2b3e52577434a081dcb8fab2ff0ecd0a.ppt
- Количество слайдов: 30
![L 17 - Thermodynamics [2] n Science that studies the relationships between heat and L 17 - Thermodynamics [2] n Science that studies the relationships between heat and](https://present5.com/presentation/2b3e52577434a081dcb8fab2ff0ecd0a/image-1.jpg) L 17 - Thermodynamics [2] n Science that studies the relationships between heat and work and the conversion of one into the other n it applies to all living and non-living things n it predicts the direction of natural processes § why ice melts (rather than getting colder!) § why gases expand to fill entire volumes 1
L 17 - Thermodynamics [2] n Science that studies the relationships between heat and work and the conversion of one into the other n it applies to all living and non-living things n it predicts the direction of natural processes § why ice melts (rather than getting colder!) § why gases expand to fill entire volumes 1
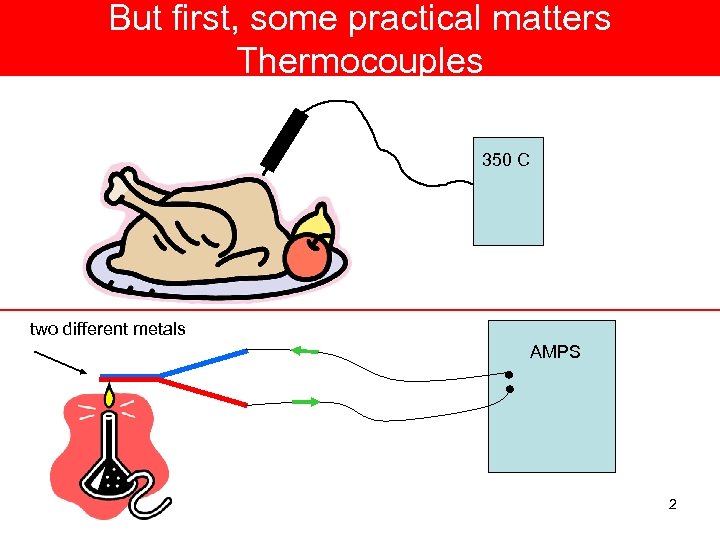 But first, some practical matters Thermocouples 350 C two different metals AMPS 2
But first, some practical matters Thermocouples 350 C two different metals AMPS 2
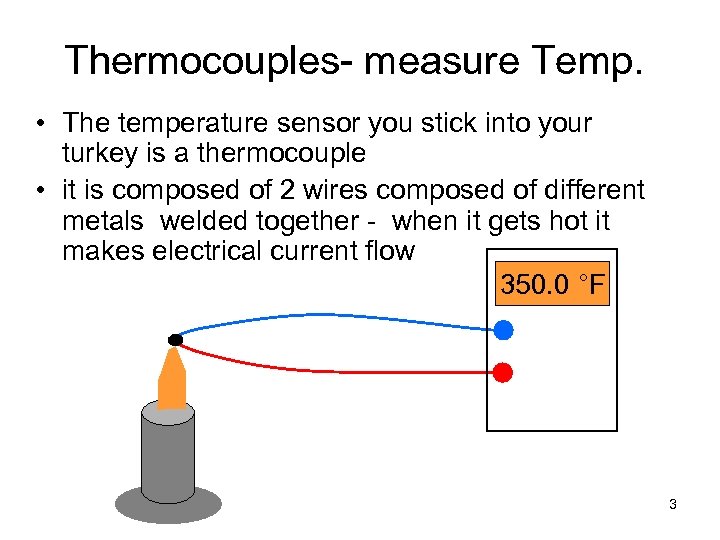 Thermocouples- measure Temp. • The temperature sensor you stick into your turkey is a thermocouple • it is composed of 2 wires composed of different metals welded together - when it gets hot it makes electrical current flow 350. 0 °F 3
