3f08d05f8dd187987f9d3e6fc649e58b.ppt
- Количество слайдов: 68

Laboratory Information Management Systems Douglas Perry, Ph. D. IU School of Informatics

Laboratory Information § The sole product of any laboratory, serving any purpose, in any industry, is information 2

Laboratory Informatics Defined § The specialized application of information technology to optimize and extend laboratory operations 3
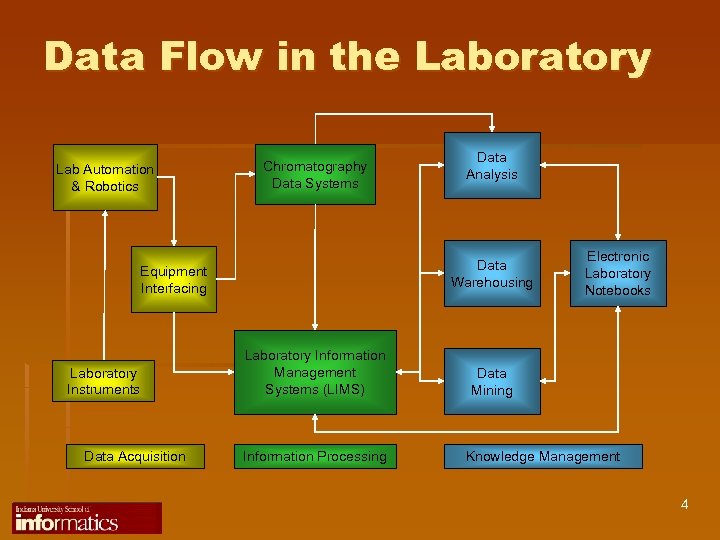
Data Flow in the Laboratory Lab Automation & Robotics Chromatography Data Systems Data Warehousing Equipment Interfacing Laboratory Instruments Data Acquisition Data Analysis Laboratory Information Management Systems (LIMS) Information Processing Electronic Laboratory Notebooks Data Mining Knowledge Management 4
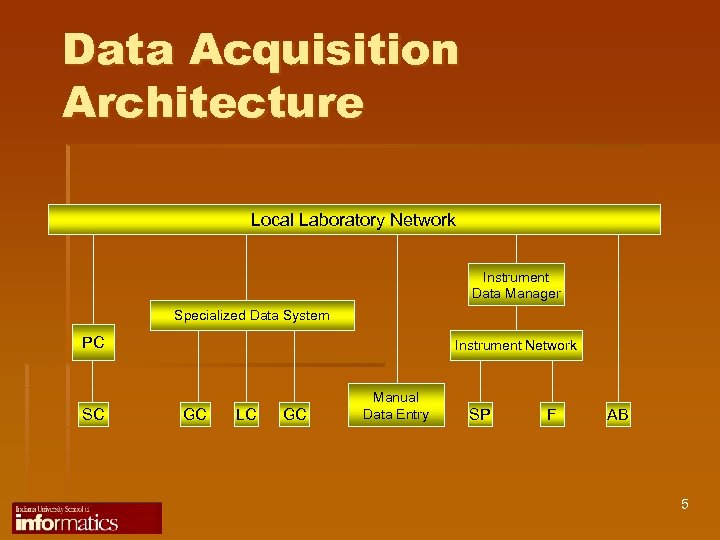
Data Acquisition Architecture Local Laboratory Network Instrument Data Manager Specialized Data System PC SC Instrument Network GC LC GC Manual Data Entry SP F AB 5
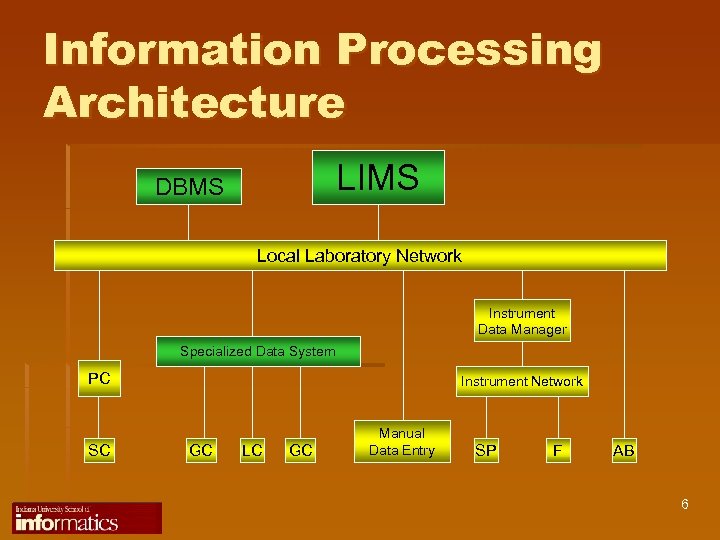
Information Processing Architecture LIMS DBMS Local Laboratory Network Instrument Data Manager Specialized Data System PC SC Instrument Network GC LC GC Manual Data Entry SP F AB 6
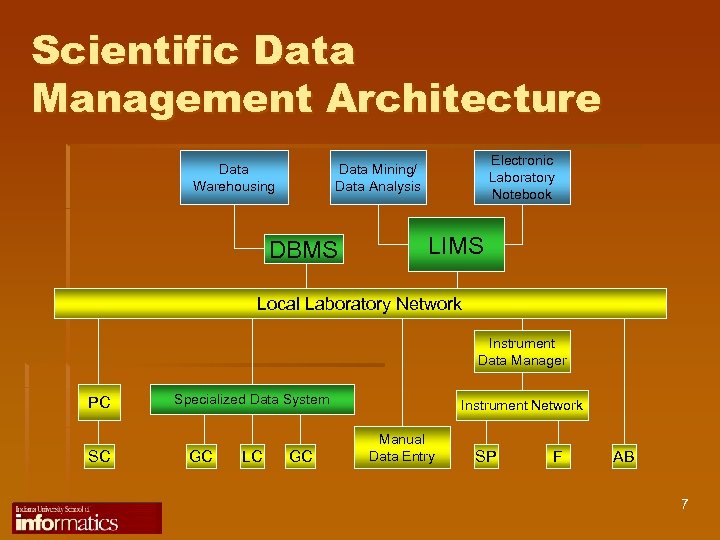
