Gogoleva N.V..ppt
- Количество слайдов: 21
 Kurnakov Institute of General and Inorganic Chemistry RAS Ph. D. Gogoleva Natalya Vyacheslavovna, M. A. Shmelev, M. A. Kiskin, Zh. V. Dobrokhotova, G. G. Aleksandrov, A. A. Sidorov and I. L. Eremenko CARBOXYLATE Cd COMPLEXES. PERSPECTIVES OF SYNTHESIS OF NEW HOMO- AND HETEROMETALLIC COMPOUNDS Novosibirsk, 5 -9 October 2015
Kurnakov Institute of General and Inorganic Chemistry RAS Ph. D. Gogoleva Natalya Vyacheslavovna, M. A. Shmelev, M. A. Kiskin, Zh. V. Dobrokhotova, G. G. Aleksandrov, A. A. Sidorov and I. L. Eremenko CARBOXYLATE Cd COMPLEXES. PERSPECTIVES OF SYNTHESIS OF NEW HOMO- AND HETEROMETALLIC COMPOUNDS Novosibirsk, 5 -9 October 2015
![► Introduction Carboxylate complexes of Zn и Cd Nanomaterials [1] Luminescence [3] Bioactivity [2] ► Introduction Carboxylate complexes of Zn и Cd Nanomaterials [1] Luminescence [3] Bioactivity [2]](https://present5.com/presentation/-104014181_425097612/image-2.jpg) ► Introduction Carboxylate complexes of Zn и Cd Nanomaterials [1] Luminescence [3] Bioactivity [2] Typical coordination numbers 3 d-metals 2+ CN=4 CN=5 CN=6 CN=7 Cd 2+ CN=8 CN=9 (rare) 2 [1] U. Kumar, J. Thomas, M. Agrawal, N. Thirupathi (2011) Inorg. Chim. Acta, 370, 122. [2] S. Bhattacharyya et al. (2014) J. Inorg. Biochem. , 140, 131. [3] Y. -X. Chi, S. -Y. Niu, Z. -L. Wang, J. Jin. (2008) Eur. J. Inorg. Chem. , 2336. 2
► Introduction Carboxylate complexes of Zn и Cd Nanomaterials [1] Luminescence [3] Bioactivity [2] Typical coordination numbers 3 d-metals 2+ CN=4 CN=5 CN=6 CN=7 Cd 2+ CN=8 CN=9 (rare) 2 [1] U. Kumar, J. Thomas, M. Agrawal, N. Thirupathi (2011) Inorg. Chim. Acta, 370, 122. [2] S. Bhattacharyya et al. (2014) J. Inorg. Biochem. , 140, 131. [3] Y. -X. Chi, S. -Y. Niu, Z. -L. Wang, J. Jin. (2008) Eur. J. Inorg. Chem. , 2336. 2
![► Introduction [1] [2] [3] Carboxylate anions Trimethylacetate Di-tert-butylbenzoate [1] C. Escobedo-Martinez, M. C. ► Introduction [1] [2] [3] Carboxylate anions Trimethylacetate Di-tert-butylbenzoate [1] C. Escobedo-Martinez, M. C.](https://present5.com/presentation/-104014181_425097612/image-3.jpg) ► Introduction [1] [2] [3] Carboxylate anions Trimethylacetate Di-tert-butylbenzoate [1] C. Escobedo-Martinez, M. C. Lozada, D. Gnecco, R. G. Enriquez, M. Soriano-Garcia, W. F. Reynolds (2012) J. Chem. Cryst. , 42, 794 [2] Yu-Xian Chi, Shu-Yun Niu, Zhao-Long Wang, Jing Jin (2008) Gaodeng Xuexiao Huaxue Xuebao(Chin. )(Chem. J. Chin. Univ. (Chinese Edition)), 29, 1081 [3] Zhang Wen-Xing, Ma Chang-Qin, Li Jian, Wang Xu-Ning, Yu Zheng-Gang, Jiang De-Hua (1998) Jiegou Huaxue(Chin. J. Struct. Chem. ) , 17, 57 3
► Introduction [1] [2] [3] Carboxylate anions Trimethylacetate Di-tert-butylbenzoate [1] C. Escobedo-Martinez, M. C. Lozada, D. Gnecco, R. G. Enriquez, M. Soriano-Garcia, W. F. Reynolds (2012) J. Chem. Cryst. , 42, 794 [2] Yu-Xian Chi, Shu-Yun Niu, Zhao-Long Wang, Jing Jin (2008) Gaodeng Xuexiao Huaxue Xuebao(Chin. )(Chem. J. Chin. Univ. (Chinese Edition)), 29, 1081 [3] Zhang Wen-Xing, Ma Chang-Qin, Li Jian, Wang Xu-Ning, Yu Zheng-Gang, Jiang De-Hua (1998) Jiegou Huaxue(Chin. J. Struct. Chem. ) , 17, 57 3
