Бажина ЕС_ICFM-2015.ppt
- Количество слайдов: 23
 KURNAKOV INSTITUTE OF GENERAL AND INORGANIC CHEMISTRY RUSSIAN ACADEMY OF SCIENCES E. S. Bazhina, M. A. Kiskin, G. G. Aleksandrov, A. A. Sidorov, I. L. Eremenko POLYNUCLEAR ARCHITECTURES BASED ON FRAGMENT OF OXOVANADIUM(IV) AND TWO CHELATED SUBSTITUTED MALONIC ACID ANIONS ICFM-2015 г. Novosibirsk, October 5 -9, 2015
KURNAKOV INSTITUTE OF GENERAL AND INORGANIC CHEMISTRY RUSSIAN ACADEMY OF SCIENCES E. S. Bazhina, M. A. Kiskin, G. G. Aleksandrov, A. A. Sidorov, I. L. Eremenko POLYNUCLEAR ARCHITECTURES BASED ON FRAGMENT OF OXOVANADIUM(IV) AND TWO CHELATED SUBSTITUTED MALONIC ACID ANIONS ICFM-2015 г. Novosibirsk, October 5 -9, 2015
 PROPERTIES AND PROSPECTS OF USING UNUSUAL MAGNETIC PROPERTIES PEROVSKITE-RELATED MIXED METAL OXIDES [I. Gil de Muro et al, Inorg. Chem. , 1998, 37, 3243; I. Gil de Muro et al, J. Chem. Soc. , Dalton Trans. , 2000, 3360] PHOTOLUMINESCENT PROPERTIES [I. Gil de Muro et al, Polyhedron, 2004, 23, 929; C. Ruiz-Perez et al, Inorg. Chim. Acta, 2000, 298, 202] DESIGN OF METAL-ORGANIC FRAMEWORK SOLIDS (MOF) [B. Dey et al, Inorg. Chim. Acta, 2010, 363, 981] HETEROMETALLIC POLYNUCLEAR COMPOUNDS ANTITUMOR AND ANTIDIABETIC PROPERTIES ANTIBACTERIAL AND ANTIFUNGAL ACTIVITIES [D. Lebowhl, R. Canetta, Eur. J. Cancer, 1998, 34, 1522; M. Sutradhar et al, Inorg. Chim. Acta, 2011, 368, 13] HETEROGENEOUS CATALYSTS NANOSTRUCTURED METAL OXIDES [D. Ghoshal et al, Inorg. Chim. Acta, 2005, 358, 1027]
PROPERTIES AND PROSPECTS OF USING UNUSUAL MAGNETIC PROPERTIES PEROVSKITE-RELATED MIXED METAL OXIDES [I. Gil de Muro et al, Inorg. Chem. , 1998, 37, 3243; I. Gil de Muro et al, J. Chem. Soc. , Dalton Trans. , 2000, 3360] PHOTOLUMINESCENT PROPERTIES [I. Gil de Muro et al, Polyhedron, 2004, 23, 929; C. Ruiz-Perez et al, Inorg. Chim. Acta, 2000, 298, 202] DESIGN OF METAL-ORGANIC FRAMEWORK SOLIDS (MOF) [B. Dey et al, Inorg. Chim. Acta, 2010, 363, 981] HETEROMETALLIC POLYNUCLEAR COMPOUNDS ANTITUMOR AND ANTIDIABETIC PROPERTIES ANTIBACTERIAL AND ANTIFUNGAL ACTIVITIES [D. Lebowhl, R. Canetta, Eur. J. Cancer, 1998, 34, 1522; M. Sutradhar et al, Inorg. Chim. Acta, 2011, 368, 13] HETEROGENEOUS CATALYSTS NANOSTRUCTURED METAL OXIDES [D. Ghoshal et al, Inorg. Chim. Acta, 2005, 358, 1027]
 DICARBOXYLIC ACIDS OXALIC ACID SUCCINIC ACID PIMELIC ACID MALONIC ACID GLUTARIC ACID SUBERIC ACID … ADIPIC ACID AZELAIC ACID
