ca095ac6c0e355ff51be2ed454182d6e.ppt
- Количество слайдов: 45
 KYPROS: A KAN STUDY METHODS, RESULTS AND NEXT STEPS William G. Elder, Ph. D Niki Munk, Ph. D, LMT UK Family and Community Medicine IU School of Health and Rehabilitation Sciences Support: National Center for Complementary and Alternative Medicine (NCCAM) grant #R 21 AT 004544 National Center for Advancing Translational Sciences. National Institutes of Health (NIH) grant #UL 1 TR 000117 MAY 9, 2014
KYPROS: A KAN STUDY METHODS, RESULTS AND NEXT STEPS William G. Elder, Ph. D Niki Munk, Ph. D, LMT UK Family and Community Medicine IU School of Health and Rehabilitation Sciences Support: National Center for Complementary and Alternative Medicine (NCCAM) grant #R 21 AT 004544 National Center for Advancing Translational Sciences. National Institutes of Health (NIH) grant #UL 1 TR 000117 MAY 9, 2014
 KYPROS Kentuck. Y Pain Research Outcomes Studies
KYPROS Kentuck. Y Pain Research Outcomes Studies
 KYPROS: INTRODUCTION First “real world” study to examine massage therapy (MT) and progressive muscle relaxation therapy (PMR) for patients with CLBP when referred in conjunction with usual care from PCPs. Evaluate for improvements in health-related outcomes. Demonstrate the feasibility of this type of study.
KYPROS: INTRODUCTION First “real world” study to examine massage therapy (MT) and progressive muscle relaxation therapy (PMR) for patients with CLBP when referred in conjunction with usual care from PCPs. Evaluate for improvements in health-related outcomes. Demonstrate the feasibility of this type of study.
 KYPROS RESULTS Clinical massage therapy (CMT) delivered during a 12 week period resulted in significant statistical and clinical improvement of functional health related outcomes for chronic low back pain (CLBP) patients of primary care providers. Feasibility demonstrated with many lessons learned.
KYPROS RESULTS Clinical massage therapy (CMT) delivered during a 12 week period resulted in significant statistical and clinical improvement of functional health related outcomes for chronic low back pain (CLBP) patients of primary care providers. Feasibility demonstrated with many lessons learned.
 PRESENTATION OBJECTIVES 1. Recognition and thanks 2. Discuss practice based research and study methodology § What makes practice based research unique? § What conclusions does it permit? 3. Describe study results for benefit of treatment of CLBP patients 4. Examine next steps of this research
PRESENTATION OBJECTIVES 1. Recognition and thanks 2. Discuss practice based research and study methodology § What makes practice based research unique? § What conclusions does it permit? 3. Describe study results for benefit of treatment of CLBP patients 4. Examine next steps of this research
 OBJECTIVE 1 - THANK YOU! Community Faculty § Development of research question § Development of methodology § Maureen Flannery, MD § David Greene, MD Primary Care Providers (PCPs) § 67 PCPs consented to participate in the study. § 10 were in rural areas § 48 PCPs returned at least one pocketcard in 18 sites that participated
OBJECTIVE 1 - THANK YOU! Community Faculty § Development of research question § Development of methodology § Maureen Flannery, MD § David Greene, MD Primary Care Providers (PCPs) § 67 PCPs consented to participate in the study. § 10 were in rural areas § 48 PCPs returned at least one pocketcard in 18 sites that participated
 KYPROS KAN PRACTICES Thomas J. Burchett, MD Lexington Family Medicine Winchester Medical Associates Chevy Chase Primary Care Family Practice Associates Kentucky Clinic South Baptist Internal Medicine – Leestown Baptist Internal Medicine – Furlow and Associates Baptist Internal Medicine – Beaumont Center Midway Center for Integrative Medicine Winchester Family Practice Enlow and Shahzad UK Family Medical Center @ Kentucky Clinic Baptist Family Physicians at Tates Creek Baptist Family Physicians in Scott County Berea Primary Care Clinic White House Clinic - Richmond White House Clinic - Berea
KYPROS KAN PRACTICES Thomas J. Burchett, MD Lexington Family Medicine Winchester Medical Associates Chevy Chase Primary Care Family Practice Associates Kentucky Clinic South Baptist Internal Medicine – Leestown Baptist Internal Medicine – Furlow and Associates Baptist Internal Medicine – Beaumont Center Midway Center for Integrative Medicine Winchester Family Practice Enlow and Shahzad UK Family Medical Center @ Kentucky Clinic Baptist Family Physicians at Tates Creek Baptist Family Physicians in Scott County Berea Primary Care Clinic White House Clinic - Richmond White House Clinic - Berea

 OBJECTIVE 2 Practice Based Research & KYPROS Methodology
OBJECTIVE 2 Practice Based Research & KYPROS Methodology
 KYPROS: Design: Two-armed, repeated measures, observational trial and feasibility study. “Real world study” AIM of REAL WORLD STUDIES: Do the interventions of interest work in practice?
KYPROS: Design: Two-armed, repeated measures, observational trial and feasibility study. “Real world study” AIM of REAL WORLD STUDIES: Do the interventions of interest work in practice?
 WHAT FACTORS AFFECT TREATMENT CHOICE? Efficacy
WHAT FACTORS AFFECT TREATMENT CHOICE? Efficacy
 EFFICACY OF CMT Examined extensively § High quality Cochrane systematic review § Massage therapies are beneficial for CLBP. § Recommended more studies to assess impact on functioning and quality of life. § Recent high quality RCT § Massage effects for CLBP last up to fifty-two weeks § Very strict exclusion criteria. Furlan AD, Imamura M, Dryden T, Irvin E. Massage for low-back pain. The Cochrane database of systematic reviews. 2008(4) Cherkin DC, Sherman KJ, Kahn J, Wellman R, Cook AJ, Johnson E, et al. A comparison of the effects of 2 types of massage and usual care on chronic low back pain: a randomized, controlled trial. Ann Inter Med. 2011; 155(1): 1 -9.
