L 25 - Nuc Physics 1.pptx
- Количество слайдов: 28

L 25 - NUCLEAR PHYSICS 1 By the end of this lecture you should: • Know that the nucleus of the atom is made of protons and neutrons (collectively called nucleons and part of a group of particles called baryons) and that nucleons are regarded as made up of quarks • Appreciate that it is the strong nuclear force which keeps the nucleus together • Understand that radioactive decay is a spontaneous (or random) process involving the emission of particles from the nucleus. Therefore the mathematics which models radioactivity deal with large numbers of particles. i. e. it is a statistical theory • Know the nature of the four main particles involved in radioactive decay Be able to define activity, decay constant, half-life and isotope • Be able to perform a rigorous mathematical treatment of simple situations, involving the above concepts
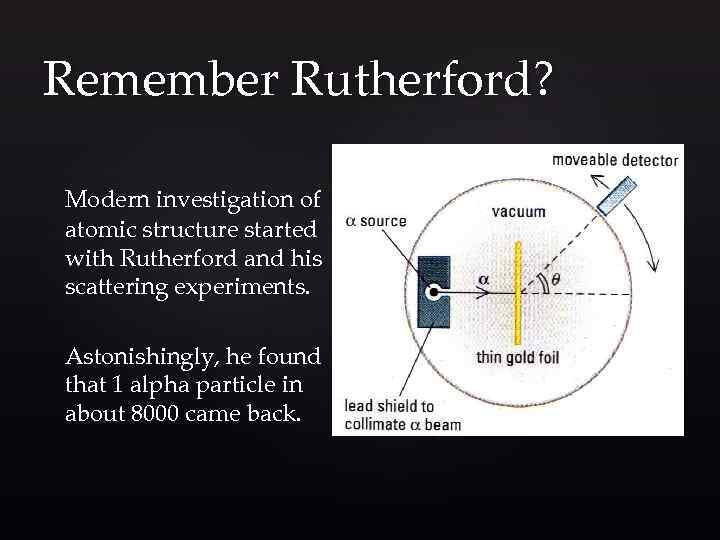
Remember Rutherford? Modern investigation of atomic structure started with Rutherford and his scattering experiments. Astonishingly, he found that 1 alpha particle in about 8000 came back.
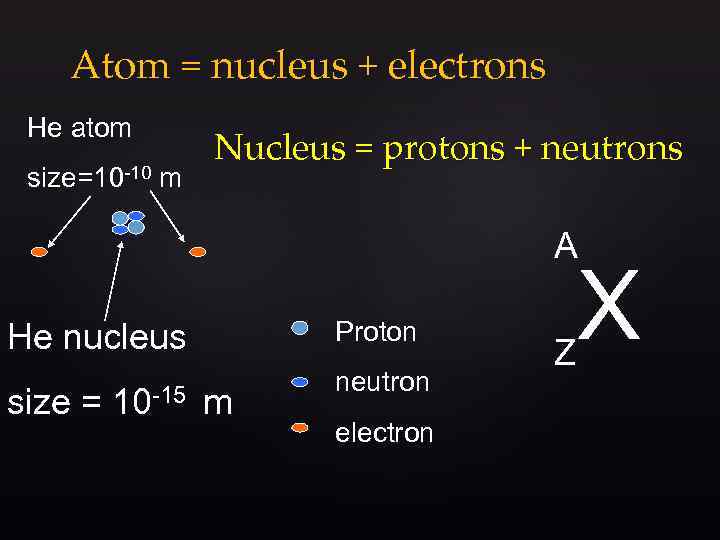
Atom = nucleus + electrons He atom size=10 -10 m Nucleus = protons + neutrons A Proton He nucleus size = 10 -15 m neutron electron Z X

