA is useful in diagnosis of ANCA – associated vasculitides (GPA, EGPA, microscopic polyangiitis) • C-ANCA – GPA • P-ANCA – EGPA, microscopic polyangiitis

ANCA • c-ANCA is seen in 90% of GPA (Wegener’s granulomatosis) • p-ANCA is associated with microscopic polyangiitis, EGPA (Churg-Strauss), and Ulcerative Colitis • Consider vasculitis (ANCA) if patient has: – Fever of unknown origin – Palpable purpura, vasculitis urticaria, or dermal necrosis – Mononeuritis multiplex – Unexplained arthritis, myositis, or serositis – Unexplained pulmonary, CV or renal disease
Complement • The most frequent clinical parameters used for judging complement activation – C 3, C 4 • C 3, C 4 are APR • C 3, C 4 consumption is associated with immune complexes diseases • In SLE low levels reflect disease activity and in lupus nephritis normalization is associated with better outcomes
Antiphospholipid antibodies (APLA) • Anti-cardiolipin antibodies (ELIZA) – Ig. G – better related to procoagulant activity compared to Ig. M/Ig. A • β 2 glycoprotein-1 Ig. G and Ig. M • Lac - functional assay for the lupus anticoagulant (LA) phenomenon (prolonged a. PTT/d. RVVT) not corrected by control plasma but shortened by adding excess phospholipid

Examples • 24 y woman presents with weakness, nausea, ptechia and echymozes
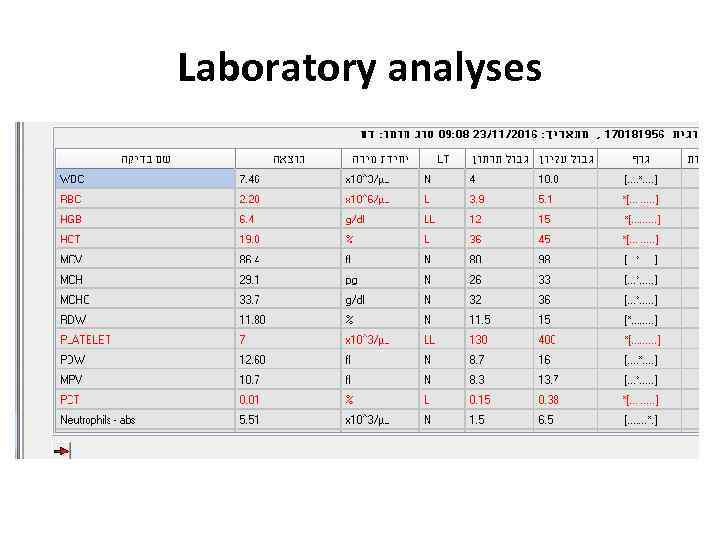
Laboratory analyses
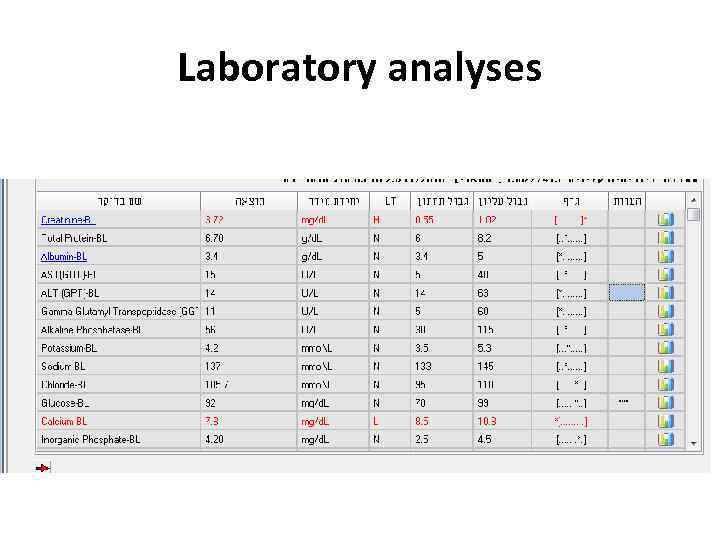