EFFICACY OF CMT Examined extensively § High quality Cochrane systematic review § Massage therapies are beneficial for CLBP. § Recommended more studies to assess impact on functioning and quality of life. § Recent high quality RCT § Massage effects for CLBP last up to fifty-two weeks § Very strict exclusion criteria. Furlan AD, Imamura M, Dryden T, Irvin E. Massage for low-back pain. The Cochrane database of systematic reviews. 2008(4) Cherkin DC, Sherman KJ, Kahn J, Wellman R, Cook AJ, Johnson E, et al. A comparison of the effects of 2 types of massage and usual care on chronic low back pain: a randomized, controlled trial. Ann Inter Med. 2011; 155(1): 1 -9.
 WHAT FACTORS AFFECT TREATMENT CHOICE? Efficacy Previous experience with the treatment Local norms Side and adverse effects Condition severity Comorbid conditions Concurrent treatments Continuity Other patient characteristics Patient expectations Availability and costs All of these, plus research design issues, add up to an expectation that a treatment will work in practice
WHAT FACTORS AFFECT TREATMENT CHOICE? Efficacy Previous experience with the treatment Local norms Side and adverse effects Condition severity Comorbid conditions Concurrent treatments Continuity Other patient characteristics Patient expectations Availability and costs All of these, plus research design issues, add up to an expectation that a treatment will work in practice
 LEVEL OF EVIDENCE AMERICAN COLLEGE OF PHYSICIANS AND AMERICAN SPINE SOCIETY GUIDELINES For nonspecific acute LBP advise patients to remain active and perform selfcare; medication selection should consider the poor long-term efficacy and safety data for opioids, with acetaminophen and NSAIDs as first-line medication options. For patients not improving with self-care, the guidelines recommend addition of nonpharmacological approaches Chou, Quaseem, Snow, Cassey, Cross, Sheckle. Ann Int Med, 2007
LEVEL OF EVIDENCE AMERICAN COLLEGE OF PHYSICIANS AND AMERICAN SPINE SOCIETY GUIDELINES For nonspecific acute LBP advise patients to remain active and perform selfcare; medication selection should consider the poor long-term efficacy and safety data for opioids, with acetaminophen and NSAIDs as first-line medication options. For patients not improving with self-care, the guidelines recommend addition of nonpharmacological approaches Chou, Quaseem, Snow, Cassey, Cross, Sheckle. Ann Int Med, 2007
 LEVEL OF EVIDENCE AMERICAN COLLEGE OF PHYSICIANS AND AMERICAN SPINE SOCIETY GUIDELINES For CLBP, Intensive interdisciplinary rehabilitation, exercise therapy, acupuncture, massage therapy, spinal manipulation, yoga, cognitive-behavioral therapy, or progressive muscle relaxation (PMR). However, the guideline authors and others expressed concern that the effectiveness of these approaches had not yet been evaluated in primary care settings and, thus placed this recommendation at a lower tier (weak recommendation, moderate evidence) in comparison, for example, to prescribing NSAIDS. Chou, Quaseem, Snow, Cassey, Cross, Sheckle. Ann Int Med, 2007
LEVEL OF EVIDENCE AMERICAN COLLEGE OF PHYSICIANS AND AMERICAN SPINE SOCIETY GUIDELINES For CLBP, Intensive interdisciplinary rehabilitation, exercise therapy, acupuncture, massage therapy, spinal manipulation, yoga, cognitive-behavioral therapy, or progressive muscle relaxation (PMR). However, the guideline authors and others expressed concern that the effectiveness of these approaches had not yet been evaluated in primary care settings and, thus placed this recommendation at a lower tier (weak recommendation, moderate evidence) in comparison, for example, to prescribing NSAIDS. Chou, Quaseem, Snow, Cassey, Cross, Sheckle. Ann Int Med, 2007
 LEVEL OF EVIDENCE AMERICAN COLLEGE OF PHYSICIANS AND AMERICAN SPINE SOCIETY GUIDELINES However, Guideline authors and others expressed concern that the effectiveness of these approaches had not yet been evaluated in primary care settings. Placed this recommendation at a lower tier (weak recommendation, moderate evidence) in comparison, for example, to prescribing NSAIDS. Chou, Quaseem, Snow, Cassey, Cross, Sheckle. Ann Int Med, 2007
LEVEL OF EVIDENCE AMERICAN COLLEGE OF PHYSICIANS AND AMERICAN SPINE SOCIETY GUIDELINES However, Guideline authors and others expressed concern that the effectiveness of these approaches had not yet been evaluated in primary care settings. Placed this recommendation at a lower tier (weak recommendation, moderate evidence) in comparison, for example, to prescribing NSAIDS. Chou, Quaseem, Snow, Cassey, Cross, Sheckle. Ann Int Med, 2007
 GOAL OF OUR RESEARCH AND ITS METHODOLOGY Examine CMT to see if merits higher level of evidence? Make new treatments available to offer CLBP patients.
GOAL OF OUR RESEARCH AND ITS METHODOLOGY Examine CMT to see if merits higher level of evidence? Make new treatments available to offer CLBP patients.
 EFFICACY AND EFFECTIVENESS Efficacy § In controlled conditions, does a specific input result in a specific outcome? Effectiveness § Does a treatment work in practice? Effect § Both of these deal with effect. § That is, what is the effect of X on Y? The question is where and under what conditions?
EFFICACY AND EFFECTIVENESS Efficacy § In controlled conditions, does a specific input result in a specific outcome? Effectiveness § Does a treatment work in practice? Effect § Both of these deal with effect. § That is, what is the effect of X on Y? The question is where and under what conditions?