Some common atoms #e #p #n § Hydrogen 1 1 0 § Deuterium 1 1 1 § Tritium 1 1 2 § Helium- 4 2 2 2 § Carbon-12 6 6 6 § Carbon-14 6 6 8 Uranium-238 92 92 146 Symbol 1 1 H
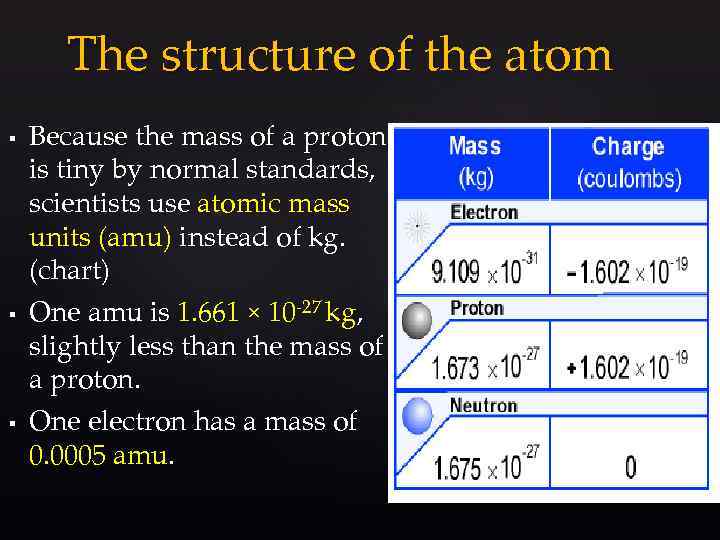
The structure of the atom § § § Because the mass of a proton is tiny by normal standards, scientists use atomic mass units (amu) instead of kg. (chart) One amu is 1. 661 × 10 -27 kg, slightly less than the mass of a proton. One electron has a mass of 0. 0005 amu.

The structure of the atom § § A neutral atom has a total charge of zero. Because the number of electrons equals the number of protons in a complete atom they tend to stay neutral because electric forces are very strong.
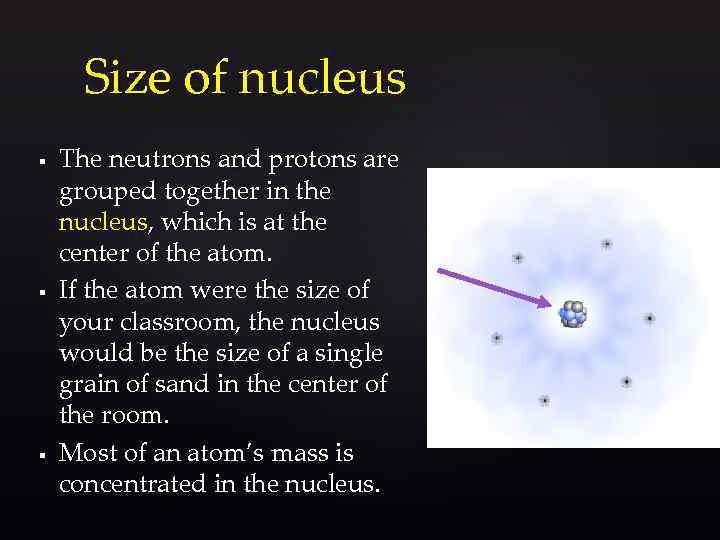
Size of nucleus § § § The neutrons and protons are grouped together in the nucleus, which is at the center of the atom. If the atom were the size of your classroom, the nucleus would be the size of a single grain of sand in the center of the room. Most of an atom’s mass is concentrated in the nucleus.

The Strong Force What are strong forces? The nucleus of helium contains two protons. They are both positively charged and will repel each other. So why don’t protons go flying out the atom and stay bound in a helium nucleus? There must be another force that holds them together. This force is the Strong Nuclear Force.

Strong Nuclear Force § § Holds protons and neutrons together in a nucleus Strongest force. About 20 times stronger than EM force Short range - only extends a little beyond size of proton (10 -15 m) Mostly attractive but only affects particles like protons and neutrons. Electrons do not “feel” this force
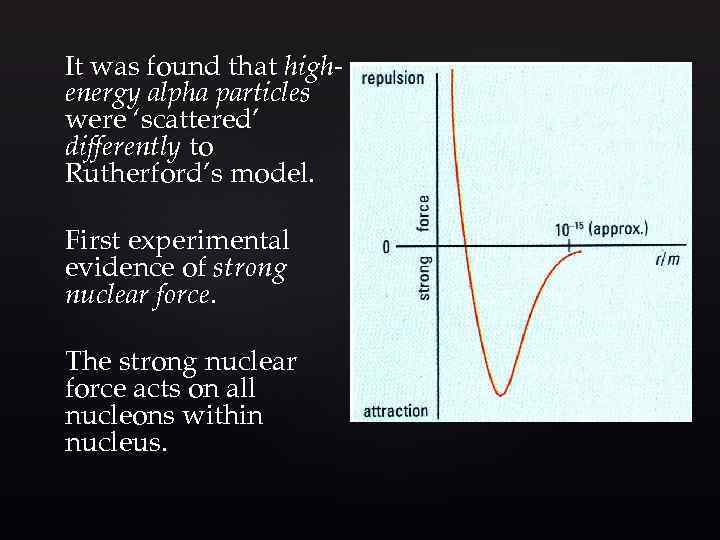
It was found that highenergy alpha particles were ‘scattered’ differently to Rutherford’s model. First experimental evidence of strong nuclear force. The strong nuclear force acts on all nucleons within nucleus.