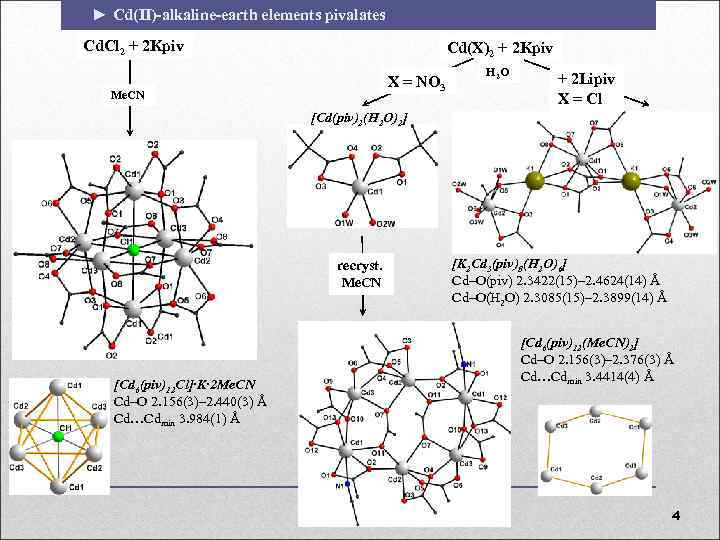 ► Cd(II)-alkaline-earth elements pivalates Cd. Cl 2 + 2 Kpiv Cd(X)2 + 2 Kpiv X = NO 3 Me. CN H 2 O + 2 Lipiv X = Cl [Cd(piv)2(H 2 O)2] recryst. Me. CN [Cd 6(piv)12 Cl]∙K∙ 2 Me. CN Cd–O 2. 156(3)– 2. 440(3) Å Cd…Cdmin 3. 984(1) Å [K 2 Cd 3(piv)8(H 2 O)6] Cd–O(piv) 2. 3422(15)– 2. 4624(14) Å Cd–O(H 2 O) 2. 3085(15)– 2. 3899(14) Å [Cd 6(piv)12(Me. CN)2] Cd–O 2. 156(3)– 2. 376(3) Å Cd…Cdmin 3. 4414(4) Å 4
► Cd(II)-alkaline-earth elements pivalates Cd. Cl 2 + 2 Kpiv Cd(X)2 + 2 Kpiv X = NO 3 Me. CN H 2 O + 2 Lipiv X = Cl [Cd(piv)2(H 2 O)2] recryst. Me. CN [Cd 6(piv)12 Cl]∙K∙ 2 Me. CN Cd–O 2. 156(3)– 2. 440(3) Å Cd…Cdmin 3. 984(1) Å [K 2 Cd 3(piv)8(H 2 O)6] Cd–O(piv) 2. 3422(15)– 2. 4624(14) Å Cd–O(H 2 O) 2. 3085(15)– 2. 3899(14) Å [Cd 6(piv)12(Me. CN)2] Cd–O 2. 156(3)– 2. 376(3) Å Cd…Cdmin 3. 4414(4) Å 4
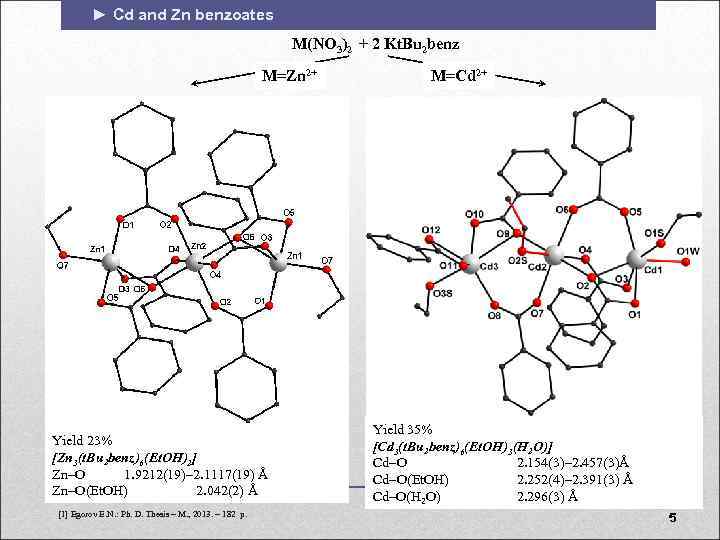 ► Cd and Zn benzoates M(NO 3)2 + 2 Kt. Bu 2 benz M=Zn 2+ Yield 23% [Zn 3(t. Bu 2 benz)6(Et. OH)2] Zn O 1. 9212(19)– 2. 1117(19) Å Zn O(Et. OH) 2. 042(2) Å [1] Egorov E. N. : Ph. D. Thesis – М. , 2013. – 182 p. M=Cd 2+ Yield 35% [Cd 3(t. Bu 2 benz)6(Et. OH)3(H 2 O)] Cd O 2. 154(3)– 2. 457(3)Å Cd O(Et. OH) 2. 252(4)– 2. 391(3) Å Cd–O(H 2 O) 2. 296(3) Å 5
► Cd and Zn benzoates M(NO 3)2 + 2 Kt. Bu 2 benz M=Zn 2+ Yield 23% [Zn 3(t. Bu 2 benz)6(Et. OH)2] Zn O 1. 9212(19)– 2. 1117(19) Å Zn O(Et. OH) 2. 042(2) Å [1] Egorov E. N. : Ph. D. Thesis – М. , 2013. – 182 p. M=Cd 2+ Yield 35% [Cd 3(t. Bu 2 benz)6(Et. OH)3(H 2 O)] Cd O 2. 154(3)– 2. 457(3)Å Cd O(Et. OH) 2. 252(4)– 2. 391(3) Å Cd–O(H 2 O) 2. 296(3) Å 5
![► Homometallic Cd(II) pivalates [Cd(piv)2]n + 2 L hexane Me. CN L = ihin ► Homometallic Cd(II) pivalates [Cd(piv)2]n + 2 L hexane Me. CN L = ihin](https://present5.com/presentation/-104014181_425097612/image-6.jpg) ► Homometallic Cd(II) pivalates [Cd(piv)2]n + 2 L hexane Me. CN L = ihin [Cd 2(piv)4]∙ 2 Et 3 N Cd. . . Cd 3. 497(1) Å [Cd 3(Et 3 N)2(piv)6] Cd. . . Cd 3. 562(1)Å L = ampy L = lut L = cdpy [Cd(ihin)3(piv)2] [Cd 2(phtr)2(piv)4] Cd. . . Cd 3. 686(1) Å [Cd 2(ampy)2(piv)4] [Cd 2(lut)2(piv)4] Cd. . . Cd 3. 758(1) Å [Cd 2(cdp)2(piv)4] Cd. . . Cd 3. 105(1) Å 6
► Homometallic Cd(II) pivalates [Cd(piv)2]n + 2 L hexane Me. CN L = ihin [Cd 2(piv)4]∙ 2 Et 3 N Cd. . . Cd 3. 497(1) Å [Cd 3(Et 3 N)2(piv)6] Cd. . . Cd 3. 562(1)Å L = ampy L = lut L = cdpy [Cd(ihin)3(piv)2] [Cd 2(phtr)2(piv)4] Cd. . . Cd 3. 686(1) Å [Cd 2(ampy)2(piv)4] [Cd 2(lut)2(piv)4] Cd. . . Cd 3. 758(1) Å [Cd 2(cdp)2(piv)4] Cd. . . Cd 3. 105(1) Å 6
