38a1a9b3b47d5aa7c20faf1634c01d48.ppt
- Количество слайдов: 14

Laboratory Assessment Tool-LAT Philippe Dubois Phom Penh, Cambodia April 2 -12, 2013
Overview The target audience is any stakeholder performing laboratory assessments: national health authorities, multilateral agencies, Non. Governmental Organizations , laboratory managers, etc. This document describes a general process for assessing laboratories and provides two questionnaires to help assess national laboratory systems (Annex 1) and individual laboratories (Annex 2). http: //www. who. int/ihr/lyon/hls/en/index. html

1. 1 Rationale « Laboratory services are an essential and fundamental part of all health systems. Reliable and timely laboratory tests are at the centre of the efficient treatment of patients. Moreover, prevention and management of infectious and noncommunicable diseases requires accurate laboratory diagnostic information. Many therapeutic decisions rely heavily on data from health laboratories and, at the time of disease outbreaks or other public health events, laboratories are at the very heart of the public health investigation and response mechanisms. Today’s world cannot afford unreliable laboratory results, wasting precious time, precious samples, and too often, precious lives. »

« Moreover, the International Health Regulations (IHR), adopted by the World Health Assembly in 2005, have placed specific responsibilities on WHO Member States for building and strengthening national capacities for the surveillance, detection, assessment, early notification and response to disease outbreaks and other emergencies of potential public health concern 5. Laboratories are obviously playing a critical role in this surveillance and response process. In this framework, monitoring and evaluation of laboratory capacity require a standardized approach and methodology. »
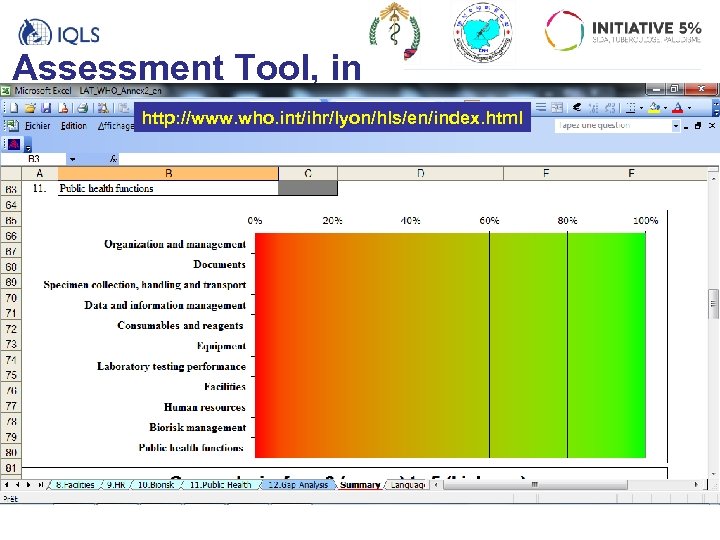
Assessment Tool, in Excel http: //www. who. int/ihr/lyon/hls/en/index. html
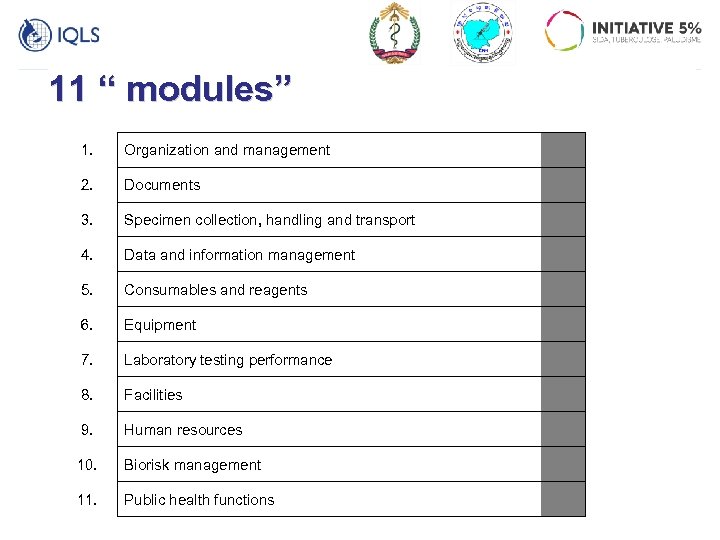
11 “ modules” 1. Organization and management 2. Documents 3. Specimen collection, handling and transport 4. Data and information management 5. Consumables and reagents 6. Equipment 7. Laboratory testing performance 8. Facilities 9. Human resources 10. Biorisk management 11. Public health functions
Each module addresses 20 to 40 topics
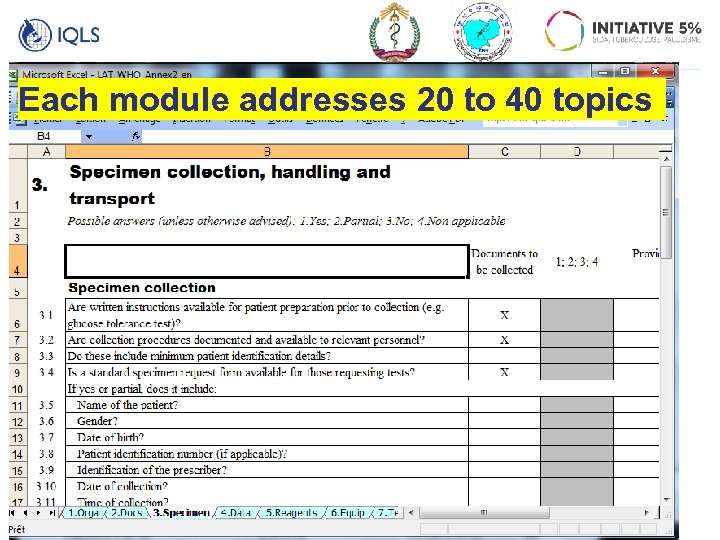
Each module addresses 20 to 40 topics
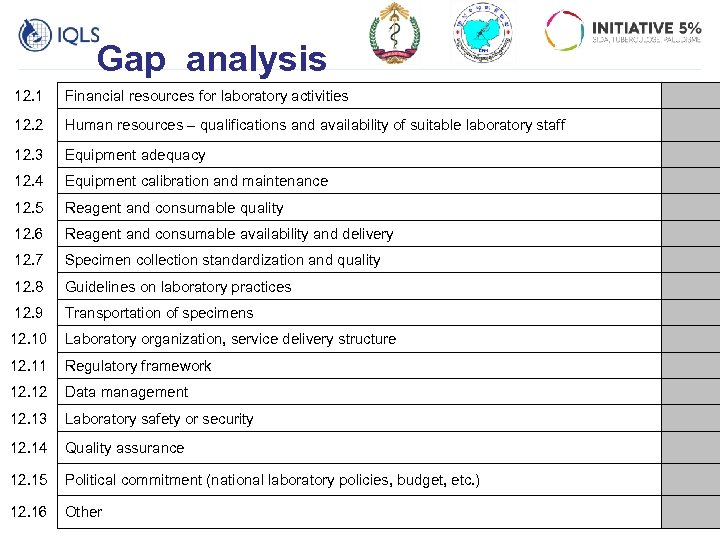
Gap analysis 12. 1 Financial resources for laboratory activities 12. 2 Human resources – qualifications and availability of suitable laboratory staff 12. 3 Equipment adequacy 12. 4 Equipment calibration and maintenance 12. 5 Reagent and consumable quality 12. 6 Reagent and consumable availability and delivery 12. 7 Specimen collection standardization and quality 12. 8 Guidelines on laboratory practices 12. 9 Transportation of specimens 12. 10 Laboratory organization, service delivery structure 12. 11 Regulatory framework 12. 12 Data management 12. 13 Laboratory safety or security 12. 14 Quality assurance 12. 15 Political commitment (national laboratory policies, budget, etc. ) 12. 16 Other