How do we ‘do’ nuclear physics?
LHC – from above

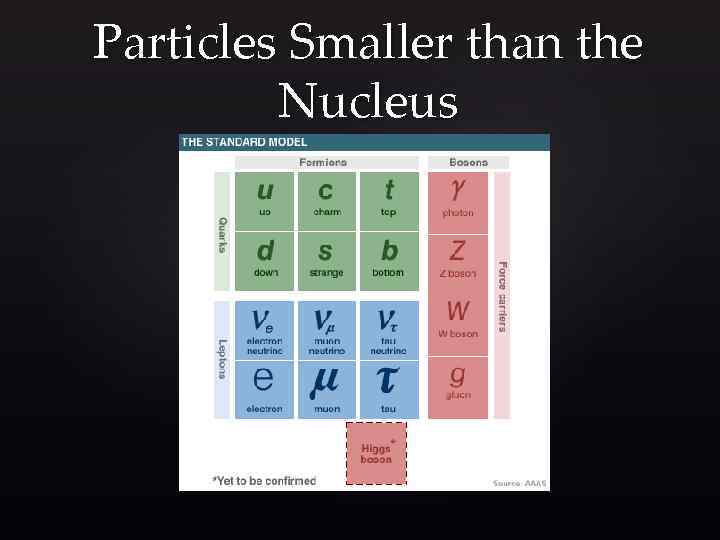
Particles Smaller than the Nucleus
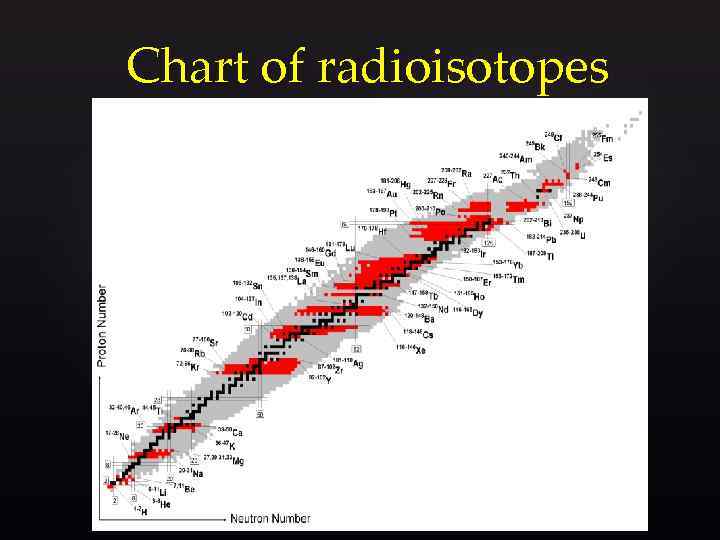
Chart of radioisotopes
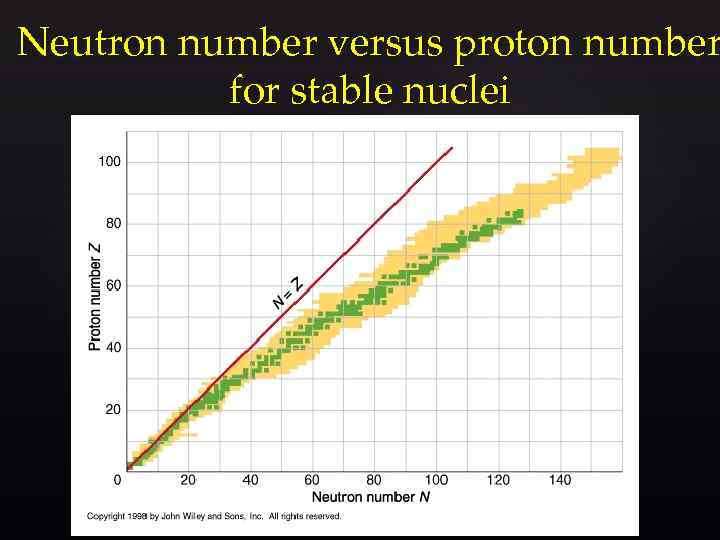
Neutron number versus proton number for stable nuclei
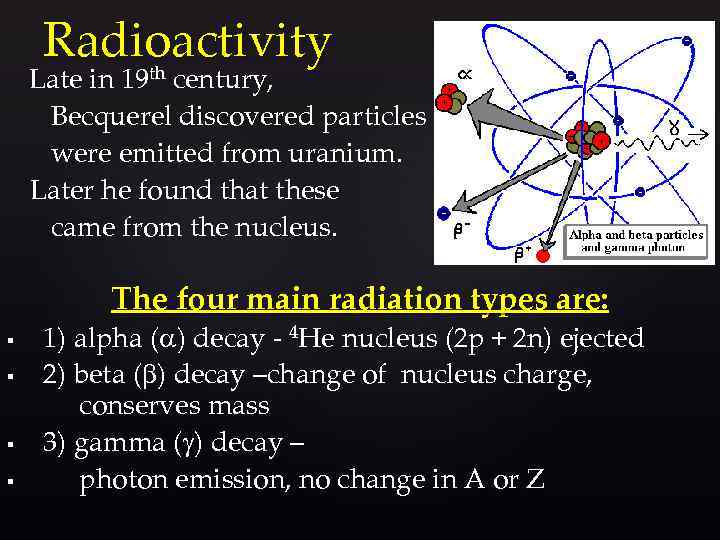
Radioactivity Late in 19 th century, Becquerel discovered particles were emitted from uranium. Later he found that these came from the nucleus. The four main radiation types are: § § 1) alpha (a) decay - 4 He nucleus (2 p + 2 n) ejected 2) beta ( ) decay –change of nucleus charge, conserves mass 3) gamma (g) decay – photon emission, no change in A or Z

