2d23bcc3428fd7ced036f2e7b8da3433.ppt
- Количество слайдов: 49
 KS 4: Useful Materials From Metal Ores © Boardworks Ltd 2001
KS 4: Useful Materials From Metal Ores © Boardworks Ltd 2001
 Getting Metals From Ores Most metals do not occur naturally (native). They have to be extracted from metal containing rocks (ores. ) 1. First substances other than the metal compound are removed (concentration. ) 2. Next the metal itself is extracted from its compound (reduction). © Boardworks Ltd 2001
Getting Metals From Ores Most metals do not occur naturally (native). They have to be extracted from metal containing rocks (ores. ) 1. First substances other than the metal compound are removed (concentration. ) 2. Next the metal itself is extracted from its compound (reduction). © Boardworks Ltd 2001
 Extraction of Metals and Energy Changes • • • The more vigorously an element forms compounds the harder it will be to get back that element from its compounds. Eg. Magnesium gives out lots of heat when it combines with oxygen. This means we will have to put lots of energy back to extract magnesium from magnesium oxide. It will be hard to extract. © Boardworks Ltd 2001
Extraction of Metals and Energy Changes • • • The more vigorously an element forms compounds the harder it will be to get back that element from its compounds. Eg. Magnesium gives out lots of heat when it combines with oxygen. This means we will have to put lots of energy back to extract magnesium from magnesium oxide. It will be hard to extract. © Boardworks Ltd 2001
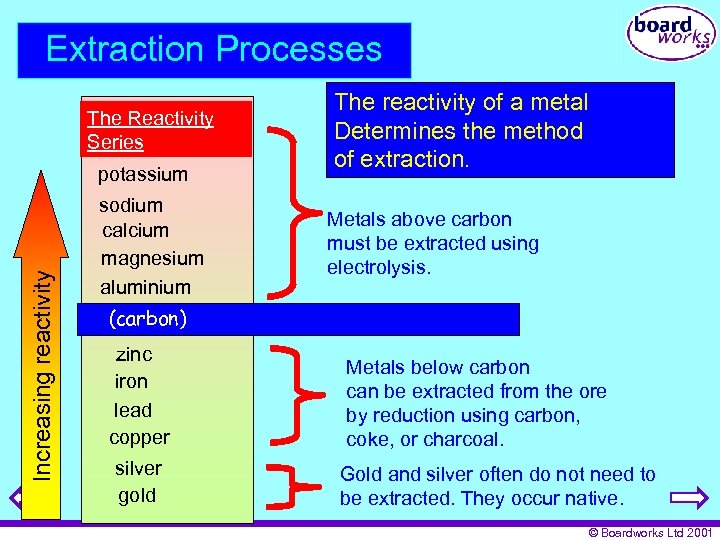 Extraction Processes The Reactivity Series Increasing reactivity potassium sodium calcium magnesium aluminium The reactivity of a metal Determines the method of extraction. Metals above carbon must be extracted using electrolysis. (carbon) zinc iron lead copper silver gold Metals below carbon can be extracted from the ore by reduction using carbon, coke, or charcoal. Gold and silver often do not need to be extracted. They occur native. © Boardworks Ltd 2001
Extraction Processes The Reactivity Series Increasing reactivity potassium sodium calcium magnesium aluminium The reactivity of a metal Determines the method of extraction. Metals above carbon must be extracted using electrolysis. (carbon) zinc iron lead copper silver gold Metals below carbon can be extracted from the ore by reduction using carbon, coke, or charcoal. Gold and silver often do not need to be extracted. They occur native. © Boardworks Ltd 2001
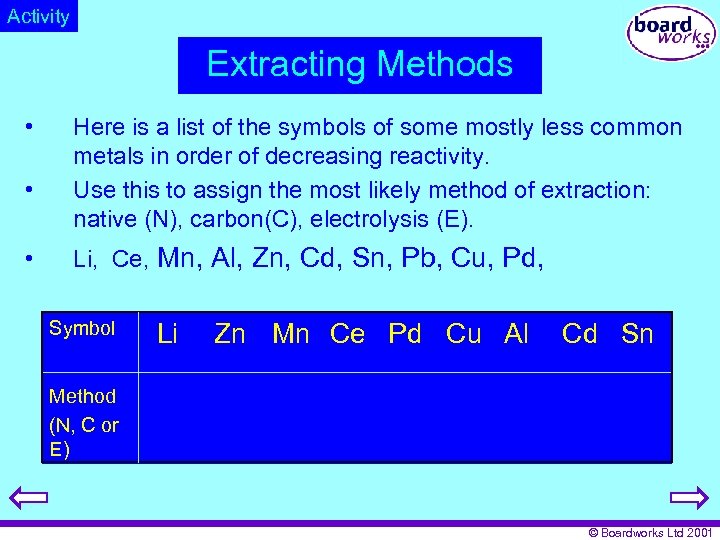 Activity Extracting Methods • • • Here is a list of the symbols of some mostly less common metals in order of decreasing reactivity. Use this to assign the most likely method of extraction: native (N), carbon(C), electrolysis (E). Li, Ce, Mn, Al, Zn, Cd, Sn, Pb, Cu, Pd, Symbol Li Zn Mn Ce Pd Cu Al Cd Sn Method (N, C or E) © Boardworks Ltd 2001
Activity Extracting Methods • • • Here is a list of the symbols of some mostly less common metals in order of decreasing reactivity. Use this to assign the most likely method of extraction: native (N), carbon(C), electrolysis (E). Li, Ce, Mn, Al, Zn, Cd, Sn, Pb, Cu, Pd, Symbol Li Zn Mn Ce Pd Cu Al Cd Sn Method (N, C or E) © Boardworks Ltd 2001
 Extracting Gold • Because gold occurs native its extraction is a low-tech affair that simply involves finding it! © Boardworks Ltd 2001
Extracting Gold • Because gold occurs native its extraction is a low-tech affair that simply involves finding it! © Boardworks Ltd 2001
 Iron • • Iron is a moderately reactive metal. Iron ore is plentiful and relatively easily reduced to iron metal by heating with coal (carbon). It is therefore cheap. It is strong and malleable (non-brittle). Iron is the most commonly used metal. © Boardworks Ltd 2001