Thermocouples- measure Temp. • The temperature sensor you stick into your turkey is a thermocouple • it is composed of 2 wires composed of different metals welded together - when it gets hot it makes electrical current flow 350. 0 °F 3
 Thermocouples protect you! • a thermocouple is used in gas heaters and dryers to protect against explosions • a thermocouple is placed in the pilot light • as long as the pilot light is on, thermocouple is hot and current flows • a circuit senses the current and allows the main gas valve to open • if the pilot light is out, the circuit prevents the main gas valve from opening 4
Thermocouples protect you! • a thermocouple is used in gas heaters and dryers to protect against explosions • a thermocouple is placed in the pilot light • as long as the pilot light is on, thermocouple is hot and current flows • a circuit senses the current and allows the main gas valve to open • if the pilot light is out, the circuit prevents the main gas valve from opening 4
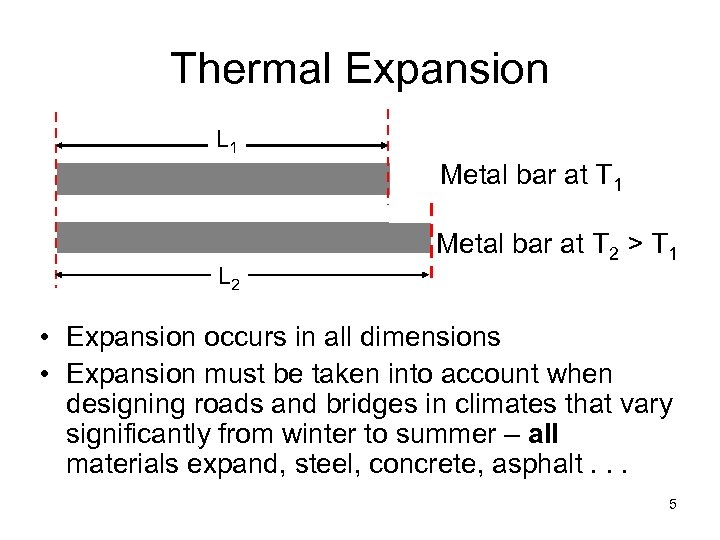 Thermal Expansion L 1 Metal bar at T 1 L 2 Metal bar at T 2 > T 1 • Expansion occurs in all dimensions • Expansion must be taken into account when designing roads and bridges in climates that vary significantly from winter to summer – all materials expand, steel, concrete, asphalt. . . 5
Thermal Expansion L 1 Metal bar at T 1 L 2 Metal bar at T 2 > T 1 • Expansion occurs in all dimensions • Expansion must be taken into account when designing roads and bridges in climates that vary significantly from winter to summer – all materials expand, steel, concrete, asphalt. . . 5
