55976f31ac9b16762ef3a49d25e08557.ppt
- Количество слайдов: 35

Labeled Immunoassay

Immunoassay • An immunoassay is a test that uses antibody and antigen complexes as a means of generating a measurable result. • An antibody: antigen complex is also known as an immuno-complex. “Immuno” refers to an immune response that causes the body to generate antibodies, and “assay” refers to a test. • The assay takes advantage of the specific binding of an antibody to its antigen. • The antibodies used must have a high affinity for the antigen.
Immunoassay • Both the presence of antigen or antibodies can be measured. • Example, when detecting infection the presence of antibody against the pathogen is measured. • For measuring hormones such as insulin, the insulin acts as the antigen. • For numerical results, the response of the fluid being measured must be compared to standards of a known concentration. • This is usually done through the plotting of a standard curve on a graph paper, and then the quantity of the unknown is found from the curve.

History and Background • • In the year 1959, Drs. Rosalyn Yalow & Soloman Berson invented the radioimmunoassay, which applied the use of radioisotopes in the measurement of insulin. The RIA is the predecessor of modern immunoassays. Dr. Rosalyn Yalow became the first female to win a Nobel Prize with her work on the radioimmunoassay.
Immunoassay • The first immunoassays were described for the measurement of insulin and thyroxine, respectively. • There are now hundreds of immunoassays for scores of analytes including: • • hormones, tumor markers, drugs, antibodies, and cardiac markers • covering the fields of • • • endocrinology, oncology, hematology, toxicology, serology, infectious diseases • Developments in antibodies, labels, and automation have resulted in highly specific and sensitive assays.

Labeled immunoassays • Labeled immunoassays are designed for antigens and antibodies that may be • small in size • or present in very low concentrations. • The presence of such antigens or antibody is determined indirectly by using a labeled reactant to detect whether or not specific binding has taken place.

Constituents of Labeled assay • For detection of an analyte, the following are usually a part of the assay: 1. 2. 3. 4. Labeled and nonlabeled analytes Specific antibody Standards or calibrators A method to separate the bound from free components 5. A method for detection of the label.
1 - Labeled analyte • A labeled reactant is used to detect whether or not specific binding has taken place. • The label used in immunoassay: • must not alter the reactivity of the molecule, • and it should remain stable for the shelf life of the reagent. • Labels attached to analytes and antibodies may be: • radioactive, usually iodine-125 (radioimmunoassay and immunoradiometric assays), • enzymes such as alkaline phosphatase and horseradish peroxidase, (enzyme immunoassay or immunometric assay, or enzyme-linked immunosorbent assay [ELISA]), • chemiluminescent (e. g. , acridinium ester), • or fluorescent (e. g. , fluorscein).
Methods of coupling indicator labels to antigen or antibody • Different methods by which the indicator label may be coupled to antigen or antibody. • For example, the radioactive isotope iodine is covalently linked to tyrosine residues present on antibodies and most antigens.
Methods of coupling indicator labels to antigen or antibody • Fluorochromes or enzymes may be coupled to antigens or antibodies using glutaraldhyde, a bifunctional reagent that covalently cross links two aminoacids together. • Alternatively enzymes can be linked to streptavidin for use in systems where biotin has been attached to either antigen or antibody.

The biotin-Streptavidin/ Avidin indicator label system • Biotin is a vitamin that can bind tightly to either avidin or streptavidin. • Avidin & streptavidin are proteins. • The natural attraction of these two proteins for one another is a property that has been exploited to facilitate coupling of indicator molecules to antigens or antibodies.
