 L 22 – Modern Physics 2 At the end of this lecture you should: • Have a knowledge of the current model of the atom • Have an idea of how the knowledge of that structure developed historically • Understand the main points of Rutherford's scattering experiment to probe the atom, alpha particle • Understand the meaning of the chemical symbols in the periodic table and what isotopes are • Know about quantized orbits in the Bohr atom • Know emission spectra and absorption spectra, and how they arise due to electrons jumping between shells in an atom • Be able to define ground state, excited state, ionization.
L 22 – Modern Physics 2 At the end of this lecture you should: • Have a knowledge of the current model of the atom • Have an idea of how the knowledge of that structure developed historically • Understand the main points of Rutherford's scattering experiment to probe the atom, alpha particle • Understand the meaning of the chemical symbols in the periodic table and what isotopes are • Know about quantized orbits in the Bohr atom • Know emission spectra and absorption spectra, and how they arise due to electrons jumping between shells in an atom • Be able to define ground state, excited state, ionization.
 Tests on Monday Jan 30 th • Test on Monday January 30 th. It will be instead of the Lecture. (11 a. m-12 noon) Questions on three topics: – Diffraction and Refraction (CW 8) – Mechanical and Stationary Waves (CW 9) – Modern Physics 1 and 2 (CW 10) • To prepare for this test, be able to do all CW and PSC questions on these topics.
Tests on Monday Jan 30 th • Test on Monday January 30 th. It will be instead of the Lecture. (11 a. m-12 noon) Questions on three topics: – Diffraction and Refraction (CW 8) – Mechanical and Stationary Waves (CW 9) – Modern Physics 1 and 2 (CW 10) • To prepare for this test, be able to do all CW and PSC questions on these topics.
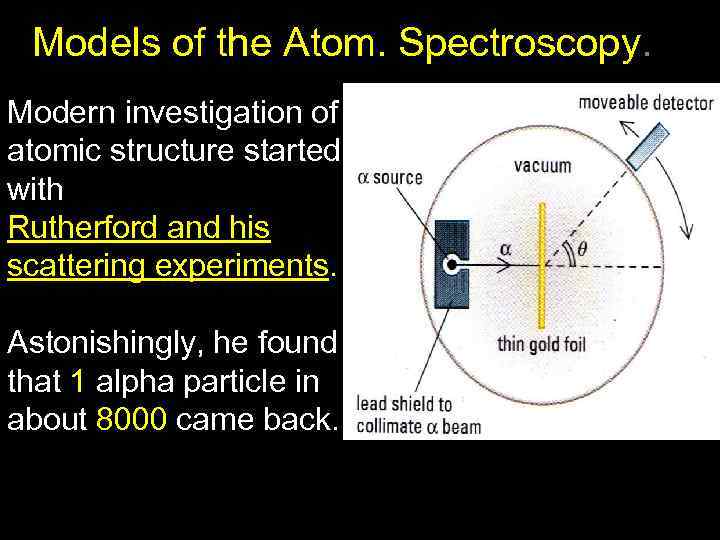 Models of the Atom. Spectroscopy. Modern investigation of atomic structure started with Rutherford and his scattering experiments. Astonishingly, he found that 1 alpha particle in about 8000 came back.
Models of the Atom. Spectroscopy. Modern investigation of atomic structure started with Rutherford and his scattering experiments. Astonishingly, he found that 1 alpha particle in about 8000 came back.