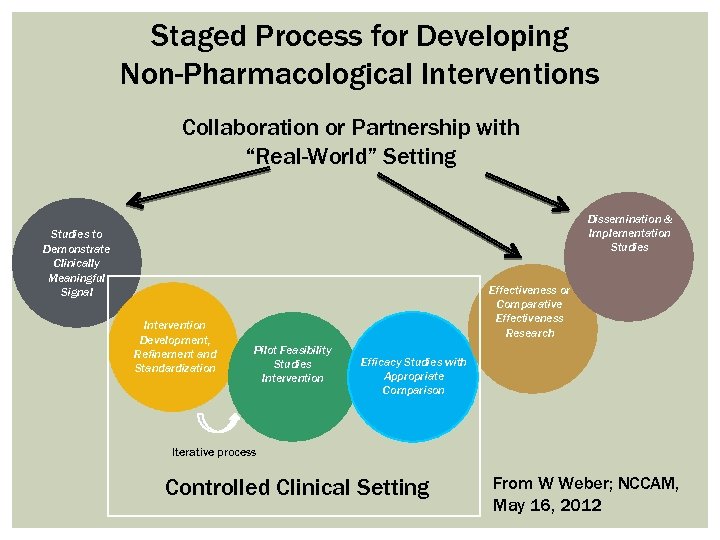 Staged Process for Developing Non-Pharmacological Interventions Collaboration or Partnership with “Real-World” Setting Dissemination & Implementation Studies to Demonstrate Clinically Meaningful Signal Intervention Development, Refinement and Standardization Effectiveness or Comparative Effectiveness Research Pilot Feasibility Studies Intervention Efficacy Studies with Appropriate Comparison Iterative process Controlled Clinical Setting From W Weber; NCCAM, May 16, 2012
Staged Process for Developing Non-Pharmacological Interventions Collaboration or Partnership with “Real-World” Setting Dissemination & Implementation Studies to Demonstrate Clinically Meaningful Signal Intervention Development, Refinement and Standardization Effectiveness or Comparative Effectiveness Research Pilot Feasibility Studies Intervention Efficacy Studies with Appropriate Comparison Iterative process Controlled Clinical Setting From W Weber; NCCAM, May 16, 2012
 EXPLANATORY VS. PRAGMATIC Explanatory § What are the effects of an intervention under ideal circumstances? § Efficacy Pragmatic § What are the effects of an intervention under usual circumstances where applied? § Effectiveness
EXPLANATORY VS. PRAGMATIC Explanatory § What are the effects of an intervention under ideal circumstances? § Efficacy Pragmatic § What are the effects of an intervention under usual circumstances where applied? § Effectiveness
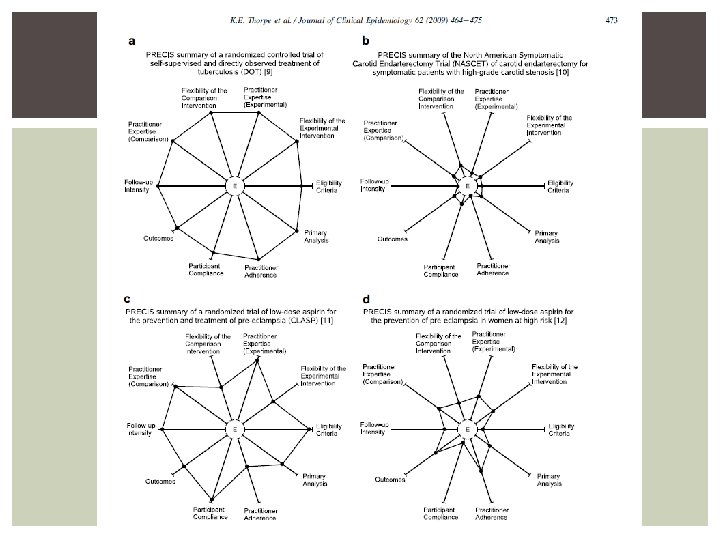
 EXAMPLE OF AN EXPLANATORY TRIAL “Among patients with angiographicallyconfirmed, symptomatic 70 -99% stenosis of a carotid artery, can the addition of carotid endarterectomy (performed by an expert vascular or neurosurgeon with an excellent track record) to best medical therapy, vs. best medical therapy alone, reduce the risk of major or fatal stroke over the next two years of rigorous follow-up? ” (NASCET: NEJM 1991; 325: 445 -53) From: http: //support-collaboration. org/precis. pdf
EXAMPLE OF AN EXPLANATORY TRIAL “Among patients with angiographicallyconfirmed, symptomatic 70 -99% stenosis of a carotid artery, can the addition of carotid endarterectomy (performed by an expert vascular or neurosurgeon with an excellent track record) to best medical therapy, vs. best medical therapy alone, reduce the risk of major or fatal stroke over the next two years of rigorous follow-up? ” (NASCET: NEJM 1991; 325: 445 -53) From: http: //support-collaboration. org/precis. pdf
 EXPLANATORY TRIAL: PROS AND CONS Advantage If negative, you can abandon the treatment (it won’t work anywhere) Disadvantage If positive, you still don’t know whether it will work in usual health care conditions From: http: //support-collaboration. org/precis. pdf
EXPLANATORY TRIAL: PROS AND CONS Advantage If negative, you can abandon the treatment (it won’t work anywhere) Disadvantage If positive, you still don’t know whether it will work in usual health care conditions From: http: //support-collaboration. org/precis. pdf
 EXAMPLE OF AN PRAGMATIC TRIAL Among women at 12 -32 weeks gestation whose clinicians thought they were at sufficient risk for pre-eclampsia or IUGR to be uncertain whether they should be prescribed low-dose aspirin, does simply prescribing aspirin (compared with placebo), and with no study follow-up visits, reduce the risk of a composite of bad outcomes for her baby? (CLASP: Lancet 1994; 343: 619 -29) From: http: //support-collaboration. org/precis. pdf
EXAMPLE OF AN PRAGMATIC TRIAL Among women at 12 -32 weeks gestation whose clinicians thought they were at sufficient risk for pre-eclampsia or IUGR to be uncertain whether they should be prescribed low-dose aspirin, does simply prescribing aspirin (compared with placebo), and with no study follow-up visits, reduce the risk of a composite of bad outcomes for her baby? (CLASP: Lancet 1994; 343: 619 -29) From: http: //support-collaboration. org/precis. pdf
