L 30 - Nuclear Physics 2 Updated.pptx
- Количество слайдов: 25
 L 30 - Nuclear Physics 2 OUTLINE 1. 2. 3. 4. 5. 1 Review of Radioactive decay Calculations in Nuclear Reactions Mass defect Binding energy Fission and fusion including: Nuclear reactor
L 30 - Nuclear Physics 2 OUTLINE 1. 2. 3. 4. 5. 1 Review of Radioactive decay Calculations in Nuclear Reactions Mass defect Binding energy Fission and fusion including: Nuclear reactor
 Announcement CW Test 4 th March. Wednesday 4 4: 00 pm – 5. 00 pm Lectures and PSCs 23 -30 CW 12 -15 2
Announcement CW Test 4 th March. Wednesday 4 4: 00 pm – 5. 00 pm Lectures and PSCs 23 -30 CW 12 -15 2
 1. 1 – Review of Radioactivity • 3
1. 1 – Review of Radioactivity • 3
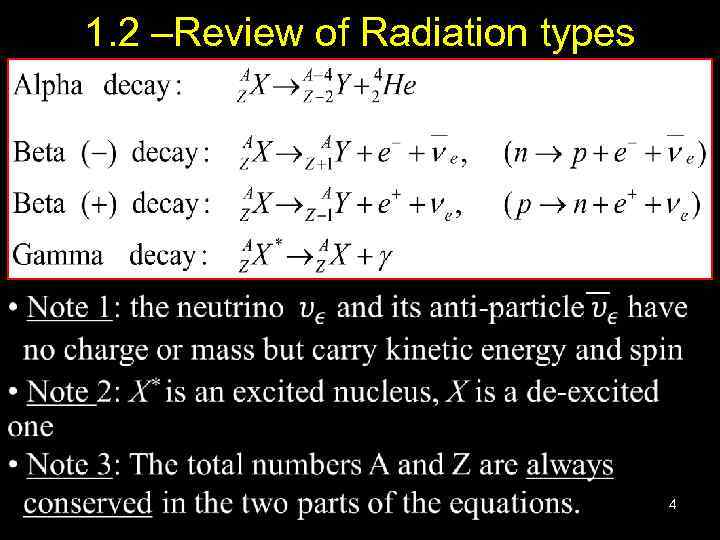 1. 2 –Review of Radiation types 4
1. 2 –Review of Radiation types 4
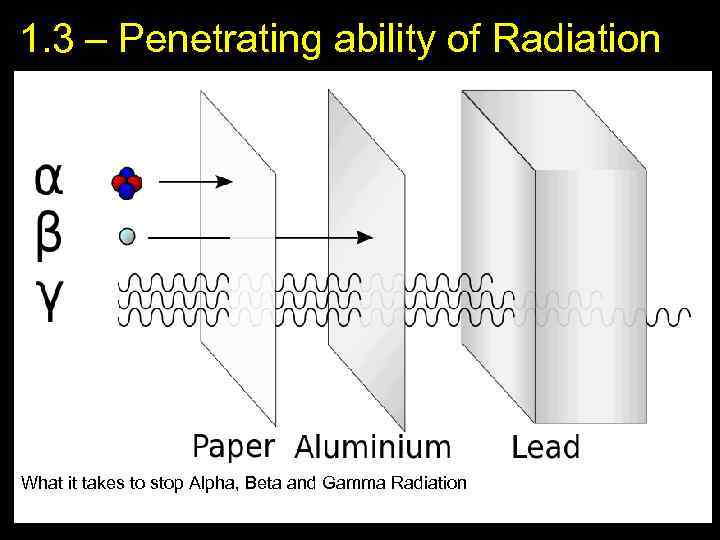 1. 3 – Penetrating ability of Radiation What it takes to stop Alpha, Beta and Gamma Radiation 5
1. 3 – Penetrating ability of Radiation What it takes to stop Alpha, Beta and Gamma Radiation 5
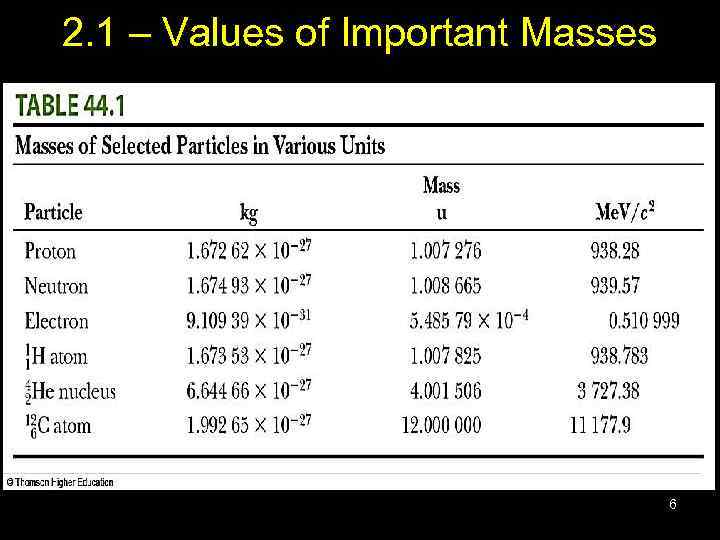 2. 1 – Values of Important Masses 6
2. 1 – Values of Important Masses 6
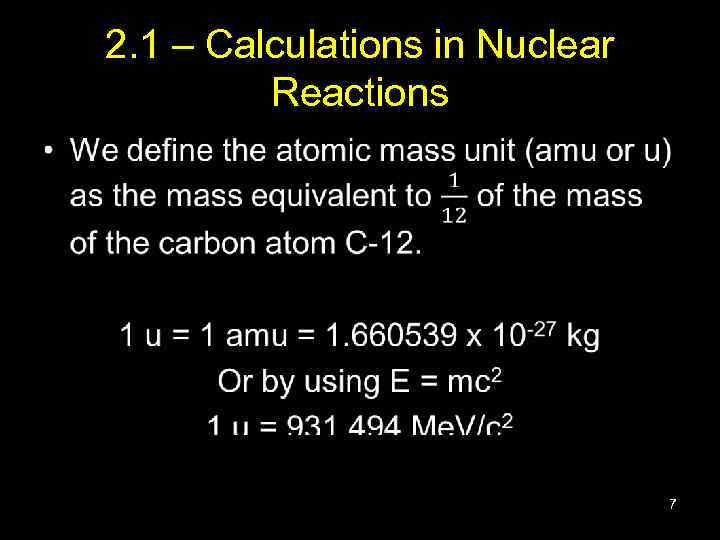 2. 1 – Calculations in Nuclear Reactions • 7
2. 1 – Calculations in Nuclear Reactions • 7