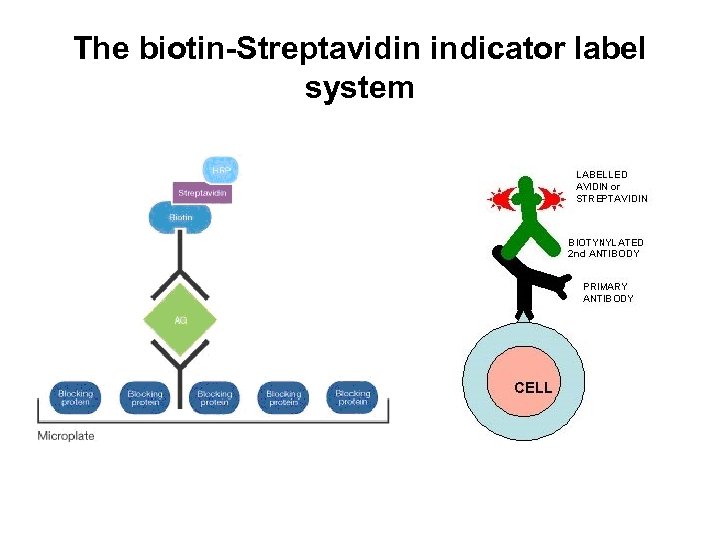
The biotin-Streptavidin indicator label system

Production of Antibodies • The production of antibodies is an important process in the use of immunoassays because it is the antibody-antigen complexes form the basic. • Antibodies can be called monoclonal or polyclonal, depending upon the technique used to produce them.
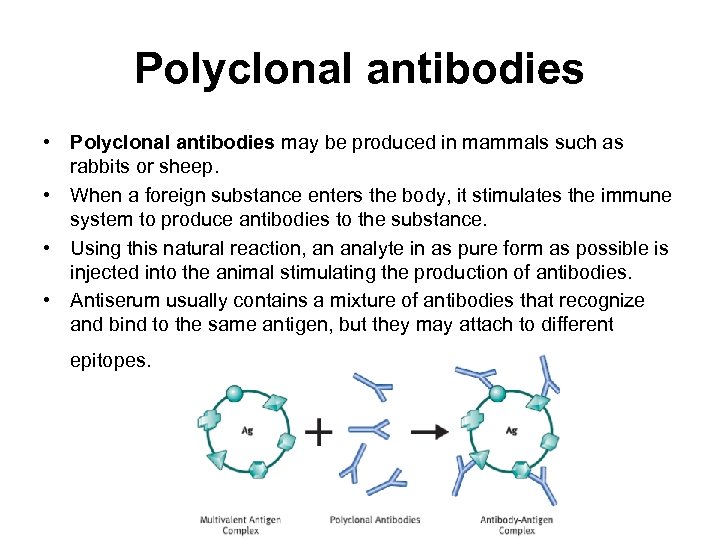
Polyclonal antibodies • Polyclonal antibodies may be produced in mammals such as rabbits or sheep. • When a foreign substance enters the body, it stimulates the immune system to produce antibodies to the substance. • Using this natural reaction, an analyte in as pure form as possible is injected into the animal stimulating the production of antibodies. • Antiserum usually contains a mixture of antibodies that recognize and bind to the same antigen, but they may attach to different epitopes.