![► Cd(II)-Li pivalates [M(piv)2]n + ½ Lipiv + L M=Zn 2+ L=2, 4 -lut ► Cd(II)-Li pivalates [M(piv)2]n + ½ Lipiv + L M=Zn 2+ L=2, 4 -lut](https://present5.com/presentation/-104014181_425097612/image-7.jpg) ► Cd(II)-Li pivalates [M(piv)2]n + ½ Lipiv + L M=Zn 2+ L=2, 4 -lut Yield 77% [Zn 2 Li 2(lut)2(piv)6] Zn–O 1. 934(2)– 1. 955(2) Å Zn–N 2. 072(2) Å Li–O 1. 882(5)– 1. 981(5) Å Zn. . . Li 3. 180(4) Å Also M=Co 2+, Mn 2+, Fe 2+ … M=Cd 2+ Yield 57% [Cd 2 Li 2(bpy)2(piv)6]∙ 4 Me. CN Cd–O 2. 1989(16)– 2. 3956(15) Å Cd–N 2. 3542(17) – 2. 3818(17) Å Li–O 1. 904(4)– 1. 997(4)Å Cd. . . Li 3. 138(3) Å Yield 72% [Cd 2 Li 2(lut)2(piv)6] Cd–O 2. 1740(16) – 2. 4943(18) Å Cd–N 2. 2832(18) – 2. 2991(18) Å Li–O 1. 920(4) – 1. 985(4) Å Cd. . . Li 3. 062(4) – 3. 065(4) Å L=2, 2’-bpy L=2, 4 -lut 7
► Cd(II)-Li pivalates [M(piv)2]n + ½ Lipiv + L M=Zn 2+ L=2, 4 -lut Yield 77% [Zn 2 Li 2(lut)2(piv)6] Zn–O 1. 934(2)– 1. 955(2) Å Zn–N 2. 072(2) Å Li–O 1. 882(5)– 1. 981(5) Å Zn. . . Li 3. 180(4) Å Also M=Co 2+, Mn 2+, Fe 2+ … M=Cd 2+ Yield 57% [Cd 2 Li 2(bpy)2(piv)6]∙ 4 Me. CN Cd–O 2. 1989(16)– 2. 3956(15) Å Cd–N 2. 3542(17) – 2. 3818(17) Å Li–O 1. 904(4)– 1. 997(4)Å Cd. . . Li 3. 138(3) Å Yield 72% [Cd 2 Li 2(lut)2(piv)6] Cd–O 2. 1740(16) – 2. 4943(18) Å Cd–N 2. 2832(18) – 2. 2991(18) Å Li–O 1. 920(4) – 1. 985(4) Å Cd. . . Li 3. 062(4) – 3. 065(4) Å L=2, 2’-bpy L=2, 4 -lut 7
![► Cd(II)-alkaline-earth elements pivalates [M(piv)2]n + ½ Mg(NO 3)2∙ 6 H 2 O + ► Cd(II)-alkaline-earth elements pivalates [M(piv)2]n + ½ Mg(NO 3)2∙ 6 H 2 O +](https://present5.com/presentation/-104014181_425097612/image-8.jpg) ► Cd(II)-alkaline-earth elements pivalates [M(piv)2]n + ½ Mg(NO 3)2∙ 6 H 2 O + Kpiv + 2, 4 -lut M=Cd 2+ M=Co 2+ M=Cd 2+ Excess Kpiv Yield 45% [Cd 2 Mg(Lut)2(piv)6] Cd–O 2. 156(2)– 2. 218(2) Å Cd–N 2. 325(2) Å Mg–O 2. 057(2)– 2. 083(2) Å Cd. . . Mg 3. 4534(2) Å Replace Mg – Ca Yield 49% [Cd 2 Ca(lut)2(piv)6] Cd–O 2. 151(3)– 2. 239(3) Å [Ca. Cd 2 K 2(Me. CN)4(piv)8]n Cd–N 2. 326(3) Å Cd–O 2. 212(4)– 2. 313(4) Å Ca–O 2. 291(3)– 2. 326(3) Å Ca–O 2. 295(4)– 2. 337(4) Å Cd. . . Ca 3. 6281(4) Å Cd. . . Ca 3. 6824(5) Å Yield 64% [Co 2 Mg(lut)2(piv)6] Co–O 1. 928(3)– 1. 978(4) Å Co–N 2. 080(3) Å Mg–O 2. 040(3)– 2. 084(3) Å Co. . . Mg 3. 598(1) Å Replace Mg – Sr Yield 52% [Cd 2 Sr(Lut)2(piv)6] Cd–O 2. 148(6)– 2. 274(7) Å Cd–N 2. 323(6) Å Sr–O 2. 444(6)– 2. 465(6) Å Cd. . . Sr 3. 7525(6) Å 8
► Cd(II)-alkaline-earth elements pivalates [M(piv)2]n + ½ Mg(NO 3)2∙ 6 H 2 O + Kpiv + 2, 4 -lut M=Cd 2+ M=Co 2+ M=Cd 2+ Excess Kpiv Yield 45% [Cd 2 Mg(Lut)2(piv)6] Cd–O 2. 156(2)– 2. 218(2) Å Cd–N 2. 325(2) Å Mg–O 2. 057(2)– 2. 083(2) Å Cd. . . Mg 3. 4534(2) Å Replace Mg – Ca Yield 49% [Cd 2 Ca(lut)2(piv)6] Cd–O 2. 151(3)– 2. 239(3) Å [Ca. Cd 2 K 2(Me. CN)4(piv)8]n Cd–N 2. 326(3) Å Cd–O 2. 212(4)– 2. 313(4) Å Ca–O 2. 291(3)– 2. 326(3) Å Ca–O 2. 295(4)– 2. 337(4) Å Cd. . . Ca 3. 6281(4) Å Cd. . . Ca 3. 6824(5) Å Yield 64% [Co 2 Mg(lut)2(piv)6] Co–O 1. 928(3)– 1. 978(4) Å Co–N 2. 080(3) Å Mg–O 2. 040(3)– 2. 084(3) Å Co. . . Mg 3. 598(1) Å Replace Mg – Sr Yield 52% [Cd 2 Sr(Lut)2(piv)6] Cd–O 2. 148(6)– 2. 274(7) Å Cd–N 2. 323(6) Å Sr–O 2. 444(6)– 2. 465(6) Å Cd. . . Sr 3. 7525(6) Å 8