DICARBOXYLIC ACIDS OXALIC ACID SUCCINIC ACID PIMELIC ACID MALONIC ACID GLUTARIC ACID SUBERIC ACID … ADIPIC ACID AZELAIC ACID
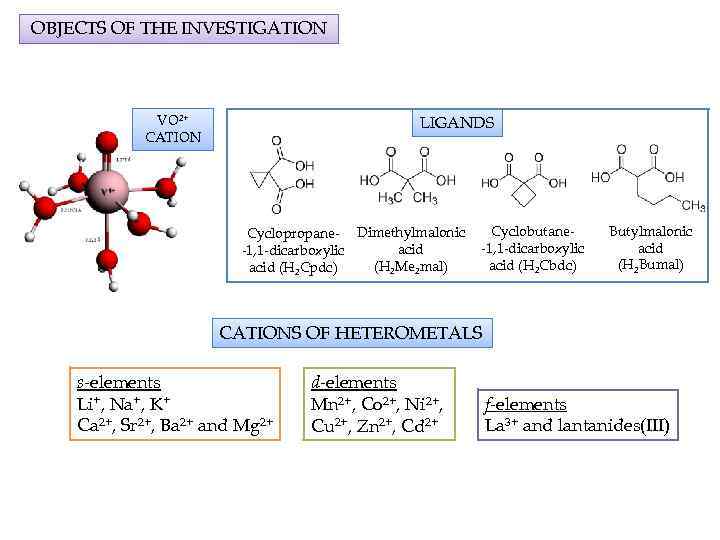 OBJECTS OF THE INVESTIGATION VO 2+ CATION LIGANDS Cyclopropane- Dimethylmalonic acid -1, 1 -dicarboxylic (H 2 Me 2 mal) acid (H 2 Cpdc) Cyclobutane-1, 1 -dicarboxylic acid (H 2 Cbdc) Butylmalonic acid (H 2 Bumal) CATIONS OF HETEROMETALS s-elements Li+, Na+, K+ Ca 2+, Sr 2+, Ba 2+ and Mg 2+ d-elements Mn 2+, Co 2+, Ni 2+, Cu 2+, Zn 2+, Cd 2+ f-elements La 3+ and lantanides(III)
OBJECTS OF THE INVESTIGATION VO 2+ CATION LIGANDS Cyclopropane- Dimethylmalonic acid -1, 1 -dicarboxylic (H 2 Me 2 mal) acid (H 2 Cpdc) Cyclobutane-1, 1 -dicarboxylic acid (H 2 Cbdc) Butylmalonic acid (H 2 Bumal) CATIONS OF HETEROMETALS s-elements Li+, Na+, K+ Ca 2+, Sr 2+, Ba 2+ and Mg 2+ d-elements Mn 2+, Co 2+, Ni 2+, Cu 2+, Zn 2+, Cd 2+ f-elements La 3+ and lantanides(III)
 COMPOUNDS (VIVO)-Ba C. N. = 6 C. N. = 5 VOSO 4+ 2 H 2 L + 2 Ba(OH)2 L = Cpdc {[Ba 2(VO)2 (Cpdc)4(H 2 O)8]· 2 H 2 O}n (yield 58. 9 %) 3 D-FRAMEWORK L = Me 2 mal H 2 O {[Ba(VO)(Me 2 mal)2(H 2 O)]·H 2 O}n (yield 40. 1 %) 2 D-LAYERS E. S. Bazhina, M. E. Nikiforova, G. G. Aleksandrov at al, Russ. Chem. Bull. , Int. Ed. , 2011, 60, 797. E. S. Bazhina, G. G. Aleksandrov, A. S. Bogomyakov et al, Polyhedron, 2013, 77, 47. L = Bumal {[Ba 3(VO)3(Bumal)6(H 2 O)13]· 4 H 2 O}n (yield 57. 7 %) 2 D-LAYERS V=O V–O (H 2 O) V–O (L) Ba–O 1. 58(2)– 1. 610(7) Å 2. 212(7)– 2. 37(2) Å 1. 954(5)– 2. 02(2) Å 2. 61(2)– 3. 01(2) Å
COMPOUNDS (VIVO)-Ba C. N. = 6 C. N. = 5 VOSO 4+ 2 H 2 L + 2 Ba(OH)2 L = Cpdc {[Ba 2(VO)2 (Cpdc)4(H 2 O)8]· 2 H 2 O}n (yield 58. 9 %) 3 D-FRAMEWORK L = Me 2 mal H 2 O {[Ba(VO)(Me 2 mal)2(H 2 O)]·H 2 O}n (yield 40. 1 %) 2 D-LAYERS E. S. Bazhina, M. E. Nikiforova, G. G. Aleksandrov at al, Russ. Chem. Bull. , Int. Ed. , 2011, 60, 797. E. S. Bazhina, G. G. Aleksandrov, A. S. Bogomyakov et al, Polyhedron, 2013, 77, 47. L = Bumal {[Ba 3(VO)3(Bumal)6(H 2 O)13]· 4 H 2 O}n (yield 57. 7 %) 2 D-LAYERS V=O V–O (H 2 O) V–O (L) Ba–O 1. 58(2)– 1. 610(7) Å 2. 212(7)– 2. 37(2) Å 1. 954(5)– 2. 02(2) Å 2. 61(2)– 3. 01(2) Å