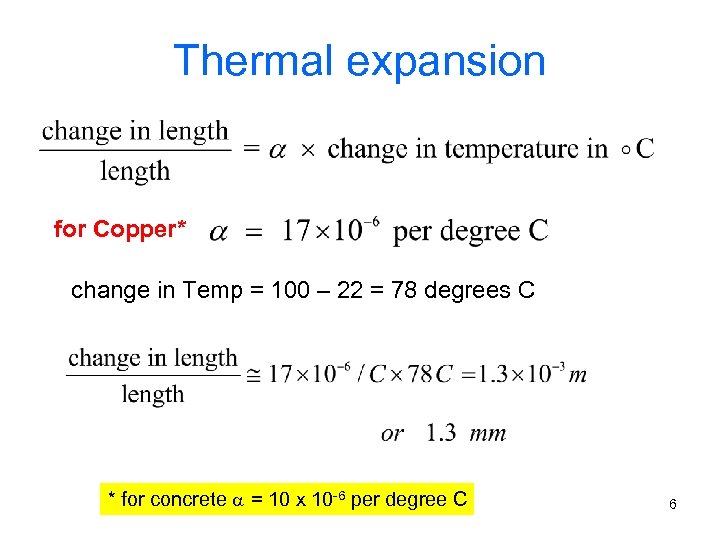 Thermal expansion for Copper* change in Temp = 100 – 22 = 78 degrees C * for concrete = 10 x 10 -6 per degree C 6
Thermal expansion for Copper* change in Temp = 100 – 22 = 78 degrees C * for concrete = 10 x 10 -6 per degree C 6
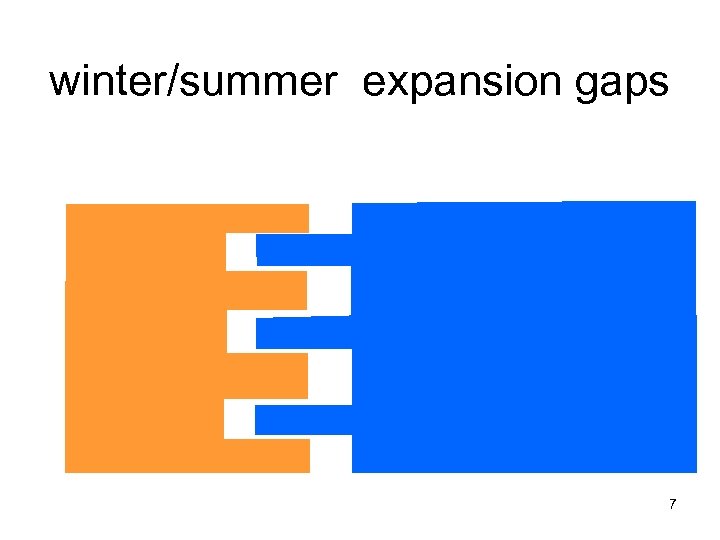 winter/summer expansion gaps 7
winter/summer expansion gaps 7
 expansion gaps on bridges 8
expansion gaps on bridges 8
 Thermal Expansion problems 9
Thermal Expansion problems 9
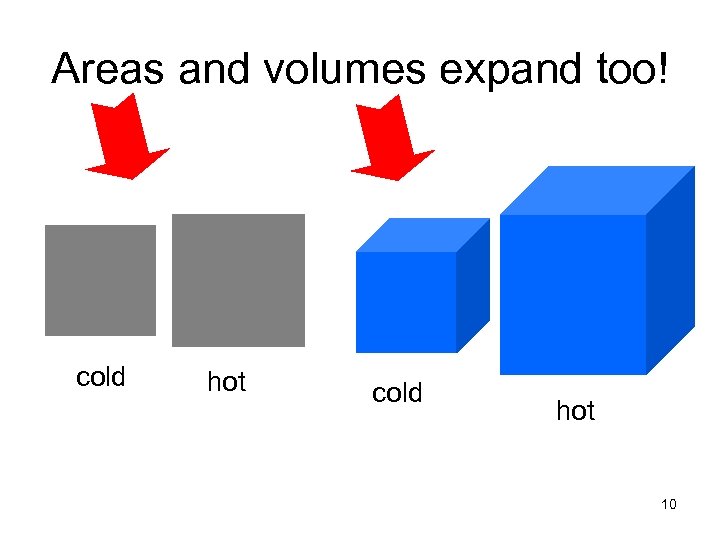 Areas and volumes expand too! cold hot 10
Areas and volumes expand too! cold hot 10
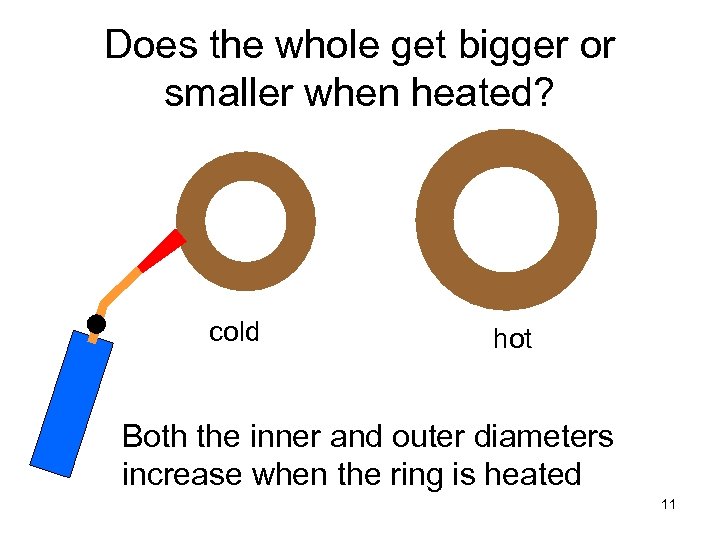 Does the whole get bigger or smaller when heated? cold hot Both the inner and outer diameters increase when the ring is heated 11
Does the whole get bigger or smaller when heated? cold hot Both the inner and outer diameters increase when the ring is heated 11
 12
12
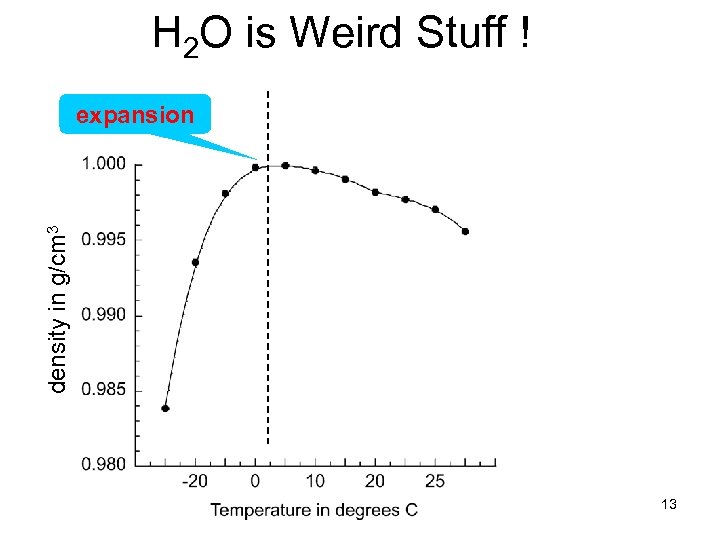 H 2 O is Weird Stuff ! density in g/cm 3 expansion 13
H 2 O is Weird Stuff ! density in g/cm 3 expansion 13
 14
14
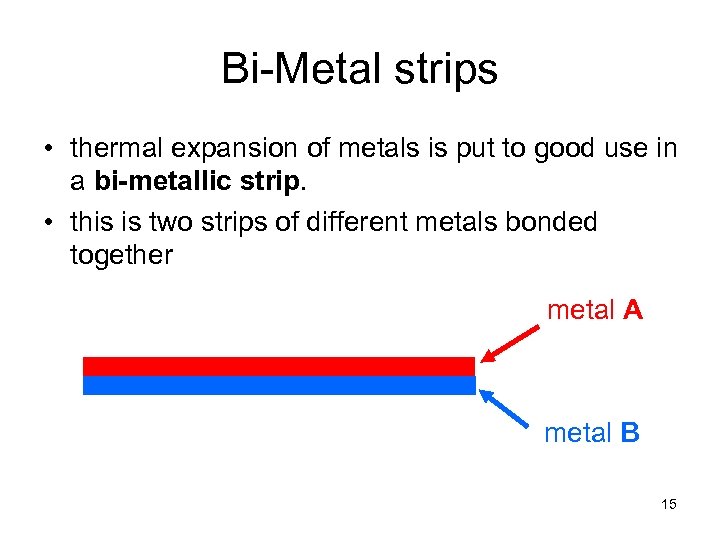 Bi-Metal strips • thermal expansion of metals is put to good use in a bi-metallic strip. • this is two strips of different metals bonded together metal A metal B 15
Bi-Metal strips • thermal expansion of metals is put to good use in a bi-metallic strip. • this is two strips of different metals bonded together metal A metal B 15