Scientific Data Management Architecture Data Warehousing Electronic Laboratory Notebook Data Mining/ Data Analysis DBMS LIMS Local Laboratory Network Instrument Data Manager PC SC Chromatography Data System Specialized Data System GC LC GC Instrument Network Manual Data Entry SP F AB 7
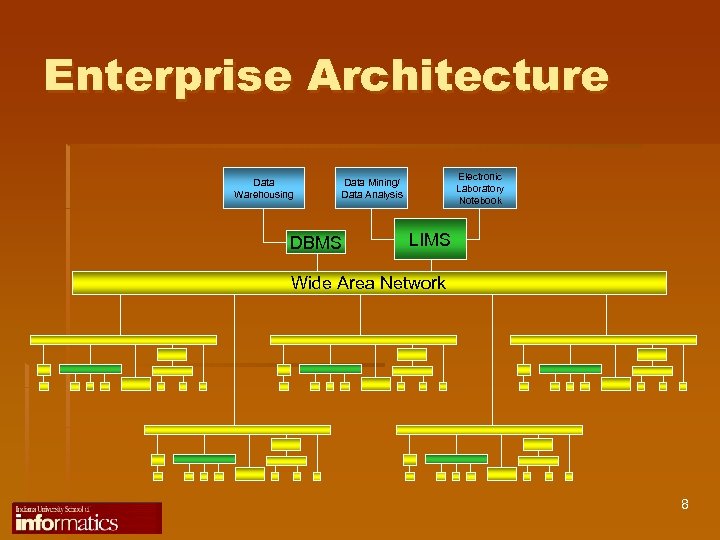
Enterprise Architecture Data Warehousing Electronic Laboratory Notebook Data Mining/ Data Analysis DBMS LIMS Wide Area Network 8
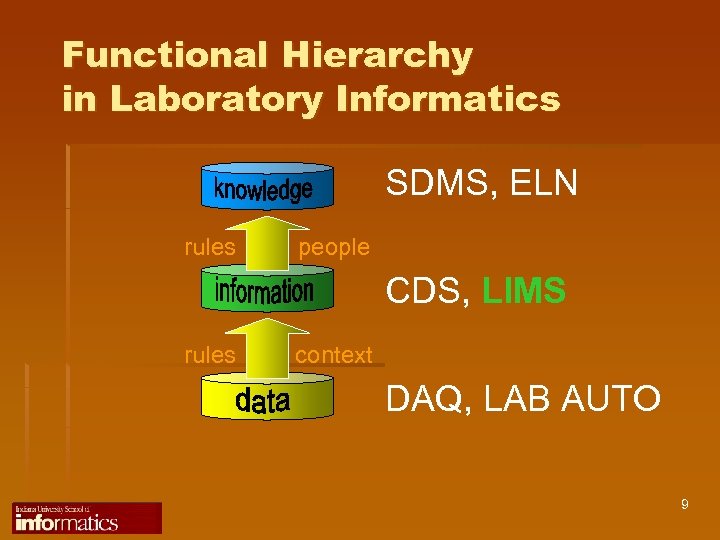
Functional Hierarchy in Laboratory Informatics SDMS, ELN rules people CDS, LIMS rules context DAQ, LAB AUTO 9
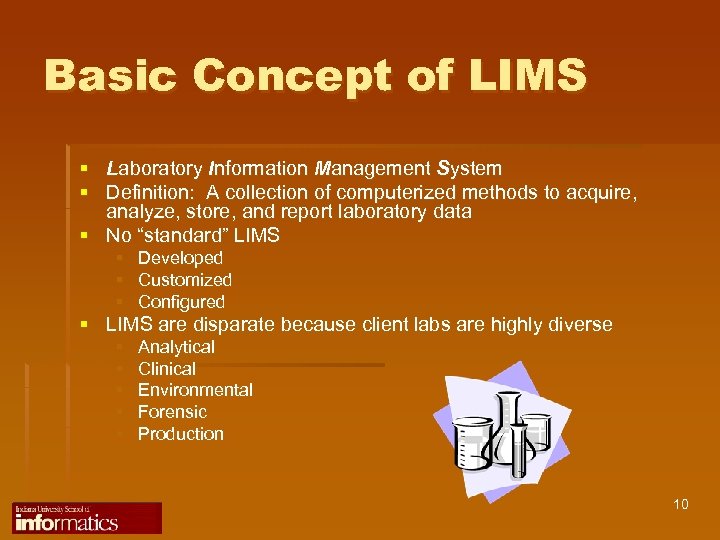
Basic Concept of LIMS § Laboratory Information Management System § Definition: A collection of computerized methods to acquire, analyze, store, and report laboratory data § No “standard” LIMS § § § Developed Customized Configured § LIMS are disparate because client labs are highly diverse § § § Analytical Clinical Environmental Forensic Production 10
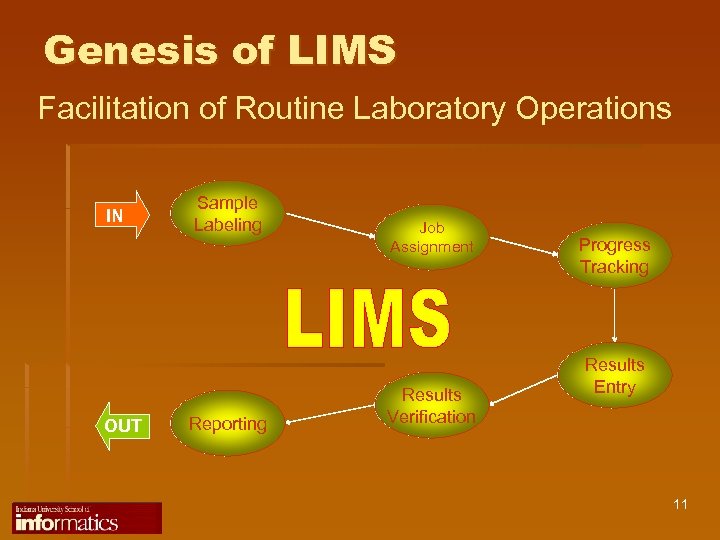
Genesis of LIMS Facilitation of Routine Laboratory Operations IN OUT Sample Labeling Reporting Job Assignment Results Verification Progress Tracking Results Entry 11
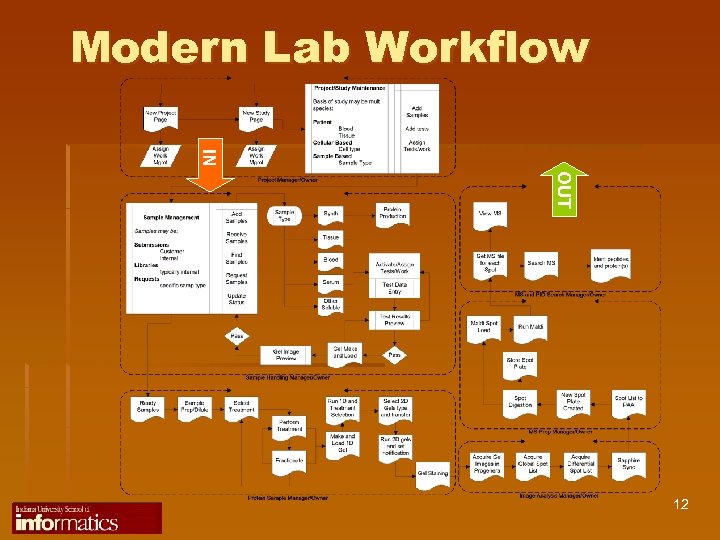
Modern Lab Workflow IN OUT 12