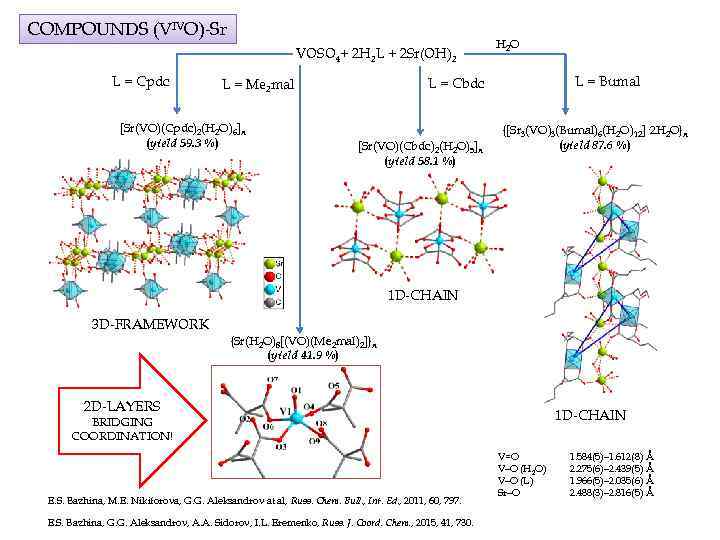 COMPOUNDS (VIVO)-Sr VOSO 4+ 2 H 2 L + 2 Sr(OH)2 L = Cpdc L = Bumal L = Cbdc L = Me 2 mal [Sr(VO)(Cpdc)2(H 2 O)6]n (yield 59. 3 %) H 2 O [Sr(VO)(Cbdc)2(H 2 O)5]n (yield 58. 1 %) {[Sr 3(VO)3(Bumal)6(H 2 O)12]· 2 H 2 O}n (yield 87. 6 %) 1 D-CHAIN 3 D-FRAMEWORK {Sr(H 2 O)8[(VO)(Me 2 mal)2]}n (yield 41. 9 %) 2 D-LAYERS 1 D-CHAIN BRIDGING COORDINATION! E. S. Bazhina, M. E. Nikiforova, G. G. Aleksandrov at al, Russ. Chem. Bull. , Int. Ed. , 2011, 60, 797. E. S. Bazhina, G. G. Aleksandrov, A. A. Sidorov, I. L. Eremenko, Russ. J. Coord. Chem. , 2015, 41, 730. V=O V–O (H 2 O) V–O (L) Sr–O 1. 584(5)– 1. 612(8) Å 2. 275(6)– 2. 439(5) Å 1. 966(5)– 2. 035(6) Å 2. 488(3)– 2. 816(5) Å
COMPOUNDS (VIVO)-Sr VOSO 4+ 2 H 2 L + 2 Sr(OH)2 L = Cpdc L = Bumal L = Cbdc L = Me 2 mal [Sr(VO)(Cpdc)2(H 2 O)6]n (yield 59. 3 %) H 2 O [Sr(VO)(Cbdc)2(H 2 O)5]n (yield 58. 1 %) {[Sr 3(VO)3(Bumal)6(H 2 O)12]· 2 H 2 O}n (yield 87. 6 %) 1 D-CHAIN 3 D-FRAMEWORK {Sr(H 2 O)8[(VO)(Me 2 mal)2]}n (yield 41. 9 %) 2 D-LAYERS 1 D-CHAIN BRIDGING COORDINATION! E. S. Bazhina, M. E. Nikiforova, G. G. Aleksandrov at al, Russ. Chem. Bull. , Int. Ed. , 2011, 60, 797. E. S. Bazhina, G. G. Aleksandrov, A. A. Sidorov, I. L. Eremenko, Russ. J. Coord. Chem. , 2015, 41, 730. V=O V–O (H 2 O) V–O (L) Sr–O 1. 584(5)– 1. 612(8) Å 2. 275(6)– 2. 439(5) Å 1. 966(5)– 2. 035(6) Å 2. 488(3)– 2. 816(5) Å
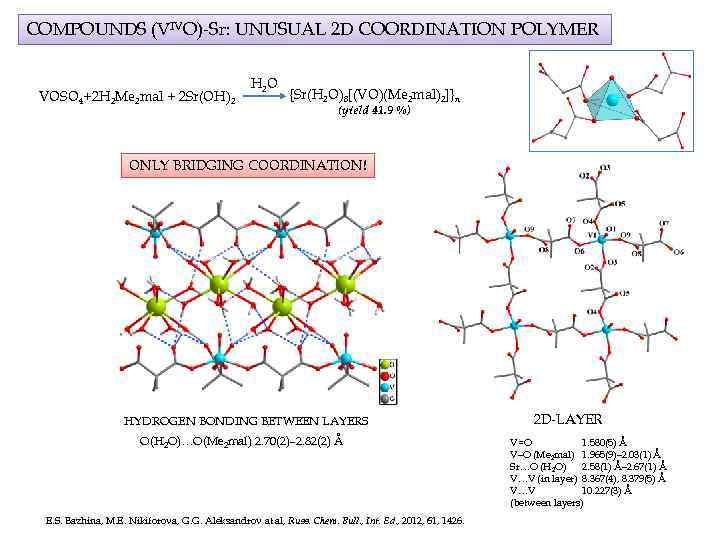 COMPOUNDS (VIVO)-Sr: UNUSUAL 2 D COORDINATION POLYMER VOSO 4+2 H 2 Me 2 mal + 2 Sr(OH)2 H 2 O {Sr(H 2 O)8[(VO)(Me 2 mal)2]}n (yield 41. 9 %) ONLY BRIDGING COORDINATION! HYDROGEN BONDING BETWEEN LAYERS O(H 2 O)…O(Me 2 mal) 2. 70(2)– 2. 82(2) Å E. S. Bazhina, M. E. Nikiforova, G. G. Aleksandrov at al, Russ. Chem. Bull. , Int. Ed. , 2012, 61, 1426. 2 D-LAYER V=O 1. 580(5) Å V–O (Me 2 mal) 1. 965(9)– 2. 03(1) Å Sr…O (H 2 O) 2. 58(1) Å– 2. 67(1) Å V…V (in layer) 8. 367(4), 8. 379(5) Å V…V 10. 227(3) Å (between layers)
COMPOUNDS (VIVO)-Sr: UNUSUAL 2 D COORDINATION POLYMER VOSO 4+2 H 2 Me 2 mal + 2 Sr(OH)2 H 2 O {Sr(H 2 O)8[(VO)(Me 2 mal)2]}n (yield 41. 9 %) ONLY BRIDGING COORDINATION! HYDROGEN BONDING BETWEEN LAYERS O(H 2 O)…O(Me 2 mal) 2. 70(2)– 2. 82(2) Å E. S. Bazhina, M. E. Nikiforova, G. G. Aleksandrov at al, Russ. Chem. Bull. , Int. Ed. , 2012, 61, 1426. 2 D-LAYER V=O 1. 580(5) Å V–O (Me 2 mal) 1. 965(9)– 2. 03(1) Å Sr…O (H 2 O) 2. 58(1) Å– 2. 67(1) Å V…V (in layer) 8. 367(4), 8. 379(5) Å V…V 10. 227(3) Å (between layers)
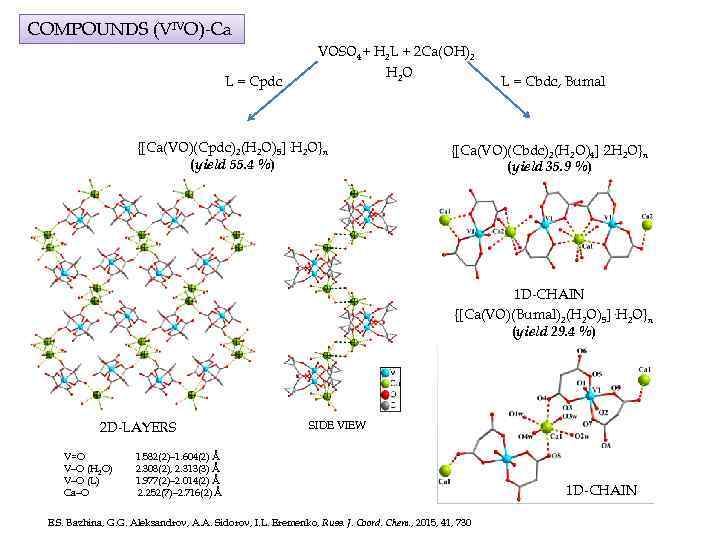 COMPOUNDS (VIVO)-Ca L = Cpdc VOSO 4+ H 2 L + 2 Ca(OH)2 H 2 O {[Ca(VO)(Cpdc)2(H 2 O)5]·H 2 O}n (yield 55. 4 %) L = Cbdc, Bumal {[Ca(VO)(Cbdc)2(H 2 O)4]· 2 H 2 O}n (yield 35. 9 %) 1 D-CHAIN {[Ca(VO)(Bumal)2(H 2 O)5]·H 2 O}n (yield 29. 4 %) 2 D-LAYERS V=O V–O (H 2 O) V–O (L) Ca–O SIDE VIEW 1. 582(2)– 1. 604(2) Å 2. 308(2), 2. 313(3) Å 1. 977(2)– 2. 014(2) Å 2. 252(7)– 2. 716(2) Å E. S. Bazhina, G. G. Aleksandrov, A. A. Sidorov, I. L. Eremenko, Russ. J. Coord. Chem. , 2015, 41, 730 1 D-CHAIN