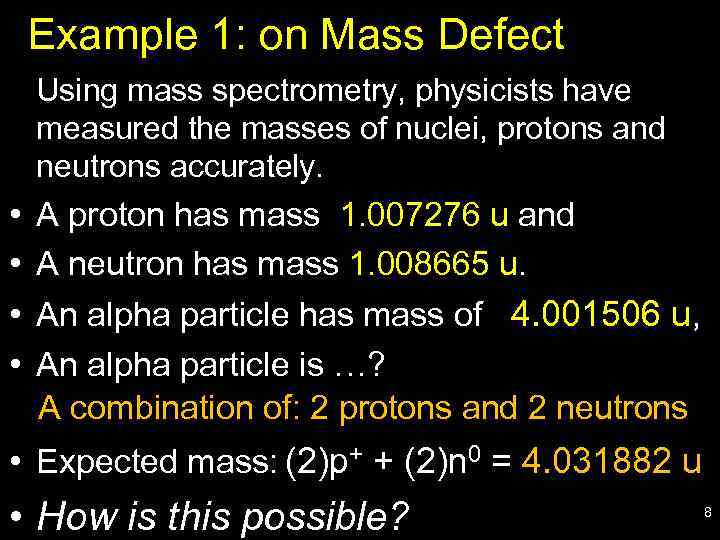 Example 1: on Mass Defect Using mass spectrometry, physicists have measured the masses of nuclei, protons and neutrons accurately. • • A proton has mass 1. 007276 u and A neutron has mass 1. 008665 u. An alpha particle has mass of 4. 001506 u, An alpha particle is …? A combination of: 2 protons and 2 neutrons • Expected mass: (2)p+ + (2)n 0 = 4. 031882 u • How is this possible? 8
Example 1: on Mass Defect Using mass spectrometry, physicists have measured the masses of nuclei, protons and neutrons accurately. • • A proton has mass 1. 007276 u and A neutron has mass 1. 008665 u. An alpha particle has mass of 4. 001506 u, An alpha particle is …? A combination of: 2 protons and 2 neutrons • Expected mass: (2)p+ + (2)n 0 = 4. 031882 u • How is this possible? 8
 3. 1 - Mass defect In any type of nuclear transformation, reactants products (sum of rest masses of reactants) (sum of rest masses of products) MASS DEFECT Or, put another way ΔM (mass defect) =MREACTANTS – MPRODUCTS (Eq. 5) 9
3. 1 - Mass defect In any type of nuclear transformation, reactants products (sum of rest masses of reactants) (sum of rest masses of products) MASS DEFECT Or, put another way ΔM (mass defect) =MREACTANTS – MPRODUCTS (Eq. 5) 9
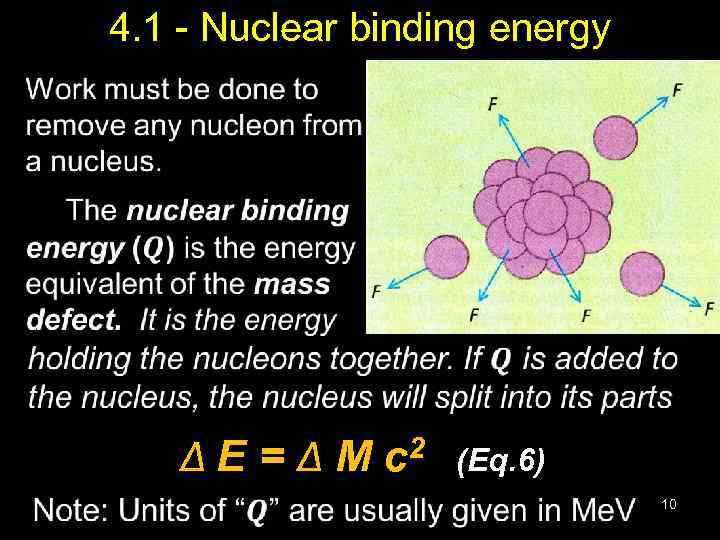 4. 1 - Nuclear binding energy • Δ E = Δ M c 2 (Eq. 6) 10
4. 1 - Nuclear binding energy • Δ E = Δ M c 2 (Eq. 6) 10
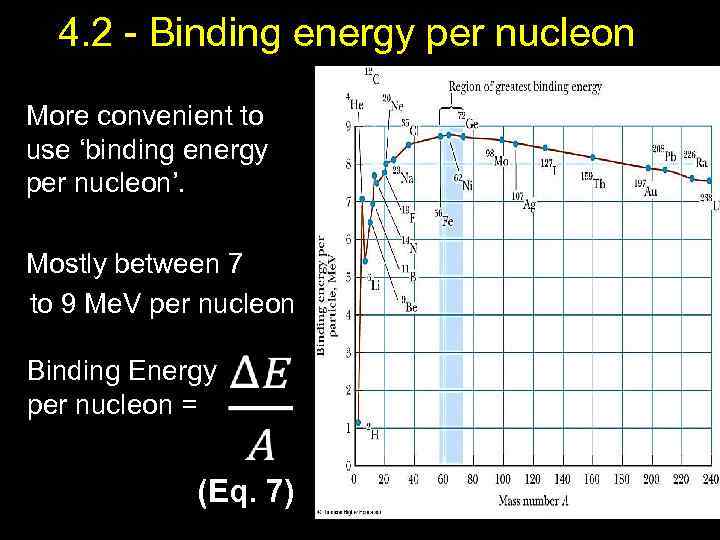 4. 2 - Binding energy per nucleon More convenient to use ‘binding energy per nucleon’. Mostly between 7 to 9 Me. V per nucleon Binding Energy per nucleon = (Eq. 7) 11