Challenge and Opportunity 1988 2003 1 experiment 1 gene 10, 000 genes 10 data 10, 000 data 13
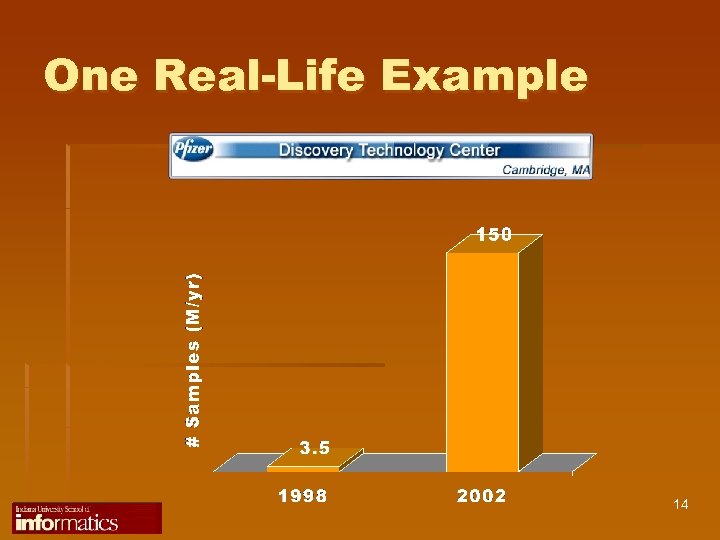
One Real-Life Example 14
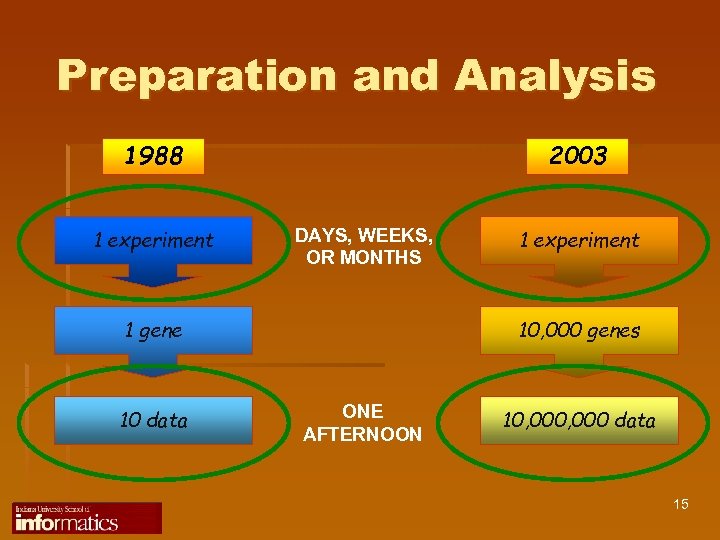
Preparation and Analysis 1988 1 experiment 2003 DAYS, WEEKS, OR MONTHS 1 gene 10 data 1 experiment 10, 000 genes ONE AFTERNOON 10, 000 data 15

Universal Need for LIMS § Regardless of focus, all labs need: § § Quality assurance and control Error reduction Fast sample turnaround Management of information 16

Increasing Need for LIMS: Information Management § Advances in instrument automation § Robotics for sample processing § Microarray technology § Increased government regulations § Gx. P: GLP, GMP, GCP § Demands of enterprise resource planning § CRM, MRP, MES 17

Increasing Need for LIMS: Quality Assurance & Control § § Quality assurance (QA) Quality control (QC) Statistical process control (SPC) ISO 9000 18

Increasing Need for LIMS: Error Reduction § Data entry restriction § Acceptable parameters § Drop-down lists § Range checking § Customer specifications § Internal controls § Sample log-in § Bar code reader § Automatic calculations 19
Increasing Need for LIMS: Sample Turnaround § § Automated data entry Automatic calculations Rapid data retrieval Automatic reporting 20
Types of Data Used in LIMS § § § Alphanumeric Descriptive Limits Numeric TDU Stamp 21

Types of Laboratories Using LIMS § § § Research & Development labs Analytical labs Manufacturing labs 22

Research & Development Laboratories § Objective § Support pure or applied research § Characteristics § § § Small, autonomous Diverse, non-routine tests Low sample volume Flexible operations High internal security Low, circumscribed data flow 23

LIMS requirements for R&D Labs § Flexibility § Sample types, tests, methods, reports § Traceability § Audit trails, on-the-fly notation § Security § Very limited access, but with lateral authorization § Time § Usually not an issue 24

Analytical Laboratories § Objective § Provide a service (information) § Characteristics § Large, organization-dependent § Routine tests § High sample volume § Client-driven operations § High, narrow data flow 25

LIMS Requirements for Analytical Labs § Tracking § Samples, orders, reports § Scheduling § Tests, equipment maintenance § Quality assurance § Validation, QA/QC § Data access and sharing § Instrument interfacing § Client-centered reporting, Co. A 26

Manufacturing Laboratories § Objective § Assure product specifications § Statistical process control § Characteristics § Ongoing testing: raw materials, process, final product, stability § Dynamic, demanding environment § High, wide data flow § Fast turnaround 27

LIMS Requirements for Manufacturing Labs § Rapid sample turnaround § Automation, bar-code entry § Connectivity § MRP, ERP, CRM § Statistical analysis § Statistical process control § Flexible reporting § Diverse information demands 28
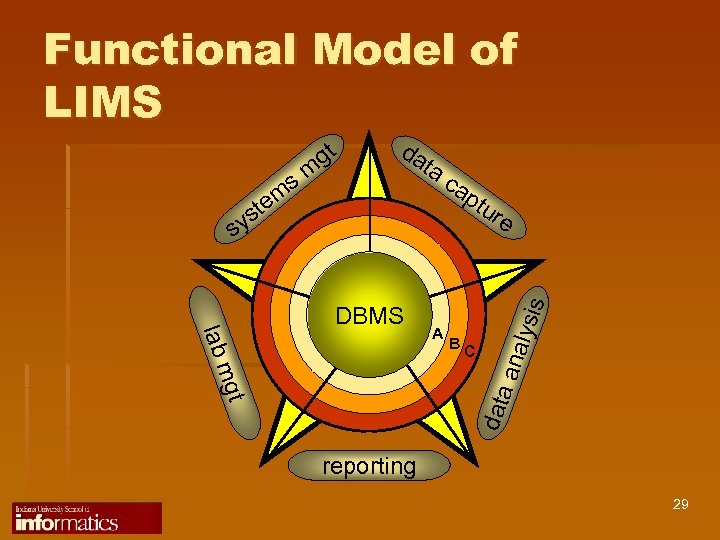
Functional Model of LIMS DBMS ta ca ptu re lab B C mgt data A lysis m ste sy sm da ana gt reporting 29
Data Capture § Sample identification § Log-In, reading, labeling § Work scheduling § Test initiation, test assignment § Data acquisition § Interfacing, instrument control 30
Data Analysis § Data transfer § Buffer tapping, file transfer § Data processing § Conversion, reduction, specification review, statistical analysis 31