COMPOUNDS (VIVO)-Ca L = Cpdc VOSO 4+ H 2 L + 2 Ca(OH)2 H 2 O {[Ca(VO)(Cpdc)2(H 2 O)5]·H 2 O}n (yield 55. 4 %) L = Cbdc, Bumal {[Ca(VO)(Cbdc)2(H 2 O)4]· 2 H 2 O}n (yield 35. 9 %) 1 D-CHAIN {[Ca(VO)(Bumal)2(H 2 O)5]·H 2 O}n (yield 29. 4 %) 2 D-LAYERS V=O V–O (H 2 O) V–O (L) Ca–O SIDE VIEW 1. 582(2)– 1. 604(2) Å 2. 308(2), 2. 313(3) Å 1. 977(2)– 2. 014(2) Å 2. 252(7)– 2. 716(2) Å E. S. Bazhina, G. G. Aleksandrov, A. A. Sidorov, I. L. Eremenko, Russ. J. Coord. Chem. , 2015, 41, 730 1 D-CHAIN
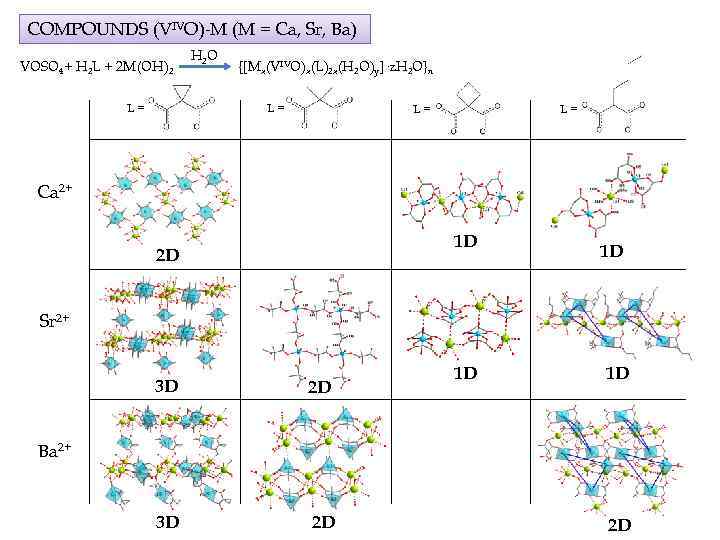 COMPOUNDS (VIVO)-M (M = Ca, Sr, Ba) VOSO 4+ H 2 L + 2 M(OH)2 L= H 2 O {[Mx(VIVO)x(L)2 x(H 2 O)y]·z. H 2 O}n L= L= L= Ca 2+ 1 D 2 D 1 D Sr 2+ 3 D 2 D 1 D 1 D Ba 2+ 2 D
COMPOUNDS (VIVO)-M (M = Ca, Sr, Ba) VOSO 4+ H 2 L + 2 M(OH)2 L= H 2 O {[Mx(VIVO)x(L)2 x(H 2 O)y]·z. H 2 O}n L= L= L= Ca 2+ 1 D 2 D 1 D Sr 2+ 3 D 2 D 1 D 1 D Ba 2+ 2 D
 COMPOUNDS (VIVO)-K VOSO 4 + 2 H 2 L +3 K 2 CO 3 L = Me 2 mal ? L = Bumal H 2 O ? L = Cbdc [K 4(VO)2(Cbdc)4(H 2 O)4]n (yield 36. 7 %) Formation of K 6[VV 10 O 28] crystals 3 D-FRAMEWORK V=O V–O (Cbdc) K–O (H 2 O) K–O (V=O) K–O (Cbdc) 1. 600(4), 1. 602(4) Å 1. 939(4)– 2. 002(4) Å 2. 664(5)– 2. 836(5) Å 2. 806(4), 2. 808(4) Å 2. 565(4)– 3. 038(4) Å
COMPOUNDS (VIVO)-K VOSO 4 + 2 H 2 L +3 K 2 CO 3 L = Me 2 mal ? L = Bumal H 2 O ? L = Cbdc [K 4(VO)2(Cbdc)4(H 2 O)4]n (yield 36. 7 %) Formation of K 6[VV 10 O 28] crystals 3 D-FRAMEWORK V=O V–O (Cbdc) K–O (H 2 O) K–O (V=O) K–O (Cbdc) 1. 600(4), 1. 602(4) Å 1. 939(4)– 2. 002(4) Å 2. 664(5)– 2. 836(5) Å 2. 806(4), 2. 808(4) Å 2. 565(4)– 3. 038(4) Å
 COMPOUNDS (VIVO)-Na VOSO 4 + 2 H 2 L +3 Na 2 CO 3 L = Me 2 mal [Na 4(VO)2(Me 2 mal)4(H 2 O)]n (yield 74. 6 %) 3 D-FRAMEWORK L = Bumal L = Cbdc {[Na 6(VO)3(Bumal)6(H 2 O)15]· 3 H 2 O}n [Na 2(VO)(Cbdc)2(H 2 Cbdc)(H 2 O)3]n (yield 26. 4 %) (yield 57. 9 %) 2 D-LAYERS V=O V–O (H 2 O) V–O (L) Na–O 1. 578(4)– 1. 603(4) Å 2. 272(2)– 2. 445(3) Å 1. 956(2)– 2. 018(3) Å 2. 272(2)– 2. 701(4) Å E. S. Bazhina, G. G. Aleksandrov, M. A. Kiskin at al, Russ. Chem. Bull. , Int. Ed. , 2014, 63, 1475 2 D-LAYERS