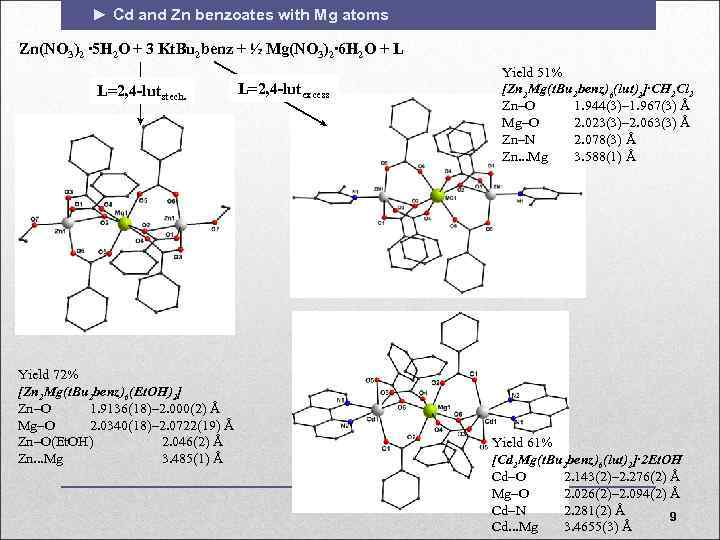 ► Cd and Zn benzoates with Mg atoms Zn(NO 3)2 ∙ 5 H 2 O + 3 Kt. Bu 2 benz + ½ Mg(NO 3)2∙ 6 H 2 O + L L=2, 4 -lutstech. Yield 72% [Zn 2 Mg(t. Bu 2 benz)6(Et. OH)2] Zn O 1. 9136(18)– 2. 000(2) Å Mg O 2. 0340(18)– 2. 0722(19) Å Zn O(Et. OH) 2. 046(2) Å Zn. . . Mg 3. 485(1) Å L=2, 4 -lutexcess Yield 51% [Zn 2 Mg(t. Bu 2 benz)6(lut)2]∙CH 2 Cl 2 Zn O 1. 944(3)– 1. 967(3) Å Mg O 2. 023(3)– 2. 063(3) Å Zn N 2. 078(3) Å Zn. . . Mg 3. 588(1) Å Yield 61% [Cd 2 Mg(t. Bu 2 benz)6(lut)2]∙ 2 Et. OH Cd O 2. 143(2)– 2. 276(2) Å Mg O 2. 026(2)– 2. 094(2) Å Cd N 2. 281(2) Å 9 Cd. . . Mg 3. 4655(3) Å
► Cd and Zn benzoates with Mg atoms Zn(NO 3)2 ∙ 5 H 2 O + 3 Kt. Bu 2 benz + ½ Mg(NO 3)2∙ 6 H 2 O + L L=2, 4 -lutstech. Yield 72% [Zn 2 Mg(t. Bu 2 benz)6(Et. OH)2] Zn O 1. 9136(18)– 2. 000(2) Å Mg O 2. 0340(18)– 2. 0722(19) Å Zn O(Et. OH) 2. 046(2) Å Zn. . . Mg 3. 485(1) Å L=2, 4 -lutexcess Yield 51% [Zn 2 Mg(t. Bu 2 benz)6(lut)2]∙CH 2 Cl 2 Zn O 1. 944(3)– 1. 967(3) Å Mg O 2. 023(3)– 2. 063(3) Å Zn N 2. 078(3) Å Zn. . . Mg 3. 588(1) Å Yield 61% [Cd 2 Mg(t. Bu 2 benz)6(lut)2]∙ 2 Et. OH Cd O 2. 143(2)– 2. 276(2) Å Mg O 2. 026(2)– 2. 094(2) Å Cd N 2. 281(2) Å 9 Cd. . . Mg 3. 4655(3) Å
![► Cd(II)-alkaline-earth elements pivalates [M(piv)2]n + ½ Mgpiv 2 + L M=Ni 2+ L=2, ► Cd(II)-alkaline-earth elements pivalates [M(piv)2]n + ½ Mgpiv 2 + L M=Ni 2+ L=2,](https://present5.com/presentation/-104014181_425097612/image-10.jpg) ► Cd(II)-alkaline-earth elements pivalates [M(piv)2]n + ½ Mgpiv 2 + L M=Ni 2+ L=2, 2’-bpy M=Cd 2+ L=phen Yield 77% [Cd 2(bpy)2(piv)4] Cd–O 2. 263(4)– 2. 515(4) Å Cd–N 2. 355(3)– 2. 368(3) Å Yield 64% [Ni 2 Mg(lut)2(piv)6] Ni–O 1. 987(2)– 2. 191(2) Å Ni–N 2. 045(2)– 2. 056(3) Å Mg–O 2. 071(2)– 2. 107(2) Å Ni. . . Mg 3. 442(1)– 3. 491(1) Å [1] A. A. Sidorov, M. E. Nikiforova, N. V. Zauzolkova, E. N. Zorina, M. A. Kiskin, V. V. Zyuzin, E. F. Zharikova, S. V. Savilov, Zh. V. Dobrokhotova, V. M. Novotortsev, I. L. Eremenko (2010) Izv. Vyssh. Uchebn. Zaved. , Khim. Tek. (Russ. ) (Bull. Colleg. , Chem. Technol. ), 53, 69 [Cd 2(phen)2(piv)4] (*) *Russ. Chem. Bull. , 2015, 12, in press 10
► Cd(II)-alkaline-earth elements pivalates [M(piv)2]n + ½ Mgpiv 2 + L M=Ni 2+ L=2, 2’-bpy M=Cd 2+ L=phen Yield 77% [Cd 2(bpy)2(piv)4] Cd–O 2. 263(4)– 2. 515(4) Å Cd–N 2. 355(3)– 2. 368(3) Å Yield 64% [Ni 2 Mg(lut)2(piv)6] Ni–O 1. 987(2)– 2. 191(2) Å Ni–N 2. 045(2)– 2. 056(3) Å Mg–O 2. 071(2)– 2. 107(2) Å Ni. . . Mg 3. 442(1)– 3. 491(1) Å [1] A. A. Sidorov, M. E. Nikiforova, N. V. Zauzolkova, E. N. Zorina, M. A. Kiskin, V. V. Zyuzin, E. F. Zharikova, S. V. Savilov, Zh. V. Dobrokhotova, V. M. Novotortsev, I. L. Eremenko (2010) Izv. Vyssh. Uchebn. Zaved. , Khim. Tek. (Russ. ) (Bull. Colleg. , Chem. Technol. ), 53, 69 [Cd 2(phen)2(piv)4] (*) *Russ. Chem. Bull. , 2015, 12, in press 10