4. 2 - Binding energy per nucleon More convenient to use ‘binding energy per nucleon’. Mostly between 7 to 9 Me. V per nucleon Binding Energy per nucleon = (Eq. 7) 11
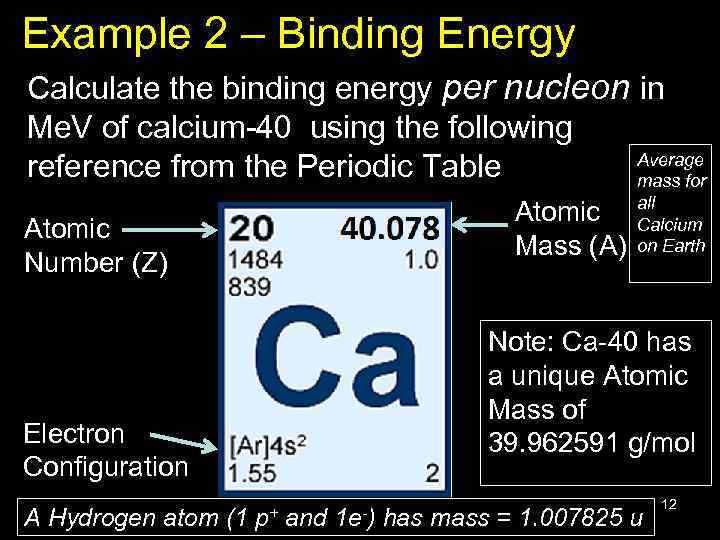 Example 2 – Binding Energy Calculate the binding energy per nucleon in Me. V of calcium-40 using the following Average reference from the Periodic Table mass for Atomic Mass (A) Atomic Number (Z) Note: Ca-40 has a unique Atomic Mass of 39. 962591 g/mol Electron Configuration A Hydrogen atom (1 all Calcium on Earth p+ and 1 e-) has mass = 1. 007825 u 12
Example 2 – Binding Energy Calculate the binding energy per nucleon in Me. V of calcium-40 using the following Average reference from the Periodic Table mass for Atomic Mass (A) Atomic Number (Z) Note: Ca-40 has a unique Atomic Mass of 39. 962591 g/mol Electron Configuration A Hydrogen atom (1 all Calcium on Earth p+ and 1 e-) has mass = 1. 007825 u 12
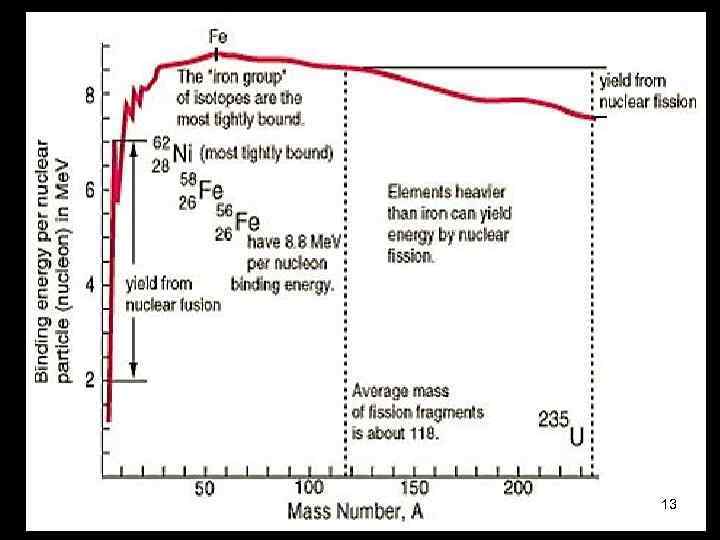 13
13
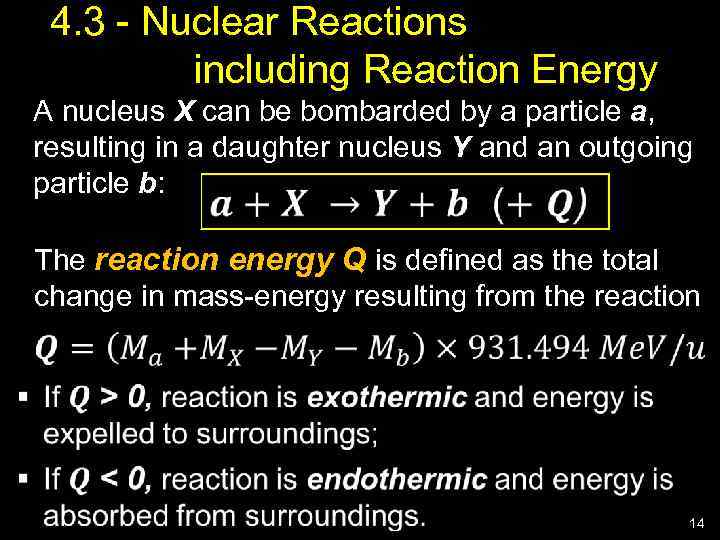 4. 3 - Nuclear Reactions including Reaction Energy A nucleus X can be bombarded by a particle a, resulting in a daughter nucleus Y and an outgoing particle b: The reaction energy Q is defined as the total change in mass-energy resulting from the reaction 14