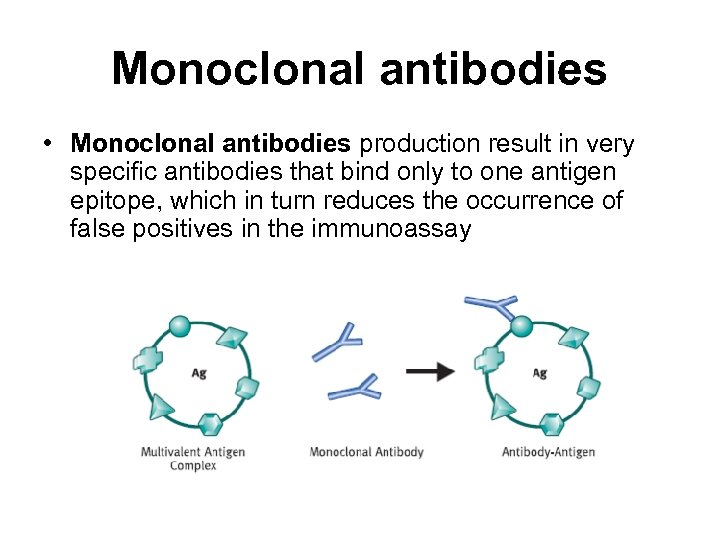
Monoclonal antibodies • Monoclonal antibodies production result in very specific antibodies that bind only to one antigen epitope, which in turn reduces the occurrence of false positives in the immunoassay
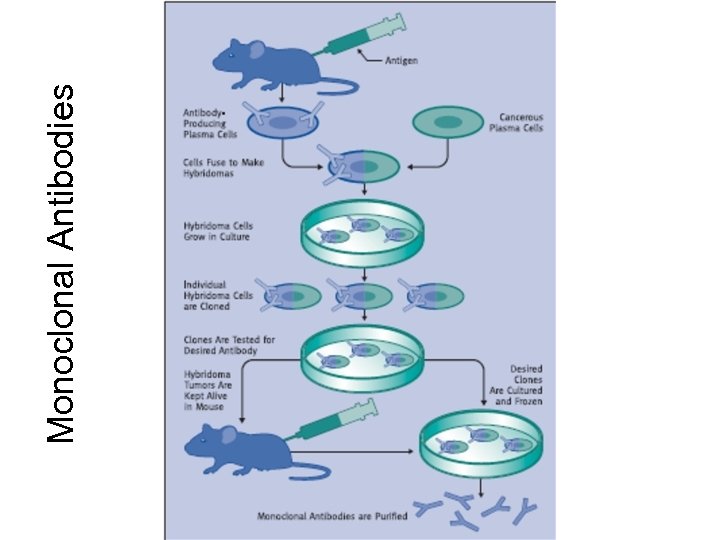
Monoclonal Antibodies
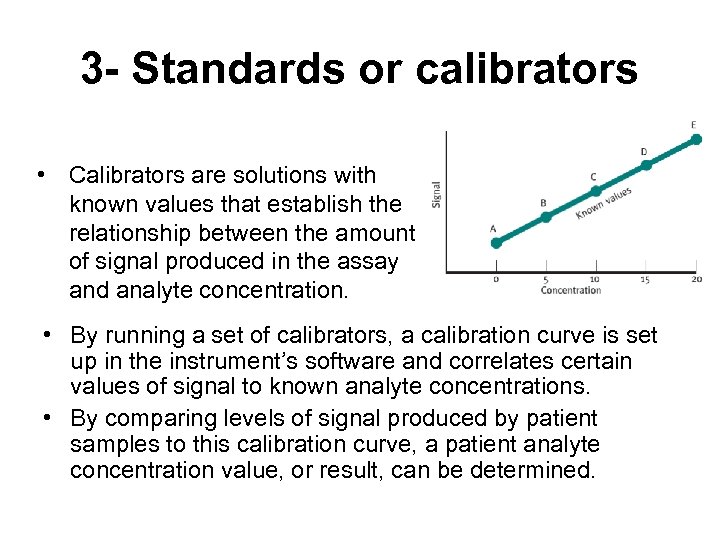
3 - Standards or calibrators • Calibrators are solutions with known values that establish the relationship between the amount of signal produced in the assay and analyte concentration. • By running a set of calibrators, a calibration curve is set up in the instrument’s software and correlates certain values of signal to known analyte concentrations. • By comparing levels of signal produced by patient samples to this calibration curve, a patient analyte concentration value, or result, can be determined.
4 - Separation Methods • In most assays, once the reaction between antigen and antibody has taken place, there must a way of separating reacted from unreacted analyte. • This can be accomplished by several different means. Unreacted analyte can be removed by: • Adsorption on particles such as dextran-coated charcoal, – These adsorb out the smaller unbound molecules, which are then separated from bound molecules by centrifugation or filtration. – The amount of label remaining in the supernatant provides an indirect measure of analyte present in the patient's sample.

4 - Separation Methods • Another means of separation involves precipitation of antigen-antibody complexes. – Complexes can be precipitated by adding concentrated solutions of ammonium sulfate, or ethanol • Antigen-antibody complexes can also be removed from solution by the use of a second precipitating antibody (anti-antibody).
