L25 Molecular Physics.pptx
- Количество слайдов: 27
 L 25 – Molecular Physics 1) Atomic Energy Levels 2) Molecular Bonds a) Features of Molecular Bonds b) Force and Potential Energy graphs for molecular separation c) Types of Molecular Bonds 3) Molecular Energy Levels and Molecular Spectra
L 25 – Molecular Physics 1) Atomic Energy Levels 2) Molecular Bonds a) Features of Molecular Bonds b) Force and Potential Energy graphs for molecular separation c) Types of Molecular Bonds 3) Molecular Energy Levels and Molecular Spectra
 1) Atomic Energy Levels • The principle of quantization of electron angular momentum used to calculate energy levels of the hydrogen atom apply to more complex atoms • Atoms are characterized by a set of discrete energy levels and quantum numbers • The most stable configuration for an atom (ground state) has the lowest energy • Repartition of electrons in atomic shells obeys Pauli exclusion principle 2
1) Atomic Energy Levels • The principle of quantization of electron angular momentum used to calculate energy levels of the hydrogen atom apply to more complex atoms • Atoms are characterized by a set of discrete energy levels and quantum numbers • The most stable configuration for an atom (ground state) has the lowest energy • Repartition of electrons in atomic shells obeys Pauli exclusion principle 2
 2) Molecular Bonds • The bonding mechanisms in a molecule are fundamentally due to electric forces • The forces between atoms in a molecule are related to a potential energy function • A stable molecule has a configuration for which the potential energy is minimum 3
2) Molecular Bonds • The bonding mechanisms in a molecule are fundamentally due to electric forces • The forces between atoms in a molecule are related to a potential energy function • A stable molecule has a configuration for which the potential energy is minimum 3