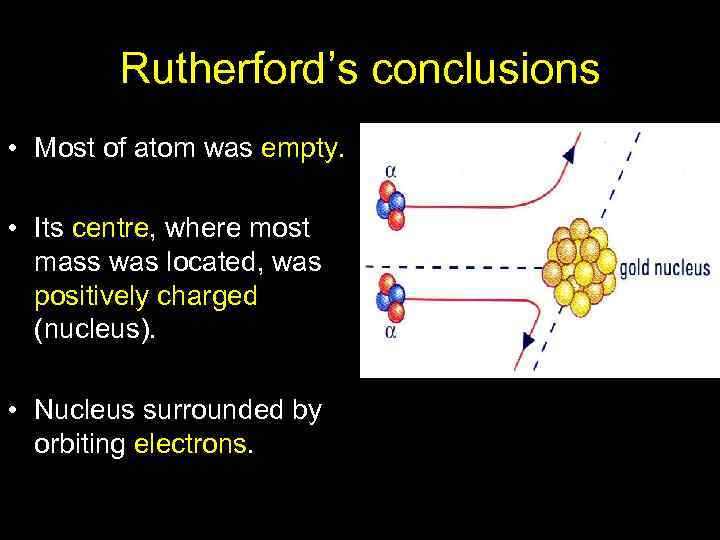 Rutherford’s conclusions • Most of atom was empty. • Its centre, where most mass was located, was positively charged (nucleus). • Nucleus surrounded by orbiting electrons.
Rutherford’s conclusions • Most of atom was empty. • Its centre, where most mass was located, was positively charged (nucleus). • Nucleus surrounded by orbiting electrons.
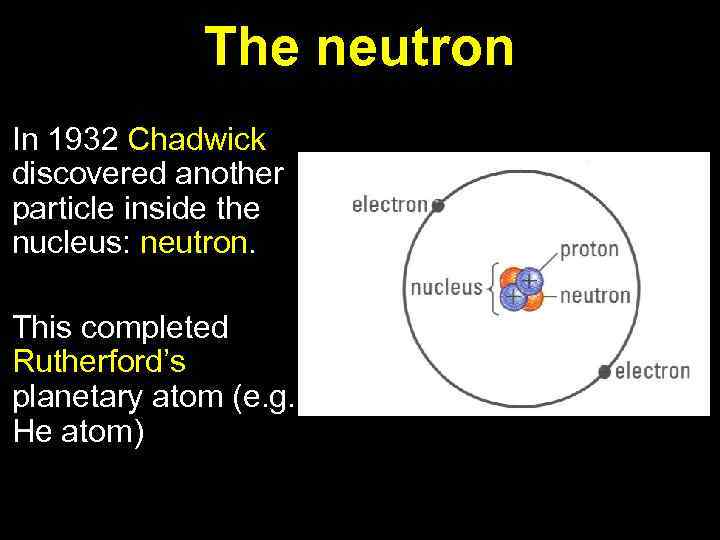 The neutron In 1932 Chadwick discovered another particle inside the nucleus: neutron. This completed Rutherford’s planetary atom (e. g. He atom)
The neutron In 1932 Chadwick discovered another particle inside the nucleus: neutron. This completed Rutherford’s planetary atom (e. g. He atom)
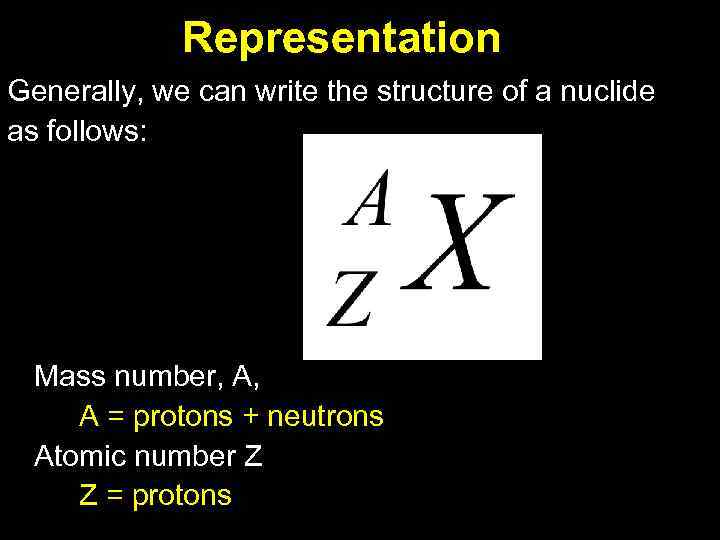 Representation Generally, we can write the structure of a nuclide as follows: Mass number, A, A = protons + neutrons Atomic number Z Z = protons
Representation Generally, we can write the structure of a nuclide as follows: Mass number, A, A = protons + neutrons Atomic number Z Z = protons
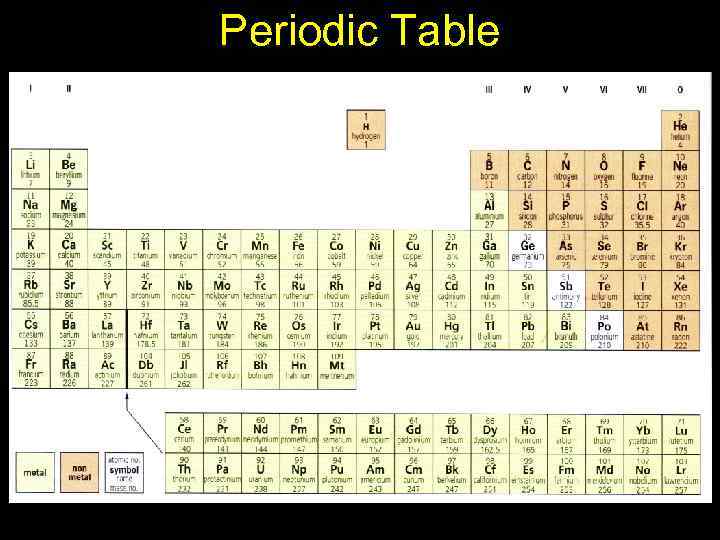 Periodic Table
Periodic Table
