62c0cd9ce3511ca66fa4e4ce027d13d4.ppt
- Количество слайдов: 47

Lab. No. 1 : Introduction to experimental pharmacology , handling of laboratory animals & drug administration

Introduction: Animals are widely used experimentally in almost every laboratory where a biological study is needed In pharmacological research, animals are being used to test the effect of drugs before their introduction to clinical trials where drugs are tested on humans In addition, animals are used in educational institutions to demonstrate the effect of drugs for teaching purposes.

• Experimental animals should always be: A. Suitably bred. B. Gently handled C. Properly used

Ethical and legal responsibility • Careful handling of animals in such a manner that they do not suffer from unnecessary pain. • Providing a good care towards the health and well being of animals. • It is important that animals are aware of the handler’s presence before attempting to restrain them, particularly if the animal is initially asleep. This will reduce stress for the animal and help to avoid bite injuries.

ANIMAL HOUSE • Diurnal light cycles in animal holding rooms (12: 12 -h light: dark cycle) • Controlled temperature (22º C) • Controlled humidity (range 30%-70%) • Free access to water ‘ad libitum’ and chow diet • Adaptation period

CAGES

Selection of an experimental animal for a particular experiment depends on: 1. Nature of the experiment. 2. Type of drug activity to be evaluated. 3. Specific character of the experimental animals

Animals can be used in: • “In Vivo” experiments when the whole intact animal is used, or in • “Ex Vivo” experiments when only a part of the animal is used. This part could be an organ (e. g. heart, uterus, trachea. etc. ), a tissue (e. g. skeletal muscle) or even some cells (e. g. blood cells).

Types of animals: • Large (monkey, pigs, dogs and cats) OR • Small (mice, rats, rabbits and frogs) ü For teaching purposes the small animals are commonly used.
Selection of species depends on many factors: • Technical issues, e. g. availability of the species in the numbers needed, health and genetic status of the species, animal size and volume of blood required • Animal husbandry issues, e. g. availability of suitable housing, ease of handling of the animals, training and level of competence of personnel • Public concern about use of certain species • Financial considerations

Types of experimental animals: • 1. Mouse • 2. Rat • 3. Guinea pig • 4. Rabbit

MOUSE

Mice • The most common mammal among laboratory animals. • Characteristics: 1. Mice are small laboratory animals weighing about 18 -30 g. 2. They are very sensitive and consume small doses of drugs. 3. They can be easily handled. 4. Highly exploring and mobile 5. Drugs are best injected intraperitoneally or intravenously into one of their superficial tail veins.

Mice • Uses in research: 1. In cancer and genetic research. 2. In acute toxicity studies. 3. In screening of various drug activities specially central nervous system (CNS) activity.
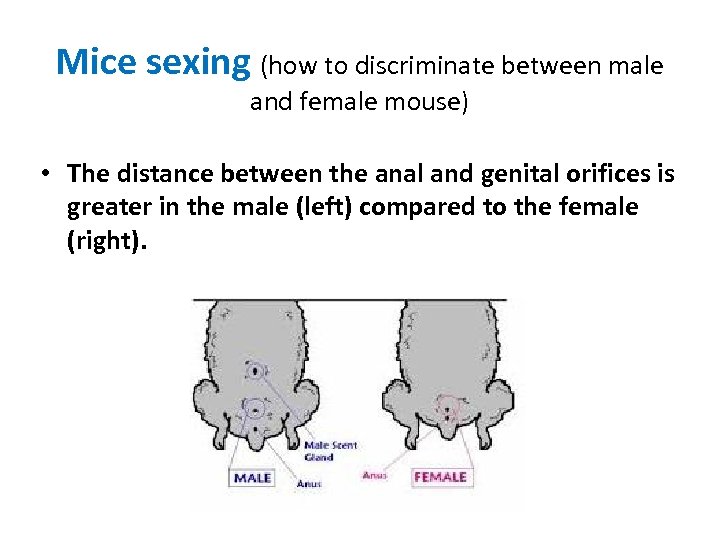
Mice sexing (how to discriminate between male and female mouse) • The distance between the anal and genital orifices is greater in the male (left) compared to the female (right).