Iron • • Iron is a moderately reactive metal. Iron ore is plentiful and relatively easily reduced to iron metal by heating with coal (carbon). It is therefore cheap. It is strong and malleable (non-brittle). Iron is the most commonly used metal. © Boardworks Ltd 2001
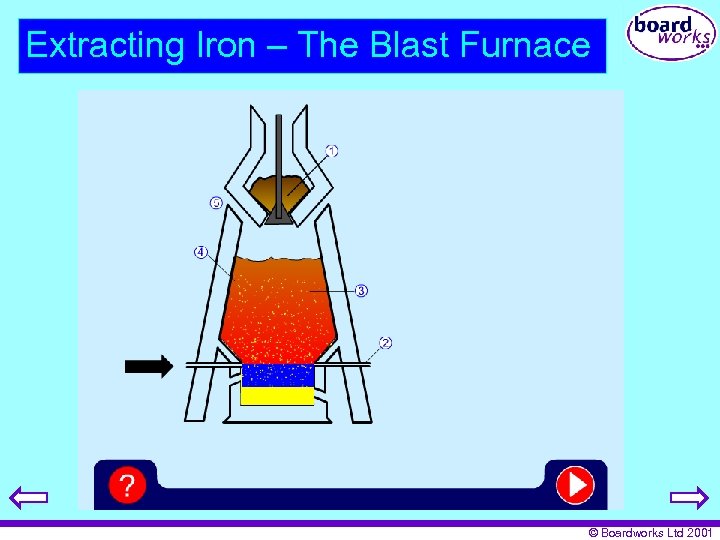 Extracting Iron – The Blast Furnace © Boardworks Ltd 2001
Extracting Iron – The Blast Furnace © Boardworks Ltd 2001
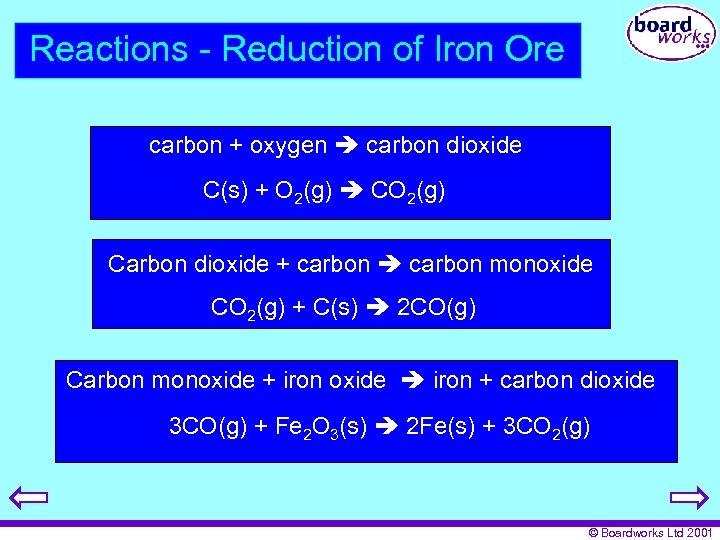 Reactions - Reduction of Iron Ore carbon + oxygen carbon dioxide C(s) + O 2(g) CO 2(g) Carbon dioxide + carbon monoxide CO 2(g) + C(s) 2 CO(g) Carbon monoxide + iron oxide iron + carbon dioxide 3 CO(g) + Fe 2 O 3(s) 2 Fe(s) + 3 CO 2(g) © Boardworks Ltd 2001
Reactions - Reduction of Iron Ore carbon + oxygen carbon dioxide C(s) + O 2(g) CO 2(g) Carbon dioxide + carbon monoxide CO 2(g) + C(s) 2 CO(g) Carbon monoxide + iron oxide iron + carbon dioxide 3 CO(g) + Fe 2 O 3(s) 2 Fe(s) + 3 CO 2(g) © Boardworks Ltd 2001
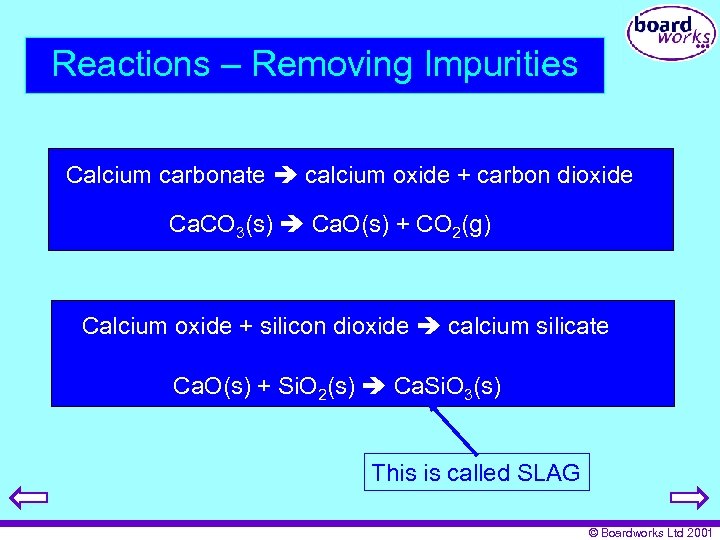 Reactions – Removing Impurities Calcium carbonate calcium oxide + carbon dioxide Ca. CO 3(s) Ca. O(s) + CO 2(g) Calcium oxide + silicon dioxide calcium silicate Ca. O(s) + Si. O 2(s) Ca. Si. O 3(s) This is called SLAG © Boardworks Ltd 2001
Reactions – Removing Impurities Calcium carbonate calcium oxide + carbon dioxide Ca. CO 3(s) Ca. O(s) + CO 2(g) Calcium oxide + silicon dioxide calcium silicate Ca. O(s) + Si. O 2(s) Ca. Si. O 3(s) This is called SLAG © Boardworks Ltd 2001
 Activity Extraction of Iron © Boardworks Ltd 2001
Activity Extraction of Iron © Boardworks Ltd 2001
 Copper • Copper is a metal of low reactivity. • It occasionally occurs native but more often occurs as copper compounds. • Heating copper compounds with carbon gives copper but this is not pure enough to use for electrical work. © Boardworks Ltd 2001
Copper • Copper is a metal of low reactivity. • It occasionally occurs native but more often occurs as copper compounds. • Heating copper compounds with carbon gives copper but this is not pure enough to use for electrical work. © Boardworks Ltd 2001
 Electrolytic Purification • The conductivity of copper is drastically reduced by tiny amounts of impurities. • Because of this most copper metal is further purified by electrolysis. • In this process impure anodes dissolve. • This dissolved copper is plated onto a cathode leaving behind impurities. © Boardworks Ltd 2001
Electrolytic Purification • The conductivity of copper is drastically reduced by tiny amounts of impurities. • Because of this most copper metal is further purified by electrolysis. • In this process impure anodes dissolve. • This dissolved copper is plated onto a cathode leaving behind impurities. © Boardworks Ltd 2001
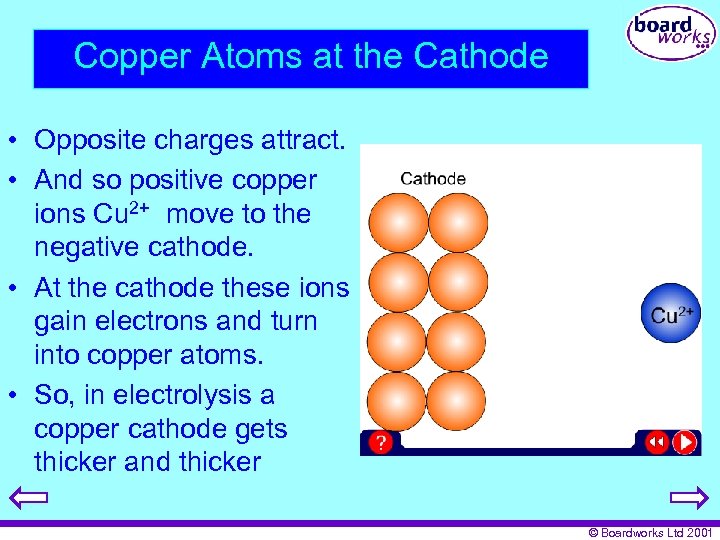 Copper Atoms at the Cathode • Opposite charges attract. • And so positive copper ions Cu 2+ move to the negative cathode. • At the cathode these ions gain electrons and turn into copper atoms. • So, in electrolysis a copper cathode gets thicker and thicker © Boardworks Ltd 2001
Copper Atoms at the Cathode • Opposite charges attract. • And so positive copper ions Cu 2+ move to the negative cathode. • At the cathode these ions gain electrons and turn into copper atoms. • So, in electrolysis a copper cathode gets thicker and thicker © Boardworks Ltd 2001