 PRAGMATIC TRIAL: PROS AND CONS Advantage If positive, it really works and you can implement the treatment just about everywhere. Disadvantage If negative, you can’t distinguish a worthless treatment from an efficacious treatment that isn’t applied/accepted widely enough. From: http: //support-collaboration. org/precis. pdf
PRAGMATIC TRIAL: PROS AND CONS Advantage If positive, it really works and you can implement the treatment just about everywhere. Disadvantage If negative, you can’t distinguish a worthless treatment from an efficacious treatment that isn’t applied/accepted widely enough. From: http: //support-collaboration. org/precis. pdf
 PRECIS PR pragmatic (to) E explanatory C continuum I indicator S summary
PRECIS PR pragmatic (to) E explanatory C continuum I indicator S summary
 PRECIS DOMAINS/COMPONENTS 1. Participant eligibility criteria 2. Intervention flexibility i. ii. Experimental intervention Comparison intervention 3. Intervention practitioner expertise i. ii. Experimental intervention practitioner Comparison intervention practitioner 4. Follow-up intensity 5. Primary trial outcome 6. Compliance/Adherence i. ii. Participant to intervention Practitioner to study protocol 7. Analysis of the primary outcome
PRECIS DOMAINS/COMPONENTS 1. Participant eligibility criteria 2. Intervention flexibility i. ii. Experimental intervention Comparison intervention 3. Intervention practitioner expertise i. ii. Experimental intervention practitioner Comparison intervention practitioner 4. Follow-up intensity 5. Primary trial outcome 6. Compliance/Adherence i. ii. Participant to intervention Practitioner to study protocol 7. Analysis of the primary outcome
 PRECIS SPOKES Each spoke is defined in terms of restrictions on an otherwise totally pragmatic trial. The more restrictive the trial, the lower its PRECIS score; the lower its PRECIS score, the smaller the population for generalizability. Informed by : http: //support-collaboration. org/precis. pdf
PRECIS SPOKES Each spoke is defined in terms of restrictions on an otherwise totally pragmatic trial. The more restrictive the trial, the lower its PRECIS score; the lower its PRECIS score, the smaller the population for generalizability. Informed by : http: //support-collaboration. org/precis. pdf
 OBJECTIVE 2: CONTINUED KYPROS Methodology Via PRECIS Context
OBJECTIVE 2: CONTINUED KYPROS Methodology Via PRECIS Context
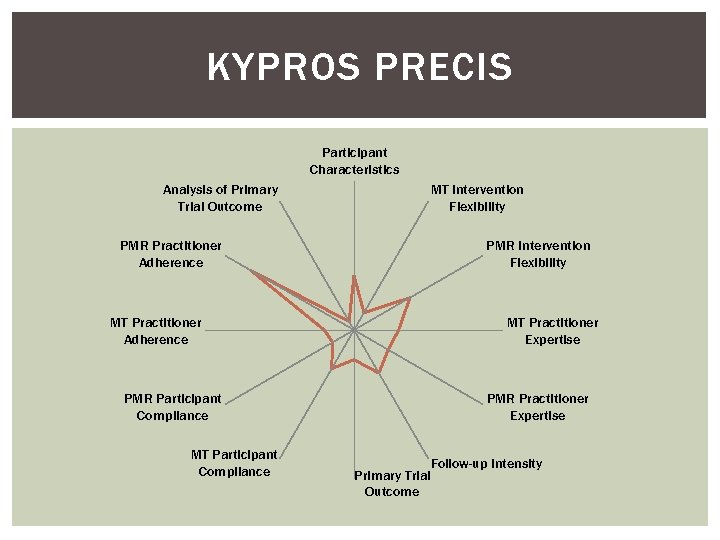 KYPROS PRECIS Participant Characteristics Analysis of Primary Trial Outcome PMR Practitioner Adherence MT Practitioner Adherence PMR Participant Compliance MT Intervention Flexibility PMR Intervention Flexibility MT Practitioner Expertise PMR Practitioner Expertise Follow-up Intensity Primary Trial Outcome
KYPROS PRECIS Participant Characteristics Analysis of Primary Trial Outcome PMR Practitioner Adherence MT Practitioner Adherence PMR Participant Compliance MT Intervention Flexibility PMR Intervention Flexibility MT Practitioner Expertise PMR Practitioner Expertise Follow-up Intensity Primary Trial Outcome
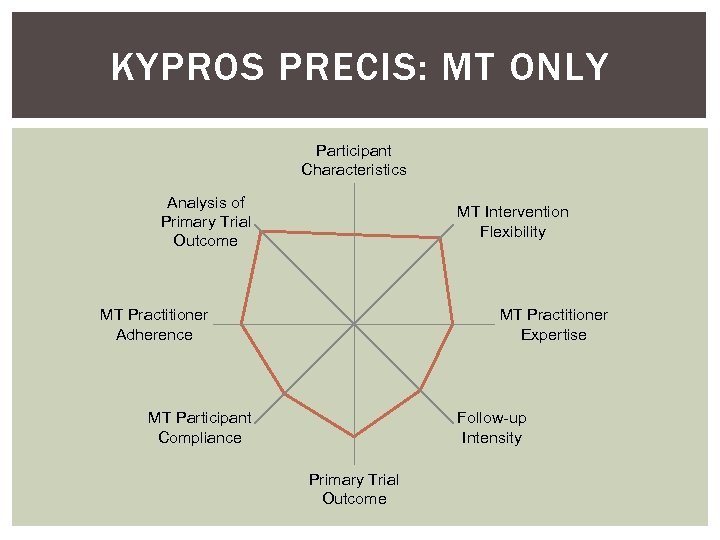 KYPROS PRECIS: MT ONLY Participant Characteristics Analysis of Primary Trial Outcome MT Intervention Flexibility MT Practitioner Adherence MT Practitioner Expertise MT Participant Compliance Follow-up Intensity Primary Trial Outcome
KYPROS PRECIS: MT ONLY Participant Characteristics Analysis of Primary Trial Outcome MT Intervention Flexibility MT Practitioner Adherence MT Practitioner Expertise MT Participant Compliance Follow-up Intensity Primary Trial Outcome
 OBJECTIVE 3 Describe study results for benefit of treatment of CLBP patients
OBJECTIVE 3 Describe study results for benefit of treatment of CLBP patients