4. 3 - Nuclear Reactions including Reaction Energy A nucleus X can be bombarded by a particle a, resulting in a daughter nucleus Y and an outgoing particle b: The reaction energy Q is defined as the total change in mass-energy resulting from the reaction 14
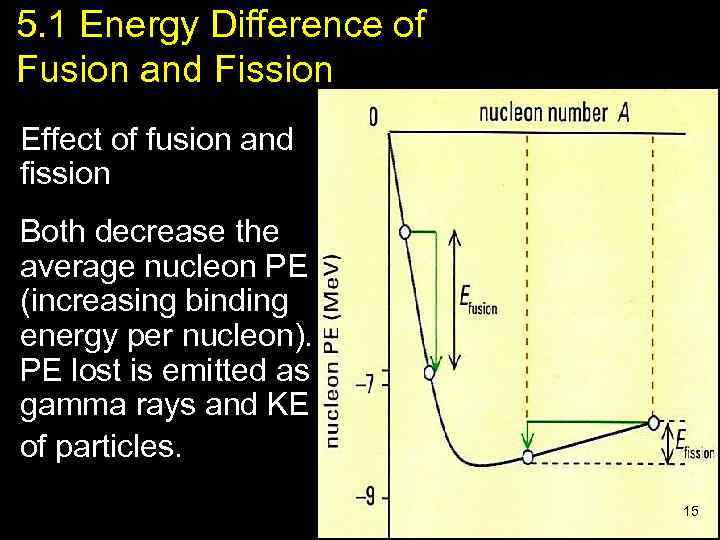 5. 1 Energy Difference of Fusion and Fission Effect of fusion and fission Both decrease the average nucleon PE (increasing binding energy per nucleon). PE lost is emitted as gamma rays and KE of particles. 15
5. 1 Energy Difference of Fusion and Fission Effect of fusion and fission Both decrease the average nucleon PE (increasing binding energy per nucleon). PE lost is emitted as gamma rays and KE of particles. 15
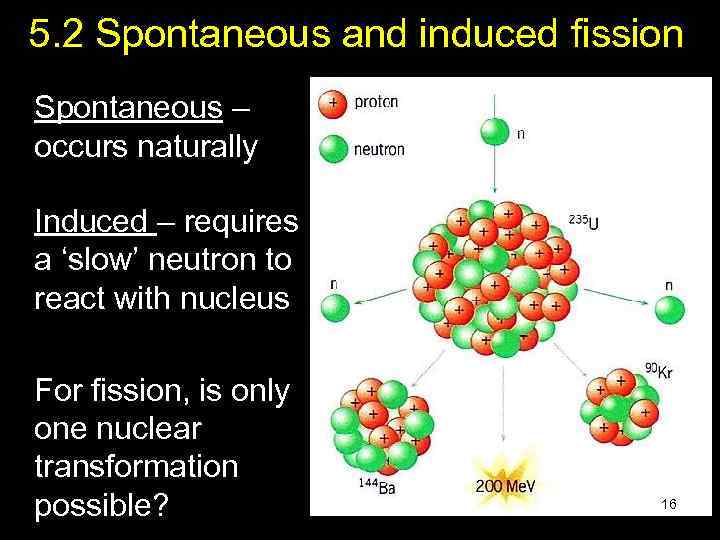 5. 2 Spontaneous and induced fission Spontaneous – occurs naturally Induced – requires a ‘slow’ neutron to react with nucleus For fission, is only one nuclear transformation possible? 16
5. 2 Spontaneous and induced fission Spontaneous – occurs naturally Induced – requires a ‘slow’ neutron to react with nucleus For fission, is only one nuclear transformation possible? 16
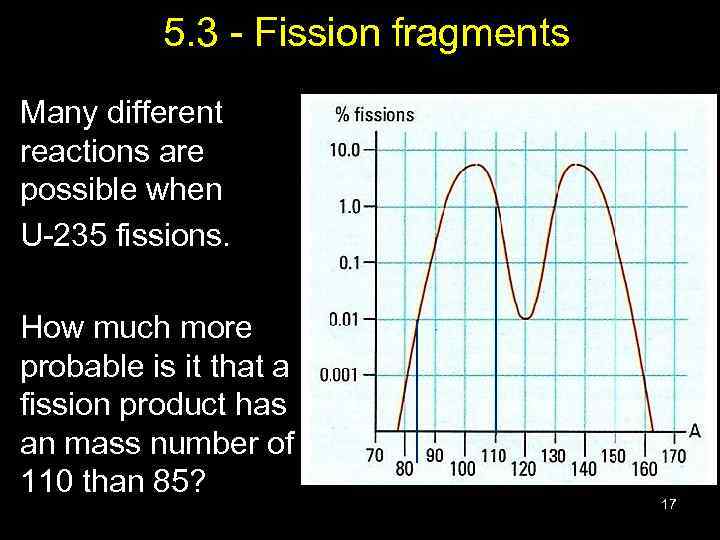 5. 3 - Fission fragments Many different reactions are possible when U-235 fissions. How much more probable is it that a fission product has an mass number of 110 than 85? 17
5. 3 - Fission fragments Many different reactions are possible when U-235 fissions. How much more probable is it that a fission product has an mass number of 110 than 85? 17