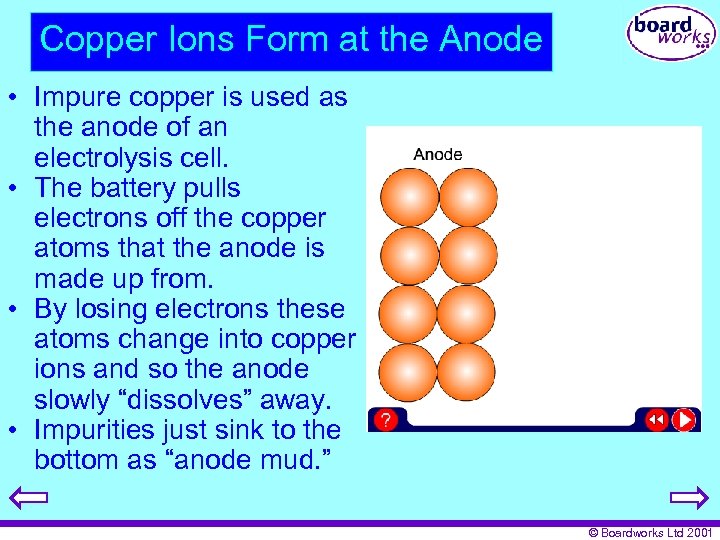 Copper Ions Form at the Anode • Impure copper is used as the anode of an electrolysis cell. • The battery pulls electrons off the copper atoms that the anode is made up from. • By losing electrons these atoms change into copper ions and so the anode slowly “dissolves” away. • Impurities just sink to the bottom as “anode mud. ” © Boardworks Ltd 2001
Copper Ions Form at the Anode • Impure copper is used as the anode of an electrolysis cell. • The battery pulls electrons off the copper atoms that the anode is made up from. • By losing electrons these atoms change into copper ions and so the anode slowly “dissolves” away. • Impurities just sink to the bottom as “anode mud. ” © Boardworks Ltd 2001
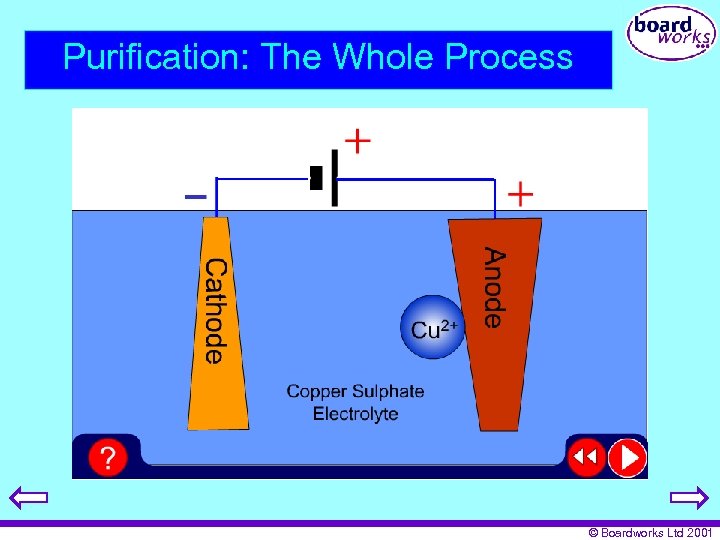 Purification: The Whole Process © Boardworks Ltd 2001
Purification: The Whole Process © Boardworks Ltd 2001
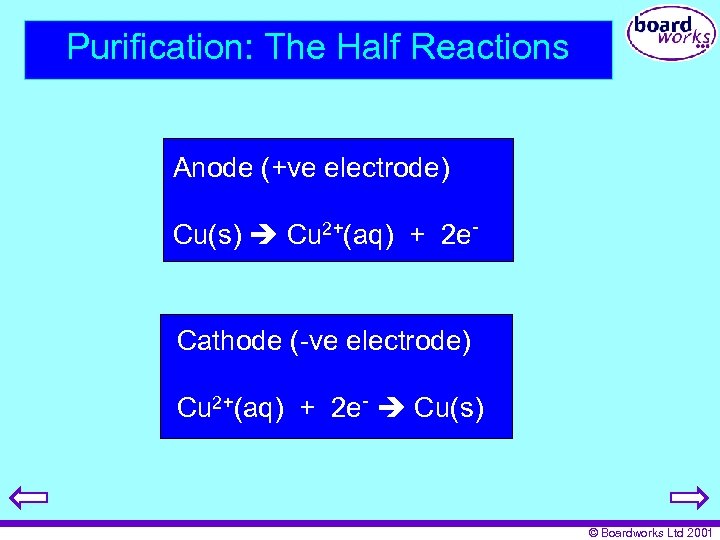 Purification: The Half Reactions Anode (+ve electrode) Cu(s) Cu 2+(aq) + 2 e- Cathode (-ve electrode) Cu 2+(aq) + 2 e- Cu(s) © Boardworks Ltd 2001
Purification: The Half Reactions Anode (+ve electrode) Cu(s) Cu 2+(aq) + 2 e- Cathode (-ve electrode) Cu 2+(aq) + 2 e- Cu(s) © Boardworks Ltd 2001
 Activity Unscramble the words to end the sentences • Copper is purified to improve its • Copper is purified by NOT CIVIC DUTY conductivity CELERY IS LOST electrolysis • Pure copper forms at the • Impurities form called • The anode will slowly DO TEACH cathode A ODD MENU anode mud dissolve DIVE LOSS • At the cathode copper ions gain CORN STEEL electrons © Boardworks Ltd 2001
Activity Unscramble the words to end the sentences • Copper is purified to improve its • Copper is purified by NOT CIVIC DUTY conductivity CELERY IS LOST electrolysis • Pure copper forms at the • Impurities form called • The anode will slowly DO TEACH cathode A ODD MENU anode mud dissolve DIVE LOSS • At the cathode copper ions gain CORN STEEL electrons © Boardworks Ltd 2001
 Activity Extracting Platinum • Platinum is a rare and expensive metal used in jewellery and also for plating the fuel nozzles in jet engines. It was first discovered by Europeans in 1735 but in South America the primitive pre. Columbian Indians had been using it for centuries. Approximately where would you place platinum in the activity series? In what form do you think platinum occurs? © Boardworks Ltd 2001
Activity Extracting Platinum • Platinum is a rare and expensive metal used in jewellery and also for plating the fuel nozzles in jet engines. It was first discovered by Europeans in 1735 but in South America the primitive pre. Columbian Indians had been using it for centuries. Approximately where would you place platinum in the activity series? In what form do you think platinum occurs? © Boardworks Ltd 2001
 Activity Purifying copper and electricity • • • Copper is purified using electrolysis. Plan an experiment to investigate factors that might affect the rate of copper production. Include: – Any factors that might affect rate. – The apparatus you would need. – A statement of how you would control variable in an investigation. – The number and range of readings. – The safety issues you would take into account. © Boardworks Ltd 2001
Activity Purifying copper and electricity • • • Copper is purified using electrolysis. Plan an experiment to investigate factors that might affect the rate of copper production. Include: – Any factors that might affect rate. – The apparatus you would need. – A statement of how you would control variable in an investigation. – The number and range of readings. – The safety issues you would take into account. © Boardworks Ltd 2001