![► Cd(II)-alkaline-earth elements pivalates [Cd(piv)2]n + ½ Mpiv 2 + 2, 4 -lut M=Mg ► Cd(II)-alkaline-earth elements pivalates [Cd(piv)2]n + ½ Mpiv 2 + 2, 4 -lut M=Mg](https://present5.com/presentation/-104014181_425097612/image-11.jpg) ► Cd(II)-alkaline-earth elements pivalates [Cd(piv)2]n + ½ Mpiv 2 + 2, 4 -lut M=Mg 2+ + 1, 10 -phen M=Ca 2+, Sr 2+ Yield 47% [Cd 2 Mg(phen)2(piv)6(H 2 O)] Cd–O 2. 103(11)– 2. 422(11) Å Cd–N 2. 319(12)– 2. 336(12) Å Mg–O 1. 987(12)– 2. 068(11) Å Mg–O(H 2 O)1. 965(9) Å Cd. . . Mg 4. 112(5) Å Yield 84% [Cd 2 Ca(phen)2(piv)6] Cd–O 2. 2150(19)– 2. 637(3) Å Cd–N 2. 354(2)– 2. 382(2) Å Ca–O 2. 286(2)– 2. 3534(18) Å Cd. . . Ca 3. 5500(5)– 3. 5527(5) Å * Russ. Chem. Bull. , 2015, 12, in press Yield 67% [Cd 2 Sr(phen)2(piv)6] Cd–O 2. 222(5)– 2. 553(5) Å Cd–N 2. 331(5)– 2. 381(5) Å Sr–O 2. 448(4)– 2. 519(4) Å Cd. . . Sr 3. 658(1) Å [1] A. Pramanik, F. R. Fronczek, R. Venkatraman, M. A. Hossain (2013) Acta Crystallogr. , Sect. E: Struct. Rep. Online, 69, m 643 11
► Cd(II)-alkaline-earth elements pivalates [Cd(piv)2]n + ½ Mpiv 2 + 2, 4 -lut M=Mg 2+ + 1, 10 -phen M=Ca 2+, Sr 2+ Yield 47% [Cd 2 Mg(phen)2(piv)6(H 2 O)] Cd–O 2. 103(11)– 2. 422(11) Å Cd–N 2. 319(12)– 2. 336(12) Å Mg–O 1. 987(12)– 2. 068(11) Å Mg–O(H 2 O)1. 965(9) Å Cd. . . Mg 4. 112(5) Å Yield 84% [Cd 2 Ca(phen)2(piv)6] Cd–O 2. 2150(19)– 2. 637(3) Å Cd–N 2. 354(2)– 2. 382(2) Å Ca–O 2. 286(2)– 2. 3534(18) Å Cd. . . Ca 3. 5500(5)– 3. 5527(5) Å * Russ. Chem. Bull. , 2015, 12, in press Yield 67% [Cd 2 Sr(phen)2(piv)6] Cd–O 2. 222(5)– 2. 553(5) Å Cd–N 2. 331(5)– 2. 381(5) Å Sr–O 2. 448(4)– 2. 519(4) Å Cd. . . Sr 3. 658(1) Å [1] A. Pramanik, F. R. Fronczek, R. Venkatraman, M. A. Hossain (2013) Acta Crystallogr. , Sect. E: Struct. Rep. Online, 69, m 643 11
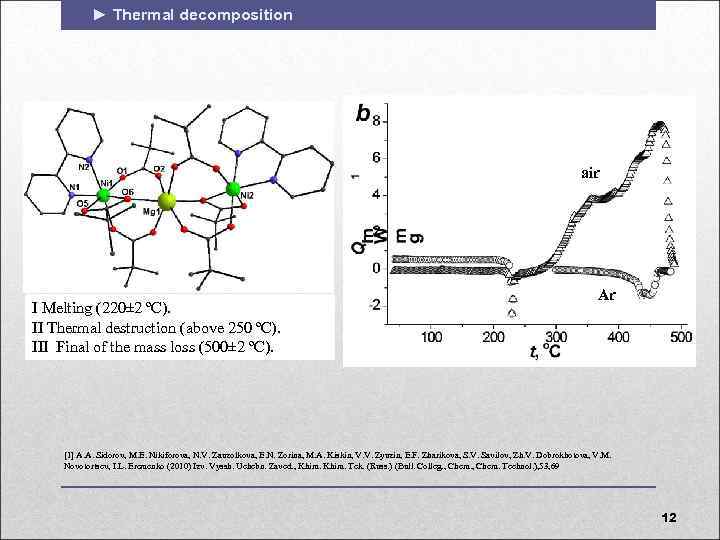 ► Thermal decomposition air I Melting (220± 2 ºС). II Thermal destruction (above 250 ºС). III Final of the mass loss (500± 2 ºС). Ar [1] A. A. Sidorov, M. E. Nikiforova, N. V. Zauzolkova, E. N. Zorina, M. A. Kiskin, V. V. Zyuzin, E. F. Zharikova, S. V. Savilov, Zh. V. Dobrokhotova, V. M. Novotortsev, I. L. Eremenko (2010) Izv. Vyssh. Uchebn. Zaved. , Khim. Tek. (Russ. ) (Bull. Colleg. , Chem. Technol. ), 53, 69 12
► Thermal decomposition air I Melting (220± 2 ºС). II Thermal destruction (above 250 ºС). III Final of the mass loss (500± 2 ºС). Ar [1] A. A. Sidorov, M. E. Nikiforova, N. V. Zauzolkova, E. N. Zorina, M. A. Kiskin, V. V. Zyuzin, E. F. Zharikova, S. V. Savilov, Zh. V. Dobrokhotova, V. M. Novotortsev, I. L. Eremenko (2010) Izv. Vyssh. Uchebn. Zaved. , Khim. Tek. (Russ. ) (Bull. Colleg. , Chem. Technol. ), 53, 69 12
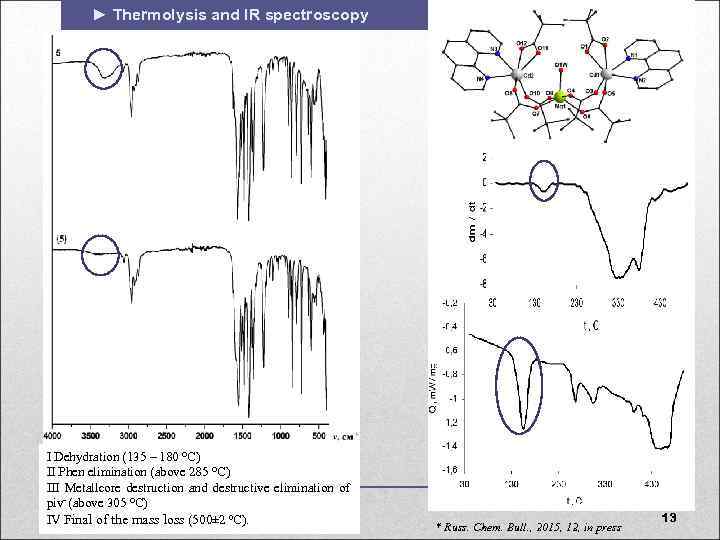 ► Thermolysis and IR spectroscopy I Dehydration (135 – 180 С) II Phen elimination (above 285 С) III Metallcore destruction and destructive elimination of piv- (above 305 С) IV Final of the mass loss (500± 2 ºС). * Russ. Chem. Bull. , 2015, 12, in press 13
► Thermolysis and IR spectroscopy I Dehydration (135 – 180 С) II Phen elimination (above 285 С) III Metallcore destruction and destructive elimination of piv- (above 305 С) IV Final of the mass loss (500± 2 ºС). * Russ. Chem. Bull. , 2015, 12, in press 13