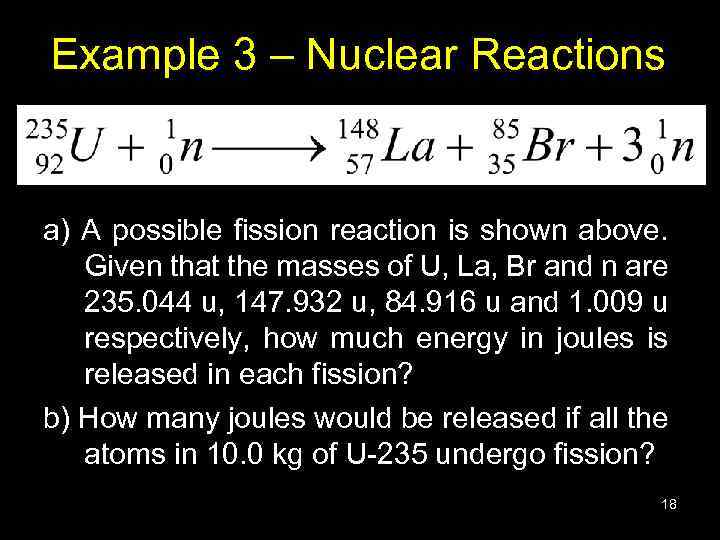 Example 3 – Nuclear Reactions a) A possible fission reaction is shown above. Given that the masses of U, La, Br and n are 235. 044 u, 147. 932 u, 84. 916 u and 1. 009 u respectively, how much energy in joules is released in each fission? b) How many joules would be released if all the atoms in 10. 0 kg of U-235 undergo fission? 18
Example 3 – Nuclear Reactions a) A possible fission reaction is shown above. Given that the masses of U, La, Br and n are 235. 044 u, 147. 932 u, 84. 916 u and 1. 009 u respectively, how much energy in joules is released in each fission? b) How many joules would be released if all the atoms in 10. 0 kg of U-235 undergo fission? 18
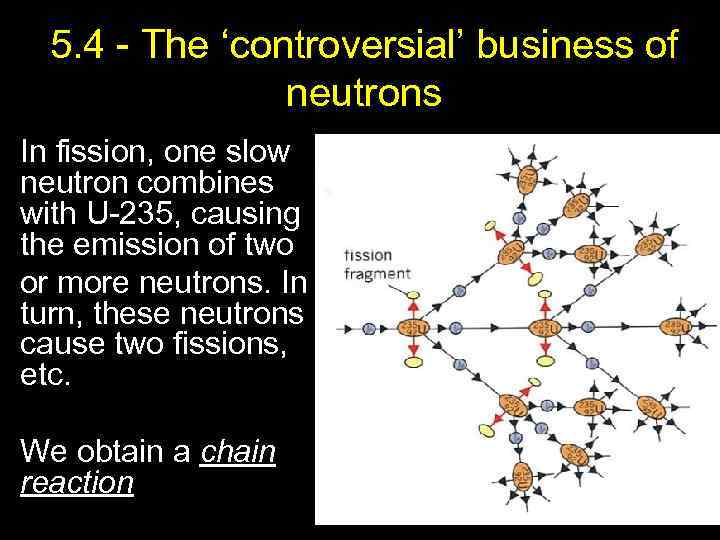 5. 4 - The ‘controversial’ business of neutrons In fission, one slow neutron combines with U-235, causing the emission of two or more neutrons. In turn, these neutrons cause two fissions, etc. We obtain a chain reaction 19
5. 4 - The ‘controversial’ business of neutrons In fission, one slow neutron combines with U-235, causing the emission of two or more neutrons. In turn, these neutrons cause two fissions, etc. We obtain a chain reaction 19
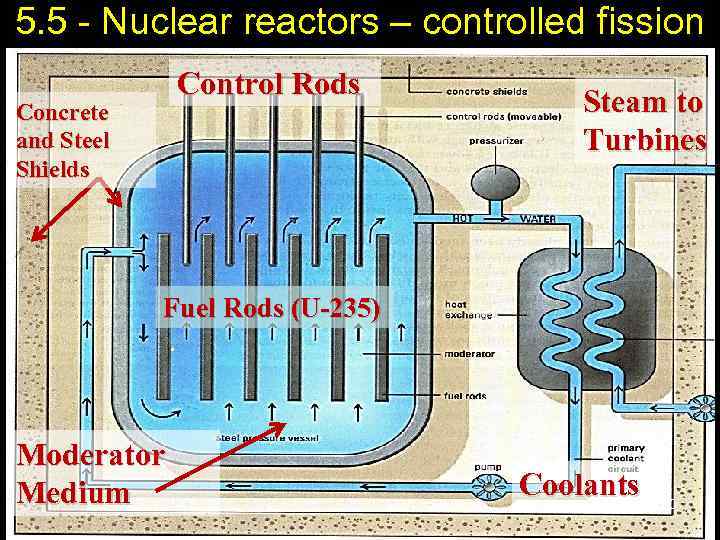 5. 5 - Nuclear reactors – controlled fission Control Rods Concrete and Steel Shields Steam to Turbines Fuel Rods (U-235) Moderator Medium Coolants 20