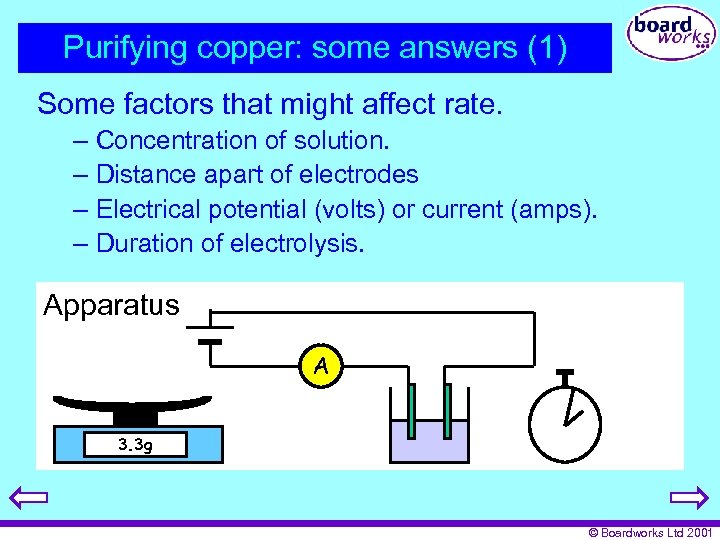 Purifying copper: some answers (1) Some factors that might affect rate. – Concentration of solution. – Distance apart of electrodes – Electrical potential (volts) or current (amps). – Duration of electrolysis. Apparatus A 3. 3 g © Boardworks Ltd 2001
Purifying copper: some answers (1) Some factors that might affect rate. – Concentration of solution. – Distance apart of electrodes – Electrical potential (volts) or current (amps). – Duration of electrolysis. Apparatus A 3. 3 g © Boardworks Ltd 2001
 Purifying copper: some answers (2) Control of variables. – Basically only change one variable at a time! Number and range of readings – Minimum of 8 -10 different values – Repeat readings at least once – Attempt a range providing 10 -fold change. Safety Issues – Check electrical, toxicity, corrosive etc. – Take appropriate measures © Boardworks Ltd 2001
Purifying copper: some answers (2) Control of variables. – Basically only change one variable at a time! Number and range of readings – Minimum of 8 -10 different values – Repeat readings at least once – Attempt a range providing 10 -fold change. Safety Issues – Check electrical, toxicity, corrosive etc. – Take appropriate measures © Boardworks Ltd 2001
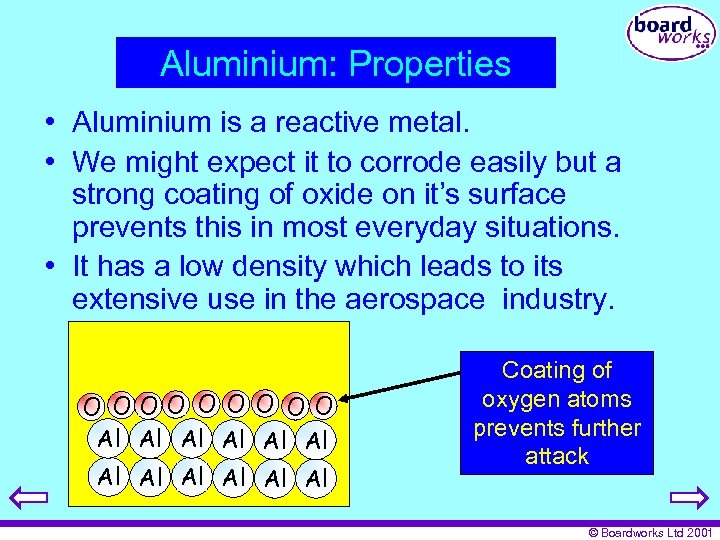 Aluminium: Properties • Aluminium is a reactive metal. • We might expect it to corrode easily but a strong coating of oxide on it’s surface prevents this in most everyday situations. • It has a low density which leads to its extensive use in the aerospace industry. O OOO O OO Al Al Al Coating of oxygen atoms prevents further attack © Boardworks Ltd 2001
Aluminium: Properties • Aluminium is a reactive metal. • We might expect it to corrode easily but a strong coating of oxide on it’s surface prevents this in most everyday situations. • It has a low density which leads to its extensive use in the aerospace industry. O OOO O OO Al Al Al Coating of oxygen atoms prevents further attack © Boardworks Ltd 2001
 Aluminium: Ores • It occurs as bauxite ore which is a form of aluminium oxide. • Because aluminium is so reactive carbon is unable to pull away the oxygen from it. • It is extracted by electrolysis of molten bauxite. Early attempts at this failed because bauxite is so hard to melt. • If cryolite is added the bauxite melts more easily. This is an essential step in the extraction process. © Boardworks Ltd 2001
Aluminium: Ores • It occurs as bauxite ore which is a form of aluminium oxide. • Because aluminium is so reactive carbon is unable to pull away the oxygen from it. • It is extracted by electrolysis of molten bauxite. Early attempts at this failed because bauxite is so hard to melt. • If cryolite is added the bauxite melts more easily. This is an essential step in the extraction process. © Boardworks Ltd 2001
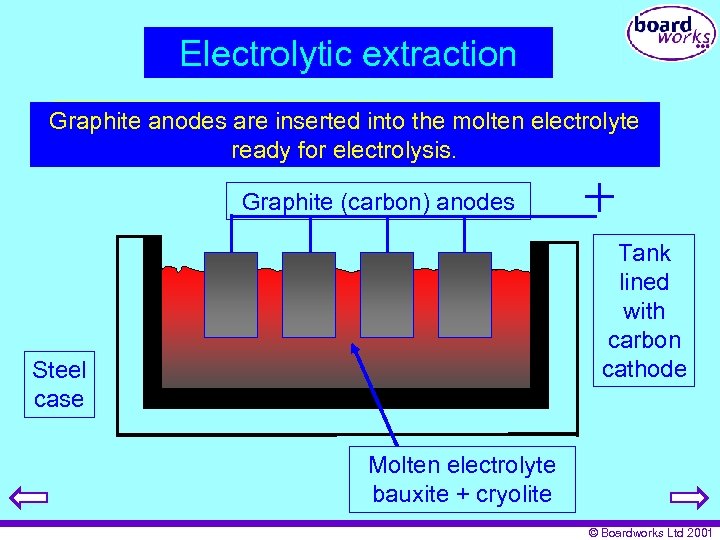 Electrolytic extraction A bauxite / are inserted into melted in steel Graphite anodescryolite mixture is the moltenaelectrolyte container containing a carbon lining. ready for electrolysis. Graphite (carbon) anodes Tank lined with carbon cathode Steel case Molten electrolyte bauxite + cryolite © Boardworks Ltd 2001
Electrolytic extraction A bauxite / are inserted into melted in steel Graphite anodescryolite mixture is the moltenaelectrolyte container containing a carbon lining. ready for electrolysis. Graphite (carbon) anodes Tank lined with carbon cathode Steel case Molten electrolyte bauxite + cryolite © Boardworks Ltd 2001
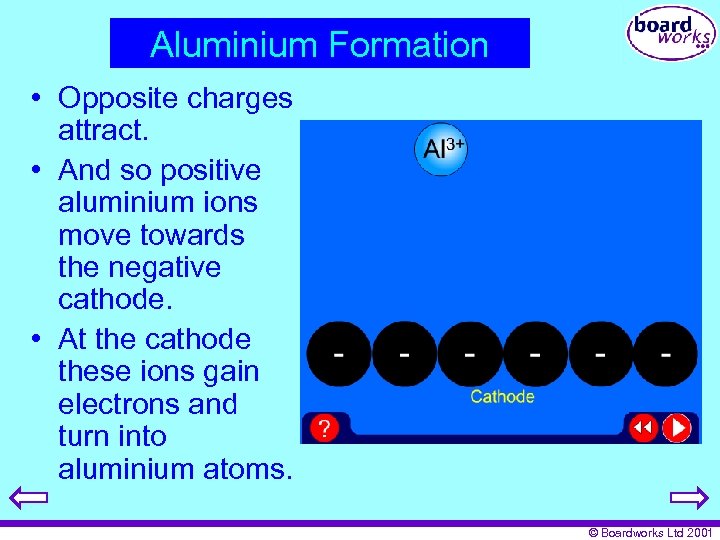 Aluminium Formation • Opposite charges attract. • And so positive aluminium ions move towards the negative cathode. • At the cathode these ions gain electrons and turn into aluminium atoms. © Boardworks Ltd 2001