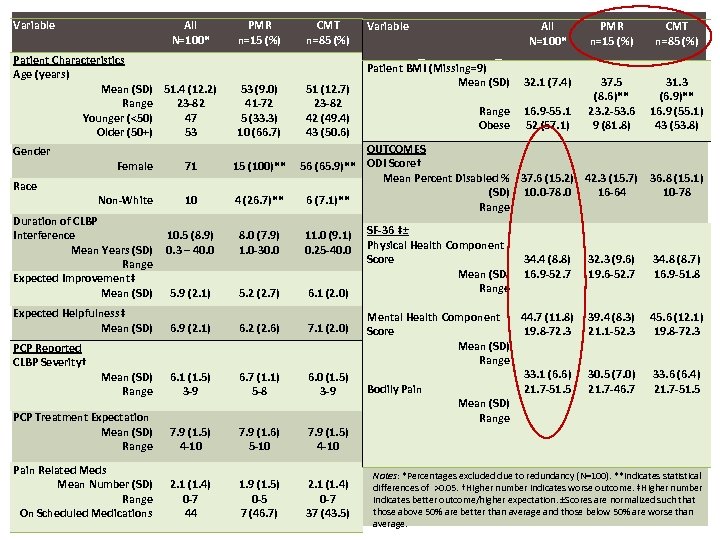 Variable All N=100* PMR n=15 (%) CMT n=85 (%) BASELINE RESULTS Patient BMI (Missing=9) Mean (SD) 32. 1 (7. 4) Range Obese 16. 9 -55. 1 52 (57. 1) 37. 5 (8. 6)** 23. 2 -53. 6 9 (81. 8) 31. 3 (6. 9)** 16. 9 (55. 1) 43 (53. 8) Patient Characteristics Age (years) Mean (SD) 51. 4 (12. 2) Range 23 -82 Younger (<50) 47 Older (50+) 53 Gender 53 (9. 0) 41 -72 5 (33. 3) 10 (66. 7) 51 (12. 7) 23 -82 42 (49. 4) 43 (50. 6) OUTCOMES 56 (65. 9)** ODI Score† Mean Percent Disabled % 37. 6 (15. 2) 42. 3 (15. 7) (SD) 10. 0 -78. 0 16 -64 6 (7. 1)** Range Female 71 15 (100)** Non-White 10 4 (26. 7)** 10. 5 (8. 9) 0. 3 – 40. 0 8. 0 (7. 9) 1. 0 -30. 0 11. 0 (9. 1) 0. 25 -40. 0 5. 9 (2. 1) 5. 2 (2. 7) 6. 1 (2. 0) 6. 9 (2. 1) 6. 2 (2. 6) 7. 1 (2. 0) Mean (SD) Range 6. 1 (1. 5) 3 -9 6. 7 (1. 1) 5 -8 6. 0 (1. 5) 3 -9 PCP Treatment Expectation Mean (SD) Range 7. 9 (1. 5) 4 -10 7. 9 (1. 6) 5 -10 7. 9 (1. 5) 4 -10 Pain Related Meds Mean Number (SD) Range On Scheduled Medications 2. 1 (1. 4) 0 -7 44 1. 9 (1. 5) 0 -5 7 (46. 7) 2. 1 (1. 4) 0 -7 37 (43. 5) Race Duration of CLBP Interference Mean Years (SD) Range Expected Improvement‡ Mean (SD) Expected Helpfulness‡ Mean (SD) PCP Reported CLBP Severity† Variable SF-36 ‡± Physical Health Component Score Mean (SD) Range 36. 8 (15. 1) 10 -78 34. 4 (8. 8) 16. 9 -52. 7 32. 3 (9. 6) 19. 6 -52. 7 34. 8 (8. 7) 16. 9 -51. 8 Mental Health Component 44. 7 (11. 8) Score 19. 8 -72. 3 Mean (SD) Range 33. 1 (6. 6) Bodily Pain 21. 7 -51. 5 Mean (SD) Range 39. 4 (8. 3) 21. 1 -52. 3 45. 6 (12. 1) 19. 8 -72. 3 30. 5 (7. 0) 21. 7 -46. 7 33. 6 (6. 4) 21. 7 -51. 5 Notes: *Percentages excluded due to redundancy (N=100). **Indicates statistical differences of >0. 05. †Higher number indicates worse outcome. ‡Higher number indicates better outcome/higher expectation. ±Scores are normalized such that those above 50% are better than average and those below 50% are worse than average.
Variable All N=100* PMR n=15 (%) CMT n=85 (%) BASELINE RESULTS Patient BMI (Missing=9) Mean (SD) 32. 1 (7. 4) Range Obese 16. 9 -55. 1 52 (57. 1) 37. 5 (8. 6)** 23. 2 -53. 6 9 (81. 8) 31. 3 (6. 9)** 16. 9 (55. 1) 43 (53. 8) Patient Characteristics Age (years) Mean (SD) 51. 4 (12. 2) Range 23 -82 Younger (<50) 47 Older (50+) 53 Gender 53 (9. 0) 41 -72 5 (33. 3) 10 (66. 7) 51 (12. 7) 23 -82 42 (49. 4) 43 (50. 6) OUTCOMES 56 (65. 9)** ODI Score† Mean Percent Disabled % 37. 6 (15. 2) 42. 3 (15. 7) (SD) 10. 0 -78. 0 16 -64 6 (7. 1)** Range Female 71 15 (100)** Non-White 10 4 (26. 7)** 10. 5 (8. 9) 0. 3 – 40. 0 8. 0 (7. 9) 1. 0 -30. 0 11. 0 (9. 1) 0. 25 -40. 0 5. 9 (2. 1) 5. 2 (2. 7) 6. 1 (2. 0) 6. 9 (2. 1) 6. 2 (2. 6) 7. 1 (2. 0) Mean (SD) Range 6. 1 (1. 5) 3 -9 6. 7 (1. 1) 5 -8 6. 0 (1. 5) 3 -9 PCP Treatment Expectation Mean (SD) Range 7. 9 (1. 5) 4 -10 7. 9 (1. 6) 5 -10 7. 9 (1. 5) 4 -10 Pain Related Meds Mean Number (SD) Range On Scheduled Medications 2. 1 (1. 4) 0 -7 44 1. 9 (1. 5) 0 -5 7 (46. 7) 2. 1 (1. 4) 0 -7 37 (43. 5) Race Duration of CLBP Interference Mean Years (SD) Range Expected Improvement‡ Mean (SD) Expected Helpfulness‡ Mean (SD) PCP Reported CLBP Severity† Variable SF-36 ‡± Physical Health Component Score Mean (SD) Range 36. 8 (15. 1) 10 -78 34. 4 (8. 8) 16. 9 -52. 7 32. 3 (9. 6) 19. 6 -52. 7 34. 8 (8. 7) 16. 9 -51. 8 Mental Health Component 44. 7 (11. 8) Score 19. 8 -72. 3 Mean (SD) Range 33. 1 (6. 6) Bodily Pain 21. 7 -51. 5 Mean (SD) Range 39. 4 (8. 3) 21. 1 -52. 3 45. 6 (12. 1) 19. 8 -72. 3 30. 5 (7. 0) 21. 7 -46. 7 33. 6 (6. 4) 21. 7 -51. 5 Notes: *Percentages excluded due to redundancy (N=100). **Indicates statistical differences of >0. 05. †Higher number indicates worse outcome. ‡Higher number indicates better outcome/higher expectation. ±Scores are normalized such that those above 50% are better than average and those below 50% are worse than average.