Reporting § § § § Client-centered reports User-defined reports Automated batch reports Tabular and graphical formats Ad hoc queries Event triggers Exportation to external IS 32

Lab Management § § Work scheduling Sample tracking Job tracking Standard Operating Protocols (SOP) § Pricing and invoicing § Cost analysis 33
Systems Management § Security § External: unauthorized access § Internal: data sabotage § Data archiving § Mirroring § Off-loading § Data warehousing § Long-term storage § Far-off retrieval 34
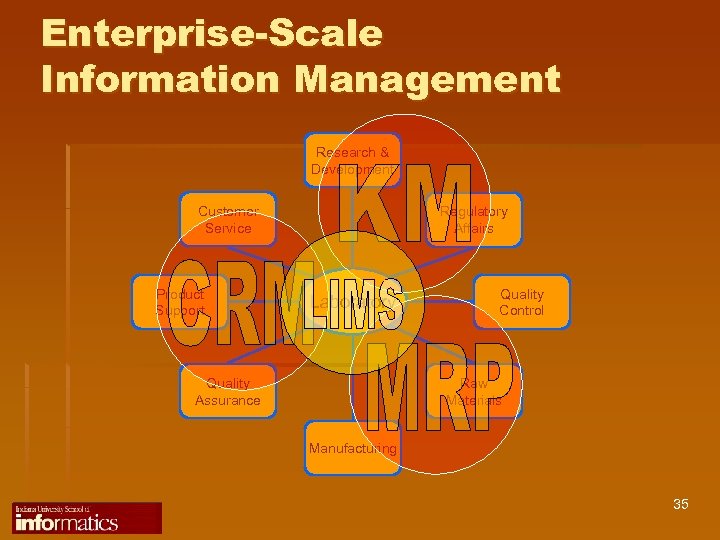
Enterprise-Scale Information Management Research & Development Customer Service Product Support Regulatory Affairs Laboratory Quality Assurance Quality Control Raw Materials Manufacturing 35
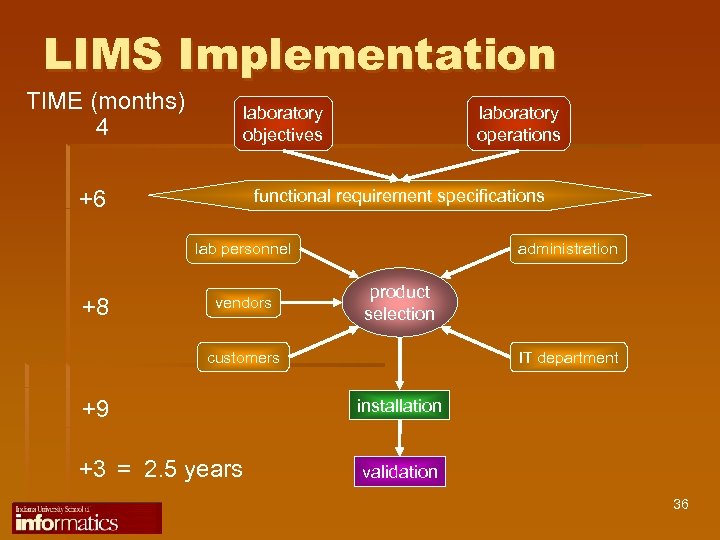
LIMS Implementation TIME (months) 4 laboratory objectives +6 laboratory operations functional requirement specifications lab personnel +8 vendors administration product selection customers IT department +9 installation +3 = 2. 5 years validation 36