Laboratory analyses
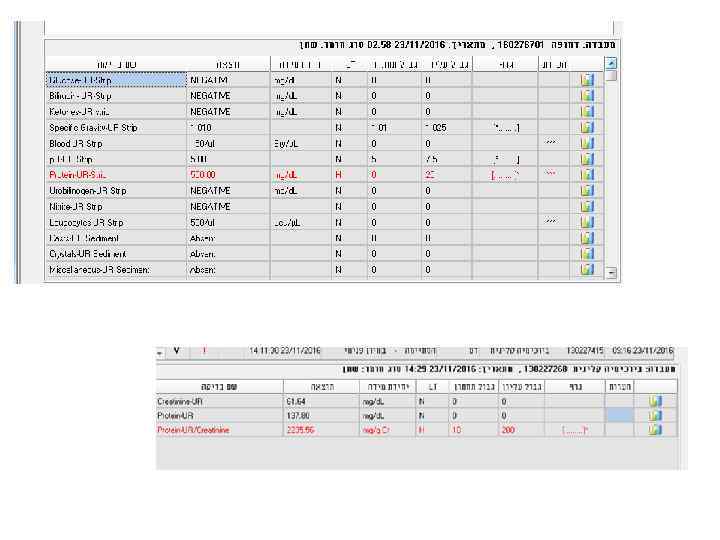
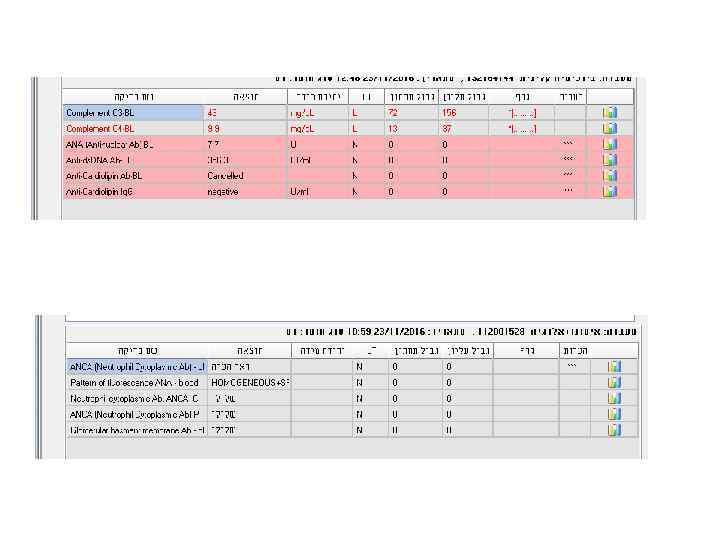

Examples • 28 y old woman presents with cough, fever, dyspnea, fatigue
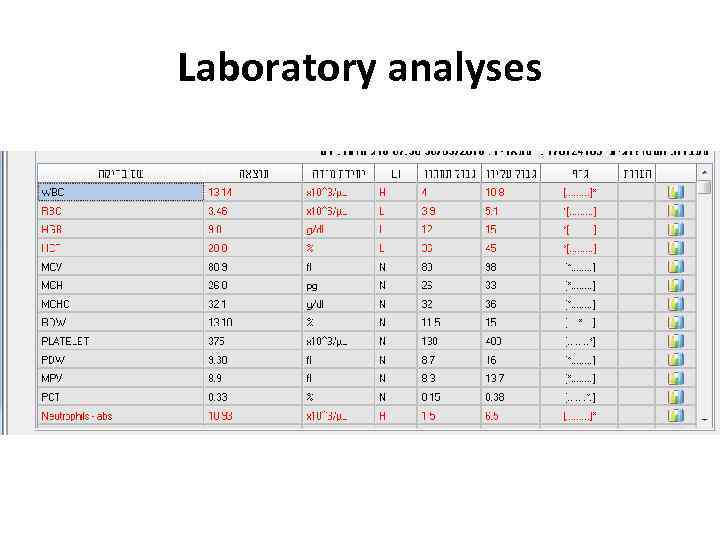
Laboratory analyses
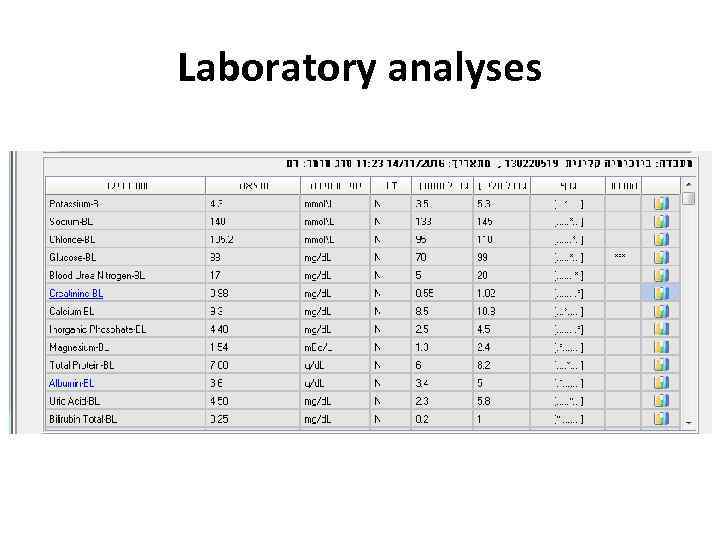
Laboratory analyses
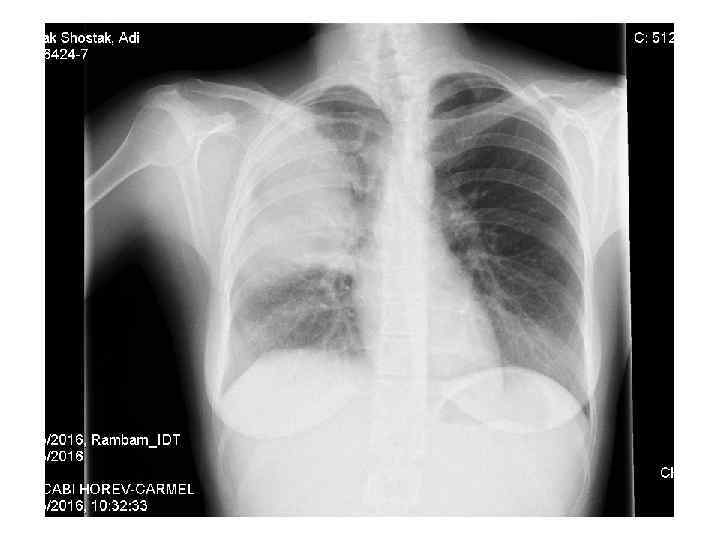
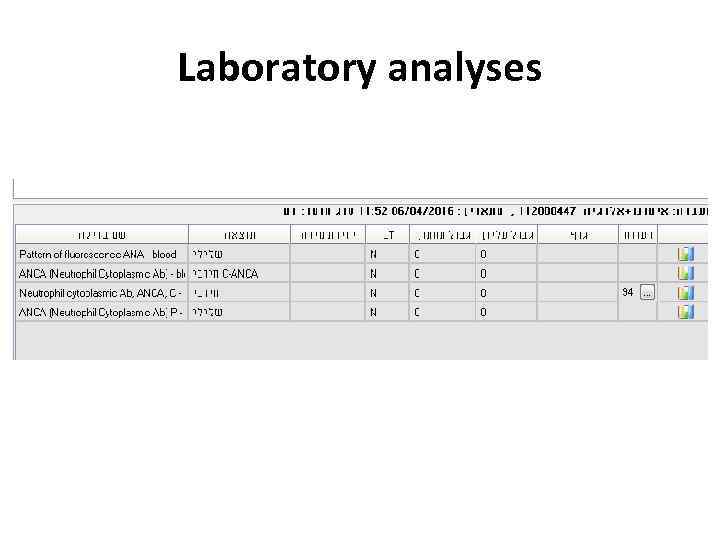
Laboratory analyses
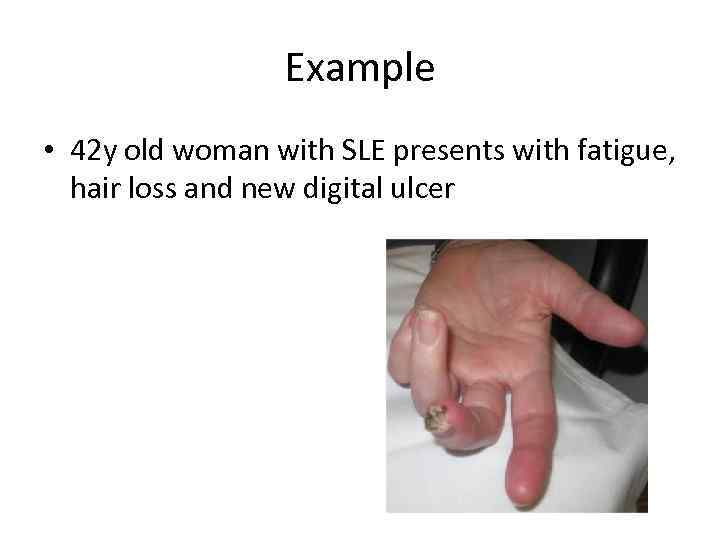