Aluminium Formation • Opposite charges attract. • And so positive aluminium ions move towards the negative cathode. • At the cathode these ions gain electrons and turn into aluminium atoms. © Boardworks Ltd 2001
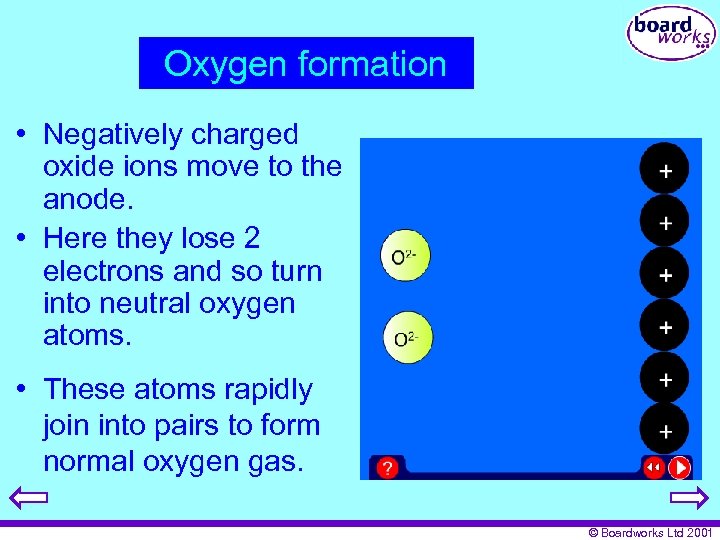 Oxygen formation • Negatively charged oxide ions move to the anode. • Here they lose 2 electrons and so turn into neutral oxygen atoms. • These atoms rapidly join into pairs to form normal oxygen gas. © Boardworks Ltd 2001
Oxygen formation • Negatively charged oxide ions move to the anode. • Here they lose 2 electrons and so turn into neutral oxygen atoms. • These atoms rapidly join into pairs to form normal oxygen gas. © Boardworks Ltd 2001
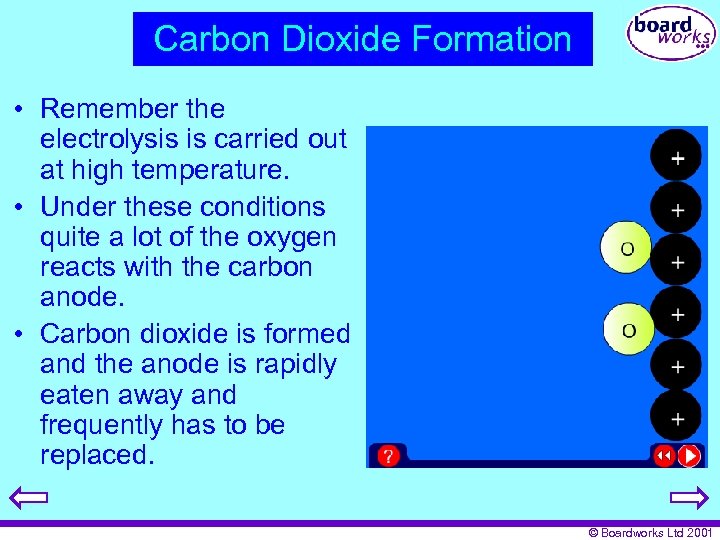 Carbon Dioxide Formation • Remember the electrolysis is carried out at high temperature. • Under these conditions quite a lot of the oxygen reacts with the carbon anode. • Carbon dioxide is formed and the anode is rapidly eaten away and frequently has to be replaced. © Boardworks Ltd 2001
Carbon Dioxide Formation • Remember the electrolysis is carried out at high temperature. • Under these conditions quite a lot of the oxygen reacts with the carbon anode. • Carbon dioxide is formed and the anode is rapidly eaten away and frequently has to be replaced. © Boardworks Ltd 2001
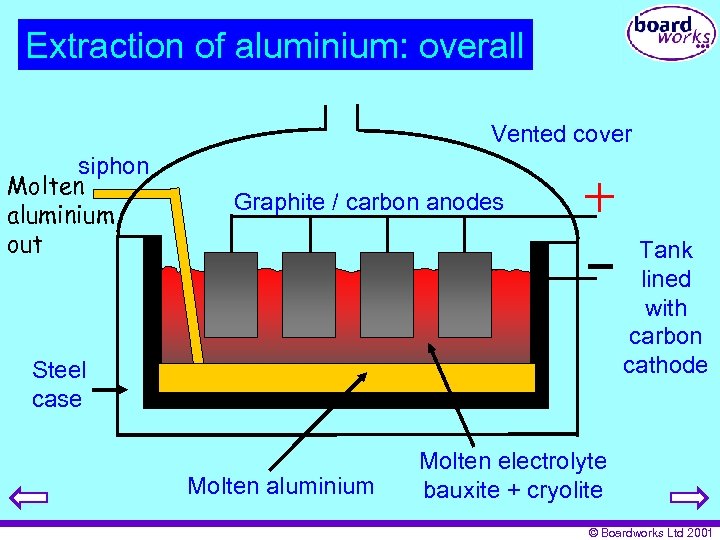 Extraction of aluminium: overall Vented cover siphon Molten aluminium out Graphite / carbon anodes Tank lined with carbon cathode Steel case Molten aluminium Molten electrolyte bauxite + cryolite © Boardworks Ltd 2001
Extraction of aluminium: overall Vented cover siphon Molten aluminium out Graphite / carbon anodes Tank lined with carbon cathode Steel case Molten aluminium Molten electrolyte bauxite + cryolite © Boardworks Ltd 2001
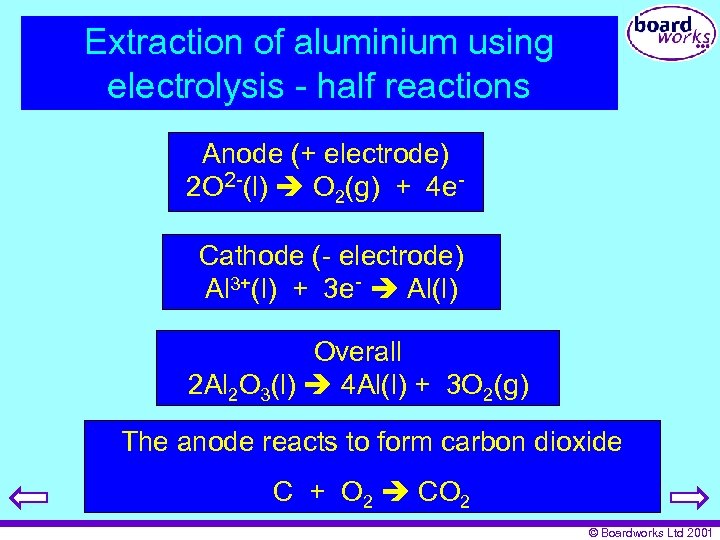 Extraction of aluminium using electrolysis - half reactions Anode (+ electrode) 2 O 2 -(l) O 2(g) + 4 e. Cathode (- electrode) Al 3+(l) + 3 e- Al(l) Overall 2 Al 2 O 3(l) 4 Al(l) + 3 O 2(g) The anode reacts to form carbon dioxide C + O 2 CO 2 © Boardworks Ltd 2001
Extraction of aluminium using electrolysis - half reactions Anode (+ electrode) 2 O 2 -(l) O 2(g) + 4 e. Cathode (- electrode) Al 3+(l) + 3 e- Al(l) Overall 2 Al 2 O 3(l) 4 Al(l) + 3 O 2(g) The anode reacts to form carbon dioxide C + O 2 CO 2 © Boardworks Ltd 2001
 Activity Unscramble the words to end the sentences • Common aluminium ore bauxite I axe tub • Added to reduce melting point cryolite City role • The electrodes are made out of Right ape graphite • Extracting aluminium is a reduction Cretin duo © Boardworks Ltd 2001
Activity Unscramble the words to end the sentences • Common aluminium ore bauxite I axe tub • Added to reduce melting point cryolite City role • The electrodes are made out of Right ape graphite • Extracting aluminium is a reduction Cretin duo © Boardworks Ltd 2001
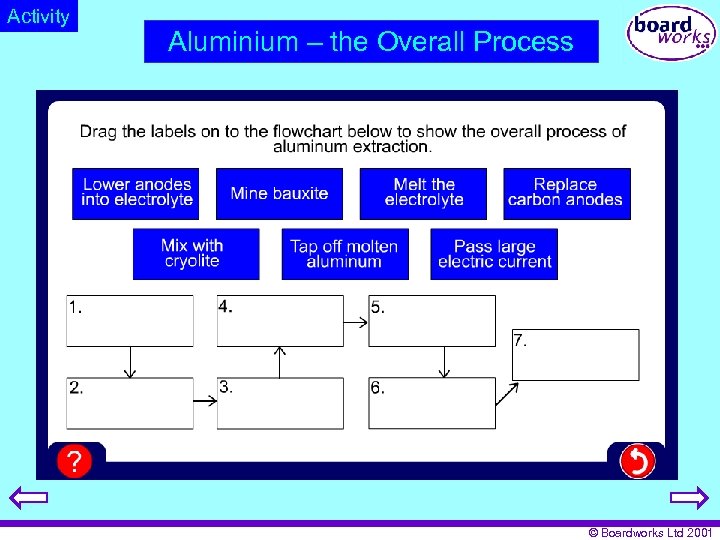 Activity Aluminium – the Overall Process © Boardworks Ltd 2001
Activity Aluminium – the Overall Process © Boardworks Ltd 2001
 A Rotten Week in the State of Chemark Since the war of 2042 world trade had been drastically reduced. Many countries have a policy of using home produced materials wherever possible because imported materials remain difficult to buy. The country of Chemark had done relatively well at using its own resources but supplies, even of home produced materials, can be unreliable. © Boardworks Ltd 2001
A Rotten Week in the State of Chemark Since the war of 2042 world trade had been drastically reduced. Many countries have a policy of using home produced materials wherever possible because imported materials remain difficult to buy. The country of Chemark had done relatively well at using its own resources but supplies, even of home produced materials, can be unreliable. © Boardworks Ltd 2001