LIMS Functionality Examples using Labware™ LIMS
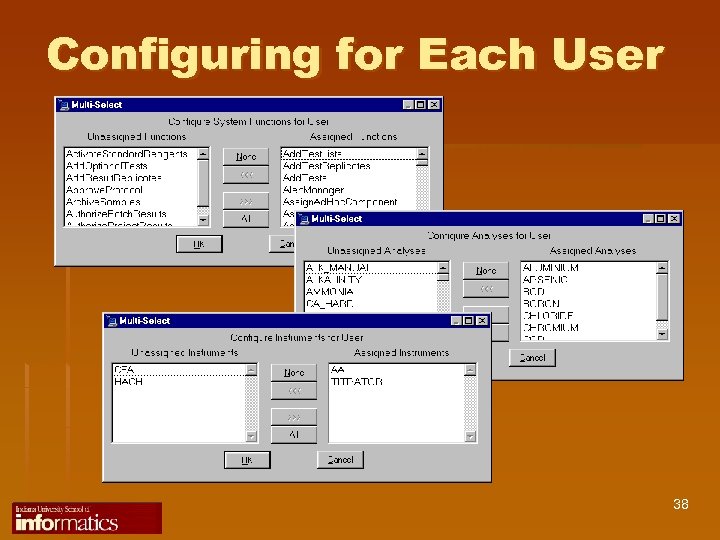
Configuring for Each User 38
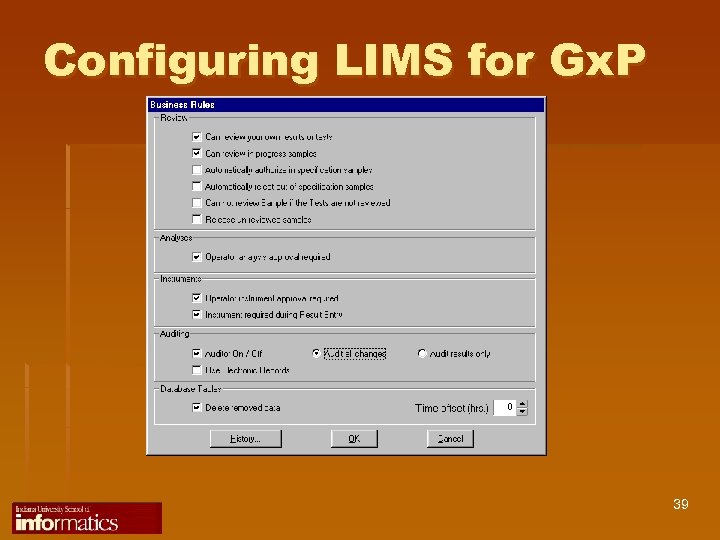
Configuring LIMS for Gx. P 39
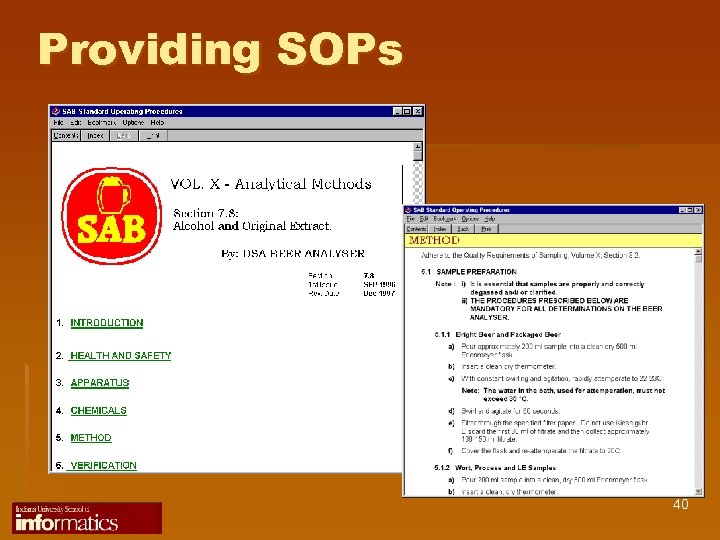
Providing SOPs 40
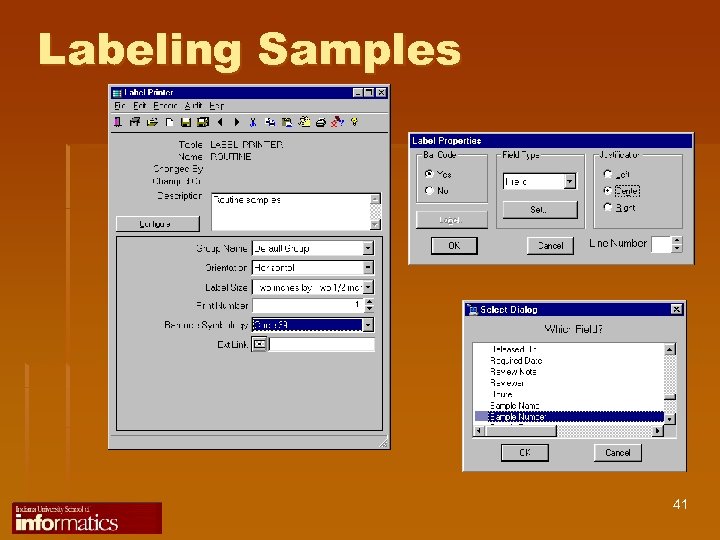
Labeling Samples 41
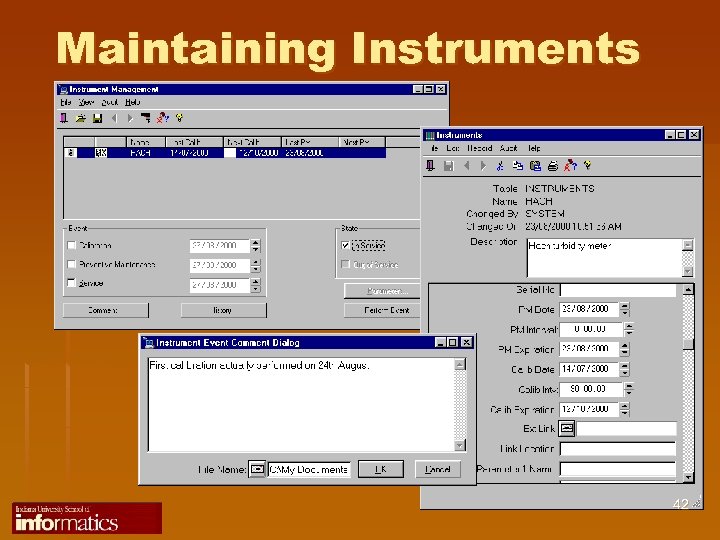
Maintaining Instruments 42
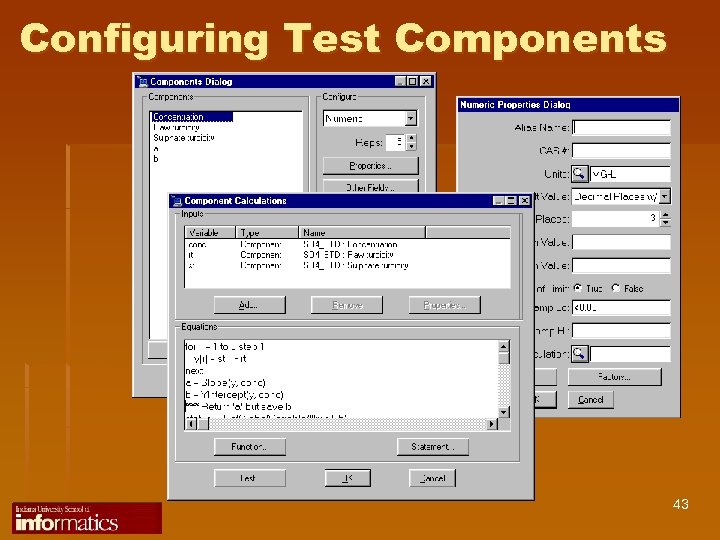
Configuring Test Components 43
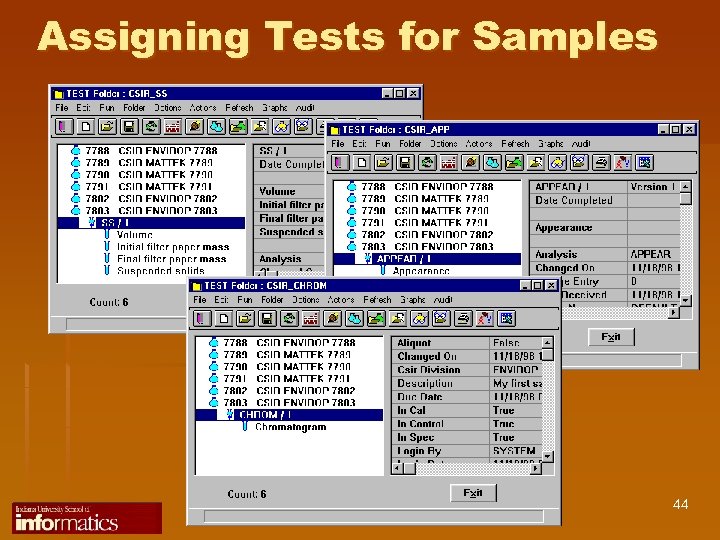
Assigning Tests for Samples 44
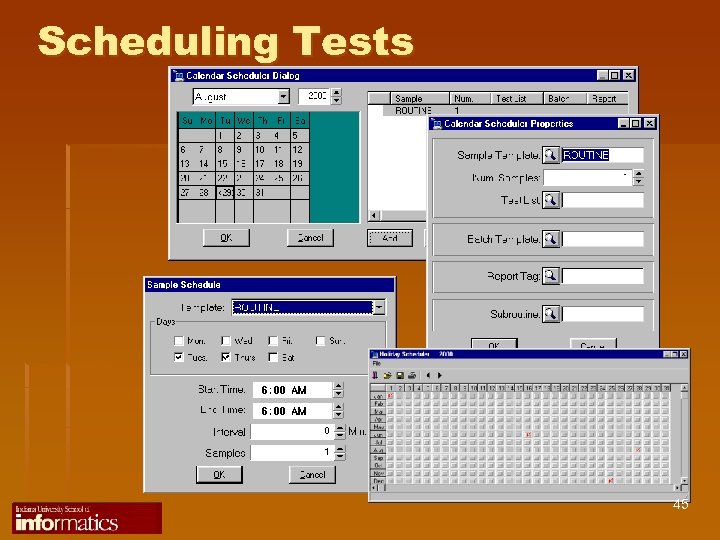