4 - Separation Methods • Currently, most immunoassays use a solidphase stage for separation. • Numerous substances, such as polystyrene test tubes, microtiter plates are used for this purpose. • Antigen or antibody is attached by physical adsorption , and when specific binding takes place, complexes remain attached to the solid phase. • This provides a simple way to separate bound and free reactants.
5 - Methods for detection of label • The last step common for all immunoassays is detection of the labeled analyte. • The method depends on the label; e. g. 125 I is easily detected in a γ-counter • Enzymes are generally used to produce coloured products from colourless substrates that can be determined easily in a spectrophotometer or colorimeter. • Automated plate readers are commercially available which make reading large numbers of samples relatively easy.

Quality Control • It is essential that quality control procedures be established. • This is done to limit random errors, such as • temperature fluctuations, • minor changes in the concentration of reagents, • and changes in detector efficiency. • A negative control and a positive control should be run. • This serves as a check on the quality of the reagents to make sure that the label is readily detectable under current testing conditions.

Types of Labeled Immunoassays 1 - Competitive and Noncompetitive Immunoassays 2 - Homogeneous and Heterogeneous Immunoassay Methods
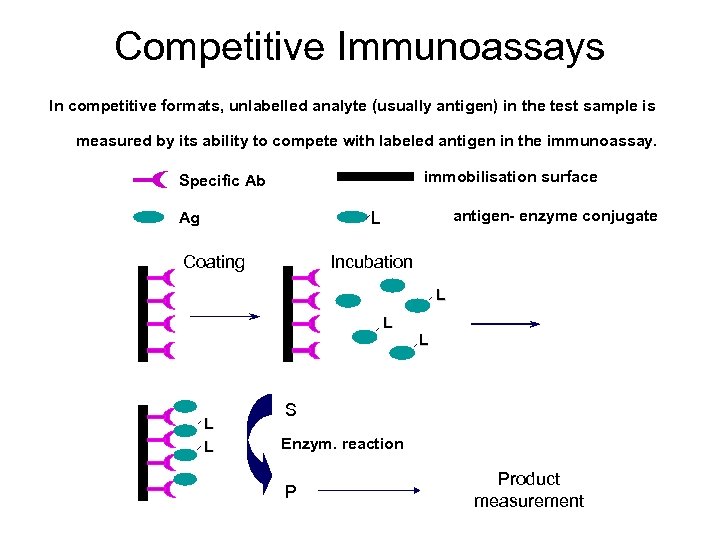