HANDLING & RESTRAINT OF MICE • • • Instructions: Gently but firmly. Wear disposable gloves Wash your hands prior to and after handling Wear a clean laboratory coat

HANDLING & RESTRAINT OF MICE • The animal should be grasped by the tail, and should then be placed on a rough surface such as a cage top while holding the tail firmly
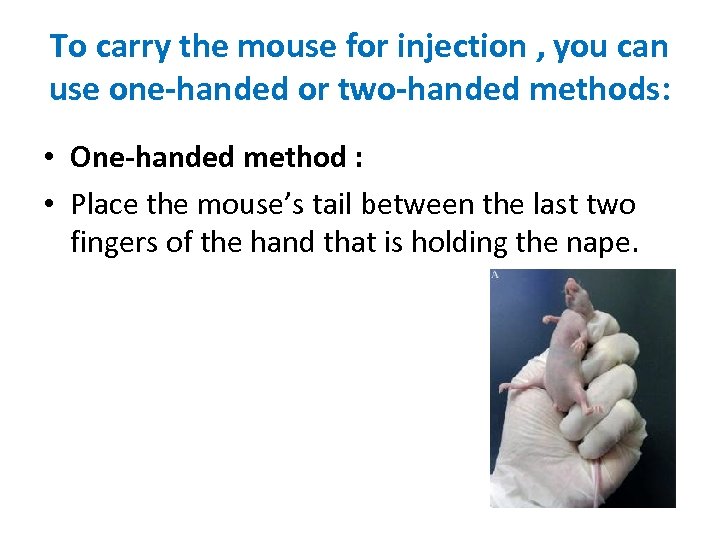
To carry the mouse for injection , you can use one-handed or two-handed methods: • One-handed method : • Place the mouse’s tail between the last two fingers of the hand that is holding the nape.
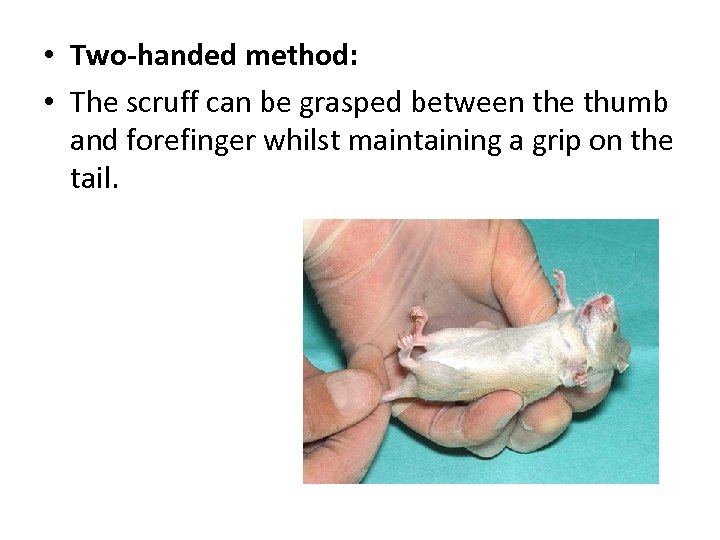
• Two-handed method: • The scruff can be grasped between the thumb and forefinger whilst maintaining a grip on the tail.
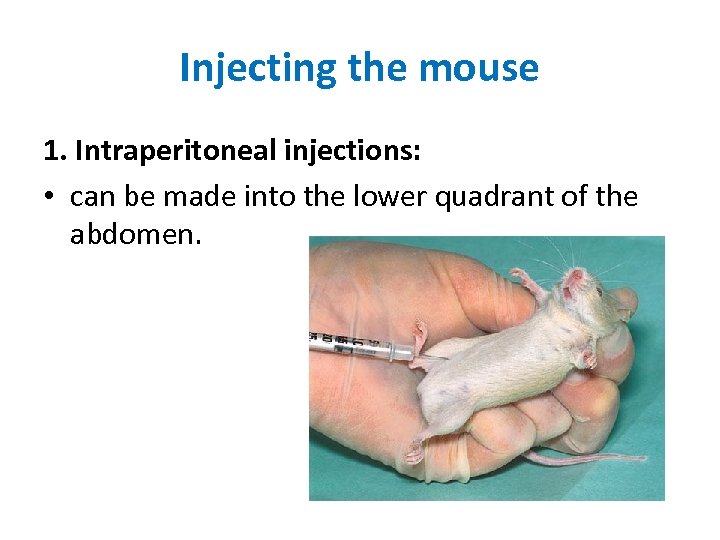
Injecting the mouse 1. Intraperitoneal injections: • can be made into the lower quadrant of the abdomen.
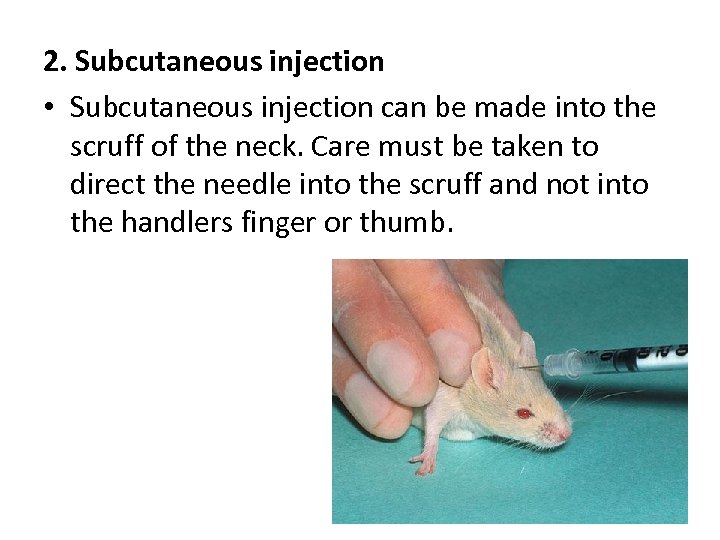