Scheduling Tests 45
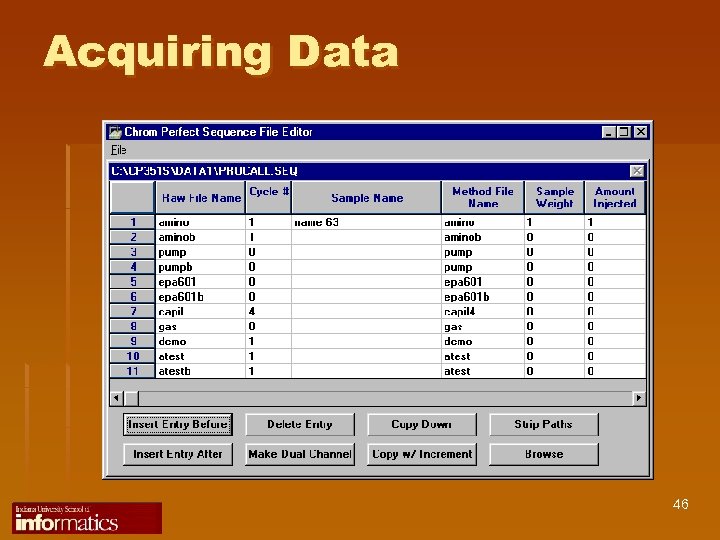
Acquiring Data 46
Capturing Data 47
Setting Result Responses 48
Retesting with Audit Trail 49
Reviewing Sample Status 50
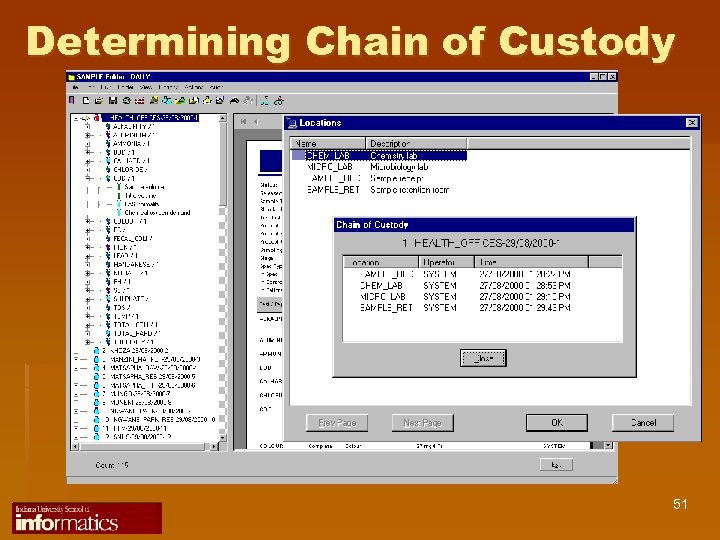
Determining Chain of Custody 51
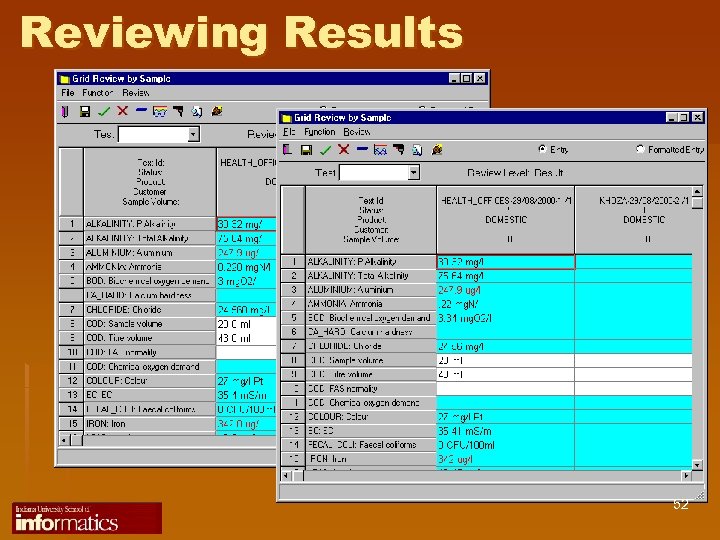
Reviewing Results 52
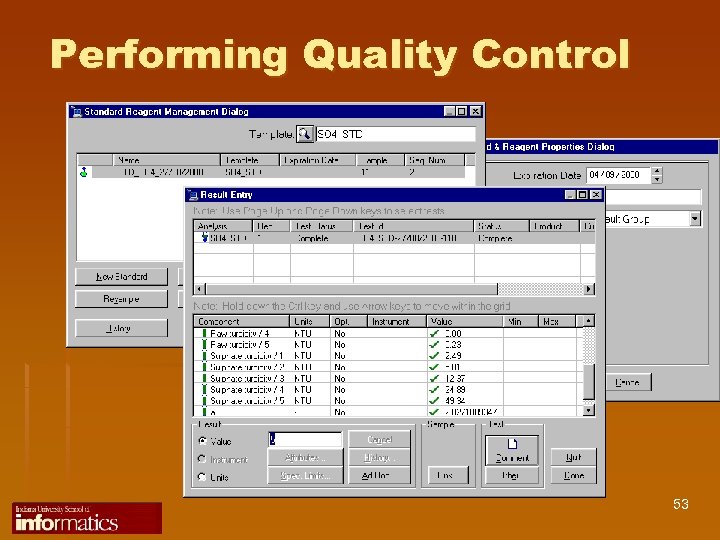
Performing Quality Control 53
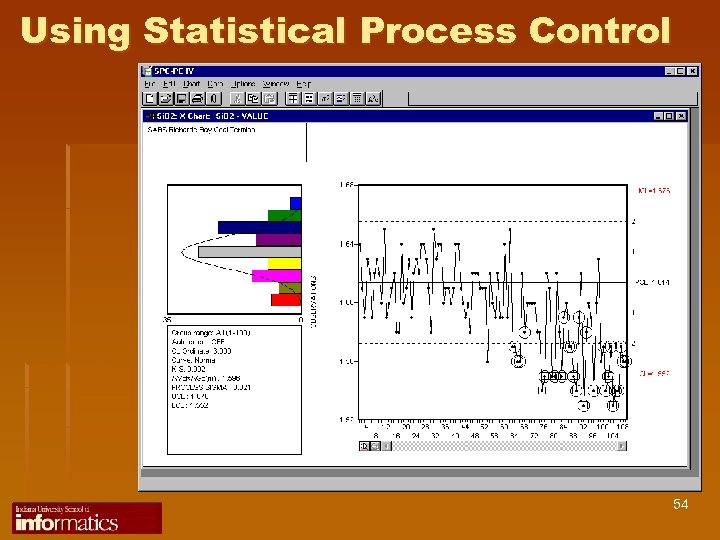
Using Statistical Process Control 54
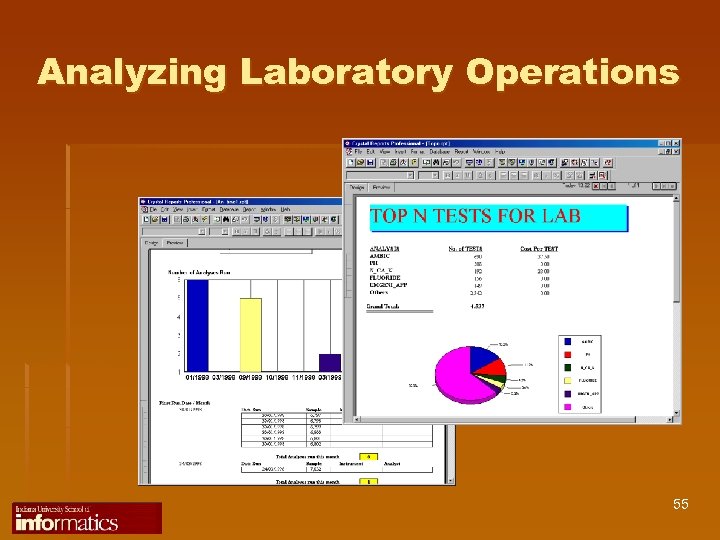
Analyzing Laboratory Operations 55
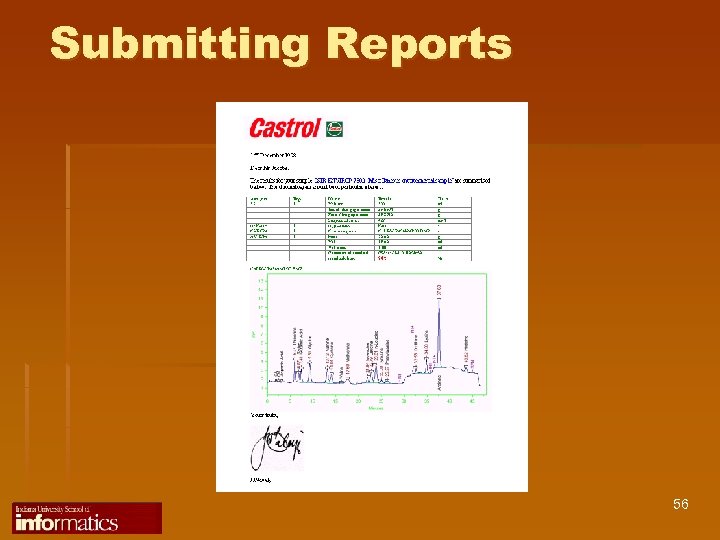