5. 5 - Nuclear reactors – controlled fission Control Rods Concrete and Steel Shields Steam to Turbines Fuel Rods (U-235) Moderator Medium Coolants 20
 5. 5 - Nuclear reactors – controlled fission Nuclear reactor is a system in which controlled nuclear chain reaction is used to liberate energy. Fuel rods - Long tube containing pellets of fissionable material, which provide fuel for nuclear reactors. Moderator – slows down the high-energy neutrons. Control rods – control the rate of the reaction (absorb neutrons without any additional reaction). Coolant - removes heat from the reactor core and transfers it to electrical generators and the environment. 21
5. 5 - Nuclear reactors – controlled fission Nuclear reactor is a system in which controlled nuclear chain reaction is used to liberate energy. Fuel rods - Long tube containing pellets of fissionable material, which provide fuel for nuclear reactors. Moderator – slows down the high-energy neutrons. Control rods – control the rate of the reaction (absorb neutrons without any additional reaction). Coolant - removes heat from the reactor core and transfers it to electrical generators and the environment. 21
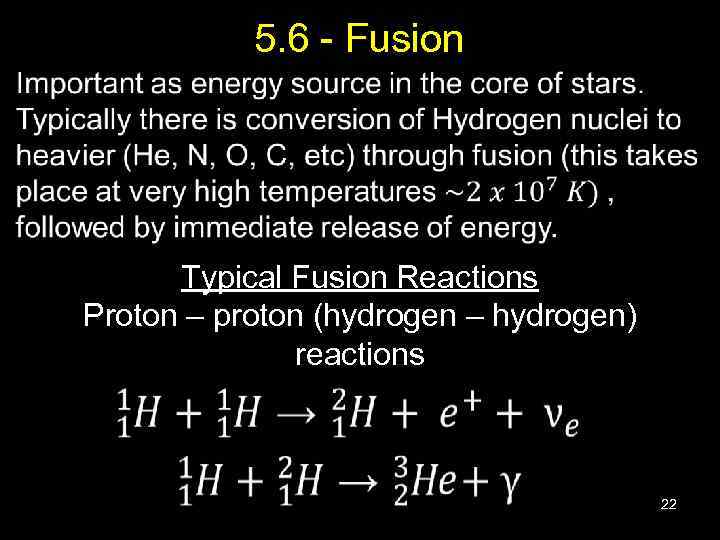 5. 6 - Fusion Typical Fusion Reactions Proton – proton (hydrogen – hydrogen) reactions 22
5. 6 - Fusion Typical Fusion Reactions Proton – proton (hydrogen – hydrogen) reactions 22