Competitive Immunoassays In competitive formats, unlabelled analyte (usually antigen) in the test sample is measured by its ability to compete with labeled antigen in the immunoassay. immobilisation surface Specific Ab antigen- enzyme conjugate L Ag Coating Incubation L L L S Enzym. reaction P Product measurement
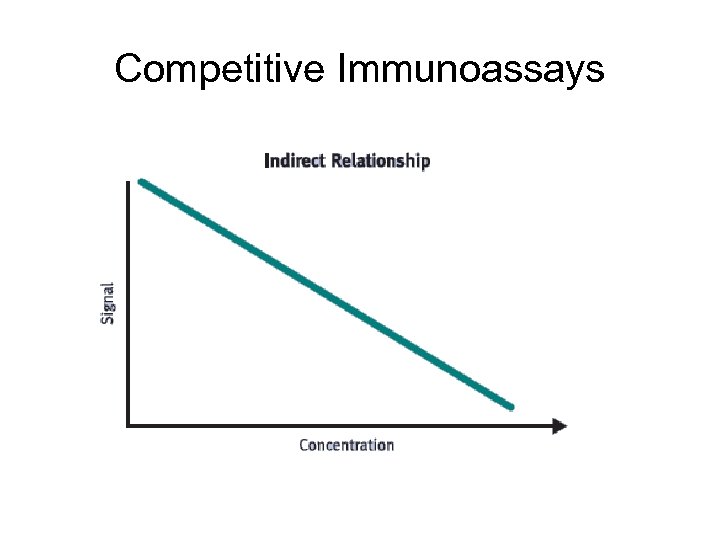
Competitive Immunoassays
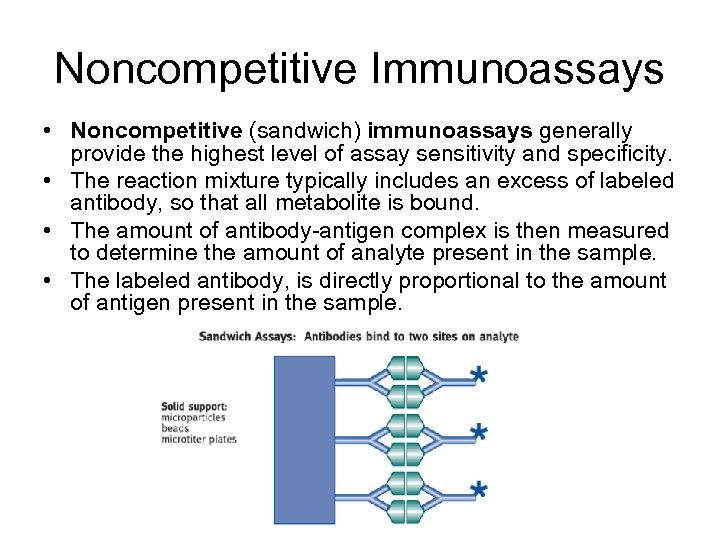
Noncompetitive Immunoassays • Noncompetitive (sandwich) immunoassays generally provide the highest level of assay sensitivity and specificity. • The reaction mixture typically includes an excess of labeled antibody, so that all metabolite is bound. • The amount of antibody-antigen complex is then measured to determine the amount of analyte present in the sample. • The labeled antibody, is directly proportional to the amount of antigen present in the sample.
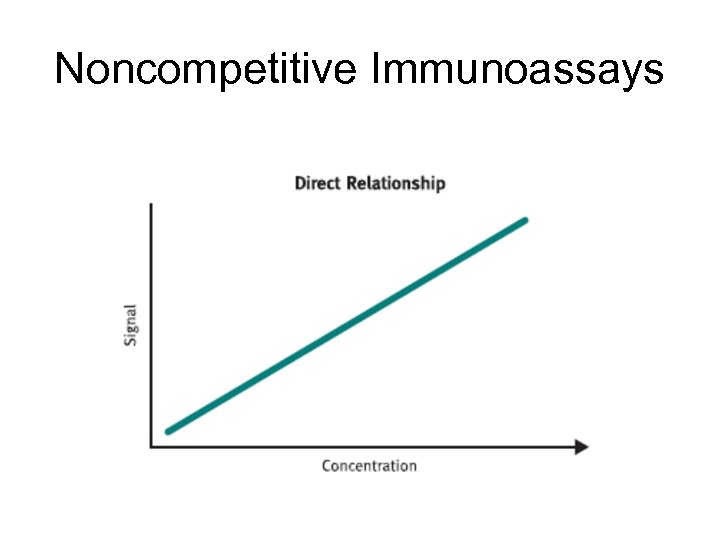
Noncompetitive Immunoassays
Homogeneous VS Heterogeneous Methods • Immunoassay methods that require separation of bound Ab-Ag* complex are referred to as heterogeneous immunoassays. • Those that do not require separation are referred to as homogeneous immunoassays. • Homogeneous methods have been generally applied to the measurement of small analytes such as abused and therapeutic drugs. • Since homogeneous methods do not require the separation of the bound Ab-Ag* from the free Ag*, they are generally much easier and faster to perform.