COMPOUNDS (VIVO)-Na VOSO 4 + 2 H 2 L +3 Na 2 CO 3 L = Me 2 mal [Na 4(VO)2(Me 2 mal)4(H 2 O)]n (yield 74. 6 %) 3 D-FRAMEWORK L = Bumal L = Cbdc {[Na 6(VO)3(Bumal)6(H 2 O)15]· 3 H 2 O}n [Na 2(VO)(Cbdc)2(H 2 Cbdc)(H 2 O)3]n (yield 26. 4 %) (yield 57. 9 %) 2 D-LAYERS V=O V–O (H 2 O) V–O (L) Na–O 1. 578(4)– 1. 603(4) Å 2. 272(2)– 2. 445(3) Å 1. 956(2)– 2. 018(3) Å 2. 272(2)– 2. 701(4) Å E. S. Bazhina, G. G. Aleksandrov, M. A. Kiskin at al, Russ. Chem. Bull. , Int. Ed. , 2014, 63, 1475 2 D-LAYERS
![COMPOUNDS (VIVO)-Li: ROLE OF THE SOLVENT H 2 O [Li 2(VO)(Me 2 mal)2]n VOSO COMPOUNDS (VIVO)-Li: ROLE OF THE SOLVENT H 2 O [Li 2(VO)(Me 2 mal)2]n VOSO](https://present5.com/presentation/-104014181_425074704/image-12.jpg) COMPOUNDS (VIVO)-Li: ROLE OF THE SOLVENT H 2 O [Li 2(VO)(Me 2 mal)2]n VOSO 4 + 2 H 2 L +2 Li 2 CO 3 L= Et. OH [Li 2(VO)(Me 2 mal)2(H 2 O)(Et. OH)]n LAYER VIEW 3 D-FRAMEWORK V=O 1. 545(9) Å V–O (Me 2 mal) 1. 963(4), 1. 966(5) Å Li…O (Me 2 mal) 1. 97(1)– 2. 18(1) Å E. S. Bazhina, G. G. Aleksandrov, M. A. Kiskin at al, Russ. Chem. Bull. , Int. Ed. , 2015, in press. 2 D-LAYERS V=O 1. 587(3) Å V–O (Me 2 mal) 1. 9423(19), 1. 9568(19) Å Li…O (Me 2 mal) 1. 865(4), 2. 044(6) Å Li…O (H 2 O, Et. OH) 1. 955(8)– 2. 006(8) Å
COMPOUNDS (VIVO)-Li: ROLE OF THE SOLVENT H 2 O [Li 2(VO)(Me 2 mal)2]n VOSO 4 + 2 H 2 L +2 Li 2 CO 3 L= Et. OH [Li 2(VO)(Me 2 mal)2(H 2 O)(Et. OH)]n LAYER VIEW 3 D-FRAMEWORK V=O 1. 545(9) Å V–O (Me 2 mal) 1. 963(4), 1. 966(5) Å Li…O (Me 2 mal) 1. 97(1)– 2. 18(1) Å E. S. Bazhina, G. G. Aleksandrov, M. A. Kiskin at al, Russ. Chem. Bull. , Int. Ed. , 2015, in press. 2 D-LAYERS V=O 1. 587(3) Å V–O (Me 2 mal) 1. 9423(19), 1. 9568(19) Å Li…O (Me 2 mal) 1. 865(4), 2. 044(6) Å Li…O (H 2 O, Et. OH) 1. 955(8)– 2. 006(8) Å
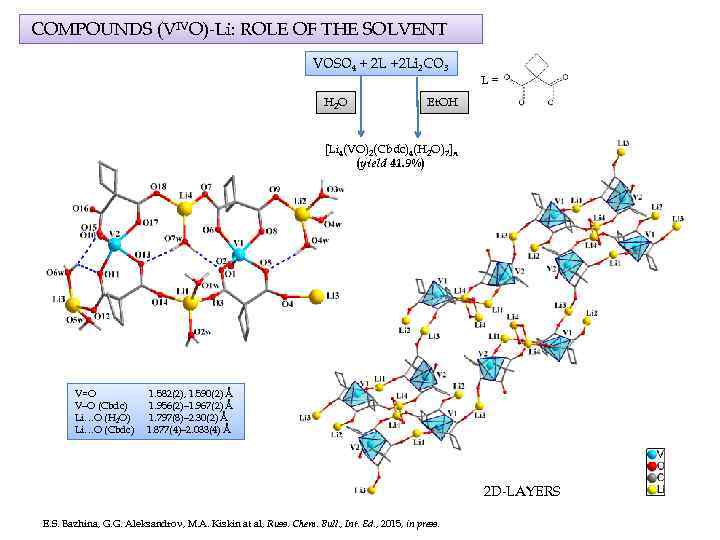 COMPOUNDS (VIVO)-Li: ROLE OF THE SOLVENT VOSO 4 + 2 L +2 Li 2 CO 3 H 2 O L= Et. OH [Li 4(VO)2(Cbdc)4(H 2 O)7]n (yield 41. 9%) V=O V–O (Cbdc) Li…O (H 2 O) Li…O (Cbdc) 1. 582(2), 1. 590(2) Å 1. 956(2)– 1. 967(2) Å 1. 797(8)– 2. 30(2) Å 1. 877(4)– 2. 033(4) Å 2 D-LAYERS E. S. Bazhina, G. G. Aleksandrov, M. A. Kiskin at al, Russ. Chem. Bull. , Int. Ed. , 2015, in press.
COMPOUNDS (VIVO)-Li: ROLE OF THE SOLVENT VOSO 4 + 2 L +2 Li 2 CO 3 H 2 O L= Et. OH [Li 4(VO)2(Cbdc)4(H 2 O)7]n (yield 41. 9%) V=O V–O (Cbdc) Li…O (H 2 O) Li…O (Cbdc) 1. 582(2), 1. 590(2) Å 1. 956(2)– 1. 967(2) Å 1. 797(8)– 2. 30(2) Å 1. 877(4)– 2. 033(4) Å 2 D-LAYERS E. S. Bazhina, G. G. Aleksandrov, M. A. Kiskin at al, Russ. Chem. Bull. , Int. Ed. , 2015, in press.