![► Synthesis of Cd–Eu pivalates x [Cd(piv)2]n + y Eu(NO 3)3 + 1, 10 ► Synthesis of Cd–Eu pivalates x [Cd(piv)2]n + y Eu(NO 3)3 + 1, 10](https://present5.com/presentation/-104014181_425097612/image-14.jpg) ► Synthesis of Cd–Eu pivalates x [Cd(piv)2]n + y Eu(NO 3)3 + 1, 10 -phen x : y = 1 : 2/3 x: y=4: 1 x: y=1: 1 cis-[Cd(NO 3)2(phen)2] [Cd 2(piv)2(NO 3)2(phen)2] trans-[Cd(NO 3)2(phen)2] [Cd(piv)2]n + L + 1/6 Eu 2 piv 6(Hpiv)7 L=2, 4 -lut + 1, 10 -phen L=2, 2’-bpy [Eu 2(phen)2(piv)6] Yield 32% [Eu. Cd 2(piv)7(H 2 O)2]∙Me. CN Cd–O 2. 214(3)– 2. 576(3) Å Cd–O(H 2 O) 2. 268(4)– 2. 308(4) Å Eu–O 2. 298(3)– 2. 552(3) Å Cd. . . Eu 3. 6614(4)– 3. 6749(4) Å Yield 17% [Eu 2(bpy)2(piv)6] Eu–N 2. 620(3)– 2. 654(3) Å Eu–O 2. 354(2)– 2. 741(2) Å 14
► Synthesis of Cd–Eu pivalates x [Cd(piv)2]n + y Eu(NO 3)3 + 1, 10 -phen x : y = 1 : 2/3 x: y=4: 1 x: y=1: 1 cis-[Cd(NO 3)2(phen)2] [Cd 2(piv)2(NO 3)2(phen)2] trans-[Cd(NO 3)2(phen)2] [Cd(piv)2]n + L + 1/6 Eu 2 piv 6(Hpiv)7 L=2, 4 -lut + 1, 10 -phen L=2, 2’-bpy [Eu 2(phen)2(piv)6] Yield 32% [Eu. Cd 2(piv)7(H 2 O)2]∙Me. CN Cd–O 2. 214(3)– 2. 576(3) Å Cd–O(H 2 O) 2. 268(4)– 2. 308(4) Å Eu–O 2. 298(3)– 2. 552(3) Å Cd. . . Eu 3. 6614(4)– 3. 6749(4) Å Yield 17% [Eu 2(bpy)2(piv)6] Eu–N 2. 620(3)– 2. 654(3) Å Eu–O 2. 354(2)– 2. 741(2) Å 14
![► Synthesis of Cd–Eu pivalates [M(piv)2]n + (NBu 4)2 chda + L + 1/6 ► Synthesis of Cd–Eu pivalates [M(piv)2]n + (NBu 4)2 chda + L + 1/6](https://present5.com/presentation/-104014181_425097612/image-15.jpg) ► Synthesis of Cd–Eu pivalates [M(piv)2]n + (NBu 4)2 chda + L + 1/6 Eu 2 piv 6(Hpiv)7 M=Cd 2+ L=2, 2’-bpy Yield 8% [Eu 2 Cd 2(chda)2(piv)6(bpy)2] Cd–O 2. 207(3)– 2. 585(3) Å Cd–N 2. 316(3)– 2. 342(3) Å Eu–O 2. 358(3)– 2. 485(3) Å Cd. . . Eu 3. 7545(6) Å M=Zn 2+ L=nbpy Yield 69% [Eu 2 Zn 2(chda)2(piv)6(nbpy)2] Zn–O 2. 032 (2)– 2. 126 (2); 2. 412 (2) Å Zn–N 2. 113 (3)– 2. 145 (3) Å Eu–O 2. 352 (2)– 2. 508 (2) Å Zn. . . Eu 3. 7926 (4) Å 15
► Synthesis of Cd–Eu pivalates [M(piv)2]n + (NBu 4)2 chda + L + 1/6 Eu 2 piv 6(Hpiv)7 M=Cd 2+ L=2, 2’-bpy Yield 8% [Eu 2 Cd 2(chda)2(piv)6(bpy)2] Cd–O 2. 207(3)– 2. 585(3) Å Cd–N 2. 316(3)– 2. 342(3) Å Eu–O 2. 358(3)– 2. 485(3) Å Cd. . . Eu 3. 7545(6) Å M=Zn 2+ L=nbpy Yield 69% [Eu 2 Zn 2(chda)2(piv)6(nbpy)2] Zn–O 2. 032 (2)– 2. 126 (2); 2. 412 (2) Å Zn–N 2. 113 (3)– 2. 145 (3) Å Eu–O 2. 352 (2)– 2. 508 (2) Å Zn. . . Eu 3. 7926 (4) Å 15
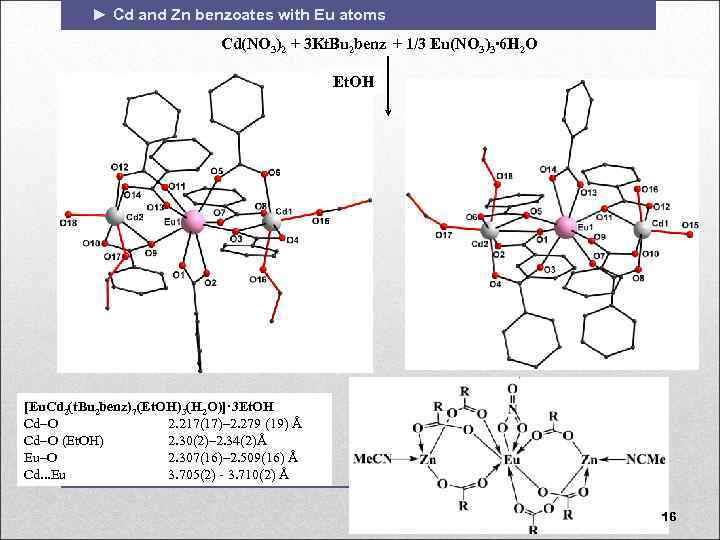 ► Cd and Zn benzoates with Eu atoms Cd(NO 3)2 + 3 Kt. Bu 2 benz + 1/3 Eu(NO 3)3∙ 6 H 2 O Et. OH [Eu. Cd 2(t. Bu 2 benz)7(Et. OH)3(H 2 O)]· 3 Et. OH Cd O 2. 217(17)– 2. 279 (19) Å Cd O (Et. OH) 2. 30(2)– 2. 34(2)Å Eu–O 2. 307(16)– 2. 509(16) Å Cd. . . Eu 3. 705(2) - 3. 710(2) Å 16
► Cd and Zn benzoates with Eu atoms Cd(NO 3)2 + 3 Kt. Bu 2 benz + 1/3 Eu(NO 3)3∙ 6 H 2 O Et. OH [Eu. Cd 2(t. Bu 2 benz)7(Et. OH)3(H 2 O)]· 3 Et. OH Cd O 2. 217(17)– 2. 279 (19) Å Cd O (Et. OH) 2. 30(2)– 2. 34(2)Å Eu–O 2. 307(16)– 2. 509(16) Å Cd. . . Eu 3. 705(2) - 3. 710(2) Å 16