 Rutherford’s model incomplete The planetary model did not correctly explain the atom. Maxwell’s theory of electromagnetism predicts that all accelerating charges should radiate. Thus…
Rutherford’s model incomplete The planetary model did not correctly explain the atom. Maxwell’s theory of electromagnetism predicts that all accelerating charges should radiate. Thus…
 Along comes Niels Bohr
Along comes Niels Bohr
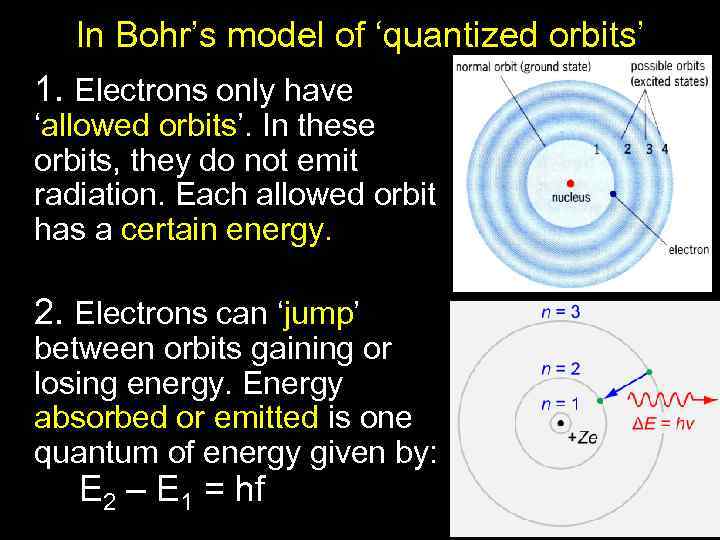 In Bohr’s model of ‘quantized orbits’ 1. Electrons only have ‘allowed orbits’. In these orbits, they do not emit radiation. Each allowed orbit has a certain energy. 2. Electrons can ‘jump’ between orbits gaining or losing energy. Energy absorbed or emitted is one quantum of energy given by: E 2 – E 1 = hf
In Bohr’s model of ‘quantized orbits’ 1. Electrons only have ‘allowed orbits’. In these orbits, they do not emit radiation. Each allowed orbit has a certain energy. 2. Electrons can ‘jump’ between orbits gaining or losing energy. Energy absorbed or emitted is one quantum of energy given by: E 2 – E 1 = hf