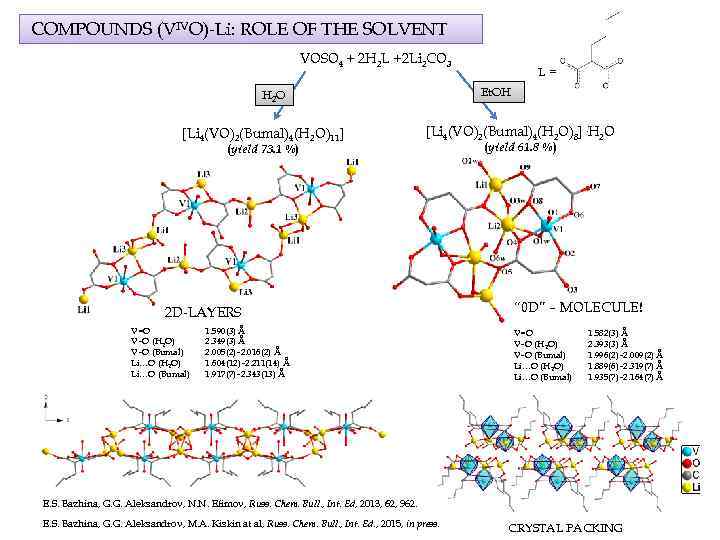 COMPOUNDS (VIVO)-Li: ROLE OF THE SOLVENT VOSO 4 + 2 H 2 L +2 Li 2 CO 3 Et. OH H 2 O [Li 4(VO)2(Bumal)4(H 2 O)11] (yield 73. 1 %) [Li 4(VO)2(Bumal)4(H 2 O)8]·H 2 O 2 D-LAYERS V=O V–O (H 2 O) V–O (Bumal) Li…O (H 2 O) Li…O (Bumal) L= 1. 590(3) Å 2. 349(3) Å 2. 005(2)– 2. 016(2) Å 1. 604(12)– 2. 211(14) Å 1. 917(7)– 2. 343(13) Å (yield 61. 8 %) “ 0 D” – MOLECULE! V=O V–O (H 2 O) V–O (Bumal) Li…O (H 2 O) Li…O (Bumal) 1. 582(3) Å 2. 393(3) Å 1. 996(2)– 2. 009(2) Å 1. 889(6)– 2. 319(7) Å 1. 935(7)– 2. 164(7) Å E. S. Bazhina, G. G. Aleksandrov, N. N. Efimov, Russ. Chem. Bull. , Int. Ed, 2013, 62, 962. E. S. Bazhina, G. G. Aleksandrov, M. A. Kiskin at al, Russ. Chem. Bull. , Int. Ed. , 2015, in press. CRYSTAL PACKING
COMPOUNDS (VIVO)-Li: ROLE OF THE SOLVENT VOSO 4 + 2 H 2 L +2 Li 2 CO 3 Et. OH H 2 O [Li 4(VO)2(Bumal)4(H 2 O)11] (yield 73. 1 %) [Li 4(VO)2(Bumal)4(H 2 O)8]·H 2 O 2 D-LAYERS V=O V–O (H 2 O) V–O (Bumal) Li…O (H 2 O) Li…O (Bumal) L= 1. 590(3) Å 2. 349(3) Å 2. 005(2)– 2. 016(2) Å 1. 604(12)– 2. 211(14) Å 1. 917(7)– 2. 343(13) Å (yield 61. 8 %) “ 0 D” – MOLECULE! V=O V–O (H 2 O) V–O (Bumal) Li…O (H 2 O) Li…O (Bumal) 1. 582(3) Å 2. 393(3) Å 1. 996(2)– 2. 009(2) Å 1. 889(6)– 2. 319(7) Å 1. 935(7)– 2. 164(7) Å E. S. Bazhina, G. G. Aleksandrov, N. N. Efimov, Russ. Chem. Bull. , Int. Ed, 2013, 62, 962. E. S. Bazhina, G. G. Aleksandrov, M. A. Kiskin at al, Russ. Chem. Bull. , Int. Ed. , 2015, in press. CRYSTAL PACKING
![COMPOUNDS (VIVO)-MII (MII = Mn, Co, Cd): 1 D-POLYMERS {[Bax(VIVO)x(L)2 x(H 2 O)y]·z. H COMPOUNDS (VIVO)-MII (MII = Mn, Co, Cd): 1 D-POLYMERS {[Bax(VIVO)x(L)2 x(H 2 O)y]·z. H](https://present5.com/presentation/-104014181_425074704/image-15.jpg) COMPOUNDS (VIVO)-MII (MII = Mn, Co, Cd): 1 D-POLYMERS {[Bax(VIVO)x(L)2 x(H 2 O)y]·z. H 2 O}n + xn. MSO 4 V=O V–O (H 2 O) V–O (L) 1. 581(3)– 1. 597(2) Å 2. 317(3)– 2. 357(2) Å 1. 965(3)– 2. 008(2) Å H 2 O {[M(VO)L 2(H 2 O)5]·H 2 O}n MII = Mn, Co, Cd; L = Me 2 mal, Bumal Co–O Mn–O Cd–O E. S. Bazhina, G. G. Aleksandrov, A. S. Bogomyakov et al, Polyhedron, 2013, 77, 47. 2. 063(2)– 2. 173(2) Å 2. 134(3)– 2. 270(4) Å 2. 239(3)– 2. 326(4) Å
COMPOUNDS (VIVO)-MII (MII = Mn, Co, Cd): 1 D-POLYMERS {[Bax(VIVO)x(L)2 x(H 2 O)y]·z. H 2 O}n + xn. MSO 4 V=O V–O (H 2 O) V–O (L) 1. 581(3)– 1. 597(2) Å 2. 317(3)– 2. 357(2) Å 1. 965(3)– 2. 008(2) Å H 2 O {[M(VO)L 2(H 2 O)5]·H 2 O}n MII = Mn, Co, Cd; L = Me 2 mal, Bumal Co–O Mn–O Cd–O E. S. Bazhina, G. G. Aleksandrov, A. S. Bogomyakov et al, Polyhedron, 2013, 77, 47. 2. 063(2)– 2. 173(2) Å 2. 134(3)– 2. 270(4) Å 2. 239(3)– 2. 326(4) Å
 COMPOUNDS (VIVO)-MII (MII = Mn, Co, Ni, Cd) WITH CBDC Solid of unknown composition H 2 O +MSO 4 VOSO 4+MSO 4 + 2 H 2 L + 2 Ba(OH)2 +MSO 4 M = Mn: yield 54. 7 % M = Co: yield 78. 9 % M = Ni: yield 40. 4 % H 2 O +Cd. SO 4 L= {[Cd(VO)L 2(H 2 O)5]·H 2 O}n (yield 48. 6%) H 2 O, Et. OH monocrystals [M(VO)(Cbdc)2(Et. OH)2(H 2 O)3]n M = Mn, Co, Ni 1 D-CHAIN V=O V–O (H 2 O) V–O (L) 1. 599(5) Å 2. 356(5) Å 1. 975(3), 2. 016(3) Å Cd–O (L) Cd–O (H 2 O) V…Cd 2. 256(4) Å 2. 234(5)– 2. 417 (3) Å 4. 8308(8) Å COMPLEXES Cu. II-Co. II AND Cu. II-Cd 1 D-CHAIN V=O V–O(L) V–O(H 2 O) 1. 569(2)– 1. 589(3) Å 1. 991(4)– 2. 028(4) Å 2. 260(4)– 2. 297(3) Å M–O (L) M–O (H 2 O) M–O (Et. OH) 2. 038(4) (Ni)-2. 228(2) (Mn) Å 2. 055(4)-2. 206(2) Å 2. 036(4)-2. 174(2) Å IONIC COMPLEX! 2 D-POLYMER!