 5. 6 Fusion continued Fusion has been used uncontrolled in Hydrogen Bombs. Often a fission detonator is used to create high temperatures and start fusion process. A challenge today is to create a controlled fusion reaction. The main problem is the high temps required to initiate fusion (this is called confinement) One interesting reaction is: 23
5. 6 Fusion continued Fusion has been used uncontrolled in Hydrogen Bombs. Often a fission detonator is used to create high temperatures and start fusion process. A challenge today is to create a controlled fusion reaction. The main problem is the high temps required to initiate fusion (this is called confinement) One interesting reaction is: 23
 Numerical Answers to Example Questions Ex 1: 0. 030376 u (28. 3 Me. V) is converted into binding energy Ex 2. binding energy of Calcium-40 = 0. 367210 u = 342. 054 Me. V = 8. 55 Me. V/nucleon (to 3 s. f. ) Ex 3. a) with mass defect 0. 178 u, each fission releases 2. 66 x 10 -11 J (166 Me. V) b) 10. 0 kg of U 235 with complete fission releases 6. 81 x 10 14 J (4. 24 x 10 27 Me. V) 24 Note: This is equivalent to 35 k tonnes of coal.
Numerical Answers to Example Questions Ex 1: 0. 030376 u (28. 3 Me. V) is converted into binding energy Ex 2. binding energy of Calcium-40 = 0. 367210 u = 342. 054 Me. V = 8. 55 Me. V/nucleon (to 3 s. f. ) Ex 3. a) with mass defect 0. 178 u, each fission releases 2. 66 x 10 -11 J (166 Me. V) b) 10. 0 kg of U 235 with complete fission releases 6. 81 x 10 14 J (4. 24 x 10 27 Me. V) 24 Note: This is equivalent to 35 k tonnes of coal.
 READING: Adams and Allday: 8. 26 to 8. 32, Serway 29. 3 -29. 6 and 30. 1 - 30. 2 Be sure to Read the Worked Examples • At the end of this lecture you should • Have met and understood how to use Einstein's equation E= mc 2 • Understand the phrases binding energy and mass defect • Understand how energy can be released from nuclear reactions by fission and fusion • Have a basic knowledge of the 'binding energy per nucleon' curve and be able to interpret it • Know what the terms critical mass, moderator, coolant and chain reaction mean in relation to production of energy from a nuclear reactor • Be aware of the main steps of hydrogen fusion in the sun 25
READING: Adams and Allday: 8. 26 to 8. 32, Serway 29. 3 -29. 6 and 30. 1 - 30. 2 Be sure to Read the Worked Examples • At the end of this lecture you should • Have met and understood how to use Einstein's equation E= mc 2 • Understand the phrases binding energy and mass defect • Understand how energy can be released from nuclear reactions by fission and fusion • Have a basic knowledge of the 'binding energy per nucleon' curve and be able to interpret it • Know what the terms critical mass, moderator, coolant and chain reaction mean in relation to production of energy from a nuclear reactor • Be aware of the main steps of hydrogen fusion in the sun 25