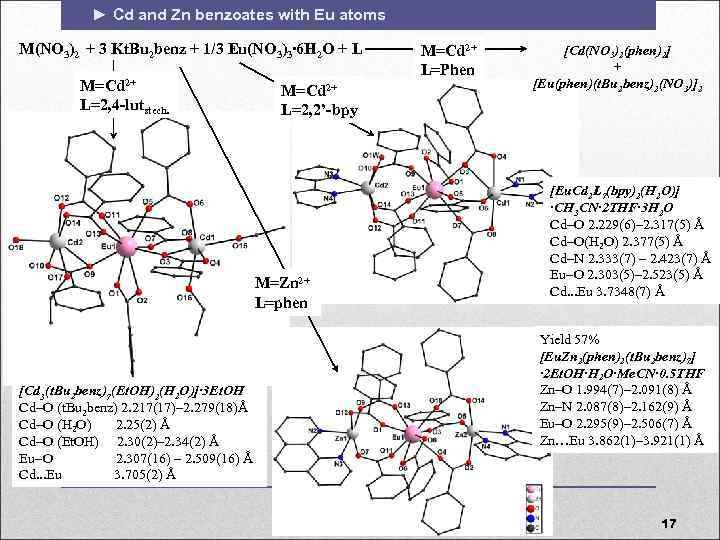 ► Cd and Zn benzoates with Eu atoms M(NO 3)2 + 3 Kt. Bu 2 benz + 1/3 Eu(NO 3)3∙ 6 H 2 O + L M=Cd 2+ L=2, 4 -lutstech. M=Cd 2+ L=2, 2’-bpy M=Zn 2+ L=phen [Cd 3(t. Bu 2 benz)7(Et. OH)2(H 2 O)]∙ 3 Et. OH Cd O (t. Bu 2 benz) 2. 217(17)– 2. 279(18)Å Cd O (H 2 O) 2. 25(2) Å Cd O (Et. OH) 2. 30(2)– 2. 34(2) Å Eu O 2. 307(16) – 2. 509(16) Å Cd. . . Eu 3. 705(2) Å M=Cd 2+ L=Phen [Cd(NO 3)2(phen)2] + [Eu(phen)(t. Bu 2 benz)2(NO 3)]2 [Eu. Cd 2 L 7(bpy)2(H 2 O)] ∙CH 3 CN∙ 2 THF∙ 3 H 2 O Cd O 2. 229(6)– 2. 317(5) Å Cd O(H 2 O) 2. 377(5) Å Cd N 2. 333(7) – 2. 423(7) Å Eu O 2. 303(5)– 2. 523(5) Å Cd. . . Eu 3. 7348(7) Å Yield 57% [Eu. Zn 2(phen)2(t. Bu 2 benz)7] ∙ 2 Et. OH∙H 2 O∙Me. CN∙ 0. 5 THF Zn–O 1. 994(7)– 2. 091(8) Å Zn–N 2. 087(8)– 2. 162(9) Å Eu–O 2. 295(9)– 2. 506(7) Å Zn…Eu 3. 862(1)– 3. 921(1) Å 17
► Cd and Zn benzoates with Eu atoms M(NO 3)2 + 3 Kt. Bu 2 benz + 1/3 Eu(NO 3)3∙ 6 H 2 O + L M=Cd 2+ L=2, 4 -lutstech. M=Cd 2+ L=2, 2’-bpy M=Zn 2+ L=phen [Cd 3(t. Bu 2 benz)7(Et. OH)2(H 2 O)]∙ 3 Et. OH Cd O (t. Bu 2 benz) 2. 217(17)– 2. 279(18)Å Cd O (H 2 O) 2. 25(2) Å Cd O (Et. OH) 2. 30(2)– 2. 34(2) Å Eu O 2. 307(16) – 2. 509(16) Å Cd. . . Eu 3. 705(2) Å M=Cd 2+ L=Phen [Cd(NO 3)2(phen)2] + [Eu(phen)(t. Bu 2 benz)2(NO 3)]2 [Eu. Cd 2 L 7(bpy)2(H 2 O)] ∙CH 3 CN∙ 2 THF∙ 3 H 2 O Cd O 2. 229(6)– 2. 317(5) Å Cd O(H 2 O) 2. 377(5) Å Cd N 2. 333(7) – 2. 423(7) Å Eu O 2. 303(5)– 2. 523(5) Å Cd. . . Eu 3. 7348(7) Å Yield 57% [Eu. Zn 2(phen)2(t. Bu 2 benz)7] ∙ 2 Et. OH∙H 2 O∙Me. CN∙ 0. 5 THF Zn–O 1. 994(7)– 2. 091(8) Å Zn–N 2. 087(8)– 2. 162(9) Å Eu–O 2. 295(9)– 2. 506(7) Å Zn…Eu 3. 862(1)– 3. 921(1) Å 17
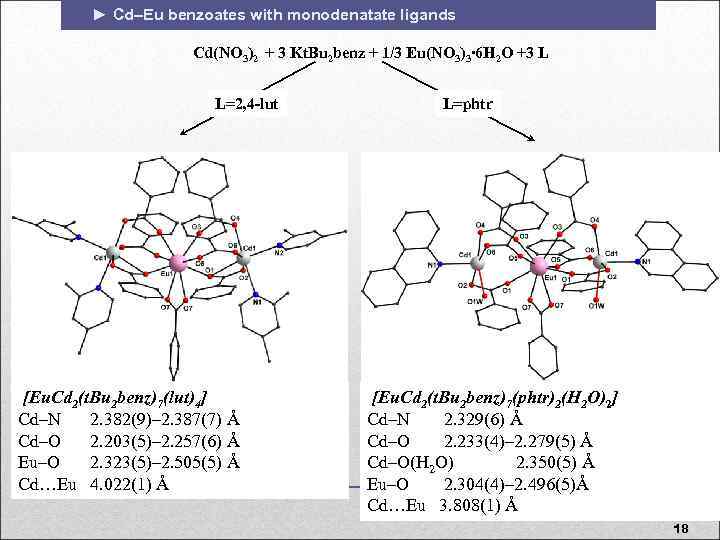 ► Cd–Eu benzoates with monodenatate ligands Cd(NO 3)2 + 3 Kt. Bu 2 benz + 1/3 Eu(NO 3)3∙ 6 H 2 O +3 L L=2, 4 -lut [Eu. Cd 2(t. Bu 2 benz)7(lut)4] Cd–N 2. 382(9)– 2. 387(7) Å Cd–O 2. 203(5)– 2. 257(6) Å Eu–O 2. 323(5)– 2. 505(5) Å Cd…Eu 4. 022(1) Å L=phtr [Eu. Cd 2(t. Bu 2 benz)7(phtr)2(H 2 O)2] Cd–N 2. 329(6) Å Cd–O 2. 233(4)– 2. 279(5) Å Cd–O(H 2 O) 2. 350(5) Å Eu–O 2. 304(4)– 2. 496(5)Å Cd…Eu 3. 808(1) Å 18 18
► Cd–Eu benzoates with monodenatate ligands Cd(NO 3)2 + 3 Kt. Bu 2 benz + 1/3 Eu(NO 3)3∙ 6 H 2 O +3 L L=2, 4 -lut [Eu. Cd 2(t. Bu 2 benz)7(lut)4] Cd–N 2. 382(9)– 2. 387(7) Å Cd–O 2. 203(5)– 2. 257(6) Å Eu–O 2. 323(5)– 2. 505(5) Å Cd…Eu 4. 022(1) Å L=phtr [Eu. Cd 2(t. Bu 2 benz)7(phtr)2(H 2 O)2] Cd–N 2. 329(6) Å Cd–O 2. 233(4)– 2. 279(5) Å Cd–O(H 2 O) 2. 350(5) Å Eu–O 2. 304(4)– 2. 496(5)Å Cd…Eu 3. 808(1) Å 18 18
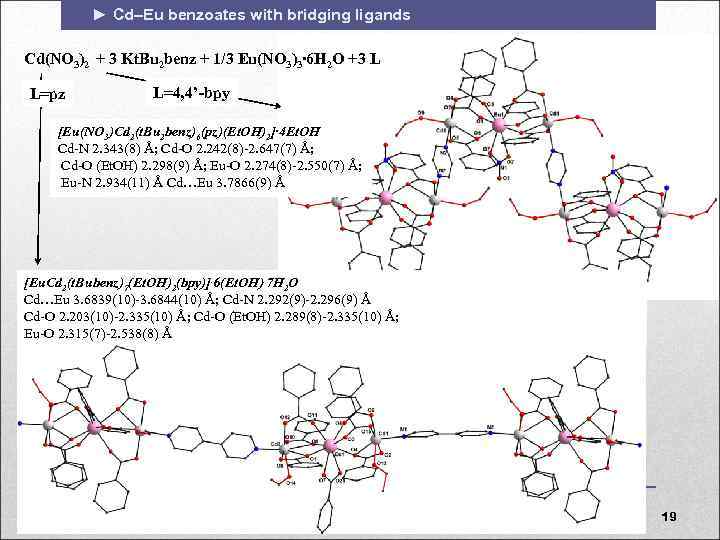 ► Cd–Eu benzoates with bridging ligands Cd(NO 3)2 + 3 Kt. Bu 2 benz + 1/3 Eu(NO 3)3∙ 6 H 2 O +3 L L=pz L=4, 4’-bpy [Eu(NO 3)Cd 2(t. Bu 2 benz)6(pz)(Et. OH)2]∙ 4 Et. OH Cd-N 2. 343(8) Å; Cd-O 2. 242(8)-2. 647(7) Å; Cd-O (Et. OH) 2. 298(9) Å; Eu-O 2. 274(8)-2. 550(7) Å; Eu-N 2. 934(11) Å Cd…Eu 3. 7866(9) Å [Eu. Cd 2(t. Bubenz)7(Et. OH)2(bpy)]. 6(Et. OH). 7 H 2 O Cd…Eu 3. 6839(10)-3. 6844(10) Å; Cd-N 2. 292(9)-2. 296(9) Å Cd-O 2. 203(10)-2. 335(10) Å; Cd-O (Et. OH) 2. 289(8)-2. 335(10) Å; Eu-O 2. 315(7)-2. 538(8) Å 19