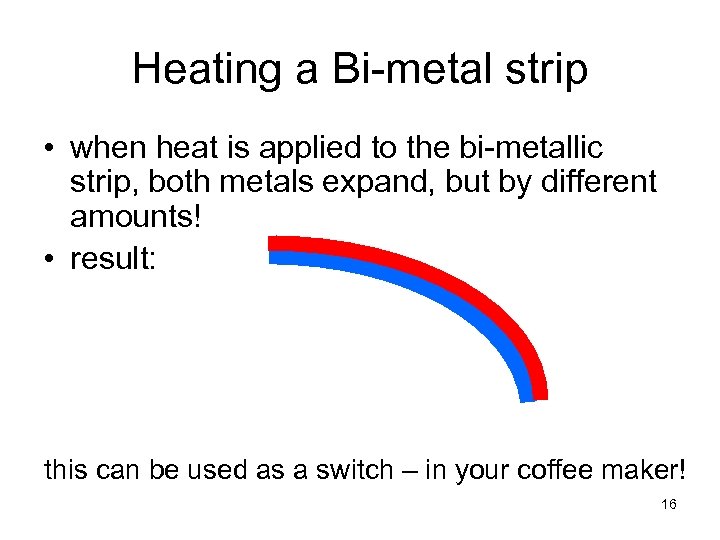 Heating a Bi-metal strip • when heat is applied to the bi-metallic strip, both metals expand, but by different amounts! • result: this can be used as a switch – in your coffee maker! 16
Heating a Bi-metal strip • when heat is applied to the bi-metallic strip, both metals expand, but by different amounts! • result: this can be used as a switch – in your coffee maker! 16
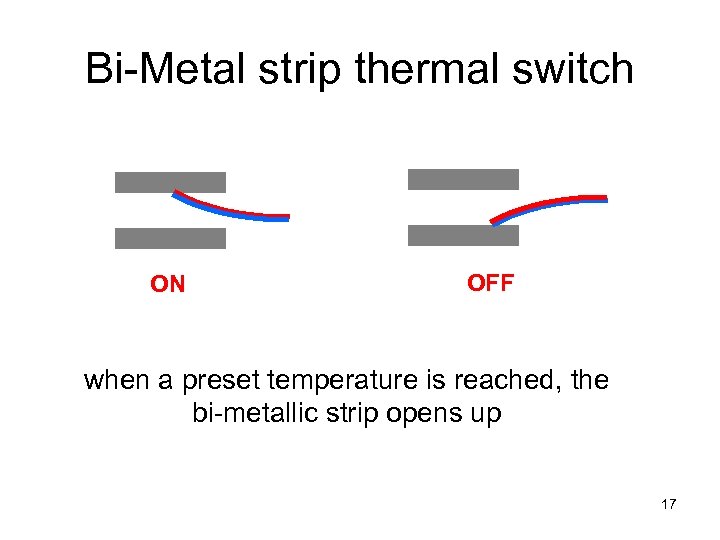 Bi-Metal strip thermal switch ON OFF when a preset temperature is reached, the bi-metallic strip opens up 17
Bi-Metal strip thermal switch ON OFF when a preset temperature is reached, the bi-metallic strip opens up 17
 Internal energy, Temperature and Heat • in a gas the molecules have energy because they are moving. • the sum of all the energies of all the molecules is the system’s internal energy • the temperature of the system is a measure of how much internal energy it has, Temperature Internal Energy 18
Internal energy, Temperature and Heat • in a gas the molecules have energy because they are moving. • the sum of all the energies of all the molecules is the system’s internal energy • the temperature of the system is a measure of how much internal energy it has, Temperature Internal Energy 18
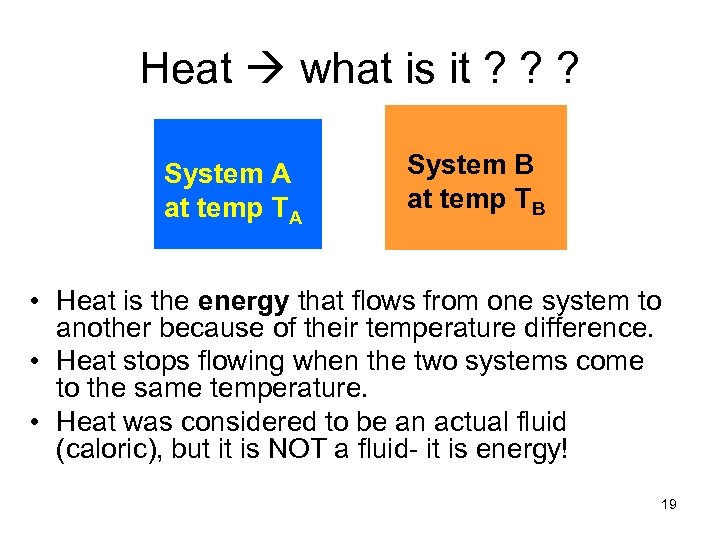 Heat what is it ? ? ? System A at temp TA System B at temp TB • Heat is the energy that flows from one system to another because of their temperature difference. • Heat stops flowing when the two systems come to the same temperature. • Heat was considered to be an actual fluid (caloric), but it is NOT a fluid- it is energy! 19