 Chemsville • The major town of Chemsville, in the state of Chemark, has 2 main industries: -Chem. Cars – manufacturing expensive cars -Chem. Comm – engaged in communications products such as newspapers, books, CDs and DVDs • It also had an 80% completed aircraft factory: Chem. Jet -Chem. Jet has orders for 22 executive jets which they are committed to delivering to a tight schedule. • There also several large farms within the borders of Chemsville producing crops and meat. © Boardworks Ltd 2001
Chemsville • The major town of Chemsville, in the state of Chemark, has 2 main industries: -Chem. Cars – manufacturing expensive cars -Chem. Comm – engaged in communications products such as newspapers, books, CDs and DVDs • It also had an 80% completed aircraft factory: Chem. Jet -Chem. Jet has orders for 22 executive jets which they are committed to delivering to a tight schedule. • There also several large farms within the borders of Chemsville producing crops and meat. © Boardworks Ltd 2001
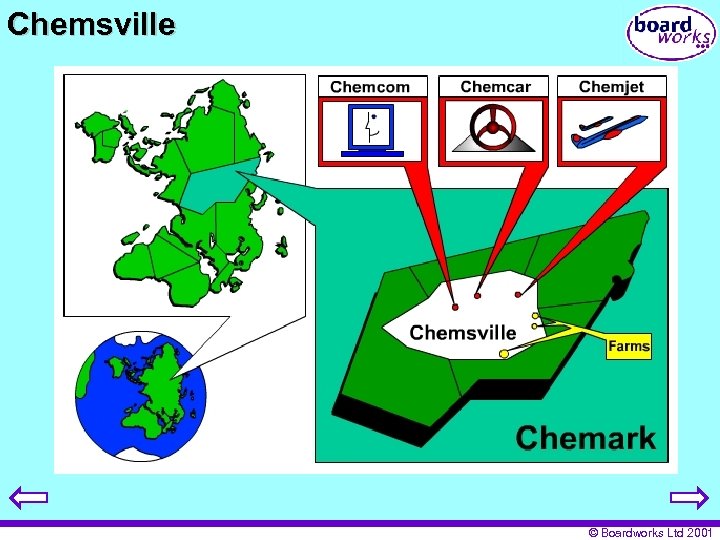 Chemsville © Boardworks Ltd 2001
Chemsville © Boardworks Ltd 2001
 Monday – Fire at Electro. Salt 1 • The week starts badly! The company that electrolyses salt in Chemark has had a major fire. The Electro. Salt factory will not open again for at least 3 months. • The mayor of Chemsville phones. She urgently wants you to produce a brief report setting out: – What rock salt is used for? – How the factory close-down may affect companies, farmers and non-industrial activities in Chemsville Draw up this report for the mayor. Include diagrams / flow charts of products from salt and suggest problems that the absence of these may cause for Chemsville companies. © Boardworks Ltd 2001
Monday – Fire at Electro. Salt 1 • The week starts badly! The company that electrolyses salt in Chemark has had a major fire. The Electro. Salt factory will not open again for at least 3 months. • The mayor of Chemsville phones. She urgently wants you to produce a brief report setting out: – What rock salt is used for? – How the factory close-down may affect companies, farmers and non-industrial activities in Chemsville Draw up this report for the mayor. Include diagrams / flow charts of products from salt and suggest problems that the absence of these may cause for Chemsville companies. © Boardworks Ltd 2001
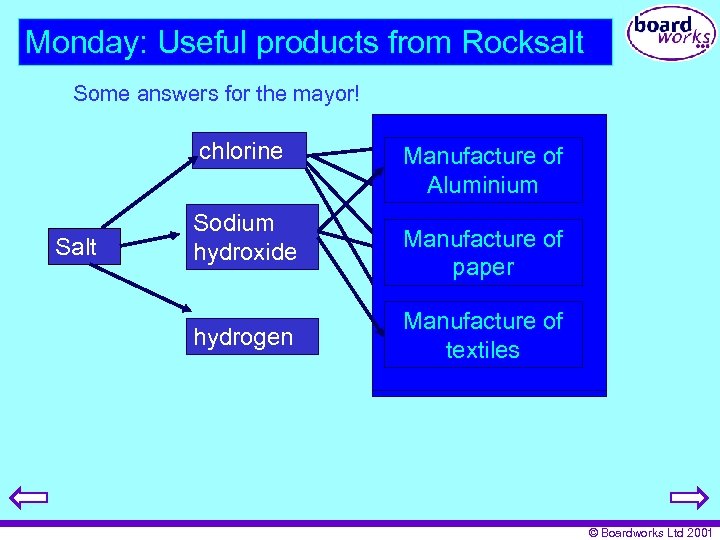 Monday: Useful products from Rocksalt Some answers for the mayor! chlorine Salt Sodium hydroxide hydrogen p. v. c. Manufacture of Aluminium Water treatment Manufacture of paper Paper bleaching Manufacture of textiles pesticides © Boardworks Ltd 2001
Monday: Useful products from Rocksalt Some answers for the mayor! chlorine Salt Sodium hydroxide hydrogen p. v. c. Manufacture of Aluminium Water treatment Manufacture of paper Paper bleaching Manufacture of textiles pesticides © Boardworks Ltd 2001
 Monday: Answers about Rocksalt Chem. Car & Chem. Jet Plastic shortages due to lack of chlorine. (Needed for wire insulation and plastic components. ) Textiles shortages (for seat covers etc) due to lack of sodium hydroxide. Aluminium shortage due to shortage of sodium hydroxide. Major problem for Chem. Car and dire problem for Chem. Jet © Boardworks Ltd 2001
Monday: Answers about Rocksalt Chem. Car & Chem. Jet Plastic shortages due to lack of chlorine. (Needed for wire insulation and plastic components. ) Textiles shortages (for seat covers etc) due to lack of sodium hydroxide. Aluminium shortage due to shortage of sodium hydroxide. Major problem for Chem. Car and dire problem for Chem. Jet © Boardworks Ltd 2001