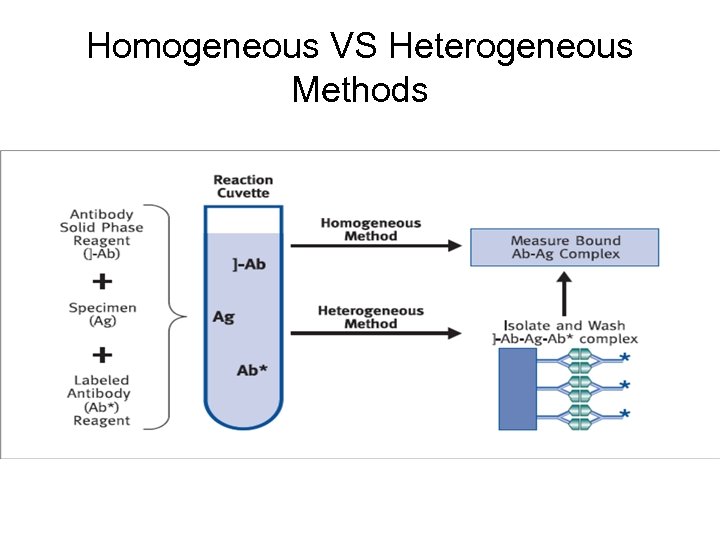
Homogeneous VS Heterogeneous Methods
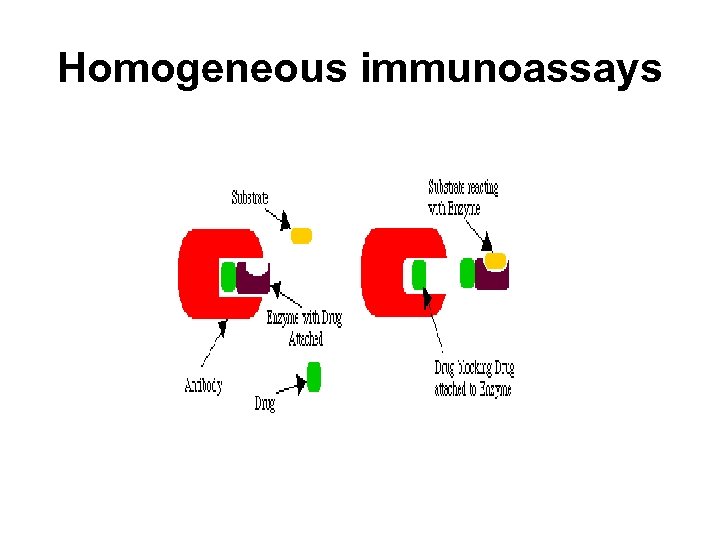
Homogeneous immunoassays
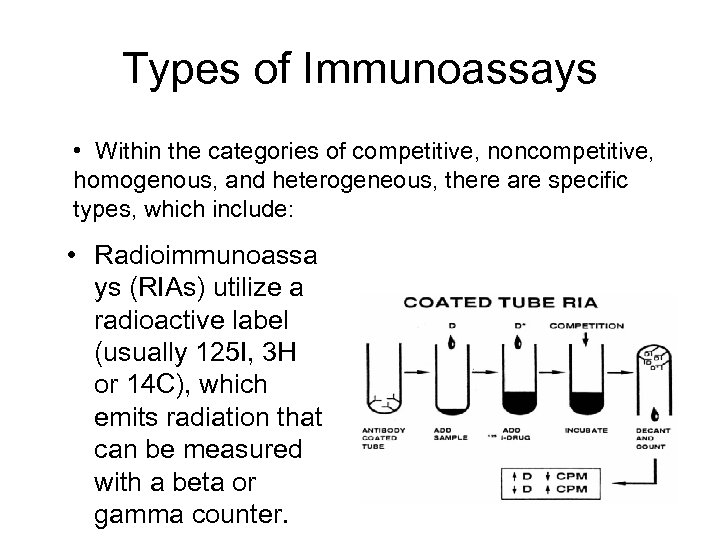
Types of Immunoassays • Within the categories of competitive, noncompetitive, homogenous, and heterogeneous, there are specific types, which include: • Radioimmunoassa ys (RIAs) utilize a radioactive label (usually 125 I, 3 H or 14 C), which emits radiation that can be measured with a beta or gamma counter.