Heat what is it ? ? ? System A at temp TA System B at temp TB • Heat is the energy that flows from one system to another because of their temperature difference. • Heat stops flowing when the two systems come to the same temperature. • Heat was considered to be an actual fluid (caloric), but it is NOT a fluid- it is energy! 19
 Heat Flow and the laws of thermodynamics • System A has a certain amount of internal energy and so does system B • If energy is transferred and the internal energy of B decreases by some amount then internal energy of A must increase by the same amount. [the first law] • If the temperature of A is less than the temperature of B then heat flows from B to A (hot to cold). [the second law] 20
Heat Flow and the laws of thermodynamics • System A has a certain amount of internal energy and so does system B • If energy is transferred and the internal energy of B decreases by some amount then internal energy of A must increase by the same amount. [the first law] • If the temperature of A is less than the temperature of B then heat flows from B to A (hot to cold). [the second law] 20
 1 st and 2 nd Laws of Thermodynamics • the 1 st law says that energy is conserved whatever energy system A gains must be accounted for by the energy that B lost. (assuming that the systems are isolated so that they do not interact with any other systems) • The 2 nd law specifies the direction of heat flow hot to cold (ice melts!) 21
1 st and 2 nd Laws of Thermodynamics • the 1 st law says that energy is conserved whatever energy system A gains must be accounted for by the energy that B lost. (assuming that the systems are isolated so that they do not interact with any other systems) • The 2 nd law specifies the direction of heat flow hot to cold (ice melts!) 21
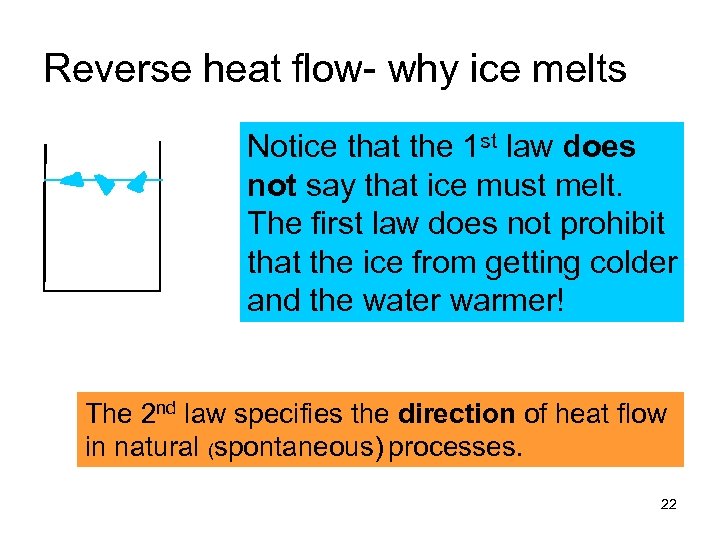 Reverse heat flow- why ice melts Notice that the 1 st law does not say that ice must melt. The first law does not prohibit that the ice from getting colder and the water warmer! The 2 nd law specifies the direction of heat flow in natural (spontaneous) processes. 22
Reverse heat flow- why ice melts Notice that the 1 st law does not say that ice must melt. The first law does not prohibit that the ice from getting colder and the water warmer! The 2 nd law specifies the direction of heat flow in natural (spontaneous) processes. 22
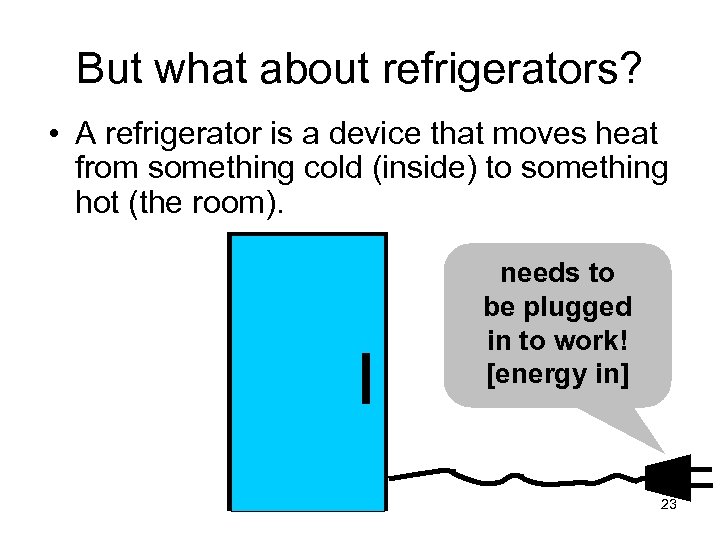 But what about refrigerators? • A refrigerator is a device that moves heat from something cold (inside) to something hot (the room). needs to be plugged in to work! [energy in] 23
But what about refrigerators? • A refrigerator is a device that moves heat from something cold (inside) to something hot (the room). needs to be plugged in to work! [energy in] 23