 Monday: Answers about Rocksalt Chem. Com Paper shortages due to lack of chlorine and sodium hydroxide Plastic shortage needed to make CDs and DVDs © Boardworks Ltd 2001
Monday: Answers about Rocksalt Chem. Com Paper shortages due to lack of chlorine and sodium hydroxide Plastic shortage needed to make CDs and DVDs © Boardworks Ltd 2001
 Monday: Useful products from Rocksalt Farmers Shortage of insecticide. Others Lack of chlorine for water treatment – health risks if untreated water © Boardworks Ltd 2001
Monday: Useful products from Rocksalt Farmers Shortage of insecticide. Others Lack of chlorine for water treatment – health risks if untreated water © Boardworks Ltd 2001
 Wednesday: Price Rises by Electro. Gen • Electro. Salt had been one of the major customers of the only Electricity company. • Loss of Electro. Salt’s custom has taken Electro. Gen into financial problems and they announce an emergency price rise of 50% for electricity. • The mayor phones. What effect will this have upon the cost of products made in Chemsville? Draw up a report for the mayor. What materials used in existing factories and in the new aircraft factory may involve major quantities of electricity? Can it’s use be avoided? © Boardworks Ltd 2001
Wednesday: Price Rises by Electro. Gen • Electro. Salt had been one of the major customers of the only Electricity company. • Loss of Electro. Salt’s custom has taken Electro. Gen into financial problems and they announce an emergency price rise of 50% for electricity. • The mayor phones. What effect will this have upon the cost of products made in Chemsville? Draw up a report for the mayor. What materials used in existing factories and in the new aircraft factory may involve major quantities of electricity? Can it’s use be avoided? © Boardworks Ltd 2001
 Wednesday: Report about Electro. Gen • Obviously there are normal running costs in all of the organisations but two vital products will be hit by the price rise and are difficult to get replace. Copper – for wiring of cars and aircraft. (Copper is refined by electrolysis which uses large amounts of electrical power. ) Aluminium – for use in cars and especially in aircraft. (Aluminium is extracted by electrolysis of molten bauxite. ) © Boardworks Ltd 2001
Wednesday: Report about Electro. Gen • Obviously there are normal running costs in all of the organisations but two vital products will be hit by the price rise and are difficult to get replace. Copper – for wiring of cars and aircraft. (Copper is refined by electrolysis which uses large amounts of electrical power. ) Aluminium – for use in cars and especially in aircraft. (Aluminium is extracted by electrolysis of molten bauxite. ) © Boardworks Ltd 2001
 Friday: Limestone shortage • It seemed things could only get better - wrong! • The director of the unfinished aircraft factory phones saying the company providing cement has been unable to get supplies because of a strike at the limestone quarry. • The aircraft factory must be finished on time. – The director suggests using an old limestone wall and clay from the foundations of the new factory to make enough cement to finish the factory off. Is this feasible / sensible? Make recommendations on how to get the cement. Who else in Chemsville may be affected by a limestone shortage? © Boardworks Ltd 2001