 2 a) Features of Molecular Bonds • The force between atoms is repulsive at very small separation distances – This repulsion is partially electrostatic and partially due to the exclusion principle – Due to the exclusion principle, some electrons in overlapping shells are forced into higher energy states – The energy of the system increases as if a repulsive force existed between the atoms • The force between the atoms is attractive at larger distances 4
2 a) Features of Molecular Bonds • The force between atoms is repulsive at very small separation distances – This repulsion is partially electrostatic and partially due to the exclusion principle – Due to the exclusion principle, some electrons in overlapping shells are forced into higher energy states – The energy of the system increases as if a repulsive force existed between the atoms • The force between the atoms is attractive at larger distances 4
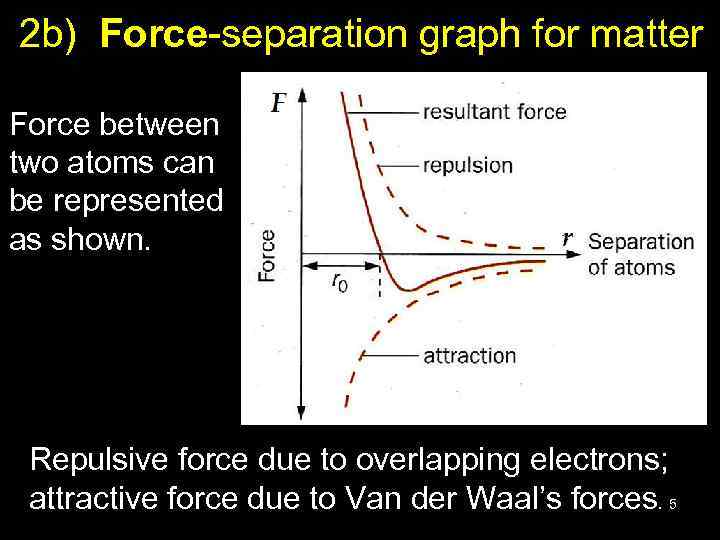 2 b) Force-separation graph for matter Force between two atoms can be represented as shown. Repulsive force due to overlapping electrons; attractive force due to Van der Waal’s forces. 5
2 b) Force-separation graph for matter Force between two atoms can be represented as shown. Repulsive force due to overlapping electrons; attractive force due to Van der Waal’s forces. 5
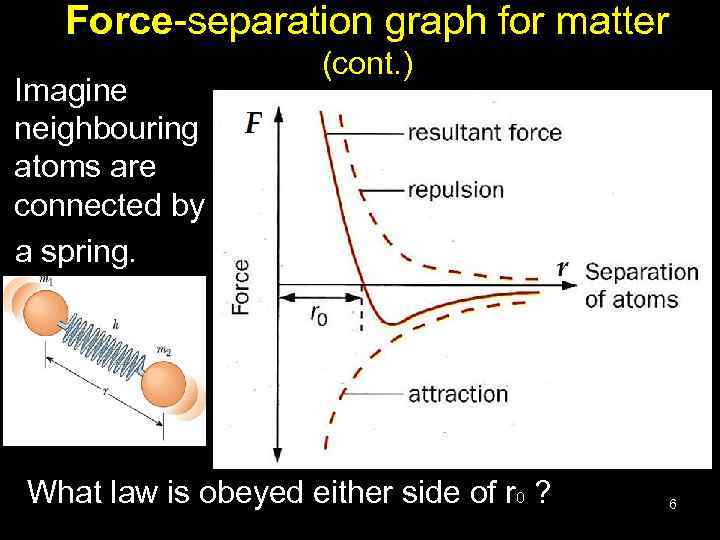 Force-separation graph for matter Imagine neighbouring atoms are connected by a spring. (cont. ) What law is obeyed either side of r 0 ? 6
Force-separation graph for matter Imagine neighbouring atoms are connected by a spring. (cont. ) What law is obeyed either side of r 0 ? 6
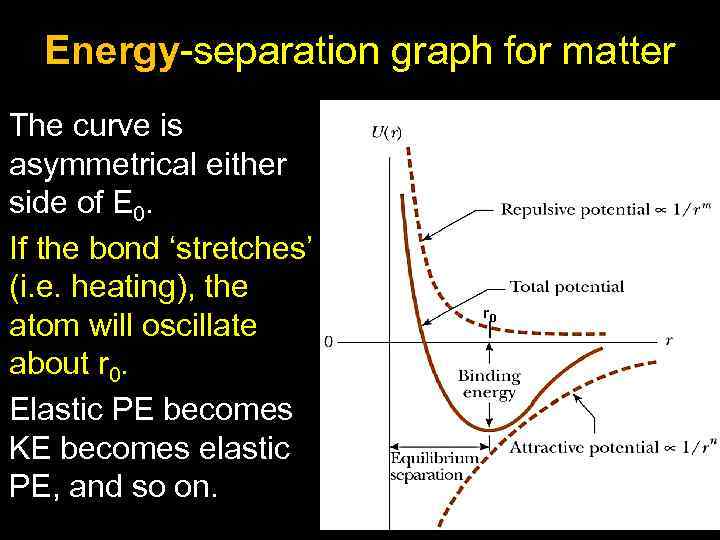 Energy-separation graph for matter The curve is asymmetrical either side of E 0. If the bond ‘stretches’ (i. e. heating), the atom will oscillate about r 0. Elastic PE becomes KE becomes elastic PE, and so on. r 0 7
Energy-separation graph for matter The curve is asymmetrical either side of E 0. If the bond ‘stretches’ (i. e. heating), the atom will oscillate about r 0. Elastic PE becomes KE becomes elastic PE, and so on. r 0 7