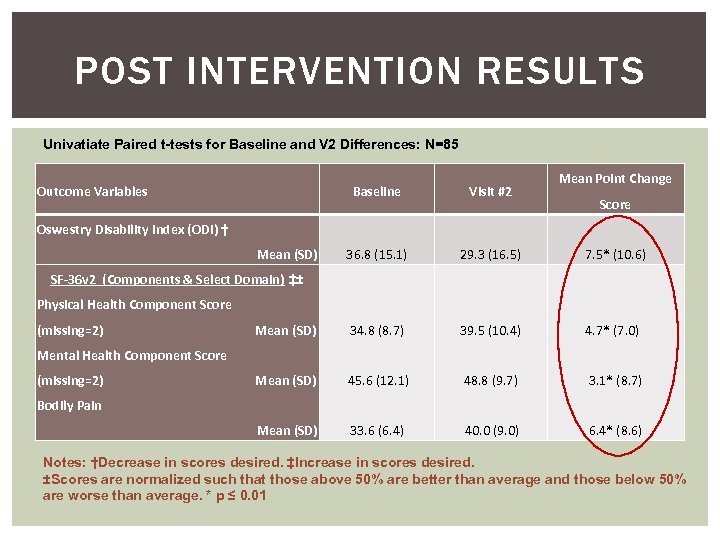 POST INTERVENTION RESULTS Univatiate Paired t-tests for Baseline and V 2 Differences: N=85 Outcome Variables Mean Point Change Baseline Visit #2 36. 8 (15. 1) 29. 3 (16. 5) 7. 5* (10. 6) 34. 8 (8. 7) 39. 5 (10. 4) 4. 7* (7. 0) Mean (SD) 45. 6 (12. 1) 48. 8 (9. 7) 3. 1* (8. 7) Mean (SD) 33. 6 (6. 4) 40. 0 (9. 0) 6. 4* (8. 6) Oswestry Disability Index (ODI) † Mean (SD) SF-36 v 2 (Components & Select Domain) ‡± Physical Health Component Score (missing=2) Mean (SD) Mental Health Component Score (missing=2) Score Bodily Pain Notes: †Decrease in scores desired. ‡Increase in scores desired. ±Scores are normalized such that those above 50% are better than average and those below 50% are worse than average. * p ≤ 0. 01
POST INTERVENTION RESULTS Univatiate Paired t-tests for Baseline and V 2 Differences: N=85 Outcome Variables Mean Point Change Baseline Visit #2 36. 8 (15. 1) 29. 3 (16. 5) 7. 5* (10. 6) 34. 8 (8. 7) 39. 5 (10. 4) 4. 7* (7. 0) Mean (SD) 45. 6 (12. 1) 48. 8 (9. 7) 3. 1* (8. 7) Mean (SD) 33. 6 (6. 4) 40. 0 (9. 0) 6. 4* (8. 6) Oswestry Disability Index (ODI) † Mean (SD) SF-36 v 2 (Components & Select Domain) ‡± Physical Health Component Score (missing=2) Mean (SD) Mental Health Component Score (missing=2) Score Bodily Pain Notes: †Decrease in scores desired. ‡Increase in scores desired. ±Scores are normalized such that those above 50% are better than average and those below 50% are worse than average. * p ≤ 0. 01
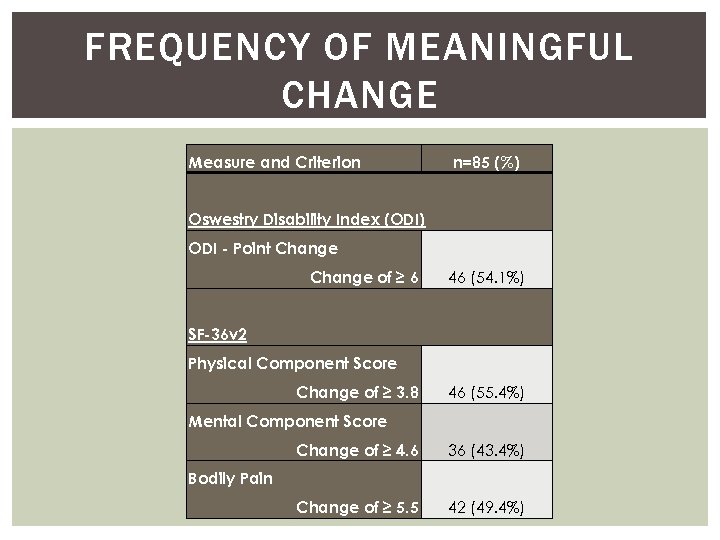 FREQUENCY OF MEANINGFUL CHANGE Measure and Criterion n=85 (%) Oswestry Disability Index (ODI) ODI - Point Change of ≥ 6 46 (54. 1%) SF-36 v 2 Physical Component Score Change of ≥ 3. 8 Mental Component Score Change of ≥ 4. 6 Bodily Pain 46 (55. 4%) 36 (43. 4%) Change of ≥ 5. 5 42 (49. 4%)
FREQUENCY OF MEANINGFUL CHANGE Measure and Criterion n=85 (%) Oswestry Disability Index (ODI) ODI - Point Change of ≥ 6 46 (54. 1%) SF-36 v 2 Physical Component Score Change of ≥ 3. 8 Mental Component Score Change of ≥ 4. 6 Bodily Pain 46 (55. 4%) 36 (43. 4%) Change of ≥ 5. 5 42 (49. 4%)
 ODI: WHAT DOES CLBP WITH DISABILITY LOOKS LIKE… ODI = 40% Disability Personal care is normal but very painful. No sitting or standing > 1 hour. < 6 hours of sleep per night. Prevents any sex life at all. Normal social life but increases pain. Pain is bad when travels are > 2 hours.