Marie and Pierre Curie Besides Becquerel, Marie and Pierre Curie were pioneers in the science of radioactivity. What is one becquerel? What is one curie?

Nuclear transformation For radioactive decay, charge and number of nucleons is conserved. So, the atomic number and the mass number are the same before and after.
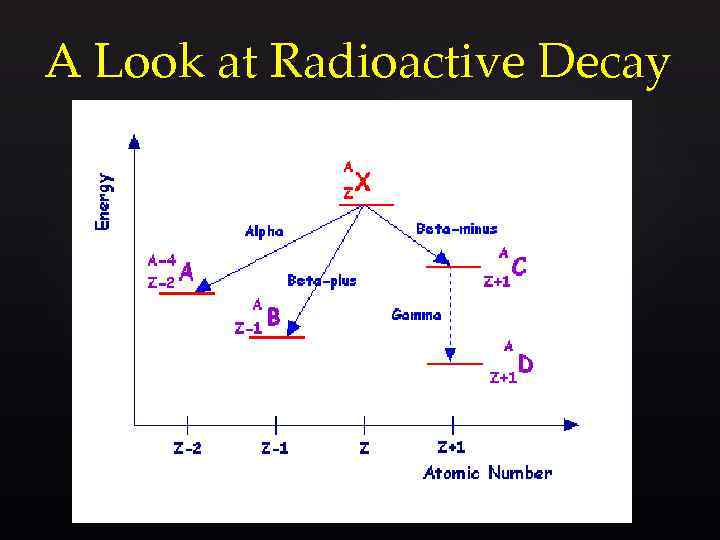
A Look at Radioactive Decay