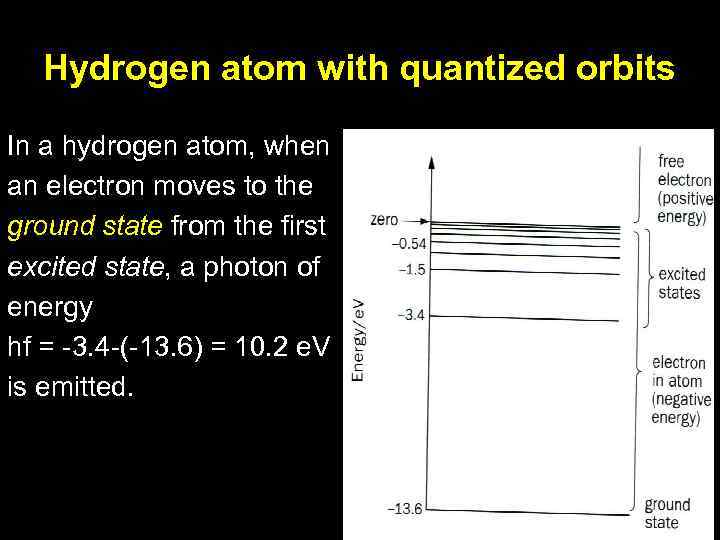 Hydrogen atom with quantized orbits In a hydrogen atom, when an electron moves to the ground state from the first excited state, a photon of energy hf = -3. 4 -(-13. 6) = 10. 2 e. V is emitted.
Hydrogen atom with quantized orbits In a hydrogen atom, when an electron moves to the ground state from the first excited state, a photon of energy hf = -3. 4 -(-13. 6) = 10. 2 e. V is emitted.
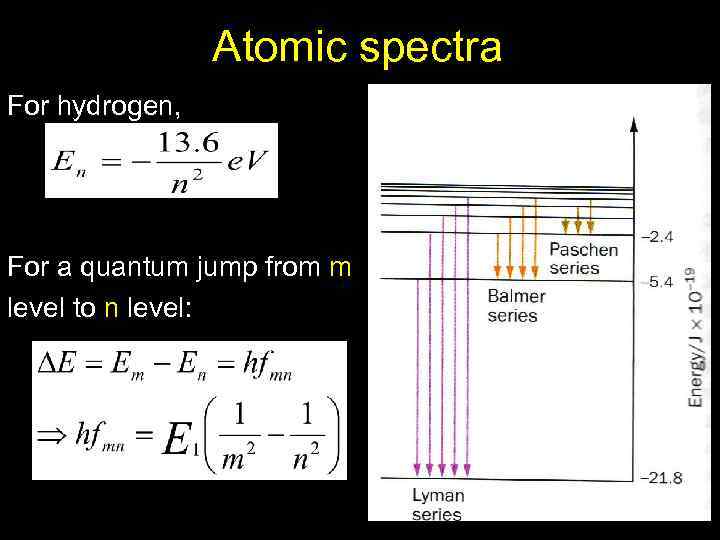 Atomic spectra For hydrogen, For a quantum jump from m level to n level:
Atomic spectra For hydrogen, For a quantum jump from m level to n level:
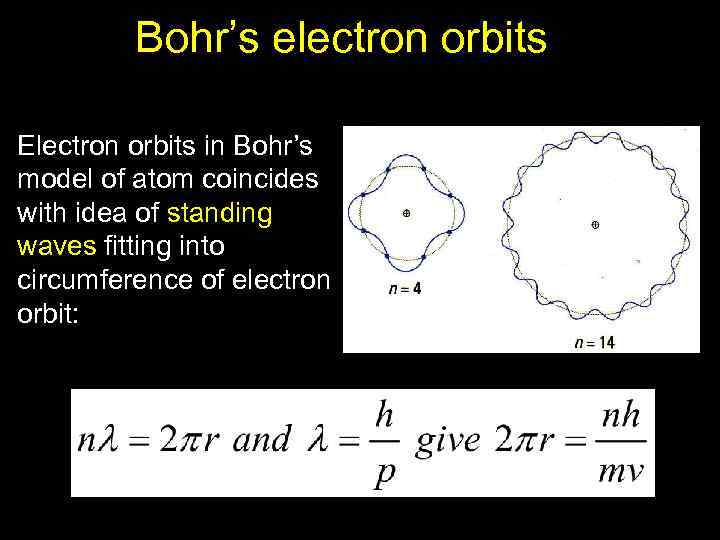 Bohr’s electron orbits Electron orbits in Bohr’s model of atom coincides with idea of standing waves fitting into circumference of electron orbit:
Bohr’s electron orbits Electron orbits in Bohr’s model of atom coincides with idea of standing waves fitting into circumference of electron orbit:
 Example 1 A) In a hydrogen atom, an electron moves from the 3 rd excited state to the 1 st excited state, is a photon emitted or absorbed? B) What is the wavelength of this photon? C) What is the minimum frequency of a photon needed to ionize a hydrogen atom in its n = 4 state?