Friday: Limestone shortage • It seemed things could only get better - wrong! • The director of the unfinished aircraft factory phones saying the company providing cement has been unable to get supplies because of a strike at the limestone quarry. • The aircraft factory must be finished on time. – The director suggests using an old limestone wall and clay from the foundations of the new factory to make enough cement to finish the factory off. Is this feasible / sensible? Make recommendations on how to get the cement. Who else in Chemsville may be affected by a limestone shortage? © Boardworks Ltd 2001
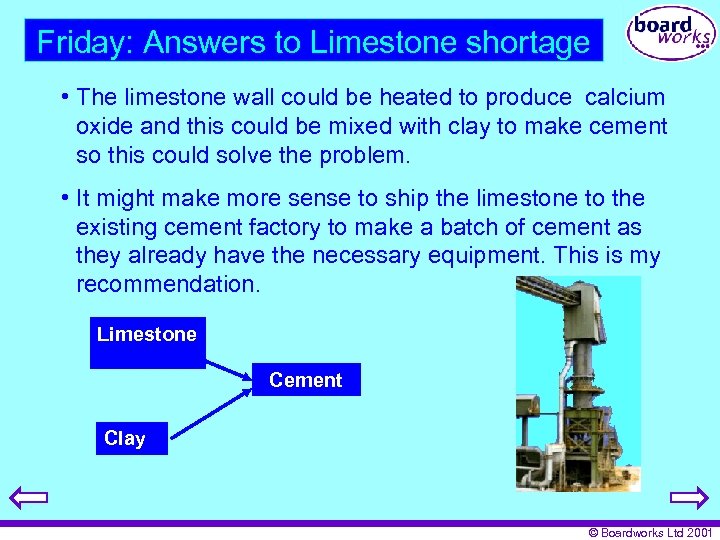 Friday: Answers to Limestone shortage • The limestone wall could be heated to produce calcium oxide and this could be mixed with clay to make cement so this could solve the problem. • It might make more sense to ship the limestone to the existing cement factory to make a batch of cement as they already have the necessary equipment. This is my recommendation. Limestone Cement Clay © Boardworks Ltd 2001
Friday: Answers to Limestone shortage • The limestone wall could be heated to produce calcium oxide and this could be mixed with clay to make cement so this could solve the problem. • It might make more sense to ship the limestone to the existing cement factory to make a batch of cement as they already have the necessary equipment. This is my recommendation. Limestone Cement Clay © Boardworks Ltd 2001
 Friday: Answers to Limestone shortage The building of the Chem. Jet factory is not the only thing that will be affected by a limestone shortage. The strike will affect manufacture of iron from which steel is made. This could have major effects on Chem. Car as many car components are made of steel. In the longer term it will also affect farmers who use limestone to neutralise their soil. © Boardworks Ltd 2001
Friday: Answers to Limestone shortage The building of the Chem. Jet factory is not the only thing that will be affected by a limestone shortage. The strike will affect manufacture of iron from which steel is made. This could have major effects on Chem. Car as many car components are made of steel. In the longer term it will also affect farmers who use limestone to neutralise their soil. © Boardworks Ltd 2001
 1. Which of the following metals is most likely to occur native? A. B. C. D. Sodium Zinc Iron Gold © Boardworks Ltd 2001
1. Which of the following metals is most likely to occur native? A. B. C. D. Sodium Zinc Iron Gold © Boardworks Ltd 2001
 2. Which of the following metals has to be extracted by electrolysis? A. B. C. D. Sodium Zinc Iron Gold © Boardworks Ltd 2001
2. Which of the following metals has to be extracted by electrolysis? A. B. C. D. Sodium Zinc Iron Gold © Boardworks Ltd 2001
 3. Which of these happens in the purification of copper? A. Copper cathode dissolves B. Copper anode gets thicker C. Copper atoms become ions at the cathode D. Copper ions become atoms by gaining electrons. © Boardworks Ltd 2001
3. Which of these happens in the purification of copper? A. Copper cathode dissolves B. Copper anode gets thicker C. Copper atoms become ions at the cathode D. Copper ions become atoms by gaining electrons. © Boardworks Ltd 2001
 4. Which of these happens in the extraction of iron? A. Carbon oxidises the iron oxide B. Combustion of carbon provides the energy for the extraction process. C. Carbon monoxide reacts with acidic impurities in the iron ore. D. The waste gas is mainly carbon monoxide © Boardworks Ltd 2001
4. Which of these happens in the extraction of iron? A. Carbon oxidises the iron oxide B. Combustion of carbon provides the energy for the extraction process. C. Carbon monoxide reacts with acidic impurities in the iron ore. D. The waste gas is mainly carbon monoxide © Boardworks Ltd 2001