2. Subcutaneous injection • Subcutaneous injection can be made into the scruff of the neck. Care must be taken to direct the needle into the scruff and not into the handlers finger or thumb.
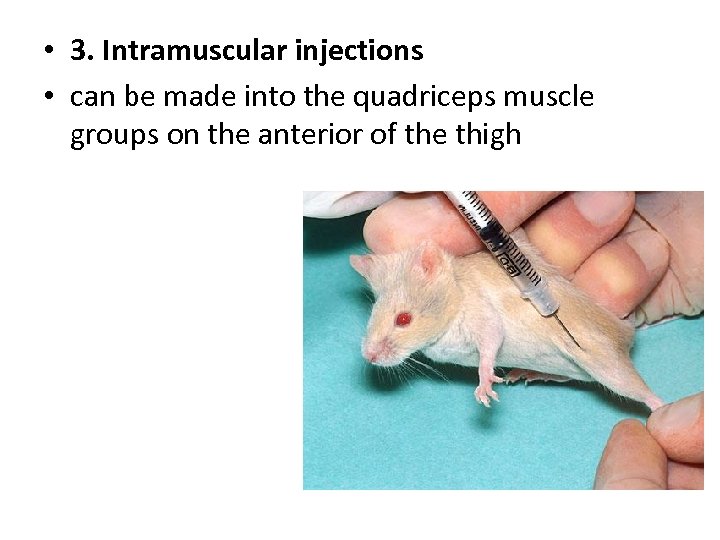
• 3. Intramuscular injections • can be made into the quadriceps muscle groups on the anterior of the thigh
GENETICALLY MODIFIED MICE • Knockout mouse (KO): • Is a genetically engineered mouse in which researchers have inactivated, or "knocked out", an existing gene • This causes changes in a mouse's phenotype (appearance, behavior, physical and biochemical characteristics) • Important animal models to study the role of genes that have been sequenced but whose functions have not been determined • …. Causing a specific gene inactivation, and observing any differences from normal behaviour or physiology, we can infer its probable function • May also be a useful experimental pathological model to test the efficacy of new drugs