ODI: WHAT DOES CLBP WITH DISABILITY LOOKS LIKE… ODI = 40% Disability Personal care is normal but very painful. No sitting or standing > 1 hour. < 6 hours of sleep per night. Prevents any sex life at all. Normal social life but increases pain. Pain is bad when travels are > 2 hours.
 ODI: WHAT CLINICAL BENEFIT LOOKS LIKE… Starting with 40% disability – 30 pt change and 75% improvement § Normal but painful personal care to normal care with no pain. § No sitting for more than an hour to sit in any chair as long as I like. § No standing for more than an hour to standing as long as I like with no pain. § < 6 hours of sleep to my sleep is never disturbed by pain. § No sex life at all due to pain to normal sex life with no extra pain. § Normal but painful social life to normal, with no pain. § Limited travels to can travel anywhere with some extra pain. Starting with 54% disability – 14 pt change and 26% improvement § Pain intensity from severe to mild. § Only lifting light to medium weights to heavy weights if conveniently placed. § Only walking 100 yards to walking ¼ mile. § Severely restricted sex life to nearly normal but painful sex life. § No 1+ hour journeys to can travel anywhere with some extra pain.
ODI: WHAT CLINICAL BENEFIT LOOKS LIKE… Starting with 40% disability – 30 pt change and 75% improvement § Normal but painful personal care to normal care with no pain. § No sitting for more than an hour to sit in any chair as long as I like. § No standing for more than an hour to standing as long as I like with no pain. § < 6 hours of sleep to my sleep is never disturbed by pain. § No sex life at all due to pain to normal sex life with no extra pain. § Normal but painful social life to normal, with no pain. § Limited travels to can travel anywhere with some extra pain. Starting with 54% disability – 14 pt change and 26% improvement § Pain intensity from severe to mild. § Only lifting light to medium weights to heavy weights if conveniently placed. § Only walking 100 yards to walking ¼ mile. § Severely restricted sex life to nearly normal but painful sex life. § No 1+ hour journeys to can travel anywhere with some extra pain.
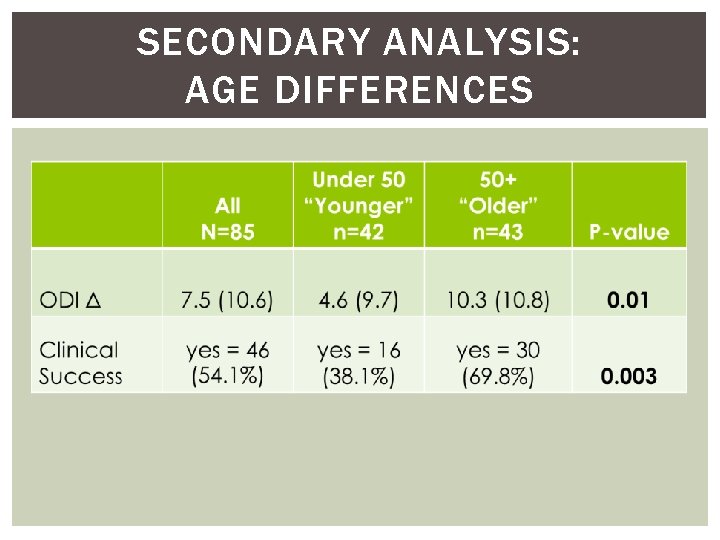 SECONDARY ANALYSIS: AGE DIFFERENCES
SECONDARY ANALYSIS: AGE DIFFERENCES
 INFORMING NEXT STEPS Starting point: Outcome Measure Oswestry Disability Index (ODI) SF-36 Physical Composite Score (missing=2) SF-36 Mental Composite Score (missing=2) SF-36 Bodily Pain Domain Score Below Normal at Baseline* N=85 83 (98%) 68 (80%) 39 (46%) 83 (98%)
INFORMING NEXT STEPS Starting point: Outcome Measure Oswestry Disability Index (ODI) SF-36 Physical Composite Score (missing=2) SF-36 Mental Composite Score (missing=2) SF-36 Bodily Pain Domain Score Below Normal at Baseline* N=85 83 (98%) 68 (80%) 39 (46%) 83 (98%)
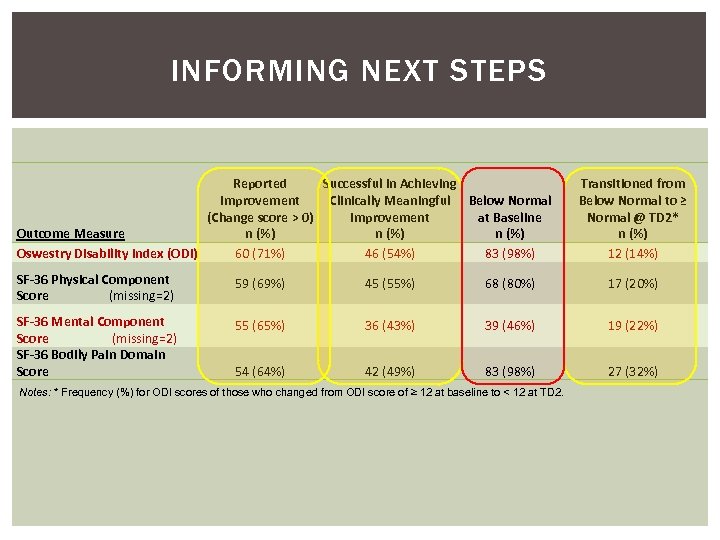 INFORMING NEXT STEPS Reported Successful in Achieving Improvement Clinically Meaningful Below Normal (Change score > 0) Improvement at Baseline Outcome Measure n (%) Oswestry Disability Index (ODI) 60 (71%) 46 (54%) 83 (98%) Transitioned from Below Normal to ≥ Normal @ TD 2* n (%) 12 (14%) SF-36 Physical Component Score (missing=2) 59 (69%) 45 (55%) 68 (80%) 17 (20%) SF-36 Mental Component Score (missing=2) SF-36 Bodily Pain Domain Score 55 (65%) 36 (43%) 39 (46%) 19 (22%) 54 (64%) 42 (49%) 83 (98%) 27 (32%) Notes: * Frequency (%) for ODI scores of those who changed from ODI score of ≥ 12 at baseline to < 12 at TD 2.