Example 1 A) In a hydrogen atom, an electron moves from the 3 rd excited state to the 1 st excited state, is a photon emitted or absorbed? B) What is the wavelength of this photon? C) What is the minimum frequency of a photon needed to ionize a hydrogen atom in its n = 4 state?
 Spectroscopy Each element has unique spectra. Analysis of these spectra allows the determination of elemental composition of source/sample. • Absorption spectra • Emission spectra
Spectroscopy Each element has unique spectra. Analysis of these spectra allows the determination of elemental composition of source/sample. • Absorption spectra • Emission spectra
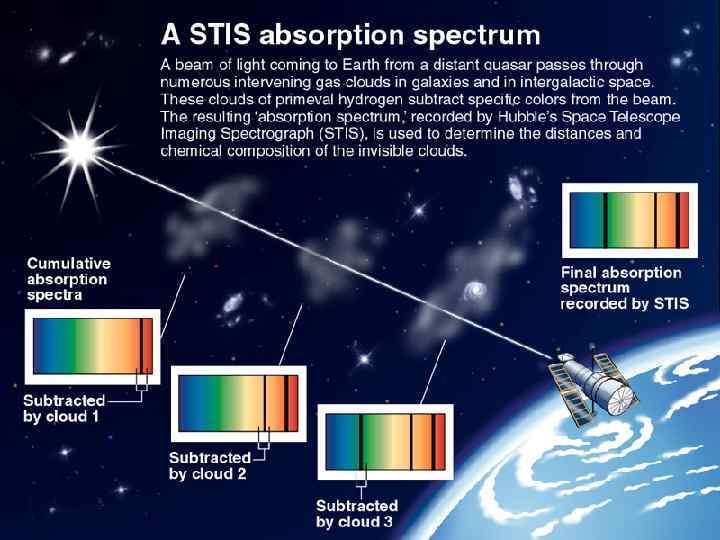
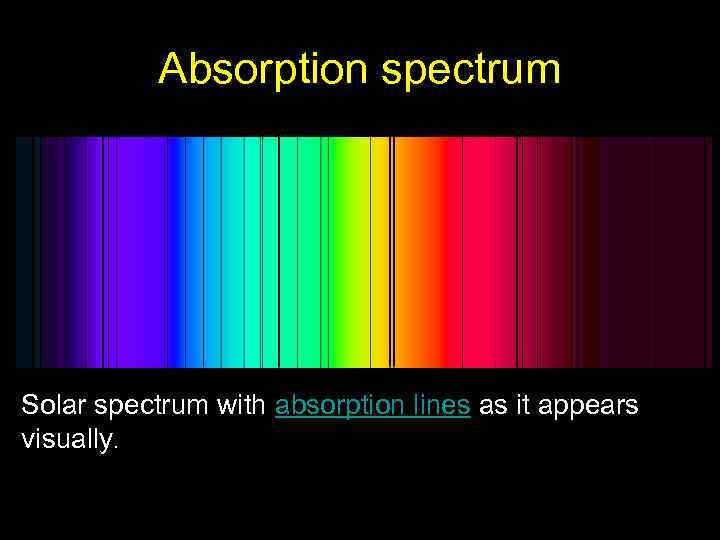 Absorption spectrum Solar spectrum with absorption lines as it appears visually.
Absorption spectrum Solar spectrum with absorption lines as it appears visually.