COMPOUNDS (VIVO)-MII (MII = Mn, Co, Ni, Cd) WITH CBDC Solid of unknown composition H 2 O +MSO 4 VOSO 4+MSO 4 + 2 H 2 L + 2 Ba(OH)2 +MSO 4 M = Mn: yield 54. 7 % M = Co: yield 78. 9 % M = Ni: yield 40. 4 % H 2 O +Cd. SO 4 L= {[Cd(VO)L 2(H 2 O)5]·H 2 O}n (yield 48. 6%) H 2 O, Et. OH monocrystals [M(VO)(Cbdc)2(Et. OH)2(H 2 O)3]n M = Mn, Co, Ni 1 D-CHAIN V=O V–O (H 2 O) V–O (L) 1. 599(5) Å 2. 356(5) Å 1. 975(3), 2. 016(3) Å Cd–O (L) Cd–O (H 2 O) V…Cd 2. 256(4) Å 2. 234(5)– 2. 417 (3) Å 4. 8308(8) Å COMPLEXES Cu. II-Co. II AND Cu. II-Cd 1 D-CHAIN V=O V–O(L) V–O(H 2 O) 1. 569(2)– 1. 589(3) Å 1. 991(4)– 2. 028(4) Å 2. 260(4)– 2. 297(3) Å M–O (L) M–O (H 2 O) M–O (Et. OH) 2. 038(4) (Ni)-2. 228(2) (Mn) Å 2. 055(4)-2. 206(2) Å 2. 036(4)-2. 174(2) Å IONIC COMPLEX! 2 D-POLYMER!
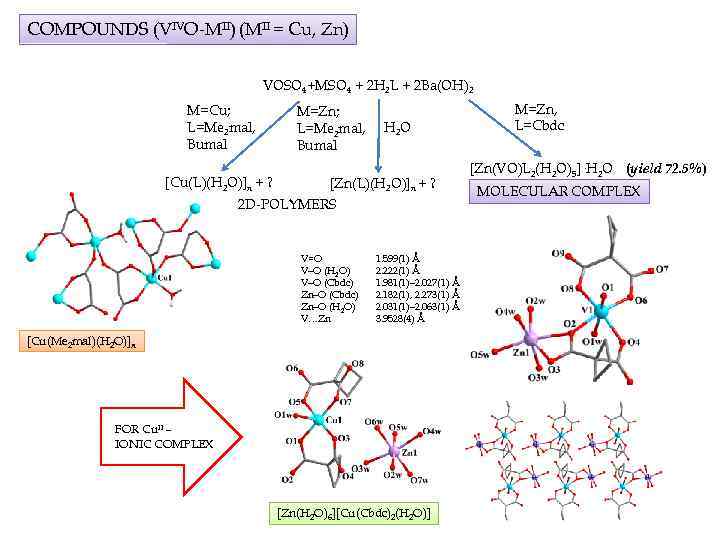 COMPOUNDS (VIVO-MII) (MII = Cu, Zn) VOSO 4+MSO 4 + 2 H 2 L + 2 Ba(OH)2 M=Cu; L=Me 2 mal, Bumal [Cu(L)(H 2 O)]n + ? M=Zn; L=Me 2 mal, Bumal H 2 O [Zn(L)(H 2 O)]n + ? 2 D-POLYMERS V=O V–O (H 2 O) V–O (Cbdc) Zn–O (H 2 O) V…Zn 1. 599(1) Å 2. 222(1) Å 1. 981(1)– 2. 027(1) Å 2. 182(1), 2. 273(1) Å 2. 031(1)– 2. 063(1) Å 3. 9528(4) Å [Cu(Me 2 mal)(H 2 O)]n FOR Cu. II – IONIC COMPLEX [Zn(H 2 O)6][Cu(Cbdc)2(H 2 O)] M=Zn, L=Cbdc [Zn(VO)L 2(H 2 O)5]·H 2 O (yield 72. 5%) MOLECULAR COMPLEX
COMPOUNDS (VIVO-MII) (MII = Cu, Zn) VOSO 4+MSO 4 + 2 H 2 L + 2 Ba(OH)2 M=Cu; L=Me 2 mal, Bumal [Cu(L)(H 2 O)]n + ? M=Zn; L=Me 2 mal, Bumal H 2 O [Zn(L)(H 2 O)]n + ? 2 D-POLYMERS V=O V–O (H 2 O) V–O (Cbdc) Zn–O (H 2 O) V…Zn 1. 599(1) Å 2. 222(1) Å 1. 981(1)– 2. 027(1) Å 2. 182(1), 2. 273(1) Å 2. 031(1)– 2. 063(1) Å 3. 9528(4) Å [Cu(Me 2 mal)(H 2 O)]n FOR Cu. II – IONIC COMPLEX [Zn(H 2 O)6][Cu(Cbdc)2(H 2 O)] M=Zn, L=Cbdc [Zn(VO)L 2(H 2 O)5]·H 2 O (yield 72. 5%) MOLECULAR COMPLEX
![COMPOUNDS (VIVO)-Mg {[Bax(VIVO)x(L)2 x(H 2 O)y]·z. H 2 O}n + xn. Mg. SO 4 COMPOUNDS (VIVO)-Mg {[Bax(VIVO)x(L)2 x(H 2 O)y]·z. H 2 O}n + xn. Mg. SO 4](https://present5.com/presentation/-104014181_425074704/image-18.jpg) COMPOUNDS (VIVO)-Mg {[Bax(VIVO)x(L)2 x(H 2 O)y]·z. H 2 O}n + xn. Mg. SO 4 L= L= H 2 O crystals unsuitable for X-ray L= 18
COMPOUNDS (VIVO)-Mg {[Bax(VIVO)x(L)2 x(H 2 O)y]·z. H 2 O}n + xn. Mg. SO 4 L= L= H 2 O crystals unsuitable for X-ray L= 18
![COMPOUNDS (VIVO)-Mg L= [K 4(VO)2(Cbdc)4(H 2 O)4]n + 2 n. Mg(NO 3)2 H 2 COMPOUNDS (VIVO)-Mg L= [K 4(VO)2(Cbdc)4(H 2 O)4]n + 2 n. Mg(NO 3)2 H 2](https://present5.com/presentation/-104014181_425074704/image-19.jpg) COMPOUNDS (VIVO)-Mg L= [K 4(VO)2(Cbdc)4(H 2 O)4]n + 2 n. Mg(NO 3)2 H 2 O {[KMg 0. 5(VO)(cbdc)2(H 2 O)7]· 3 H 2 O}n (yield 52. 8 %) [K 4(VO)2(Cbdc)4(H 2 O)4]n Mg 2+ 1 D-CHAIN 3 D-FRAMEWORK SIDE VIEW OF THE RIBBON V=O V–O (Cbdc) V–O (H 2 O) 1. 593(3) Å 1. 992(2)– 2. 021(2) Å 2. 270(3) Mg–O (Cbdc) Mg–O (H 2 O) K…O (Cbdc) 2. 094(4) Å 1. 931(4)-2. 238(5) Å 2. 732(7)-3. 023(3) Å 2. 718(2)-2. 837(2) Å