 Note: Because the curve is not symmetrical about r 0 during oscillation, the average separation of the atoms increases. This explains thermal expansion. Note: The energy needed to break the bond between atoms is U 0 (minimum potential energy). This is the energy needed to turn the matter in a gas. 8
Note: Because the curve is not symmetrical about r 0 during oscillation, the average separation of the atoms increases. This explains thermal expansion. Note: The energy needed to break the bond between atoms is U 0 (minimum potential energy). This is the energy needed to turn the matter in a gas. 8
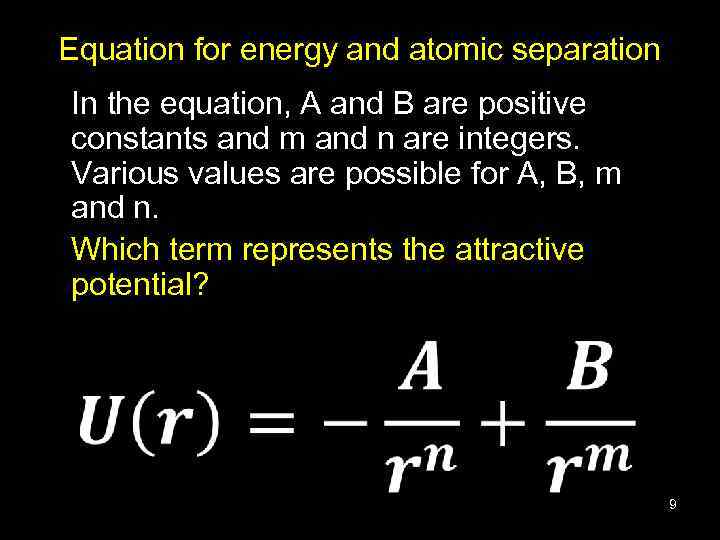 Equation for energy and atomic separation In the equation, A and B are positive constants and m and n are integers. Various values are possible for A, B, m and n. Which term represents the attractive potential? 9
Equation for energy and atomic separation In the equation, A and B are positive constants and m and n are integers. Various values are possible for A, B, m and n. Which term represents the attractive potential? 9
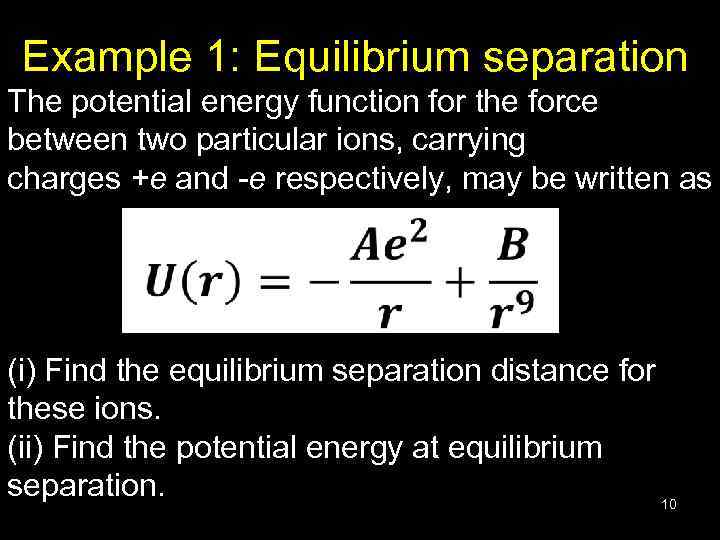 Example 1: Equilibrium separation The potential energy function for the force between two particular ions, carrying charges +e and -e respectively, may be written as (i) Find the equilibrium separation distance for these ions. (ii) Find the potential energy at equilibrium separation. 10
Example 1: Equilibrium separation The potential energy function for the force between two particular ions, carrying charges +e and -e respectively, may be written as (i) Find the equilibrium separation distance for these ions. (ii) Find the potential energy at equilibrium separation. 10
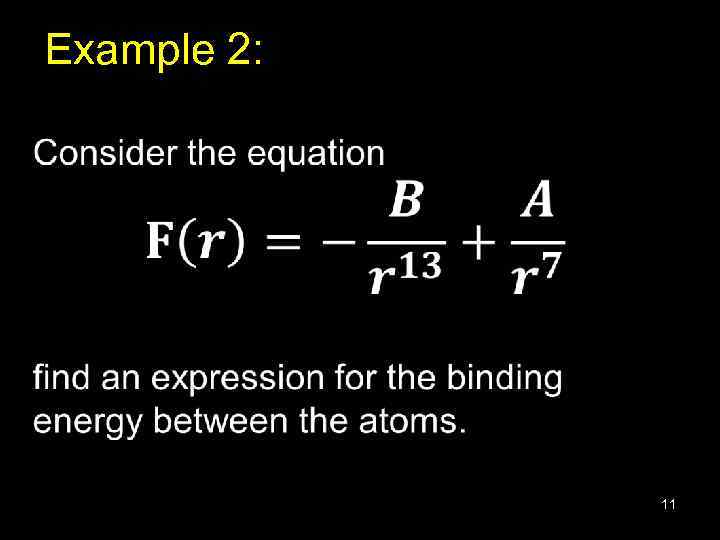 Example 2: • 11
Example 2: • 11
 3) Molecular Bonds – Types Four main models of molecular bonding: • Bonding between atoms within molecule (1) Ionic (2) Covalent • Bonding between molecule themselves (3) van der Waals (4) Hydrogen bond 12