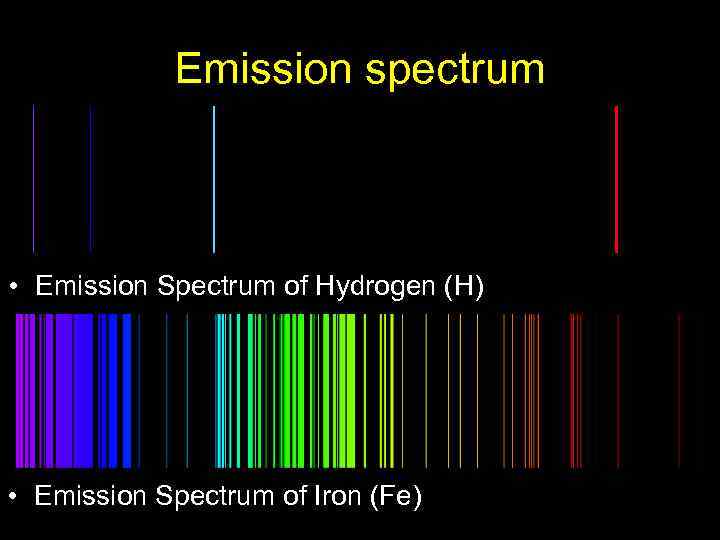 Emission spectrum • Emission Spectrum of Hydrogen (H) • Emission Spectrum of Iron (Fe)
Emission spectrum • Emission Spectrum of Hydrogen (H) • Emission Spectrum of Iron (Fe)
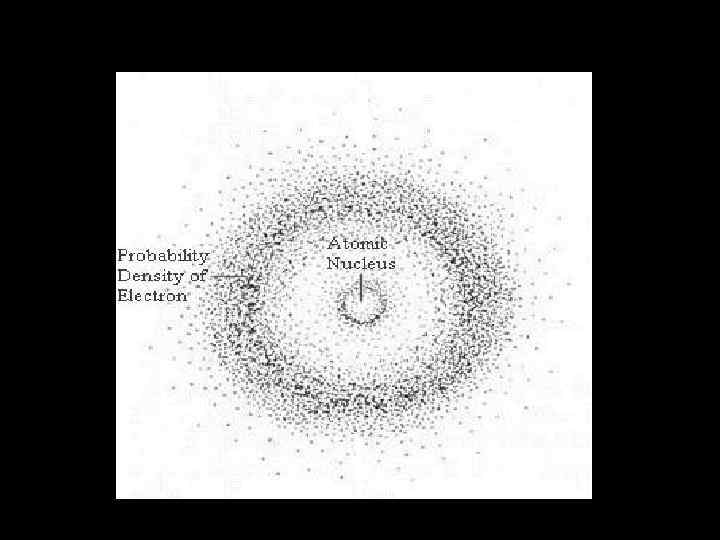
 Checklist • • • READING Adams and Allday: 8. 1, 8. 2, 8. 9, 8. 10 At the end of this lecture you should Have a knowledge of the current model of the structure of the atom Have an idea of how the knowledge of that structure developed historically Understand the main points of Rutherford's scattering experiment to probe the atom Understand the meaning of the chemical symbols in the periodic table and what isotopes are Know about quantized orbits in the Bohr atom Have a basic knowledge of what emission spectra and absorption spectra are and how they arise due to electrons jumping between shells in an atom Be able to define ground state, excited state, ionization. Have a qualitative understanding of probability waves and the Schrodinger model of the atom
Checklist • • • READING Adams and Allday: 8. 1, 8. 2, 8. 9, 8. 10 At the end of this lecture you should Have a knowledge of the current model of the structure of the atom Have an idea of how the knowledge of that structure developed historically Understand the main points of Rutherford's scattering experiment to probe the atom Understand the meaning of the chemical symbols in the periodic table and what isotopes are Know about quantized orbits in the Bohr atom Have a basic knowledge of what emission spectra and absorption spectra are and how they arise due to electrons jumping between shells in an atom Be able to define ground state, excited state, ionization. Have a qualitative understanding of probability waves and the Schrodinger model of the atom