What is the tool designed for? Mostly, a support for assessment: Produce indicators: – Initial assessment – After one year – At the end of the programme Is there any changes? This is not automatic (comparison has to be done manually) Other purpose (maybe with some refinement) – Assessments done by region/subregion – Global laboratory assessments by WHO programmes and partners – PHLs in general (self-assessment)

The assessment process One needs at least half day for a reference laboratory First: a meticulous visit of the lab (90 minutes) check fridges, freezers, incubators, shelves, staff at work, waste, general conditions of the building, temperature charts, biosafety conditions, sampling room, washing room, logbooks, QC … discussion with the staff, advice and recommendations, photos Second: filling-in the tool’s questionnaire, directly on the computer (90 -110 minutes) with the respondent Finally: conclusion, discussion, recommendations, main points to work on, copy of the file on a floppy to be kept by the Director (30 -60 minutes, sometimes more)
How indicators are calculated One or several questions are included in one indicator – Usually « yes » is the expected answer – A simple calculation transforms « yes » into 100%, and « no » into 0% – The indicator is the average of all the questions Other possibilities: – Several choices, usually 0%-50%-100% – With « na » , the assessment can be used at any level of laboratories ( « na » excludes the question from the average calculation)

In conclusion This tool is a good help for a laboratory visit, as it: Allows each component to be assessed Opens the discussion on every critical point Allows a critical review just after the assessment Allows comparison But: Is a long process

Questions? Comments? Discussions?
38a1a9b3b47d5aa7c20faf1634c01d48.ppt