3) Molecular Bonds – Types Four main models of molecular bonding: • Bonding between atoms within molecule (1) Ionic (2) Covalent • Bonding between molecule themselves (3) van der Waals (4) Hydrogen bond 12
 Ionic Bonding • An Ionic bond is an interaction between oppositely charged ionized atoms • Two atoms combine in such a way that one or more outer electrons are transferred from one atom to the other • Ionic bonds are fundamentally caused by the Coulomb attraction between oppositely charged ions • Sodium Chloride: Na. Cl (Na+ and Cl- in solution) 13
Ionic Bonding • An Ionic bond is an interaction between oppositely charged ionized atoms • Two atoms combine in such a way that one or more outer electrons are transferred from one atom to the other • Ionic bonds are fundamentally caused by the Coulomb attraction between oppositely charged ions • Sodium Chloride: Na. Cl (Na+ and Cl- in solution) 13
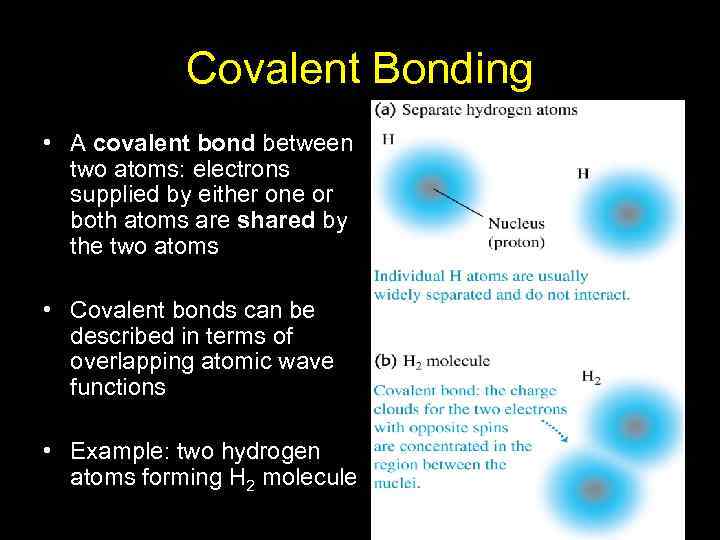 Covalent Bonding • A covalent bond between two atoms: electrons supplied by either one or both atoms are shared by the two atoms • Covalent bonds can be described in terms of overlapping atomic wave functions • Example: two hydrogen atoms forming H 2 molecule 14
Covalent Bonding • A covalent bond between two atoms: electrons supplied by either one or both atoms are shared by the two atoms • Covalent bonds can be described in terms of overlapping atomic wave functions • Example: two hydrogen atoms forming H 2 molecule 14
 Covalent Bonding, cont. • The probability is higher that the electrons associated with the atoms will be located between them • This can be modelled as if there were a fixed negative charge between the atoms, exerting attractive Coulomb forces on both nuclei • The result is an overall attractive force between the atoms, resulting in the covalent bond 15
Covalent Bonding, cont. • The probability is higher that the electrons associated with the atoms will be located between them • This can be modelled as if there were a fixed negative charge between the atoms, exerting attractive Coulomb forces on both nuclei • The result is an overall attractive force between the atoms, resulting in the covalent bond 15