 Refrigerators and the 2 nd Law • Does this violate the 2 nd law? NO, because it is not a spontaneous process • Refrigerators require energy input (work) (electricity) to operate. • Heat does not flow spontaneously from cold to hot, but it can be made to flow backwards if there is an input of WORK. • It uses electrical energy to pump heat from cold to hot. 24
Refrigerators and the 2 nd Law • Does this violate the 2 nd law? NO, because it is not a spontaneous process • Refrigerators require energy input (work) (electricity) to operate. • Heat does not flow spontaneously from cold to hot, but it can be made to flow backwards if there is an input of WORK. • It uses electrical energy to pump heat from cold to hot. 24
 How does Heat flow ? • HEAT the energy that flows from one system to another because of temperature differences. • There are 3 processes by which heat flows • convection • conduction • radiation 25
How does Heat flow ? • HEAT the energy that flows from one system to another because of temperature differences. • There are 3 processes by which heat flows • convection • conduction • radiation 25
 Convection • heat is carried from place to place by the bulk movement of either liquids or gases • does not apply to solids • when water is boiled, hot liquid rises and mixes with cooler liquid, thus the heat is transferred • Hot air rises: • want heat into lower level of house (winter) • cooled air into upper levels (summer) 26
Convection • heat is carried from place to place by the bulk movement of either liquids or gases • does not apply to solids • when water is boiled, hot liquid rises and mixes with cooler liquid, thus the heat is transferred • Hot air rises: • want heat into lower level of house (winter) • cooled air into upper levels (summer) 26
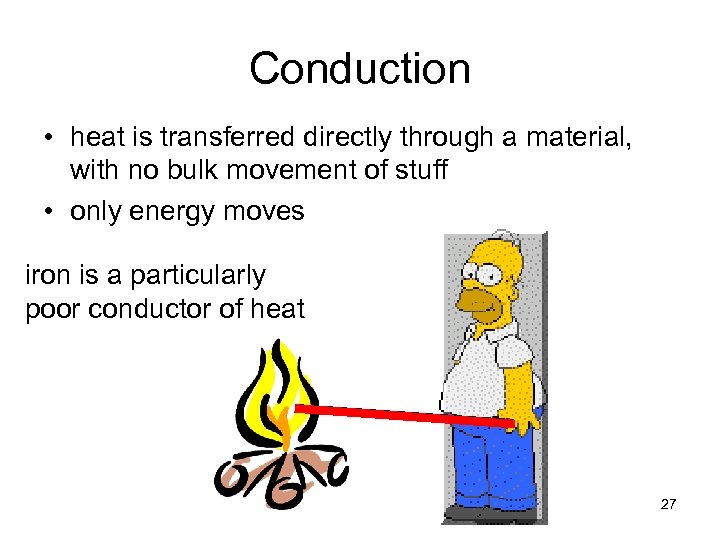 Conduction • heat is transferred directly through a material, with no bulk movement of stuff • only energy moves iron is a particularly poor conductor of heat 27
Conduction • heat is transferred directly through a material, with no bulk movement of stuff • only energy moves iron is a particularly poor conductor of heat 27
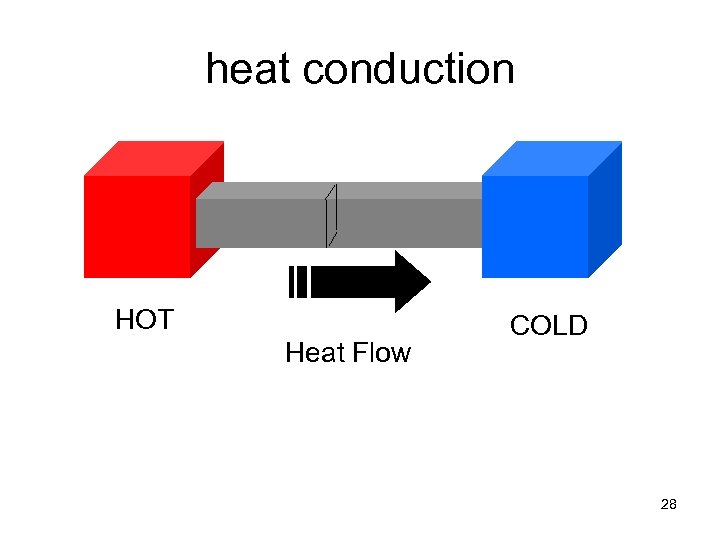 heat conduction HOT Heat Flow COLD 28
heat conduction HOT Heat Flow COLD 28
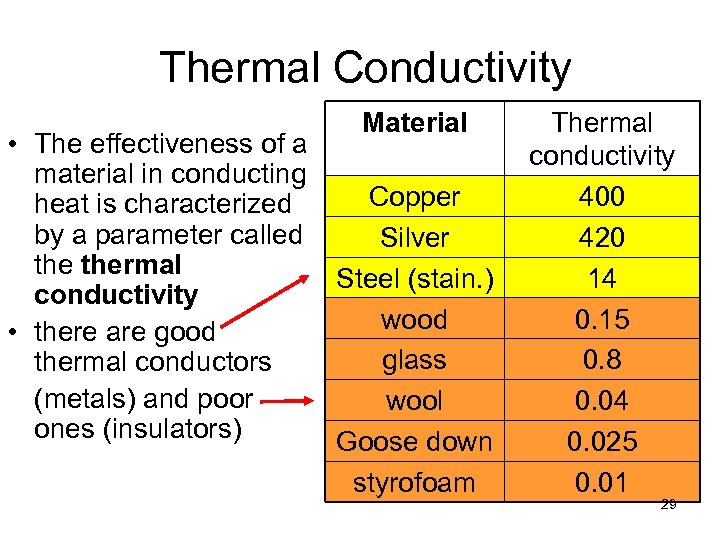 Thermal Conductivity Material • The effectiveness of a material in conducting Copper heat is characterized by a parameter called Silver thermal Steel (stain. ) conductivity wood • there are good glass thermal conductors (metals) and poor wool ones (insulators) Goose down styrofoam Thermal conductivity 400 420 14 0. 15 0. 8 0. 04 0. 025 0. 01 29
Thermal Conductivity Material • The effectiveness of a material in conducting Copper heat is characterized by a parameter called Silver thermal Steel (stain. ) conductivity wood • there are good glass thermal conductors (metals) and poor wool ones (insulators) Goose down styrofoam Thermal conductivity 400 420 14 0. 15 0. 8 0. 04 0. 025 0. 01 29
 Grandma’s silver spoons 30
Grandma’s silver spoons 30