GENETICALLY MODIFIED MICE • Knockin mouse

NUDE MOUSE • Mouse from a strain with a genetic mutation that causes a deteriorated or absent thymus, resulting in an inhibited immune system due to a greatly reduced number of T cells. • The phenotype (main outward appearance) of the mouse is a lack of body hair…. . nude" nickname. • Valuable in research! it can receive different types of tissue and tumor grafts (xenografts ), with no rejection response.

RAT
RAT • Characteristics: 1. Rats have a larger weight than mice (about 300 -500 g). 2. They can withstand long periods of experimentation under anaesthesia. 3. They can be easily handled if treated kindly. 4. Drugs can be injected intravenously (I. V) into their tail veins, subcutaneously (S. C), intramuscularly (I. M) or intraperitonealy. 5. They can be also given drugs orally by means of stomach tube. 6. Isolated preparations can be taken from them easily. (stomach, fundus, colon and uterus are the most commonly used preparations)

Proper method of handling: To initially restrain a rat, the handler should gently grasp it around the shoulders.

Proper method of handling: • The handler’s thumb can then be placed under the rat’s mandible, to prevent bites, and the rat’s hind limb scan be supported with the other hand. Restraint should be firm but not too tight as this will impede the animal’s respiration. • Note: Bites from rats are uncommon and will typically only occur if the animal is stressed or in pain.

Intravenous administration in rat
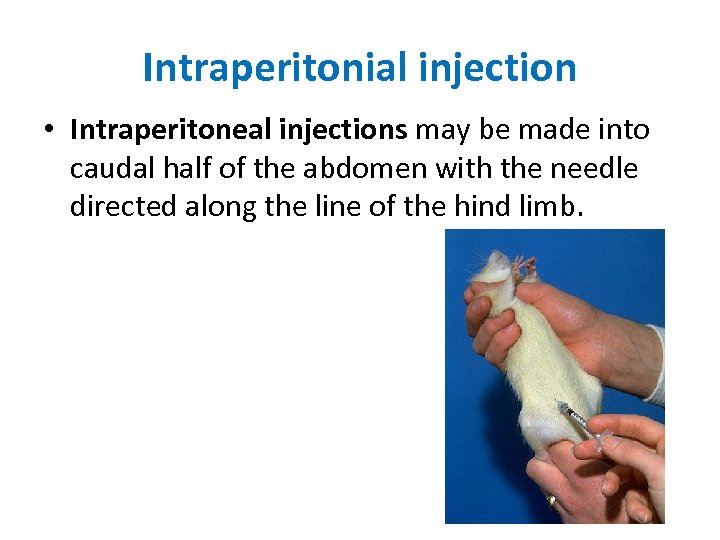
Intraperitonial injection • Intraperitoneal injections may be made into caudal half of the abdomen with the needle directed along the line of the hind limb.
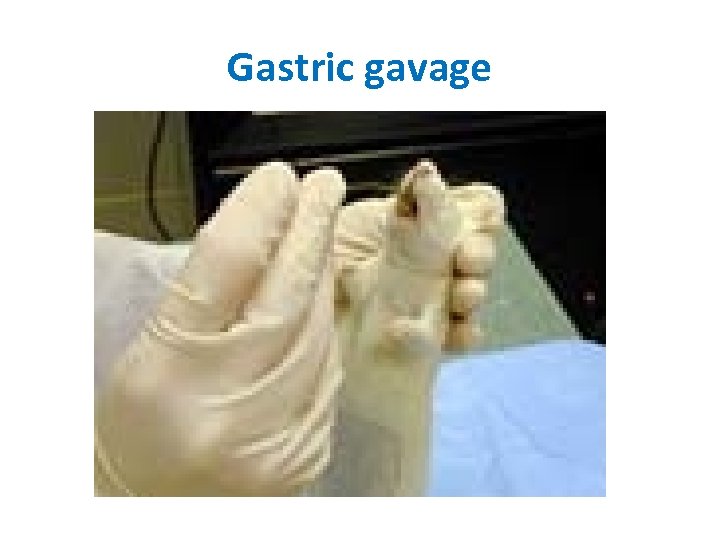
Gastric gavage

The guinea pig

The guinea pig • Characteristics: 1. very docile and tame animals. 2. very susceptible to infections and highly sensitive to action of many drugs, e. g. histamine and penicillin. 3. cannot synthesize endogenous vitamin C and this vitamin should be supplied in their diet. 4. Some isolated muscle preparations can be taken from guinea pigs, eg. Guinea pig Vas Deferens, tracheal chain or ileum. 5. Drugs can be injected intravenously (I. V) into their tail veins, subcutaneously (S. C), intramuscularly (I. M) or intraperitonealy. 6. They can be also given drugs orally by means of stomach tube.

The guinea pig • Use in research: 1. Screening for histaminic, antihistaminic drugs, respiratory or cardiovascular activity. 2. Testing the effect of drugs on their isolated muscle preparations.