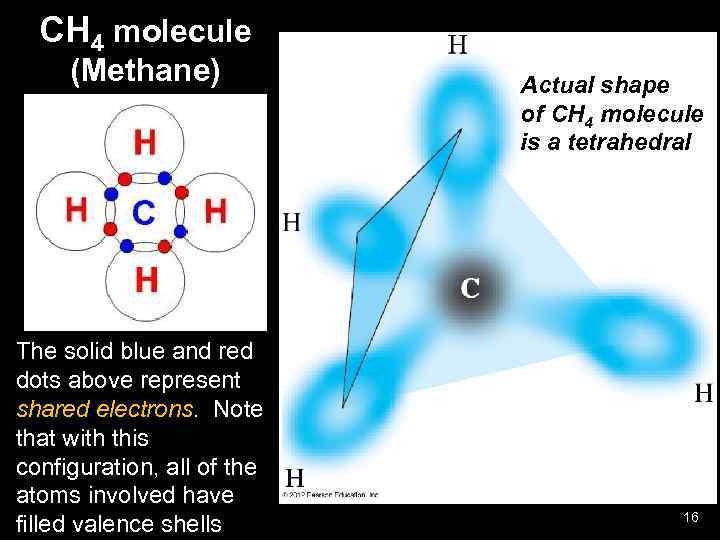 CH 4 molecule (Methane) The solid blue and red dots above represent shared electrons. Note that with this configuration, all of the atoms involved have filled valence shells Actual shape of CH 4 molecule is a tetrahedral 16
CH 4 molecule (Methane) The solid blue and red dots above represent shared electrons. Note that with this configuration, all of the atoms involved have filled valence shells Actual shape of CH 4 molecule is a tetrahedral 16
 Van der Waals Bonding • Two neutral molecules are attracted to each other by weak electrostatic forces called van der Waals forces – Atoms that do not form ionic or covalent bonds are also attracted to each other by van der Waals forces • The van der Waals force is due to the fact that the molecule has a charge distribution with positive and negative centers at different positions in the molecule 17
Van der Waals Bonding • Two neutral molecules are attracted to each other by weak electrostatic forces called van der Waals forces – Atoms that do not form ionic or covalent bonds are also attracted to each other by van der Waals forces • The van der Waals force is due to the fact that the molecule has a charge distribution with positive and negative centers at different positions in the molecule 17
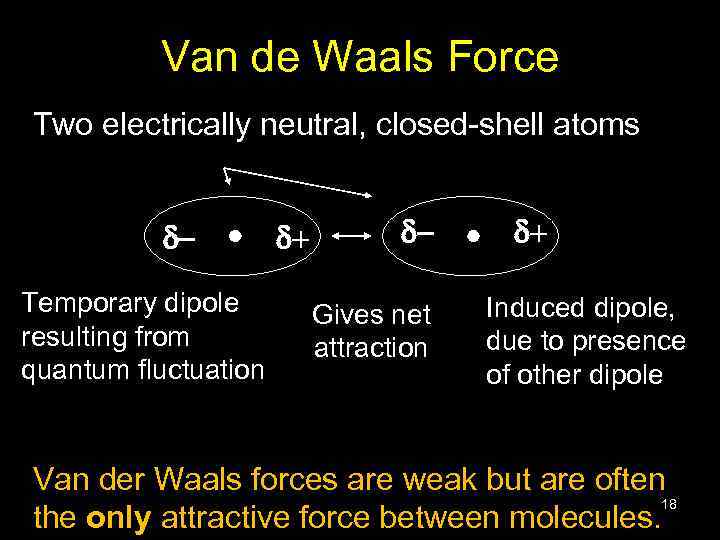 Van de Waals Force Two electrically neutral, closed-shell atoms d. Temporary dipole resulting from quantum fluctuation d+ d. Gives net attraction d+ Induced dipole, due to presence of other dipole Van der Waals forces are weak but are often 18 the only attractive force between molecules.
Van de Waals Force Two electrically neutral, closed-shell atoms d. Temporary dipole resulting from quantum fluctuation d+ d. Gives net attraction d+ Induced dipole, due to presence of other dipole Van der Waals forces are weak but are often 18 the only attractive force between molecules.