Submitting Reports 56
LIMS Functionality Examples using Lab. Vantage Sapphire™
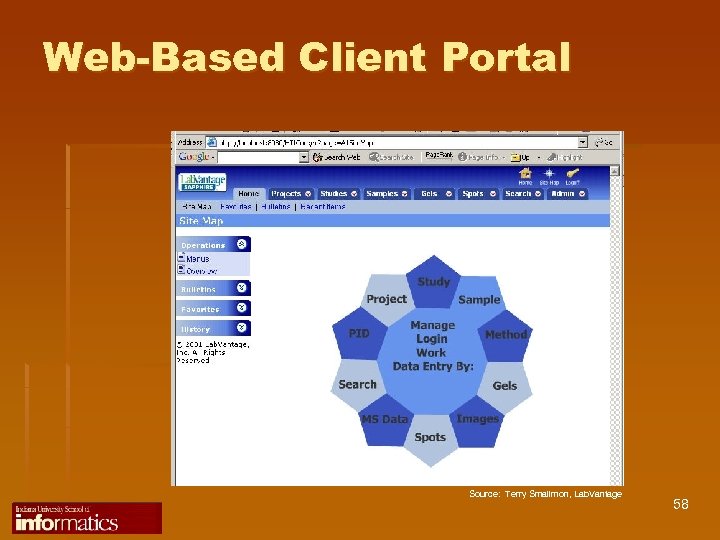
Web-Based Client Portal Source: Terry Smallmon, Lab. Vantage 58
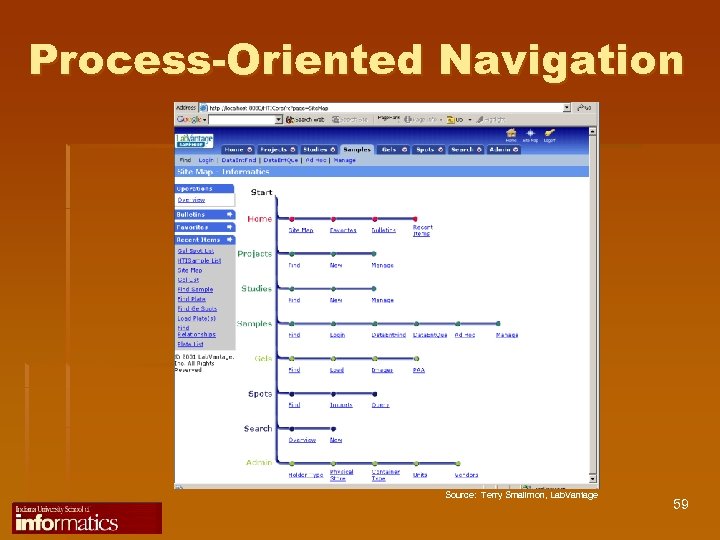
Process-Oriented Navigation Source: Terry Smallmon, Lab. Vantage 59
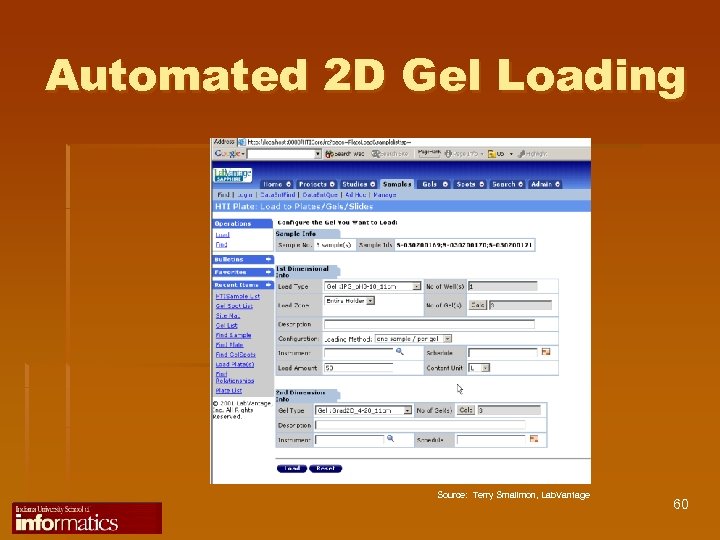
Automated 2 D Gel Loading Source: Terry Smallmon, Lab. Vantage 60
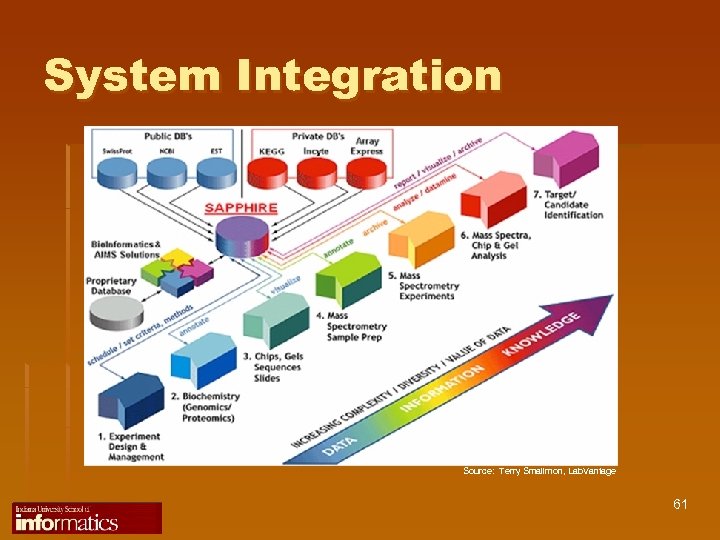
System Integration Source: Terry Smallmon, Lab. Vantage 61
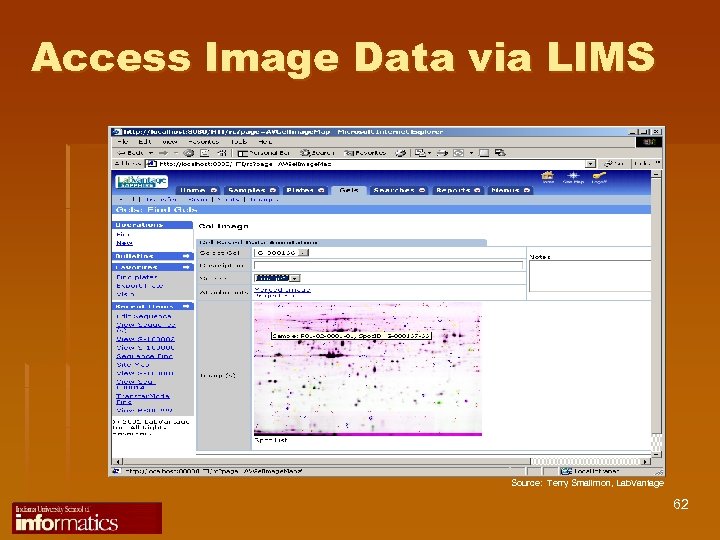