Example • 42 y old woman with SLE presents with fatigue, hair loss and new digital ulcer
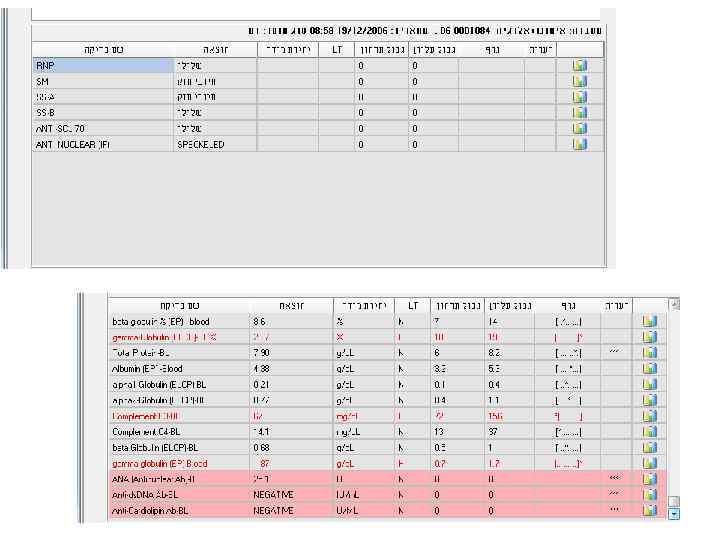
Examples
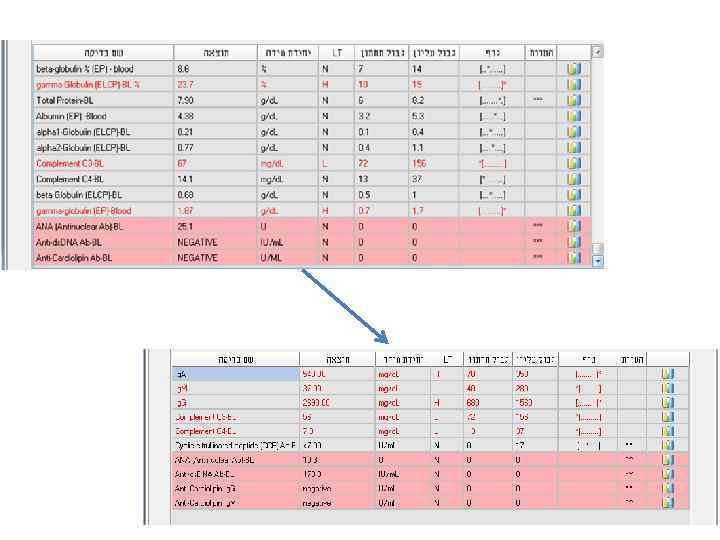
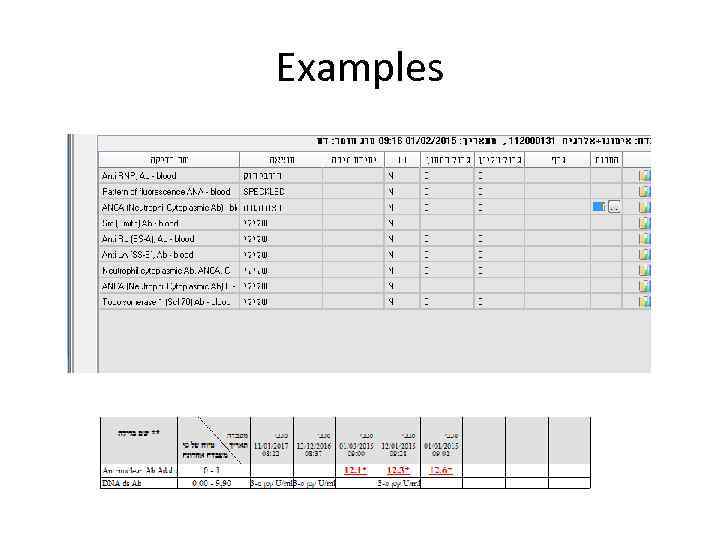
Examples
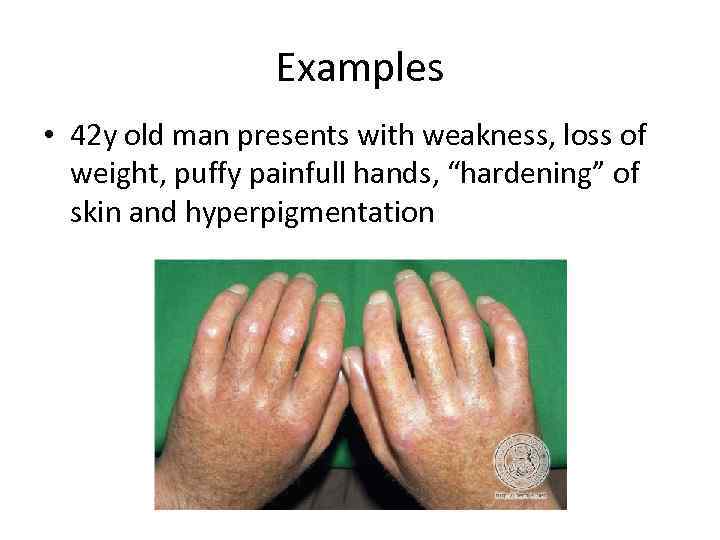
Examples • 42 y old man presents with weakness, loss of weight, puffy painfull hands, “hardening” of skin and hyperpigmentation
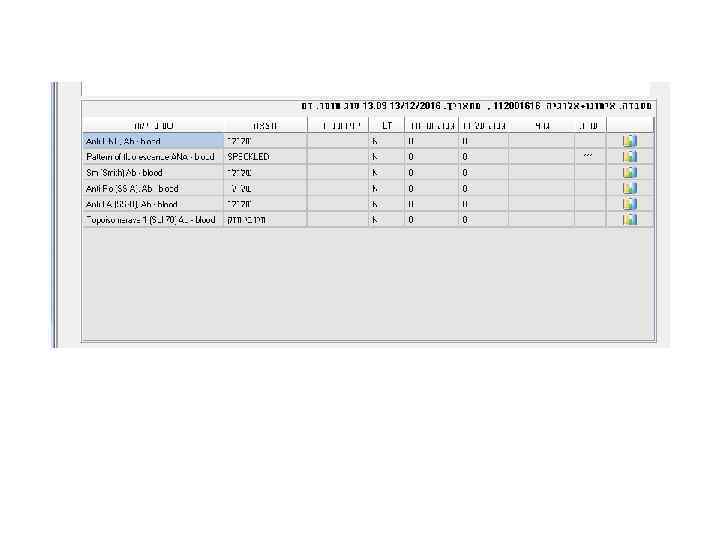
Laboratory tests in Rheumatology.ppt