 Van der Waals Bonding, cont. • As a result of this charge distribution, the molecule may act as an electric dipole • Because of the dipole electric fields, two molecules can interact such that there is an attractive force between them – Remember, this occurs even though the molecules are electrically neutral 19
Van der Waals Bonding, cont. • As a result of this charge distribution, the molecule may act as an electric dipole • Because of the dipole electric fields, two molecules can interact such that there is an attractive force between them – Remember, this occurs even though the molecules are electrically neutral 19
 Hydrogen Bonding • An hydrogen atom in a molecule can also form a hydrogen bond (weaker than other bonds) • Example: water (H 2 O): There are two covalent bonds in the molecule Electrons from the hydrogen atoms are more likely to be found near the oxygen atom than the hydrogen atoms • Bare protons at positions of the hydrogen atoms • Hence, the negative end of another molecule can come very close to the proton • Hydrogen bond strong enough to form a solid crystalline structure 20
Hydrogen Bonding • An hydrogen atom in a molecule can also form a hydrogen bond (weaker than other bonds) • Example: water (H 2 O): There are two covalent bonds in the molecule Electrons from the hydrogen atoms are more likely to be found near the oxygen atom than the hydrogen atoms • Bare protons at positions of the hydrogen atoms • Hence, the negative end of another molecule can come very close to the proton • Hydrogen bond strong enough to form a solid crystalline structure 20
 • Hydrogen bonding is a critical mechanism for the linking of biological molecules and polymers • DNA is an example 21
• Hydrogen bonding is a critical mechanism for the linking of biological molecules and polymers • DNA is an example 21
 Energy States of Molecules • Molecules also have characteristic discrete energy levels • The energy of a molecule can be divided into four categories – Electronic energy interactions between molecule’s electrons and nuclei – Translational energy (unrelated to internal structure) Due to motion of the molecule through space – Rotational energy Due to the rotation of the molecule about its center of mass – Vibrational energy Due to the vibration of the molecule’s constituent atoms 22