Access Image Data via LIMS Source: Terry Smallmon, Lab. Vantage 62
Connect Disparate Data Sources via LIMS Source: Terry Smallmon, Lab. Vantage 63
Link Results to Database via LIMS Source: Terry Smallmon, Lab. Vantage 64
Link Multiple Search Engines to Database via LIMS Source: Terry Smallmon, Lab. Vantage 65
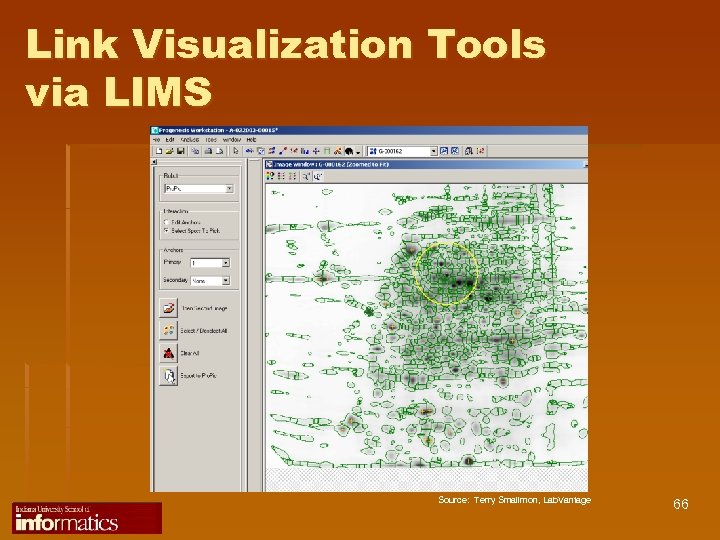
Link Visualization Tools via LIMS Source: Terry Smallmon, Lab. Vantage 66
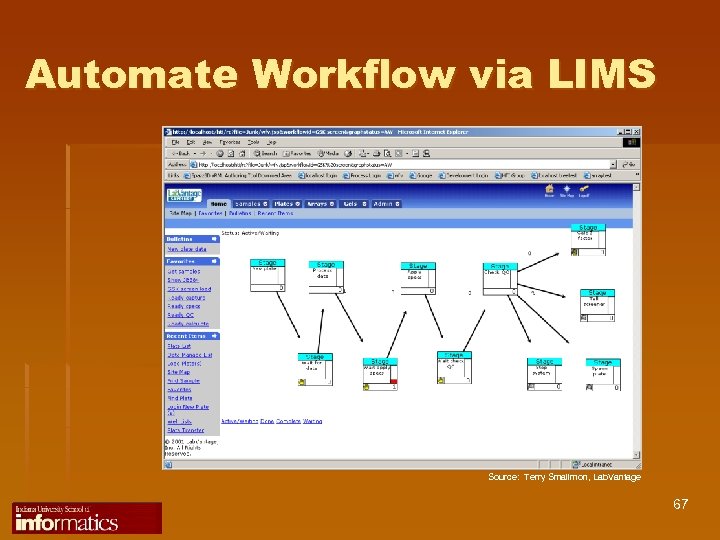
Automate Workflow via LIMS Source: Terry Smallmon, Lab. Vantage 67

Questions & Comments
3f08d05f8dd187987f9d3e6fc649e58b.ppt