INFORMING NEXT STEPS Reported Successful in Achieving Improvement Clinically Meaningful Below Normal (Change score > 0) Improvement at Baseline Outcome Measure n (%) Oswestry Disability Index (ODI) 60 (71%) 46 (54%) 83 (98%) Transitioned from Below Normal to ≥ Normal @ TD 2* n (%) 12 (14%) SF-36 Physical Component Score (missing=2) 59 (69%) 45 (55%) 68 (80%) 17 (20%) SF-36 Mental Component Score (missing=2) SF-36 Bodily Pain Domain Score 55 (65%) 36 (43%) 39 (46%) 19 (22%) 54 (64%) 42 (49%) 83 (98%) 27 (32%) Notes: * Frequency (%) for ODI scores of those who changed from ODI score of ≥ 12 at baseline to < 12 at TD 2.
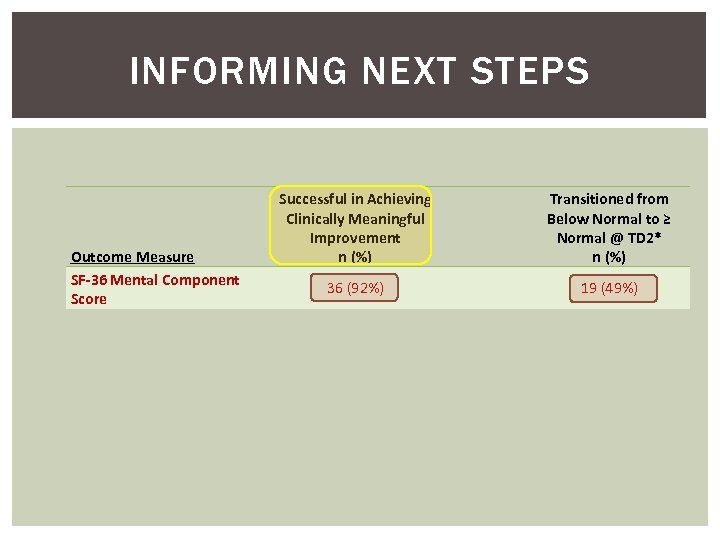 INFORMING NEXT STEPS Outcome Measure SF-36 Mental Component Score Successful in Achieving Clinically Meaningful Improvement n (%) Transitioned from Below Normal to ≥ Normal @ TD 2* n (%) 36 (92%) 19 (49%)
INFORMING NEXT STEPS Outcome Measure SF-36 Mental Component Score Successful in Achieving Clinically Meaningful Improvement n (%) Transitioned from Below Normal to ≥ Normal @ TD 2* n (%) 36 (92%) 19 (49%)
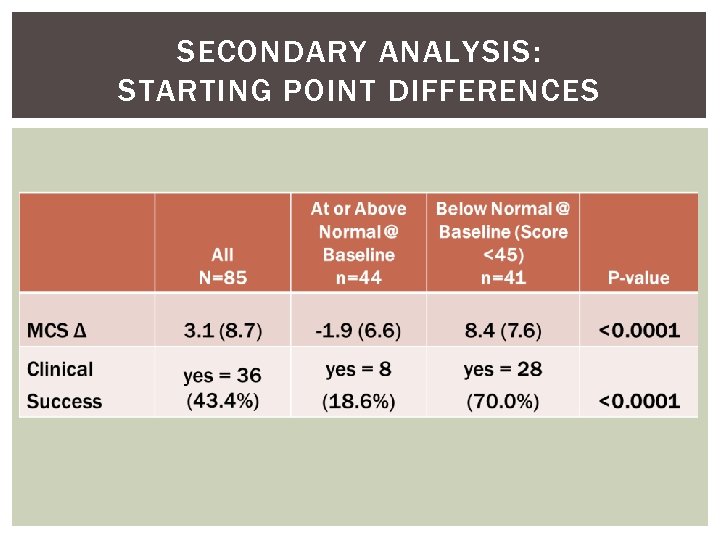 SECONDARY ANALYSIS: STARTING POINT DIFFERENCES
SECONDARY ANALYSIS: STARTING POINT DIFFERENCES
 OBJECTIVE 4 Next Steps
OBJECTIVE 4 Next Steps
 CONCLUSIONS/FUTURE PLANS Demonstrated effectiveness research feasibility. Larger study with more patient participants – clarify effectiveness and begin cost assessment § Mental health w/ CLBP § CLBP patients on scheduled medications § Medical complexity and CLBP reflective of real world R 34/R 01 Route: Feasibility (larger network/ patient complexity) - Implementation
CONCLUSIONS/FUTURE PLANS Demonstrated effectiveness research feasibility. Larger study with more patient participants – clarify effectiveness and begin cost assessment § Mental health w/ CLBP § CLBP patients on scheduled medications § Medical complexity and CLBP reflective of real world R 34/R 01 Route: Feasibility (larger network/ patient complexity) - Implementation
 THANK YOU! Questions? Acknowledgements: Maureen Flannery, MD David Green, MD Margaret Love, Ph. D Geza Bruckner, Ph. D Karen Roper, Ph. D Katie Stewart, BA, LMT Kevin Pearce, MD Contact: Bill Elder, Ph. D welder@uky. edu NIH/NCCAM Grant #1 R 21 AT 004544 -01 A 2
THANK YOU! Questions? Acknowledgements: Maureen Flannery, MD David Green, MD Margaret Love, Ph. D Geza Bruckner, Ph. D Karen Roper, Ph. D Katie Stewart, BA, LMT Kevin Pearce, MD Contact: Bill Elder, Ph. D welder@uky. edu NIH/NCCAM Grant #1 R 21 AT 004544 -01 A 2