COMPOUNDS (VIVO)-Mg L= [K 4(VO)2(Cbdc)4(H 2 O)4]n + 2 n. Mg(NO 3)2 H 2 O {[KMg 0. 5(VO)(cbdc)2(H 2 O)7]· 3 H 2 O}n (yield 52. 8 %) [K 4(VO)2(Cbdc)4(H 2 O)4]n Mg 2+ 1 D-CHAIN 3 D-FRAMEWORK SIDE VIEW OF THE RIBBON V=O V–O (Cbdc) V–O (H 2 O) 1. 593(3) Å 1. 992(2)– 2. 021(2) Å 2. 270(3) Mg–O (Cbdc) Mg–O (H 2 O) K…O (Cbdc) 2. 094(4) Å 1. 931(4)-2. 238(5) Å 2. 732(7)-3. 023(3) Å 2. 718(2)-2. 837(2) Å
![COMPOUNDS VIVO-Ln. III [K 4(VO)2(Cbdc)4(H 2 O)4]n + (4/3)n. Gd(NO 3)3 H 2 O COMPOUNDS VIVO-Ln. III [K 4(VO)2(Cbdc)4(H 2 O)4]n + (4/3)n. Gd(NO 3)3 H 2 O](https://present5.com/presentation/-104014181_425074704/image-20.jpg) COMPOUNDS VIVO-Ln. III [K 4(VO)2(Cbdc)4(H 2 O)4]n + (4/3)n. Gd(NO 3)3 H 2 O {[KGd(VO)2(Cbdc)4(H 2 O)9]· 3. 5 H 2 O}n L= STRUCTURE OF THE TRINUCLEAR FRAGMENT {V-Gd-V} V=O V–O (H 2 O) V–O (Cbdc) Gd–O (H 2 O) V…Gd 1. 581(3), 1. 588(3) Å 2. 241(3), 2. 419(3) Å 1. 976(3)– 2. 016(3) Å 2. 355(3), 2. 443(3) Å 2. 332(3)– 2. 487(3) Å 4. 5933(8), 6. 0050(7) Å K–O (Cbdc) K–O (H 2 O) 2. 739(3)-2. 965(3) Å 2. 708(3) Å
COMPOUNDS VIVO-Ln. III [K 4(VO)2(Cbdc)4(H 2 O)4]n + (4/3)n. Gd(NO 3)3 H 2 O {[KGd(VO)2(Cbdc)4(H 2 O)9]· 3. 5 H 2 O}n L= STRUCTURE OF THE TRINUCLEAR FRAGMENT {V-Gd-V} V=O V–O (H 2 O) V–O (Cbdc) Gd–O (H 2 O) V…Gd 1. 581(3), 1. 588(3) Å 2. 241(3), 2. 419(3) Å 1. 976(3)– 2. 016(3) Å 2. 355(3), 2. 443(3) Å 2. 332(3)– 2. 487(3) Å 4. 5933(8), 6. 0050(7) Å K–O (Cbdc) K–O (H 2 O) 2. 739(3)-2. 965(3) Å 2. 708(3) Å
 CONCLUSIONS Ba 2+, Sr 2+, Ca 2+ 3 D 2 D Ba 2+, Sr 2+, Ca 2+ 1 D 2 D 2 D Na+, Li+ 3 D Na+, Li+ K+, Na+, Li+ 3 D 3 D 2 D Mn 2+, Co 2+, Cd 2+ 1 D 2 D 2 D 0 D 2 D Mn 2+, Co 2+, Ni 2+, Zn 2+, Cd 2+ 1 D 1 D 0 D 1 D Mn 2+, Co 2+, Cd 2+ 1 D
CONCLUSIONS Ba 2+, Sr 2+, Ca 2+ 3 D 2 D Ba 2+, Sr 2+, Ca 2+ 1 D 2 D 2 D Na+, Li+ 3 D Na+, Li+ K+, Na+, Li+ 3 D 3 D 2 D Mn 2+, Co 2+, Cd 2+ 1 D 2 D 2 D 0 D 2 D Mn 2+, Co 2+, Ni 2+, Zn 2+, Cd 2+ 1 D 1 D 0 D 1 D Mn 2+, Co 2+, Cd 2+ 1 D
 ACKNOWLEDGMENT X-RAY HEADS Dr. Mikhail A. Kiskin Prof. Igor L. Eremenko Dr. Grigory G. Aleksandrov Cu. II COMPLEXES Prof. Alexey A. Sidorov FINANCIAL SUPPORT RUSSIAN ACADEMY OF SCIENCES COUNCIL ON GRANTS OF THE PRESIDENT OF RF PROJECTS №№ 13 -03 -00703, 13 -03 -12430 14 -03 -01116, 14 -03 -31292 Dr. Natalya V. Gogoleva МК-2917. 2014. 3, NSh-4773. 2014. 3
ACKNOWLEDGMENT X-RAY HEADS Dr. Mikhail A. Kiskin Prof. Igor L. Eremenko Dr. Grigory G. Aleksandrov Cu. II COMPLEXES Prof. Alexey A. Sidorov FINANCIAL SUPPORT RUSSIAN ACADEMY OF SCIENCES COUNCIL ON GRANTS OF THE PRESIDENT OF RF PROJECTS №№ 13 -03 -00703, 13 -03 -12430 14 -03 -01116, 14 -03 -31292 Dr. Natalya V. Gogoleva МК-2917. 2014. 3, NSh-4773. 2014. 3
 THANK YOU FOR YOUR ATTENTION! 23
THANK YOU FOR YOUR ATTENTION! 23