Energy States of Molecules • Molecules also have characteristic discrete energy levels • The energy of a molecule can be divided into four categories – Electronic energy interactions between molecule’s electrons and nuclei – Translational energy (unrelated to internal structure) Due to motion of the molecule through space – Rotational energy Due to the rotation of the molecule about its center of mass – Vibrational energy Due to the vibration of the molecule’s constituent atoms 22
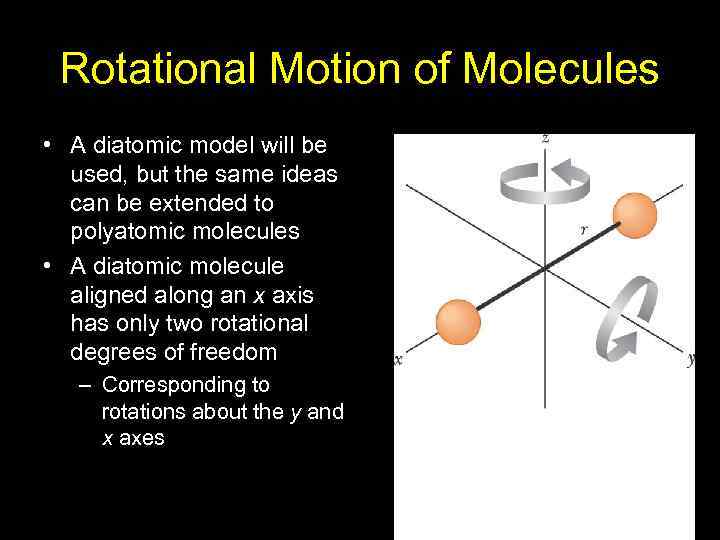 Rotational Motion of Molecules • A diatomic model will be used, but the same ideas can be extended to polyatomic molecules • A diatomic molecule aligned along an x axis has only two rotational degrees of freedom – Corresponding to rotations about the y and x axes 23
Rotational Motion of Molecules • A diatomic model will be used, but the same ideas can be extended to polyatomic molecules • A diatomic molecule aligned along an x axis has only two rotational degrees of freedom – Corresponding to rotations about the y and x axes 23
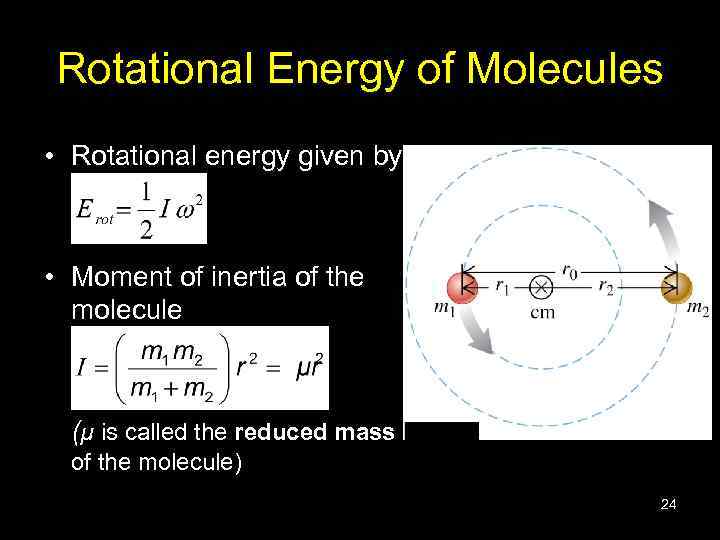 Rotational Energy of Molecules • Rotational energy given by • Moment of inertia of the molecule (µ is called the reduced mass of the molecule) 24
Rotational Energy of Molecules • Rotational energy given by • Moment of inertia of the molecule (µ is called the reduced mass of the molecule) 24
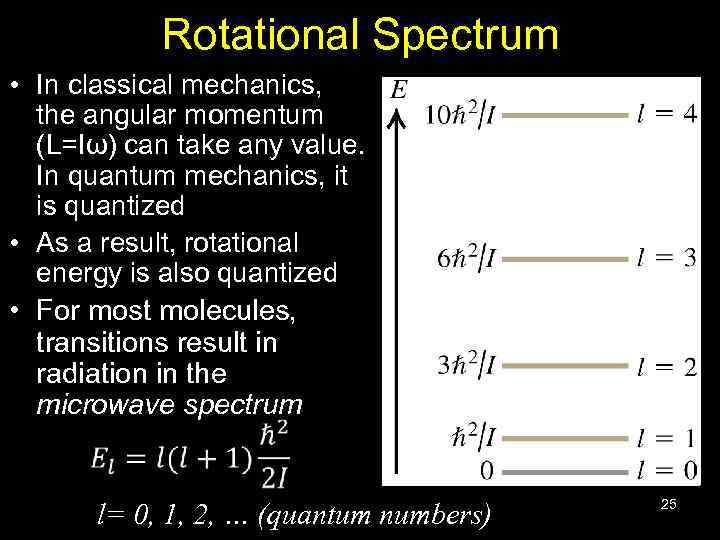 Rotational Spectrum • In classical mechanics, the angular momentum (L=Iω) can take any value. In quantum mechanics, it is quantized • As a result, rotational energy is also quantized • For most molecules, transitions result in radiation in the microwave spectrum l= 0, 1, 2, … (quantum numbers) 25
Rotational Spectrum • In classical mechanics, the angular momentum (L=Iω) can take any value. In quantum mechanics, it is quantized • As a result, rotational energy is also quantized • For most molecules, transitions result in radiation in the microwave spectrum l= 0, 1, 2, … (quantum numbers) 25
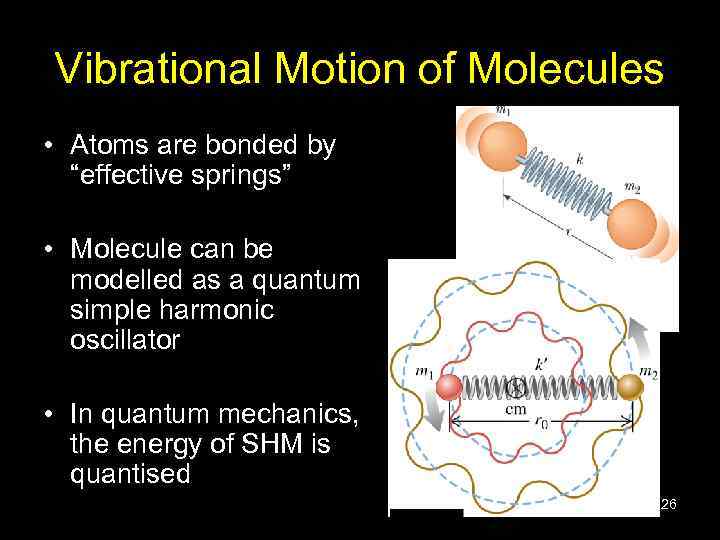 Vibrational Motion of Molecules • Atoms are bonded by “effective springs” • Molecule can be modelled as a quantum simple harmonic oscillator • In quantum mechanics, the energy of SHM is quantised 26
Vibrational Motion of Molecules • Atoms are bonded by “effective springs” • Molecule can be modelled as a quantum simple harmonic oscillator • In quantum mechanics, the energy of SHM is quantised 26
 Readings and Answers • 27
Readings and Answers • 27