► Cd–Eu benzoates with bridging ligands Cd(NO 3)2 + 3 Kt. Bu 2 benz + 1/3 Eu(NO 3)3∙ 6 H 2 O +3 L L=pz L=4, 4’-bpy [Eu(NO 3)Cd 2(t. Bu 2 benz)6(pz)(Et. OH)2]∙ 4 Et. OH Cd-N 2. 343(8) Å; Cd-O 2. 242(8)-2. 647(7) Å; Cd-O (Et. OH) 2. 298(9) Å; Eu-O 2. 274(8)-2. 550(7) Å; Eu-N 2. 934(11) Å Cd…Eu 3. 7866(9) Å [Eu. Cd 2(t. Bubenz)7(Et. OH)2(bpy)]. 6(Et. OH). 7 H 2 O Cd…Eu 3. 6839(10)-3. 6844(10) Å; Cd-N 2. 292(9)-2. 296(9) Å Cd-O 2. 203(10)-2. 335(10) Å; Cd-O (Et. OH) 2. 289(8)-2. 335(10) Å; Eu-O 2. 315(7)-2. 538(8) Å 19
 ► Conclusions ü Комплексы Cd оказались перспективны для синтеза новых типов комплексов благодаря своему высокому координационному числу: 1) за счет большего количества донорноакцепторных связей с карбоксилат-анионами, 2) за счет наличия дополнительных координированных молекул растворителя, которые могут быть замещены на Nдонорные лиганды. ü Различия в устойчивости соединений позволяют в случае кадмия кристаллизовать комплексы Cd-ЩЗЭ, изучение химии которых для 3 d-металлов было затруднено из-за синтетических трудностей. ü Большая устойчивость гомометаллических триметилацетатов Cd не дает возможности выделить молекулярные пивалаты Cd-Ln, в то время как устойчивые бензоаты могут использоваться в качестве строительных блоков для дальнейшей сборки полимеров 20
► Conclusions ü Комплексы Cd оказались перспективны для синтеза новых типов комплексов благодаря своему высокому координационному числу: 1) за счет большего количества донорноакцепторных связей с карбоксилат-анионами, 2) за счет наличия дополнительных координированных молекул растворителя, которые могут быть замещены на Nдонорные лиганды. ü Различия в устойчивости соединений позволяют в случае кадмия кристаллизовать комплексы Cd-ЩЗЭ, изучение химии которых для 3 d-металлов было затруднено из-за синтетических трудностей. ü Большая устойчивость гомометаллических триметилацетатов Cd не дает возможности выделить молекулярные пивалаты Cd-Ln, в то время как устойчивые бензоаты могут использоваться в качестве строительных блоков для дальнейшей сборки полимеров 20
 ► Acknowledgement Professor A. A. Sidorov Member of the Academy of Sciences I. L. Eremenko Ph. D. G. G. Aleksandrov Dr. M. A. Kiskin Dr. Zh. V. Dobrokhotova Student M. A. Shmelev This study was financially supported Russian Foundation of Basic Research, The Council on Grants of the President of the Russian Federation, the Russian Academy of Science 21
► Acknowledgement Professor A. A. Sidorov Member of the Academy of Sciences I. L. Eremenko Ph. D. G. G. Aleksandrov Dr. M. A. Kiskin Dr. Zh. V. Dobrokhotova Student M. A. Shmelev This study was financially supported Russian Foundation of Basic Research, The Council on Grants of the President of the Russian Federation, the Russian Academy of Science 21