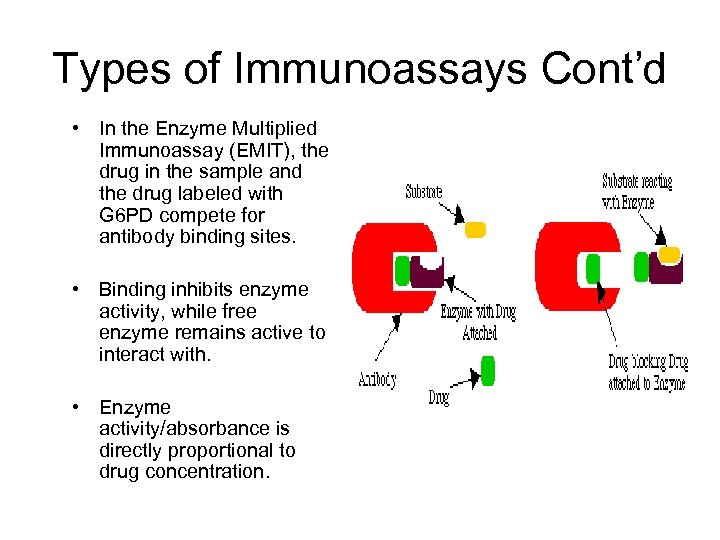
Types of Immunoassays Cont’d • In the Enzyme Multiplied Immunoassay (EMIT), the drug in the sample and the drug labeled with G 6 PD compete for antibody binding sites. • Binding inhibits enzyme activity, while free enzyme remains active to interact with. • Enzyme activity/absorbance is directly proportional to drug concentration.
Types of Immunoassays Cont’d • Enzyme linked immunosorbant assay (ELISA): competitive, heterogeneous EIA • Reaction components are absorbed or bound to the surface of a solid phase, commonly a well of a microtiter plate • Absorbance is measured using a micro-plate reader • Sample absorbance is inversely proportional to drug concentration
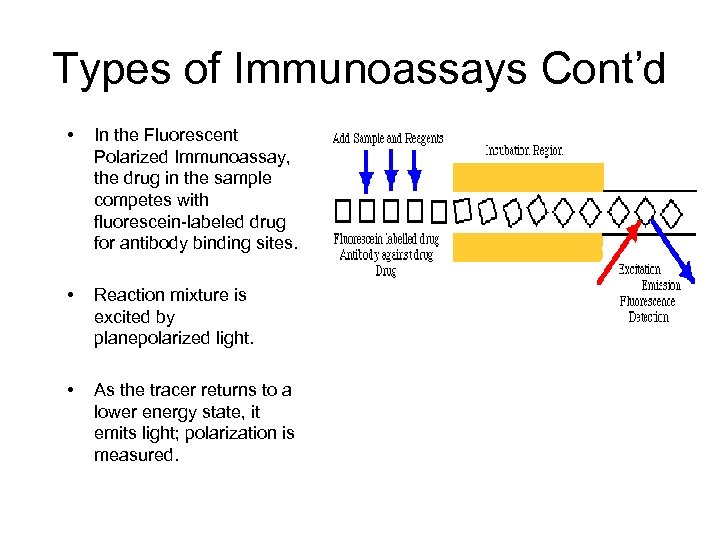
Types of Immunoassays Cont’d • In the Fluorescent Polarized Immunoassay, the drug in the sample competes with fluorescein-labeled drug for antibody binding sites. • Reaction mixture is excited by planepolarized light. • As the tracer returns to a lower energy state, it emits light; polarization is measured. • The polarization value of the sample is inversely proportional to analyte concentration.

Immunoassay Results • Qualitative – Single point calibration at a specific cutoff – Results are either ‘positive’ or ‘negative’; (i. e. above or below the cutoff) – Possible false positives; monoclonal antibodies restrict this slightly. • Quantitative – Provides numeric results that are an estimate of drug/compound concentration based on the measurement of labeled analyte in the solution, and taking into consideration the competitive/noncompetitive nature of the device. – In terms of use on drugs, this is sometimes complicated by possible cross-reactivities.
55976f31ac9b16762ef3a49d25e08557.ppt