Example 1 A radioactive atom X has an atomic number of 90 and a mass number of 230. It decays by emitting one alpha particle, one beta particle and one positron. What is the complete equation for this nuclear transformation?
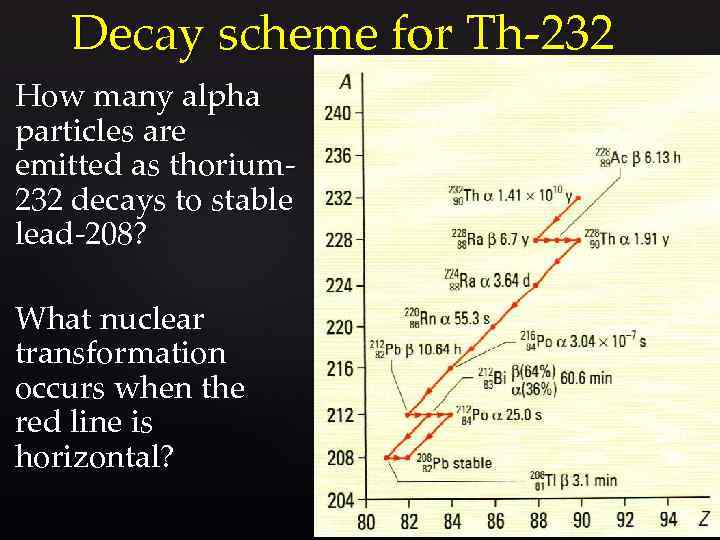
Decay scheme for Th-232 How many alpha particles are emitted as thorium 232 decays to stable lead-208? What nuclear transformation occurs when the red line is horizontal?
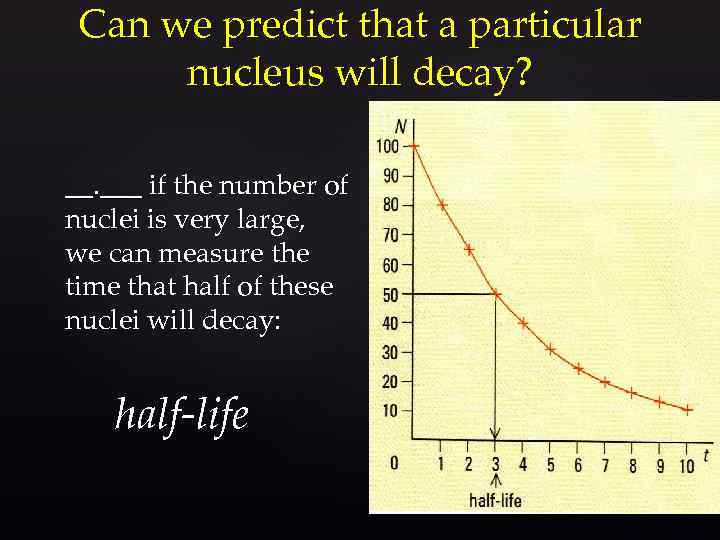
Can we predict that a particular nucleus will decay? __. ___ if the number of nuclei is very large, we can measure the time that half of these nuclei will decay: half-life

Radioactivity mathematically Assuming that both decay is random and that the rate of change of number of nuclei is proportional to the number of unstable nuclei: The “-” sign shows N is decreasing.
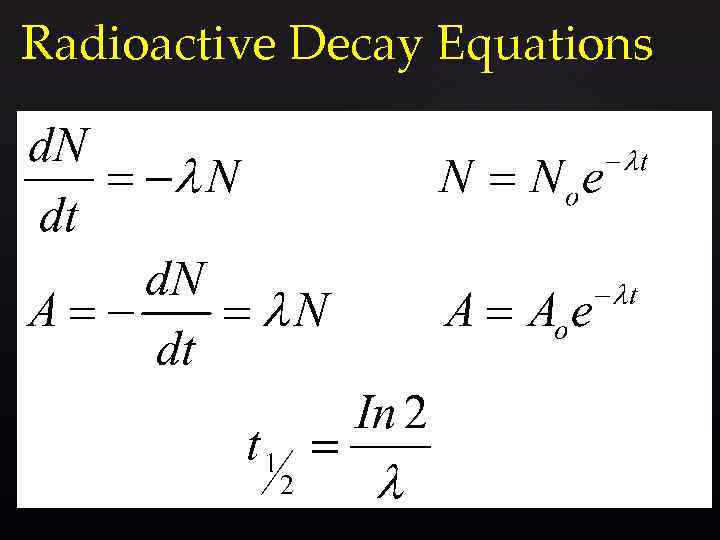
Radioactive Decay Equations

Avogadro’s number

Example 2 The activity of 1 kg of seawater containing K-40 is 12 s-1. The decay constant for K-40 is 1. 6 x 10 -17 s-1. Find: a) the half-life of K-40 b) the amount of isotope present. c) the time taken for the activity to reduce to 10 s-1

READING Adams and Allday: 8. 11 to 8. 22 inclusive, except 8. 20 Know that the nucleus of the atom is made of protons and neutrons (collectively called nucleons and part of a group of particles called baryons) and that nucleons are regarded as made up of quarks Appreciate that it is the strong nuclear force which keeps the nucleus together Understand that radioactive decay is a spontaneous (or random) process involving the emission of particles from the nucleus. Therefore the mathematics which models radioactivity deal with large numbers of particles. i. e. it is a statistical theory Know the nature of the four main particles involved in radioactive decay Be able to define activity, decay constant, half-life and isotope Be able to perform a rigorous mathematical treatment of simple situations, involving the above concepts
L 25 - Nuc Physics 1.pptx