Handling and Restraint of Guinea Pig • To initially restrain a guinea pig, the handler should be rapid and smooth, to avoid frightening the animal. • The handler’s thumb is placed beneath the jaw of the guinea pig. The hindquarters of the guinea pig are supported by the handler’s other hand

The rabbit

The rabbit • Characteristics: 1. Rabbits are very docile animals. 2. They can be easily injected intravenously into their marginal ear veins. 3. They can be also given drugs orally by means of stomach tube. 4. Some isolated muscle preparations can be taken from rabbits.
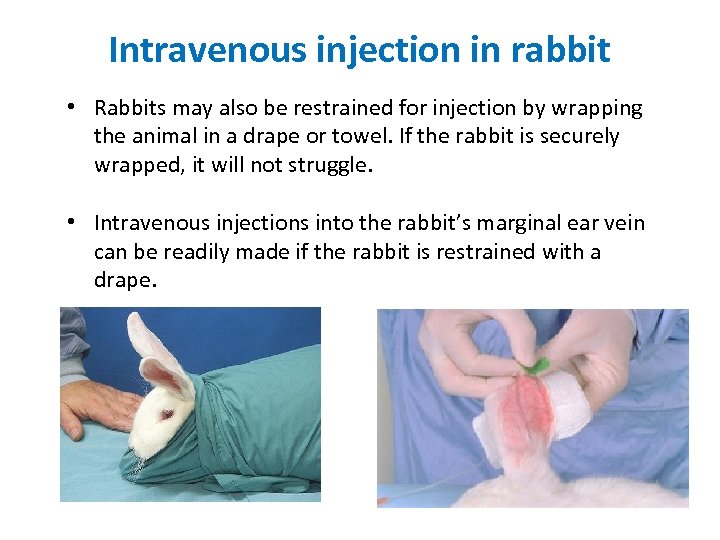
Intravenous injection in rabbit • Rabbits may also be restrained for injection by wrapping the animal in a drape or towel. If the rabbit is securely wrapped, it will not struggle. • Intravenous injections into the rabbit’s marginal ear vein can be readily made if the rabbit is restrained with a drape.

Handling and restraint of the rabbit: • Rabbits are especially susceptible to the effects of stress and should always be approached in a calm and confident manner. • The handler is restraining the rabbit firmly by the scruff with the other hand ready to support the animal’s hindquarters.
BLOOD SAMPLING IN MICE/RATS: 1. 2. 3. 4. from tail vein from orbital sinus from cardiac puncture from saphenous vein

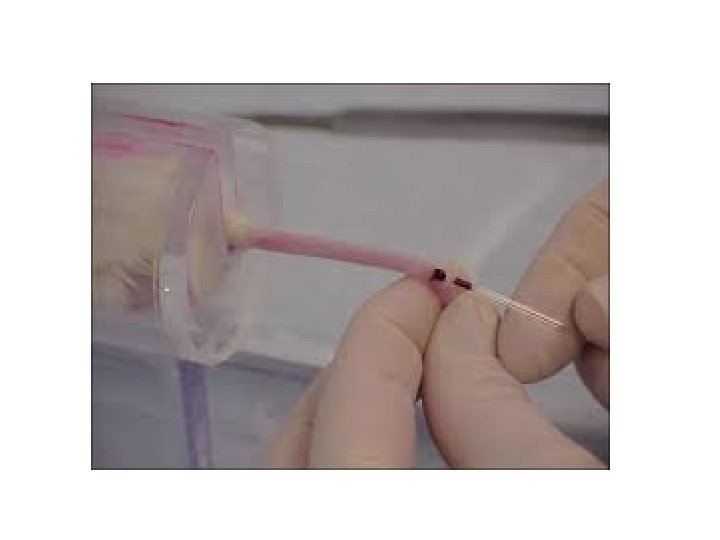
1. Blood collection from Tail vein: It may be used to collect a small volume of blood (Approximately 0. 1 ml ) 1. Carefully warm animal with heat lamp or disposable hand warmers. 2. Place animal in an appropriate restraint device. 3. Clean withdrawal site with alcohol. 4. Using a sterile scalpel blade, nick the lateral tail vein as shown in this picture. 5. Collect blood into an appropriate container. 6. Apply gentle pressure to stop bleeding.
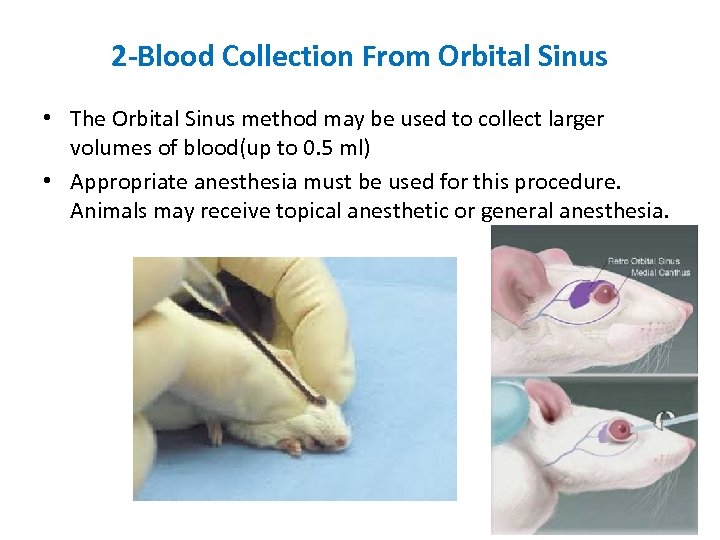
2 -Blood Collection From Orbital Sinus • The Orbital Sinus method may be used to collect larger volumes of blood(up to 0. 5 ml) • Appropriate anesthesia must be used for this procedure. Animals may receive topical anesthetic or general anesthesia.
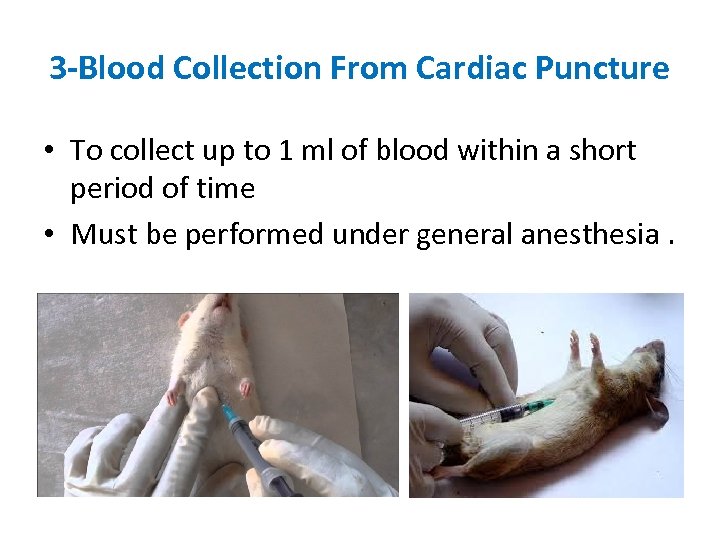
3 -Blood Collection From Cardiac Puncture • To collect up to 1 ml of blood within a short period of time • Must be performed under general anesthesia.
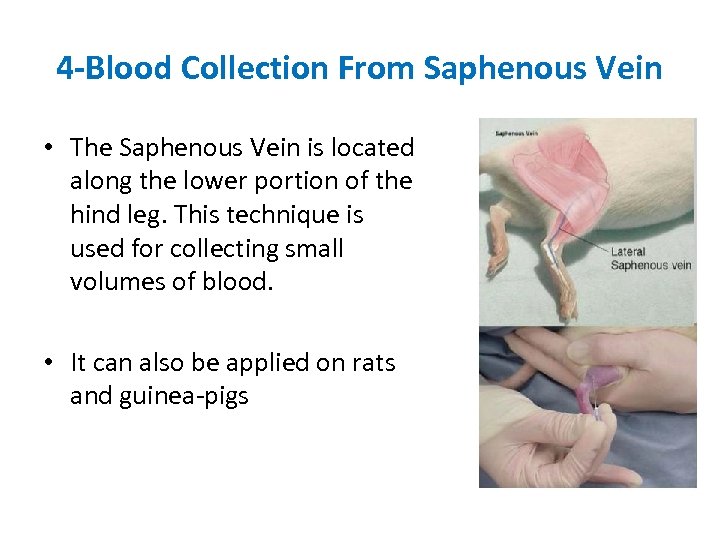
4 -Blood Collection From Saphenous Vein • The Saphenous Vein is located along the lower portion of the hind leg. This technique is used for collecting small volumes of blood. • It can also be applied on rats and guinea-pigs
62c0cd9ce3511ca66fa4e4ce027d13d4.ppt